In 2006, research on the neurotransmitter serotonin and its transporter protein (5-HTT) in the synaptic gap celebrated a series of anniversaries. Forty-five years earlier, presynaptic neurotransmitter uptake was discovered (Hertting & Axelrod, 1961). Two decades later, 5-HTT was first linked to depression (Langer et al, 1981), shortly after its identification as a target of antidepressant drugs (Raisman et al, 1979). The sequence of the transporter gene from rats was published 10 years after that (Blakely et al, 1991), which initiated an avalanche of molecular genetic studies on the regulation of emotionality. This research culminated in two reports revealing an association between variations of the 5-HTT gene (5-HTT and SLC6A4) and anxiety-related traits as well as depression (Collier et al, 1996; Lesch et al, 1996).
Since then, clinical studies have further supported the link between variants of 5-HTT and disorders in the regulation of emotion. Although modest effect sizes—typical of non-Mendelian traits—polygenic patterns of inheritance, epistatic and epigenetic interactions, and heterogeneity between studies confounded the results, 5-HTT comprises a model molecule for studying gene–environment interactions in cognitive and psychiatric neuroscience.
The demonstration in rhesus macaques that stress early in life uniquely reinforces links between variations of 5-HTT, behaviour and psychopathology seems to herald in a new era of behavioural genetics. Moreover, the discovery that 5-HTT is a susceptibility gene for depression is a first step towards explaining the molecular dimensions of personality and behaviour, identifying physiological pathways that lead to other disorders of cognitive function and emotion, and analysing the interactive effects of genes and environment in the development of disease.
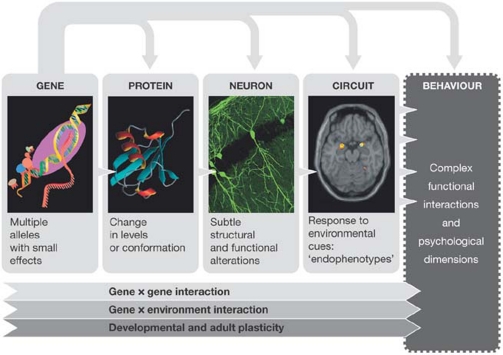
On the heels of these results from behavioural genetics, novel approaches including neurophysiology, neuropsychology and functional neuroimaging, as well as the inclusion of other phenotypes (such as higher cognitive functions, communication skills, social competence and longevity), have further strengthened the connection between 5-HTT, cognition and emotionality, and contribute to a more profound understanding of how genetic variations modulate behaviour (Fig 1). Similarly, studies in transgenic mice have underscored the central role of serotonin and its fine-tuning by the transporter protein in embryonic brain development and synaptic plasticity, particularly in brain areas related to cognitive and emotional processes.
Figure 1.
Different levels of complexity: how genes influence behaviour and psychopathological dimensions.
The potential relevance of this research for social cognition—the neurobiological processes that are used to conform with social norms and procedures—transcends the boundaries of behavioural genetics to embrace biosocial science. Steering research in this new direction raises it to the next level of complexity, with the aim of understanding the nature of these genetic variations in humans, as well as their influence on individual differences and social competence and behaviour.
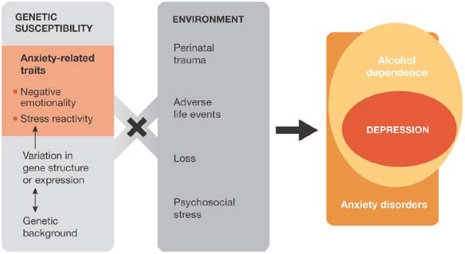
Individual differences in behavioural predisposition, which are commonly referred to as ‘personality traits', are relatively enduring, continuously distributed and substantially inheritable. Although personality results from a complex interplay of genetic and environmental factors, their relative influence is the subject of one of the longest and most contentious controversies in scientific history (McGue & Bouchard, 1998). There is now considerable evidence that personality traits (such as fearfulness, negative emotionality and stress reactivity) and environmental factors increase the risk of depression—an aetiologically heterogeneous group of brain disorders that reflect alterations in cognitive, psychomotor and emotional processes (Fig 2). Because the mode of inheritance of depression is complex, it is increasingly accepted that susceptibility is due to several genes in association with each other and in conjunction with epigenetic programming. Genes implicated in the control of cognition, emotionality and despair, and with a role in monoamine neurotransmission, such as the serotonin system, have been a rational starting point for understanding the molecular basis of this disorder.
