Abstract
Identification of optimal antigen(s) and adjuvant combination(s) to elicit potent, protective, and long-lasting immunity has been a major challenge for the development of effective vaccines against chronic viral pathogens, such as HIV-1, for which there are not yet any licensed vaccines. Here we describe the use of a novel adjuvant approach employing Carbopol 971P® NF (hereafter referred to as Carbopol971P), a cross-linked polyanionic carbomer, in combination with the Novartis proprietary oil-in-water adjuvant, MF59, as a potentially safe and effective adjuvant to augment humoral immune responses to the HIV-1 envelope glycoprotein (Env). Intramuscular immunization of small animals with recombinant Env glycoprotein (gp140) formulated in Carbopol971P plus MF59 gave significantly higher titers of binding and virus neutralizing antibodies as compared to immunization using gp140 with either MF59 or Carbopol971P alone. In addition, the antibodies generated were of higher avidity. Importantly, the use of Carbopol971P plus MF59 did not cause any serious adverse reactions or any obvious health problems in animals upon intramuscular administration. Hence, the Carbopol971P plus MF59 adjuvant formulation may provide a benefit for future vaccine applications.
Keywords: HIV, SF162, gp140, adjuvant, Carbopol, MF59
INTRODUCTION
The viral envelope (Env) glycoprotein of the Human Immunodeficiency Virus type 1 (HIV-1) is the major target for antibody-based vaccine approaches in the quest for a successful vaccine against the virus. Env glycoproteins formulated in various adjuvants have been tested in animals and humans with modest success [1–6]. Although, identification of an optimally immunogenic form of the Env glycoprotein is likely to be important in eliciting broadly protective humoral responses, this is, in itself, unlikely to be sufficient without the identification of optimally immunogenic and safe vaccine adjuvant(s) and regimen(s). The recent demonstration of vaccine efficacy in the RV144 Phase 3 HIV-1 vaccine trial in Thailand [7] using a viral vector prime-boost regimen, as compared to the lack of efficacy observed in the Vax003/004 Phase 3 trials [5, 8] with similar Env glycoproteins in relatively weakly immunogenic alum formulations, highlights the potential impact of vaccine regimen on vaccine efficacy.
In recent years, the field of adjuvants has seen some new regulatory approvals following clinical success of a few novel adjuvants [9]. How these adjuvants interact with and affect the innate and adaptive arms of the immune response is actively under investigation in several laboratories. It is interesting to note that the in vivo mechanisms of action of alum, the oldest licensed adjuvant, and MF59, an adjuvant that has been licensed for 13 years in Novartis’ FLUAD® influenza vaccine, are just now being elucidated [10–15].
MF59, an oil-in-water emulsion, is a safe and potent vaccine adjuvant [16–21]. Currently, the only approved MF59-adjuvanted vaccine is Fluad® influenza vaccine, which is indicated for use in the elderly. More recently, MF59 has been shown to be safe in a seasonal influenza vaccine in infants and children and increased vaccine efficacy from 43 to 89% [17, 22, 23]. During the 2009 H1N1 influenza pandemic, two MF59-adjuvanted vaccines (Focetria® and Celtura®, Novartis) were licensed and used safely in all age groups (down to children 6 months of age) including pregnant women. MF59 significantly improved the immunogenicity of pandemic influenza vaccines with relatively low antigen content and with fewer doses [24–27]. Moreover, the addition of MF59 to the vaccine has been shown to generate greater cross-reactivity against viral strains, even those not included in the vaccine [25, 28, 29]. Besides influenza, MF59 has also been used as adjuvant in various clinical vaccine trials including HIV [3, 30], HCV [31] and CMV [32]. Extensive pre-clinical experience using MF59 exists, and MF59 has been shown to be a potent vaccine adjuvant in a range of species, in combination with a broad range of vaccines, including recombinant proteins, viral membrane antigens, bacterial toxoids, protein–polysaccharide conjugates, peptides and virus-like particles [16, 18, 21].
For conformationally labile antigens, such as the HIV-1 Env, selection of adjuvant formulations that can best preserve critical neutralizing epitopes while improving immune responses is critical. Moreover, since some adjuvants cause localized tissue damage at the site of injection by various mechanisms, including recruitment of key immune cells, and may have systemic effects, it is important during the selection of adjuvants that tolerability considerations are not ignored.
Carbopols, hydrophilic polyanionic carbomers, are polymers of acrylic acid cross-linked with polyalkenyl ethers or divinyl glycol. Carbopols have found use in a diverse range of pharmaceutical applications ranging from controlled release solid dosage formulations to bioadhesive and topical applications [33, 34]. Particularly in vaccines, Carbopol-based adjuvant suspensions have been evaluated in veterinary vaccines since the 1970's against several pathogens, including equine influenza virus [35], porcine parvovirus [36], Staphylococcus aureus mastitis (in sheep) [37], etc. They have been shown to be well tolerated and effective when used in several mammals.