Figure 2.
Vulnerability and co-morbidity of depression: neither genes nor environment act alone.
Serotonin is a phylogenetically ancient signalling molecule. It is the most widely distributed neurotransmitter in the brain and is implicated in the regulation of emotions. The neurological circuitry of emotionality involves numerous brain structures, including the anterior cingulate cortex, amygdala, hippocampus, ventral striatum, hypothalamus and several other interconnected structures. The amygdala is the central unit where cognition, emotion and learning are processed, and it associates such stimuli with events that are either punishing or rewarding (Phelps, 2006). Serotonergic signalling pathways integrate not only basic physiological functions, but also elementary tasks of sensory processing, cognition, emotion regulation and motor activity.
Moreover, serotonin is an important regulator of early brain development and adult neuroplasticity, including cell proliferation, migration, differentiation and synaptogenesis (Di Pino et al, 2004). As it shapes various brain systems during development, it sets the stage for functions that regulate emotions throughout life. It is also a crucial modulator of emotional behaviour including anxiety and stress responses, impulsivity and aggression. The diversity of these functions is due to the capacity of serotonin to orchestrate the activity and interaction of several other neurotransmitter systems. It can therefore be viewed as a ‘team player' within this highly complex system of neural communication, which is mediated by 14 receptor subtypes.
The action of serotonin as a messenger in the brain is regulated tightly by its synthesizing and metabolizing enzymes, and, more directly, by 5-HTT, which regulates the concentration of serotonin in the synaptic gap and therefore its effect on the receiving neuron. It is likely that variations in the transporter protein itself have a profound effect on the role of serotonin as a moderator of cognition and emotionality.
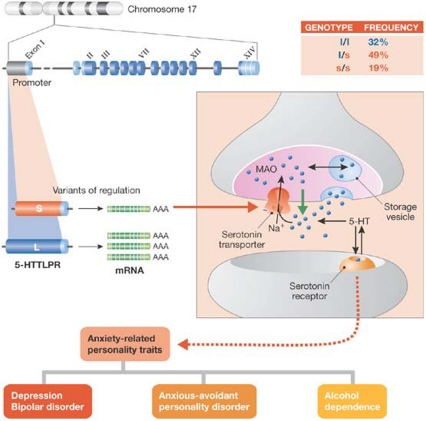
The transcriptional activity of human 5-HTT is modulated by a repetitive sequence, which has a short (s) and a long (l) variant (Fig 3). This sequence is unique to humans and simian primates; hence, the different activity of the two variants in rhesus macaques provides a unique model to study the relative contribution of genes and environmental factors to the function of serotonin, personality traits and disorders of emotion regulation. Initial reports associated the low-expressing s-variant with significantly greater levels of neuroticism—a trait related to anxiety, depression and stress reactivity (Lesch et al, 1996). The role of the s-variant in personality traits of negative emotionality was finally confirmed by meta-analyses of the published data.
Although personality results from a complex interplay of genetic and environmental factors, their relative influence is the subject of one of the longest and most contentious controversies in scientific history
Figure 3.
Allelic variation of serotonin transporter function in anxiety-related personality disorders, depression and other disorders of the regulation of emotion. LPR, linked polymorphic region; MAO, monoamine oxidase.
An association between 5-HTT and depression was first reported by Collier and co-workers (1996), and was subsequently confirmed by other studies. Similarly, the s-variant and l-variant of 5-HTT seem to have a role in a range of disorders related to regulation of emotion. Of particular interest is the role of 5-HTT in autism, which comprises a spectrum of deficits in language development and social cognition, repetitive and restricted behaviours or interests, and resistance to change. The most promising findings so far, however, indicate an association between both variants of 5-HTT and responsiveness to antidepressant treatments (Lesch & Gutknecht, 2005).
Biology has had an increasing influence on the social sciences, and biosocial concepts have paved the way for investigating human personality from an evolutionary perspective (Buss, 1991). Personality dimensions define the ability of an individual to adapt to other people in a given social context, which is crucial for long-term reproductive success. Extraversion and agreeableness allow higher mammals to form social structures ranging from pair bonds to coalitions of groups; emotional stability and conscientiousness are crucial for the maintenance of these structures, whereas openness might reflect a capacity for innovation. From an evolutionary psychology perspective, anxiety is a pervasive and innately driven form of distress that arises in response to actual or threatened exclusion from social groups (Baumeister & Tice, 1990). On the basis of this idea, Nakamura and colleagues (1997) discussed the higher prevalence of the anxiety-associated s-variant of 5-HTT among the Japanese population as a possible population-typical adaptation to prevent social exclusion in a society that is characterized by extraordinary emotional restraint and interpersonal sensitivity.
Because the genetic basis of personality and behavioural traits exists in mammals ranging from rodents to nonhuman primates, research efforts have recently focused on the latter, especially rhesus macaque monkeys. Using this model, environmental influences can be more easily controlled, and are therefore less likely to confound associations between genes and behaviour.
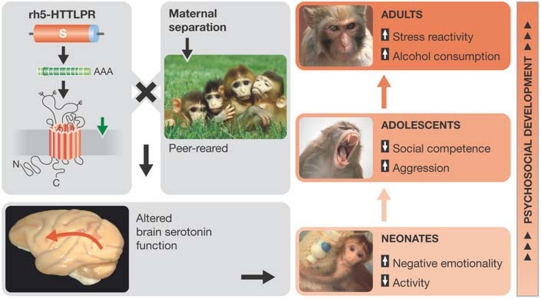
The stressful experience of maternal separation has robust and long-term consequences for the serotonin system in rhesus monkeys, as indicated by low cerebrospinal fluid (CSF) concentrations of 5-hydroxyindoleacetic acid (5-HIAA), which is the main metabolite of serotonin. This early-life trauma also causes anxiety-related and depression-related behaviours associated with deficient social adaptation and interaction throughout life. Given that rhesus monkeys also have s-variants and l-variants of 5-HTT, which is called rh5-HTT (Lesch et al, 1997), they were tested for associations between early-life stress, serotonin function and rh5-HTT genotype.
The low-activity s-variant of rh5-HTT is, indeed, predictive of CSF 5-HIAA concentrations, but early-life experiences also influence the serotonin system (Fig 4; Bennett et al, 2002). In the neonatal period—between 7 and 30 days after birth—when environmental influences are modest and least likely to confound gene–behaviour associations, infants carrying the s-variant of rh5-HTT displayed higher stress-reactivity than l-variant homozygotes (Champoux et al, 2002). The effects were more pronounced for monkeys raised in a neonatal nursery than for those reared by their mothers, which confirmed the role of the environment.
Figure 4.
Effects of maternal separation and rh5-HTT genotype on psychosocial development, including brain serotonin function, regulation of emotion, social competence, stress reactivity, behaviour and psychopathology.
Beyond the neonatal period, particularly during adolescence, further evidence for a complex interplay between the serotonin system and social success has been accrued. Impaired serotonin signalling is associated with lower rank within a social group, less competent social behaviour and greater impulsive aggression. Although subjects with low CSF 5-HIAA concentrations are no more likely to engage in competitive aggression than other monkeys, their aggressive behaviour frequently escalates to violent and hazardous intensity.
Social interaction and competition-elicited aggression are moderated by an interactive effect of the rh5-HTT genotype and the rearing environment (Barr et al, 2003). Infants that are homozygous for the l-variant are more likely to engage in rough play than carriers of the s-variant. Peer-reared s-variant carriers play less with their peers than those that are homozygous for the l-allele, whereas the rh5-HTT genotype has no effect on the incidence of social play among mother-reared monkeys. Socially dominant mother-reared l-variant carriers are more likely than their peer-reared counterparts to engage in aggression during adolescence, whereas peer-reared, but not mother-reared, monkeys carrying the s-variant show more aggressive behaviours than their homozygous l-variant counterparts. These findings indicate that peer-reared subjects with the s-variant, although unlikely to win in a competitive encounter, are more inclined to persist in aggression once it begins.