Although, carbopol compounds, such Carbopol® 934P NF, were designed for the pharmaceutical industry in the 1960’s, their regulatory acceptance has been limited because the residual solvent is benzene. Therefore, the next generation of carbopol compounds, e.g., Carbopol 71G® NF, 974P® NF, and 971P® NF were made with ethyl acetate, an acceptable solvent from a regulatory perspective, as the residual solvent.
The goal of the present study was to exploit the polyanionic and cross-linked nature of next generation Carbopols for a “controlled release” of the HIV-1 Env glycoprotein antigen, while also taking advantage of the potential adjuvant properties of Carbopols that have also been described [38, 39]. Carbopol 971P® NF (hereafter referred to as Carbopol971P) homopolymer type A was selected because of its lower degree of cross-linking and resultant lower viscosity. We also wished to determine if, upon combination with MF59, Carbopol971P might elicit improved antibody responses in comparison to responses generated using either Carbopol971P or MF59 alone. To do so, trimeric gp140 protein from the HIV-1 subtype B SF162 strain was formulated in either Carbopol971P alone, in MF59 alone, or in Carbopol971P plus MF59. Gp140 protein, when formulated in Carbopol971P plus MF59, elicited higher titers of binding and neutralizing antibodies, and higher avidity antibodies, compared to gp140 protein adjuvanted with either MF59 or Carbopol971P alone.
MATERIALS & METHODS
Proteins, adjuvants, and monoclonal antibodies
Recombinant envelope glycoprotein (Env), gp140, was derived from the subtype B CCR5-tropic strain HIV-1 SF162. The oligomeric gp140 protein contained a 30 amino acid deletion in the V2 loop region, as described previously [40], and was produced in stable CHO cell lines [40]. The gp140 protein was purified using a three-step purification process involving Galanthus Nivalis-Agarose (GNA) affinity column, cation-exchange DEAE column and a final ceramic hydroxyapatite (CHAP) column as described by Srivastava et al. [41]. Purified gp140 glycoprotein was then analyzed by SDS-PAGE (for level of purity) and immunoblots for specific reactivity (to anti-SF162 gp140 polyclonal rabbit sera). The purified gp140 protein was homogeneous with purity of >98%. Endotoxin levels in gp140 protein was measured using Endosafe® cartridges and an Endosafe®-PTSTM spectrophotometer (Charles River Laboratories International, Inc., Wilmington, MA), and found to be ≤0.05 EU/immunization dose. Using Surface Plasmon Resonance (SPR; BIAcore 3000), the binding of the gp140 protein to mAbs (b12, 2G12 and 2F5) and soluble CD4 (sCD4) were analyzed for antigenic correctness (data not shown) as previously described [42].
Carbopol® 971P NF (referred to as Carbopol 971P in this study) was purchased from Lubrizol as powder and was then resuspended in 0.1 µm filtered water (Sigma) under sterile conditions to generate a 0.5% homogenous, low viscosity suspension. The suspension was stored at 4°C until further use. A 1:1 (v/v) mix of gp140 protein and 0.5% (w/v) Carbopol971P (pH ≥3.0) was made for all in vitro evaluations. For administration in animals, a 1:1 (v/v) mix of gp140 and 0.5% (w/v) Carbopol971P was first made and the gp140 protein-Carbopol971P complex incubated for 30 minutes before addition of an equal volume of MF59, thereby keeping the final concentration of Carbopol971P suspension administered at 0.125% (w/v). For all Carbopol971P suspension for in vivo studies, endotoxin levels were measured using Endosafe® cartridges and an Endosafe®-PTSTM spectrophotometer (Charles River Laboratories International, Inc., Wilmington, MA).
Monoclonal antibodies (mAbs) b12 [43], 2G12 [44] and 2F5 [45, 46] were purchased from Polymun Scientific (Vienna, Austria). Soluble CD4 (sCD4) and CD4IgG2 (PRO 542) [47] was purchased from Progenics Pharmaceuticals (Tarrytown, NY). The mAb 17b [48] was provided by Dr. James Robinson. The mAb F425-B4e8 [49] was provided by Dr. Lisa Cavacini.