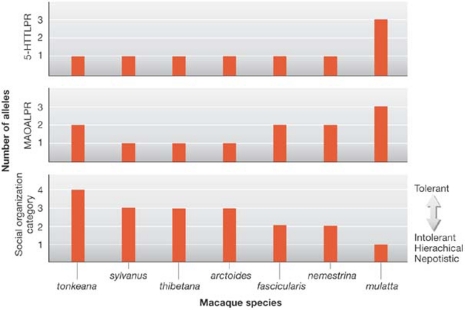
For macaques, similar to humans, survival depends on effective social functioning. Social skills facilitate access to food, protection and mates. Socially adept individuals tend to be healthier and live longer. Rhesus macaques also exhibit a variation of aggression-related behaviour that is unequalled in other primate species. On the basis of assumptions that the structure of macaque social organization is influenced more by phylogeny than by environment, and that polymorphisms in genes of the serotonin signalling pathway contribute to the variability of species-characteristic social behaviour, the variation of genes involved in the serotonin signalling pathway was determined in seven macaque species representing the entire spectrum of different social organization grades (Fig 5; Wendland et al, 2006). Macaque species with tolerant societies, relaxed dominance and high levels of conciliation were monomorphic for the rh5-HTT gene. By contrast, species that are known to have intolerant, hierarchical and nepotistic societies were polymorphic at one or more loci. The rhesus macaque, which is the most intolerant and hierarchical species, showed the greatest degree of allelic variation. These findings suggest that genetic variation of serotonin neurotransmission affects key elements of macaque social behaviour, particularly the exceptional level of interspecies variation in aggression.
Figure 5.
Degree of variation in two genes of the serotonin signalling pathway (the serotonin transporter and monoamine oxidase A), aggression and social organization in seven macaque species. This four-grade classification of social organization across several species of the genus Macaca is based on the extent and asymmetry of aggression-related behaviour within particular species (Thierry, 2000). Grade 1 species have hierarchical and nepotistic societies, as well as low levels of conciliation, whereas grade 4 species can be considered as more tolerant, displaying relaxed dominance, open relationships and high levels of conciliatory behaviour. For example, the risk of a retaliatory attack from a subordinate after an initial attack by a dominant is much higher in grade 4 species than in grade 1 species, although the retaliatory attack will probably be less severe. LPR, linked polymorphic region; MAOA, monoamine oxidase.
Taken together, the research on macaques provides evidence of an environment-dependent association between allelic variation of rh5-HTT expression and central serotonin function, and illustrates how this specific genetic variation moderates behaviour and psychosocial development (Fig 4). Because rhesus monkeys show personality and behavioural traits that parallel anxiety, depression and aggression associated with the low-activity s-variant of the human 5-HTT gene, this primate model facilitates the search for genetic mechanisms of individual differences. Indeed, the corollary of deleterious early experiences, such as inadequate maternal care in humans, seems consistent with the idea that 5-HTT influences the risk of disorders of social cognition and emotion regulation. Analogous to the findings in primates, humans with one or two low-activity s-variants of the 5-HTT gene are more likely to become depressed after adverse life events (Caspi et al, 2003). Moreover, childhood maltreatment of individuals carrying the s-allele increases the risk of depression later in life.
Serotonergic signalling pathways integrate not only basic physiological functions, but also elementary tasks of sensory processing, cognition, emotion regulation and motor activity
Biology has had an increasing influence on the social sciences, and biosocial concepts paved the way for investigating human personality from an evolutionary perspective
During the past few years, imaging techniques have become increasingly sophisticated (Fig 1), leading to the first report of an association between 5-HTT genotype and excitability of the prefrontal cortex, which implies a relationship between cognitive processing and 5-HTT function in humans (Fallgatter et al, 1999). Using functional magnetic resonance imaging (fMRI), Hariri and co-workers observed that carriers of the s-variant have greater neuronal activity of the amygdala in response to fearful stimuli (Hariri et al, 2002). Subsequent investigations focused on the modifying impact of the anterior cingulate cortex in the context of the role of 5-HTT in depression, but did not question the assumption that the neutral baseline used as the control did not itself produce changes in activation as a function of 5-HTT genotype.
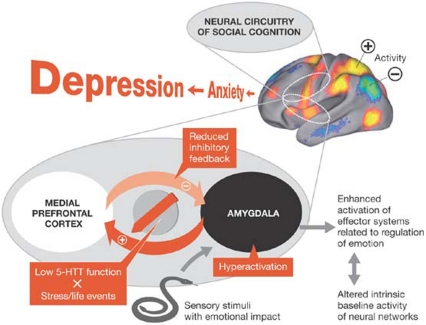
In tackling this problem, Canli and co-workers showed that allelic variations of 5-HTT are associated with differential activation in response to negative, positive and neutral stimuli in limbic, striatal and cortical regions (Canli et al, 2005). Although they confirmed greater amygdala activation to negative stimuli as compared with neutral stimuli in carriers of the s-variant of 5-HTT, this was due to decreased activation in response to neutral stimuli rather than increased activation in response to negative stimuli. These findings are consistent with the idea that the anterior cingulate cortex has an integral role in both emotional and non-emotional cognitive processes, and implies a role of serotonin transport efficiency in a wide-ranging spectrum of brain processes that control affective, cognitive and motor processes (Fig 6; Canli et al, 2006).
Figure 6.
Influence of the interaction between the 5-HTT gene and the environment on neural networks of social cognition and emotion regulation.
Although 5-HTT influences the impact of psychosocial stress on depression risk, the neural mechanisms underlying this moderator effect are still poorly understood. As a first step towards identifying these mechanisms, individuals with self-reported life stress but no history of psychopathology were investigated using multimodal fMRI. On the basis of these and other data, Canli and co-workers suggested a model in which life stress interacts with the 5-HTT genotype to influence amygdala and hippocampal resting activation, which might provoke a chronic state of negative cognitive bias (Canli et al, 2006). Although these interactions might constitute a neural mechanism for epigenetic vulnerability to depression, additional studies revealed other brain regions that are moderated by an interaction of 5-HTT genotype and life stress.
Intriguingly, these regions belong to circuits that integrate imitation-related behaviour, from which social cognition and behaviour have evolved (Iacoboni, 2005). Social cognition comprises representations of internal somatic states, interpersonal knowledge and motivations, and procedures used to decode and encode one's self in relation to other people. This complex set of processes, which must be carefully orchestrated to enable social functioning and communication-facilitated networking, has recently been associated with distinct neurocircuits in the brain. These findings might imply that social competence is subject to an interaction between 5-HTT genotype and psychosocial stress; dysfunction is thought to cause social and communication disabilities associated with autistic syndromes.
A mouse model with an inactivated 5-HTT gene—and consequently a disturbed serotonin system—has considerably advanced our understanding of the neurobiological basis of anxiety-related and depression-related behaviour (Bengel et al, 1998; Lesch & Mössner, 2006). In addition, the 5-HTT-knockout mouse provides a model to study the impact of genetic mechanisms on the development and plasticity of the brain, including regionalization of the cortex and its connectivity to subcortical structures (Di Pino et al, 2004). However, our knowledge of the machinery involved in these fine-tuning processes remains fragmentary. Finally, epigenetic programming of emotionality by maternal behaviour underscores the view that environmental influences can persistently remodel neuronal units during early development.
…the potential impact of 5-HTT variation on social cognition transcends the boundaries of behavioural genetics to embrace biosocial science and create a new social neurogenetics of behaviour
We are clearly a long way from fully understanding the evolutionary and neural mechanisms of social cognitive phenomena and social functioning. But the potential impact of 5-HTT variation on social cognition transcends the boundaries of behavioural genetics to embrace biosocial science and create a new social neurogenetics of behaviour. It has transformed biosocial science with respect to our understanding of gender-specific behaviour, marriage and the family as social institutions, freedom of will and legal responsibility, and the natural history of moral obligation (Butz & Torrey, 2006). Biosocial genomics now allows us to explore the nature of genetic variation between humans and its influence on individual differences, as well as the relative impact of genetic and environmental factors on cognition, emotion and behaviour. Its integration in behavioural genetics will eventually tear down some long-standing myths about the uniqueness of human behaviour. Although the impact of neurogenetics on social sciences has long been anticipated and represents an inevitable—albeit welcome—development, the transition from complicated correlations to useful predictions will be a challenge.