Dynamic Light Scattering
To determine the size of Carbopol971P polymers in 0.25% (w/v) suspension, 20 µl of 0.25% (w/v) suspension were added onto a 384-well plate and then analyzed by Dynamic Light Scattering (DLS) using the automated DynaPro™ Plate Reader™. (Wyatt Technology Corp., Santa Barbara, CA). For analyzing the interaction of gp140 protein with Carbopol971P, a 1:1 (v/v) of 0.5% (w/v) Carbopol971P with 1mg/ml gp140 protein was made and 20 µl of the gp140 protein-Carbopol971P complex (final Carbopol971P concentration 0.25%, w/v; final gp140 protein concentration 0.5mg/ml) was added onto a 384-well plate and then analyzed by DLS as described above.
Surface Plasmon Resonance
The binding of gp140 to ~0.1% (w/v) Carbopol971P suspension (pH ~ 3–4), was measured using surface plasmon resonance by a protocol that was described previously [50]. Responses were measured in resonance units (RU). 5000 RU of gp140 was immobilized on a CM5 sensor chip using amine coupling. HBS-EP (pH 7.2) was used as running buffer. 30 µl of 0.1% (w/v) Carbopol971P suspension (in water) was injected at 10 µl/min. Following injection, the dissociation was observed for 30 min before regeneration of the surface. The data was analyzed using BIAevaluation software 4.1 and specific binding of Carbopol971P to gp140 was determined by subtracting the mock-treated reference (control) surface from the experimental response.
In vitro stability studies
To determine the stability of gp140 proteins upon incubation with 0.5% (w/v) Carbopol971P suspension, we analyzed protein stability by PAGE/Western blot and ligand binding by ELISA. 1 µg of purified gp140 protein was added to 0.5% (w/v) Carbopol971P suspension (to make a 1:1 mixture, v/v) and incubated for 1, 2, 3 and 4 h at room temperature. Following incubation, the samples were diluted in SDS-PAGE loading buffer and analyzed via SDS-PAGE followed by Western blotting using anti-SF162 gp140 polyclonal rabbit sera.
For measurements of ligand-binding using ELISA, CD4IgG2 was used as a surrogate for CD4 receptor, mAb b12 for determining the stability of the CD4-binding site, mAb 17b for determining CD4-induced conformational changes and mAb F425- B4e8 for determining binding to V3 variable loop. Upon incubation of 10 µg gp140 protein with 0.5% Carbopol971P suspension (1:1, v/v) for 3 hours at room temperature, we analyzed the binding of the proteins to CD4IgG2, mAbs b12, 17b and F425-B4e8 using Ab D3724-based capture ELISA, as described previously [51–53]. For testing CD4-induced 17b mAb binding, a three-time molar excess of soluble CD4 (sCD4) was added to gp140 protein prior to their addition to the Ab D3724-coated plates.
Rabbit immunizations
Immunization studies were conducted at Josman LLC (Napa, CA), a research facility that is licensed through the USDA (No. 93-R-0260) and has a Public Health Service (PHS) Assurance from the NIH (No. A3404-01). Three groups of New Zealand White rabbits (5 young adult females per group) were used in the study. Rabbits were immunized with SF162 gp140 protein adjuvanted with either MF59 alone, Carbopol971P alone, or with Carbopol971P plus MF59. Four immunizations were administered intramuscularly, in the gluteus muscle (2 sites per immunization), at weeks 0, 4, 12, and 24. The protein dosage at each immunization was 50 µg. Serum samples were prepared from blood collected prior to the first immunization (pre-bleed) and at various time-points post each immunization (2wp2, 2wp3, 2wp4, 4wp4 and 15wp4) and analyzed for binding and neutralization.
For assessment of local reactogenicity associated with injection of 0.125% (w/v) Carbopol971P, visual observations of skin for edema and erythema at injection sites were performed pre-dose, immediately after immunization, and 24 h and 48 h after immunization. General observations for any obvious clinical signs were performed immediately after immunization, and 24 and 48 h post-immunization. Body-weights were also recorded before the beginning of the study, before each immunization, and 24 and 48 h following each immunization.
The study was fully approved by the Institutional Animal Care and Use Committee at Novartis (approval no. 09 NVD 044.3.3.09) in accordance with the requirements for the humane care and use of animals as set forth in the Animal Welfare Act, the ILAR Guide for the care and Use of Laboratory Animals, and all applicable local, state and federal laws and regulations.
Envelope-specific antibody ELISA and avidity measurements
Envelope-specific total antibody titers in sera from animals immunized with gp140 protein adjuvanted with MF59, gp140 protein adjuvanted with Carbopol971P, or gp140 protein adjuvanted with Carbopol971P plus MF59 were quantified by a standard ELISA assay using SF162 gp140 protein, as previously described [41]. Antibody avidity index determination was performed using an ammonium thiocyanate (NH4SCN) displacement ELISA as described elsewhere [41].
HIV-1 neutralization assays
Virus neutralization titers were measured using a well-standardized assay employing pseudoviruses and a luciferase reporter gene assay in TZM-bl cells [Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme, Inc. (Durham, NC)] as described previously [54, 55]. Briefly, a total of 200 TCID50 pseudoviruses/well were added to diluted serum samples and incubated at 37°C for 1 h. Following incubation, 10,000 cells/well in DEAE-dextran-containing media were added and incubated for 48 h at 37°C. The final concentration of DEAE-dextran was 10 µg/ml. After a 48 h incubation, 100 µl of cells was transferred to a 96-well black solid plates (Costar) for measurements of luminescence using Bright-Glo substrate solution as described by the supplier (Promega). Neutralization titers are the dilution at which relative luminescence units (RLU) were reduced by 50% compared to virus control wells after subtraction of background RLUs. HIV-1 Env pseudoviruses were prepared by co-transfection of 293T cells with expression plasmids containing full-length molecularly cloned gp160 env genes from a panel of HIV-1 isolates combined with an env-deficient HIV-1 backbone vector (pSG3Δenv) using FuGENE-6 HD (Roche Applied Sciences, Indianapolis, IN), as previously reported [55]. After 48 h, the cell culture supernatants containing the pseudoviruses were filtered through a 0.45 µm filters and stored at −80°C until use.
Statistical analysis
The Mann Whitney test was used to test for differences in ELISA titers and avidity indexes between immunization groups. For all comparisons, a two-sided P value of < 0.05 was considered statistically significant. For analysis of the ligand binding using ELISA, five parameter nonlinear least squares fit analysis was used, which is the asymmetric version of the standard sigmoidal curve. The statistical differences in EC50, if any, were determined using a paired t test. A paired t test was also used to analyze the differences in body weight drops before and after each immunization amongst animals immunized with gp140 protein with Carbopol971P plus MF59. Statistical analyses were performed using the analysis software within the GraphPad Prism package 5.01.
RESULTS
Carbopol971P suspension, at low pH, directly interacts with HIV-1 SF162 gp140 protein
Intensified efforts are now underway to screen and identify new HIV-1 Envelope (Env) glycoprotein antigens for improved elicitation of functional and neutralizing antibody responses [1, 56–64]. These efforts undoubtedly will benefit from the testing, in parallel, of novel adjuvant systems for their abilities to contribute to enhanced antigen-specific immune responses. Polyanions such as Carbopols have been shown previously to improve antibody responses and cause general immune activation [38, 39, 65, 66]. Hence, we chose to investigate the potential usefulness of a specific Carbopol, Carbopol971P, for use as an adjuvant for the HIV Env glycoprotein. We selected Carbopol971P, in particular, as opposed to other more commonly used Carbopols, due to its low specific viscosity and lower cross-linking capacity.
To generate a 0.25% (w/v) or 0.5% (w/v) Carbopol971P suspension for in vitro and in vivo studies, Carbopol971P granular powder was added to sterile water and a homogenous suspension prepared. Endotoxin levels and pH for all Carbopol971P suspensions generated for in vivo studies were measured. The endotoxin content was ≤0.1EU/ml (≤0.05 EU/immunization dose) and the pH of the suspension was ≥ 3.0. Because the Carbopol971P suspension had an acidic pH, it was predicted that HIV-1 SF162 gp140 polypeptide, having a pI of approximately 8.8, would be positively charged upon mixing with the Carbopol971P and thus, the protein would directly interact or be adsorbed on the anionic surface of the carbomer via electrostatic interactions. To determine whether it was the case, Dynamic Light Scattering (DLS) was used to measure the size of the particles in the 0.25% (w/v) Carbopol971P suspension and gp140 protein-Carbopol971P complex. We observed that particles within the 0.25% (w/v) Carbopol971P suspension had a hydrodynamic diameter of approximately 68nm. When the gp140 protein was incubated with Carbopol971P (final concentration 0.25%, w/v), we observed particles with a significantly larger average diameter, of approximately 86nm (Figure 1A). Surface Plasmon Resonance (SPR) analysis was performed to demonstrate direct binding between gp140 (immobilized on CM5 sensor chip) and low pH 0.1% (w/v) Carbopol971P (Figure 1B). We observed that under low pH conditions, Carbopol971P directly bound gp140 with considerable fast on-rate (association). We also saw that following the bulk drop-off after completion of the injection, the dissociation (off-rate) of the polymer from gp140 was slow and took greater than 1.5–2 h under physiological pH (pH 7.4 as in HBS-EP running buffer) for full-dissociation. These results confirmed that under the acidic pH condition of Carbopol971P suspension, positively-charged gp140 protein directly bound to the negatively-charged polyanionic surface of the Carbopol971P matrix and form the basis of direct interaction. Importantly, we also show that the protein was not buried inside the polymer matrix and was still available for ligand binding as confirmed using SPR (data not shown) and ELISA (Figure 2B, described below).
Figure 1.
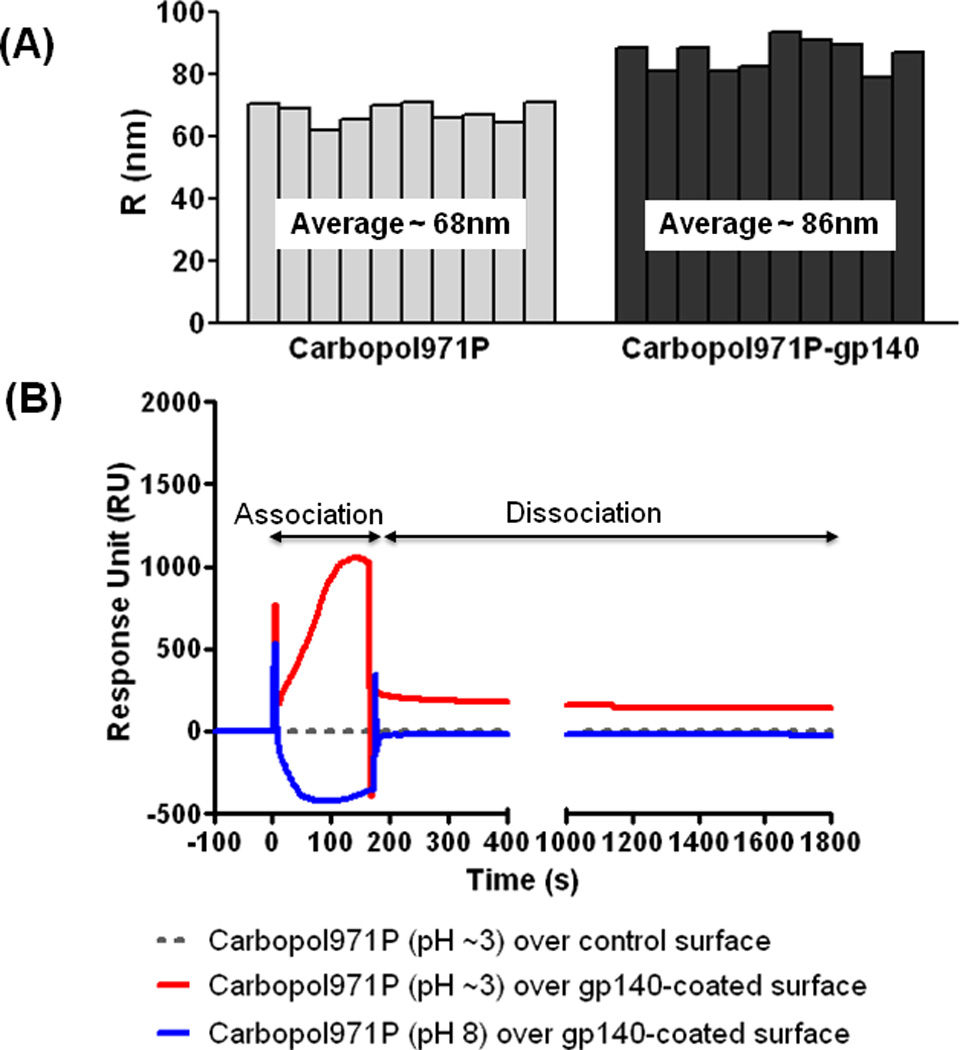
Analysis of interaction between SF162 gp140 protein and Carbopol971P. (A) Measurement of particle size of 0.25% (w/v) Carbopol971P suspension and SF162 gp140 protein-Carbopol971P (final concentration 0.25%, w/v) complex using Dynamic Light Scattering (DLS). The increased size of the gp140 protein-Carbopol971P complex (average ~86nm) in comparison to Carbopol971P alone (average ~68nm) indicates the adsorption of the positively-charged protein at pH ≥3 on negatively-charged Carbopol. (B) Direct binding of Carbopol971P suspension to SF162 gp140 protein immobilized on CM5 sensor chip. 5000RU of SF162 gp140 protein was covalently immobilized on sensor chip via amine coupling and 30 µl of 0.1% Carbopol971P suspension was injected at 10 µl/min. The increase in RU (Resonance Unit) (see association phase) indicates binding of polyanionic Carbopol971P at pH ≥3 to immobilized-gp140 (red line). Following injection, as pH alters to 7.4 (running buffer HBS-EP), a bulk-drop in RU occurs (at time = 180s) followed by very slow dissociation of the polymer from the gp140-coated surface (see dissociation phase). The dotted grey line shows the (non-) binding of Carbopol971P suspension to mock-treated control surface. The spikes at the beginning (time = 0s) and end (time = 180s) of injection/association is due to differences in pH and nature of the solvent media.
Figure 2.
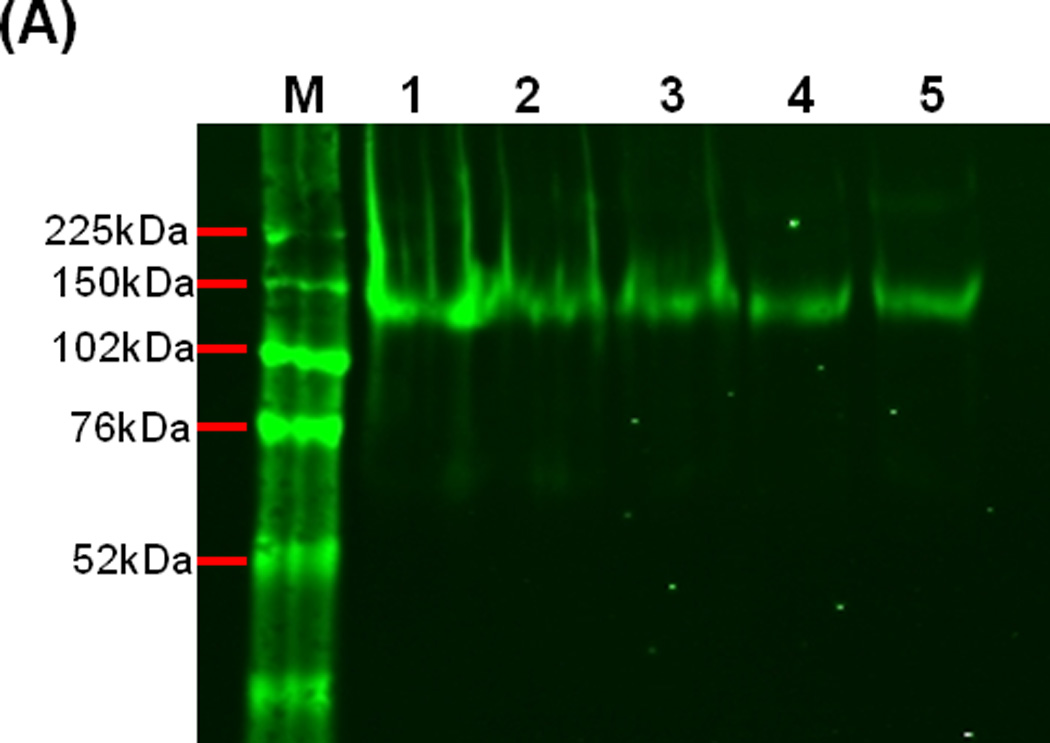
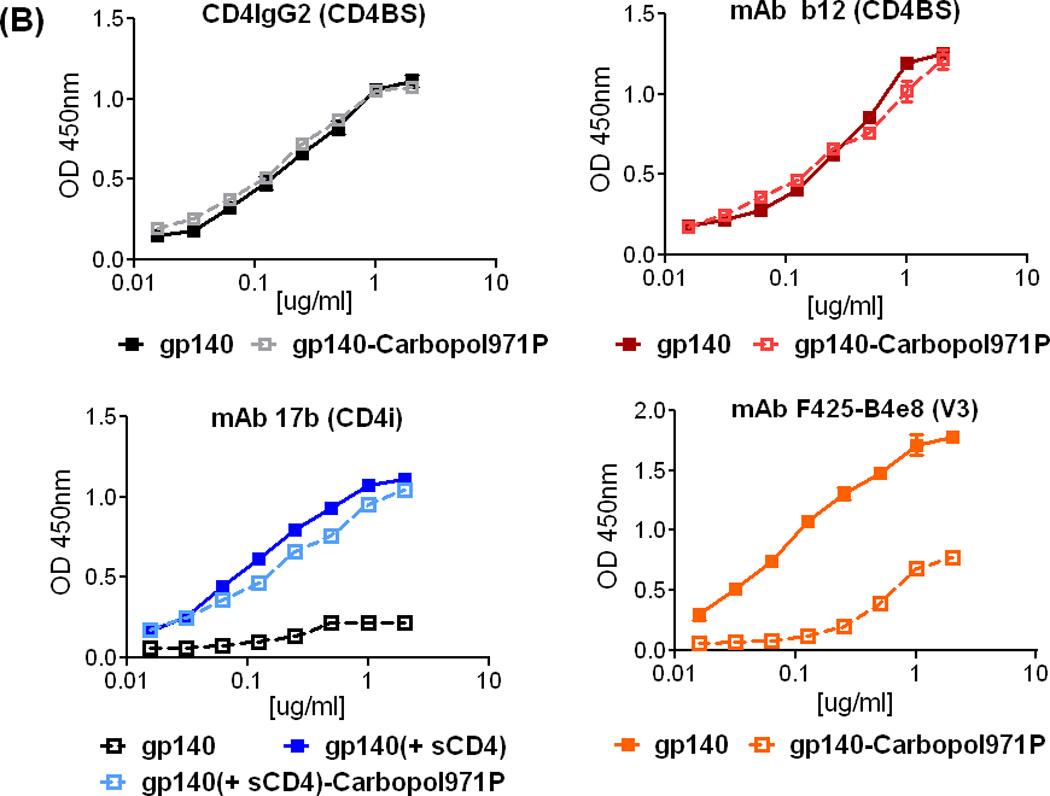
Stability of SF162 gp140 protein. (A) SDS-PAGE of SF162 gp140 protein upon incubation with 0.5% (w/v) Carbopol971P for 0 h (lane 1, control), 1 h (lane 2), 2 h (lane 3), 3 h (lane 4) and 4 h (lane 5), indicating that the protein is stable in low pH conditions in the presence of Carbopol971P. (B) Binding of CD4IgG2 (surrogate for CD4 receptor) and anti-gp120 mAbs, b12 (CD4BS, conformational), 17b (CD4-induced, CD4i) in the presence or absence of soluble CD4 (sCD4) and F425-B4e8 (V3 variable loop), to SF162 gp140 protein (control) or gp140 protein upon 3 h incubation with 0.5% (w/v) Carbopol971P. A capture ELISA using Ab D3724 was used to analyze the binding of the gp140 protein to CD4IgG2, mAbs b12, 17b (-/+ sCD4) and F425-B4e8 (see Methods section for details). For testing CD4-induced 17b mAb binding, a three-time molar excess of soluble CD4 (sCD4) was added to gp140 protein prior to their addition to the Ab D3724-coated plates.
When the Carbopol971P suspension was brought to neutral pH 7, using potassium hydroxide (KOH), and then added to HIV-1 gp140 protein to generate a gp140 protein-Carbopol971P complex and analyzed by DLS, the average diameters of the Carbopol971P alone (final concentration 0.25%, w/v) and gp140 protein-Carbopol971P (final concentration 0.25%, w/v) complex were comparable (data not shown). This indicated that once the Carbopol971P suspension was neutralized, gp140 protein did not directly interact with the polymer matrix, an observation also previously reported by G Krashias et al. [38]. Since direct interaction of the Env glycoprotein with the polyanionic matrix, that occurred at the lower pH, was desired with the hope that it might facilitate antigen delivery and immunogenicity, the previously described approach [38] was not studied here.
HIV-1 SF162 gp140 protein is stable in vitro in presence of Carbopol971P
Because the Carbopol971P suspension had low pH (≥3.0), it was important to test the stability of the Env glycoprotein antigen under such conditions. To do so, gp140 protein was added to the Carbopol971P suspension (final concentration 0.25%, w/v) and incubated at room temperature and evaluated at various time-points. Following incubation, the Env glycoprotein was analyzed by SDS-PAGE and then subjected to immunoblot analysis (described in Materials and Methods). It appeared that the gp140 protein was stable in the presence of Carbopol971P at pH ~3.0 for at least 4 h (Figure 2A), the longest time-point tested here.
The integrity and stability of the Carbopol971P-adsorbed gp140 protein was further evaluated by analyzing the ability of the protein to bind conformation-dependent anti-gp120 ligands such as sCD4 or CD4IgG2 and mAbs b12 (all binding to CD4-binding site, CD4BS), 17b (binding to the co-receptor or CD4-induced, CD4i, binding site with enhanced binding observed after Env binding to CD4 receptor) and F425-B4e8 (binding to V3 variable loop). For this analysis, SPR (data not shown) and ELISA methods were used (Figure 2B). Gp140 protein was analyzed following 3 hours incubation with Carbopol971P at room temperature. In comparison to gp140 protein alone, the gp140 protein incubated with Carbopol971P did not show any difference in binding to CD4IgG2 or mAb b12 (Figure 2B, top panels). Moreover, the gp140 protein that was incubated with Carbopol971P also maintained its conformational flexibility showing a CD4-dependent conformational upregulation of binding to the CD4i-mAb, 17b, similar to the binding observed using the control gp140 protein in presence of sCD4 (Figure 2B, bottom panel, left). The EC50s for 17b binding to gp140 (0.15 µg/ml) and gp140-Carbopol971P (0.22 µg/ml) in presence of sCD4 varied, but were not statistically different (p>0.05). Taken together, these data indicated that the gp140 protein was stable in the presence of Carbopol971P preserving critical conserved epitopes involved in receptor and co-receptor binding. However, when we analyzed the binding of anti-V3 mAb F425-B4e8 to gp140 protein alone or the gp140 protein incubated with Carbopol971P, we observed significant differences (p=0.0002) in their EC50s (Figure 2B, bottom panel, right). While the gp140 protein bound to mAb F425-B4e8 with EC50 of 0.14 µg/ml, the protein incubated with Carbopol971P bound the mAb with >4-fold higher EC50 (0.6µg/ml). It is possible that the polyanionic carbomer interacted with the protonated side chains of basic amino acids in the V3-loop region of gp140 protein and partially masked the epitope from mAb-binding. This remains to be formally demonstrated by further studies.
HIV-1 SF162 gp140 protein adjuvanted with Carbopol971P plus MF59 elicited higher levels of both binding and neutralizing antibodies
Rabbit immunogenicity studies were performed to evaluate and compare the potency of neutralizing antibody and longevity of binding antibody responses to the HIV-1 SF162 gp140 protein adjuvanted with Carbopol971P or Carbopol971P plus MF59 in comparison to the MF59 adjuvant. Groups of 5 rabbits were immunized with 50 µg of gp140 protein in either MF59 (group 1), Carbopol971P (group 2) or Carbopol971P plus MF59 (group 3) adjuvants, at weeks 0, 4, 12 and 24 (Table 1). Sera were collected pre-immunization (pre-bleed), 2-weeks post 2nd-immunization (2wp2), 2 weeks post 3rd-immunization (2wp3), 2 weeks post 4th-immunization (2wp4), 4 weeks post 4th-immunization (4wp4), 15 weeks post 4th-immunization (15wp4) and the final bleed-out time-point for analysis.
Table 1.
Evaluation of HIV-1 SF162 gp140 protein-adjuvant combinations in rabbits Group 1 immunized with gp140 protein adjuvanted with MF59, group 2 immunized with gp140 protein adjuvanted with Carbopol971P and group 3 immunized with gp140 protein adjuvanted with Carbopol971P plus MF59.
| Group (n=5) |
Immunogen | Dose (µg) |
Adjuvant | Time (weeks) |
|---|---|---|---|---|
| 1 | Gp140 protein | 50 | MF59 | 0, 4, 12, 24 |
| 2 | Gp140 protein | 50 | Carbopol971P | 0, 4, 12, 24 |
| 3 | Gp140 protein | 50 | Carbopol 971P + MF59 | 0, 4, 12, 24 |
The gp140 protein administered with MF59 adjuvant alone showed mean titers of total binding Abs as measured by ELISA of ≦106, whereas gp140 protein with Carbopol971P alone produced titers of half a log higher than MF59, at 2wp2 (p = 0.0317), 2wp3 (p = 0.0317) and 2wp4 (p = 0.0317) time-points (Figure 3). Even at later time-points when the sera were evaluated, significantly higher titers of binding antibodies were observed in the group adjuvanted with Carbopol971P than one with MF59 [4wp4 (p = 0.0317); 15wp4 (p = 0.0159)] (Figure 3). When gp140 protein was combined with the Carbopol971P plus MF59 formulation, even higher levels of binding antibodies were seen (Figure 3); half a log higher titer in comparison to gp140 adjuvanted with Carbopol971P alone that was statistically significant at all time-points evaluated, 2wp2 (p = 0.0317), 2wp3 (p = 0.0079), 2wp4 (p = 0.0317), and 15wp4 (p = 0.0159), except for the 4wp4 (p = 0.4206) time-point (Figure 3). The gp140 protein adjuvanted with Carbopol971P plus MF59 formulation also generated a log-fold higher anti-gp140 titer in comparison to gp140 protein adjuvanted with MF59 [2wp2 (p = 0.0079), 2wp3 (p = 0.0079), 2wp4 (p = 0.0079), 4wp4 (p = 0.0079) and 15wp4 (p = 0.0079)] (Figure 3). Taken together, adjuvantation using Carbopol971P plus MF59 led to significantly increased gp140-specific binding antibody titers in comparison to Carbopol971P or MF59 alone.
Figure 3.