References
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD (2003) The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav 2: 336–340 [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Tice DM (1990) Anxiety and social exclusion. J Soc Clin Psychol 9: 165–195 [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53: 649–655 [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD (2002) Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry 7: 118–122 [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT Jr, Caron MG, Peek MM, Prince HK, Bradley CC (1991) Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66–70 [DOI] [PubMed] [Google Scholar]
- Buss DM (1991) Evolutionary personality psychology. Annu Rev Psychol 42: 459–491 [DOI] [PubMed] [Google Scholar]
- Butz WP, Torrey BB (2006) Some frontiers in social science. Science 312: 1898–1900 [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP (2005) Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci USA 102: 12224–12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP (2006) Neural correlates of epigenesis. Proc Natl Acad Sci USA 103: 16033–16038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A et al. (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389 [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ (2002) Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry 7: 1058–1063 [DOI] [PubMed] [Google Scholar]
- Collier DA et al. (1996) A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry 1: 453–460 [PubMed] [Google Scholar]
- Di Pino G, Moessner R, Lesch KP, Lauder JM, Persico AM (2004) Roles for serotonin in neurodevelopment: more than just neural transmission. Curr Neuropharmacol 2: 403–417 [Google Scholar]
- Fallgatter A, Jatzke S, Bartsch A, Hamelbeck B, Lesch K (1999) Serotonin transporter promoter polymorphism influences topography of inhibitory motor control. Int J Neuropsychopharm 2: 115–120 [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR (2002) Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400–403 [DOI] [PubMed] [Google Scholar]
- Hertting G, Axelrod J (1961) Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature 192: 172–173 [DOI] [PubMed] [Google Scholar]
- Iacoboni M (2005) Neural mechanisms of imitation. Curr Opin Neurobiol 15: 632–637 [DOI] [PubMed] [Google Scholar]
- Langer SZ, Zarifian E, Briley M, Raisman R, Sechter D (1981) High-affinity binding of 3H-imipramine in brain and platelets and its relevance to the biochemistry of affective disorders. Life Sci 29: 211–220 [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L (2005) Pharmacogenetics of the serotonin transporter. Prog Neuropsychopharmacol Biol Psychiatry 29: 1062–1073 [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mössner R (2006) Inactivation of 5HT transport in mice: modeling altered 5HT homeostasis implicated in emotional dysfunction, affective disorders, and somatic syndromes. Handb Exp Pharmacol 2006: 417–456 [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531 [DOI] [PubMed] [Google Scholar]
- Lesch KP et al. (1997) The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. J Neural Transm 104: 1259–1266 [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ Jr (1998) Genetic and environmental influences on human behavioral differences. Annu Rev Neurosci 21: 1–24 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Muramatsu T, Ono Y, Matsushita S, Higuchi S, Mizushima H, Yoshimura K, Kanba S, Asai M (1997) Serotonin transporter gene regulatory region polymorphism and anxiety-related traits in the Japanese. Am J Med Genet 74: 544–545 [DOI] [PubMed] [Google Scholar]
- Phelps EA (2006) Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 57: 27–53 [DOI] [PubMed] [Google Scholar]
- Raisman R, Briley M, Langer SZ (1979) Specific tricyclic antidepressant binding sites in rat brain. Nature 281: 148–150 [DOI] [PubMed] [Google Scholar]
- Thierry B (2000) Covariation of conflict management patterns across macaque species. In Aureli F, de Waal FBM (eds), Natural Conflict Resolution, pp 106–128. Berkeley, CA, USA: University of California Press [Google Scholar]
- Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ (2006) Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet 36: 163–172 [DOI] [PubMed] [Google Scholar]



 Klaus-Peter Lesch is at the Department of Psychiatry and Psychotherapy, Molecular and Clinical Psychobiology at the University of Würzburg, Germany.E-mail:
Klaus-Peter Lesch is at the Department of Psychiatry and Psychotherapy, Molecular and Clinical Psychobiology at the University of Würzburg, Germany.E-mail: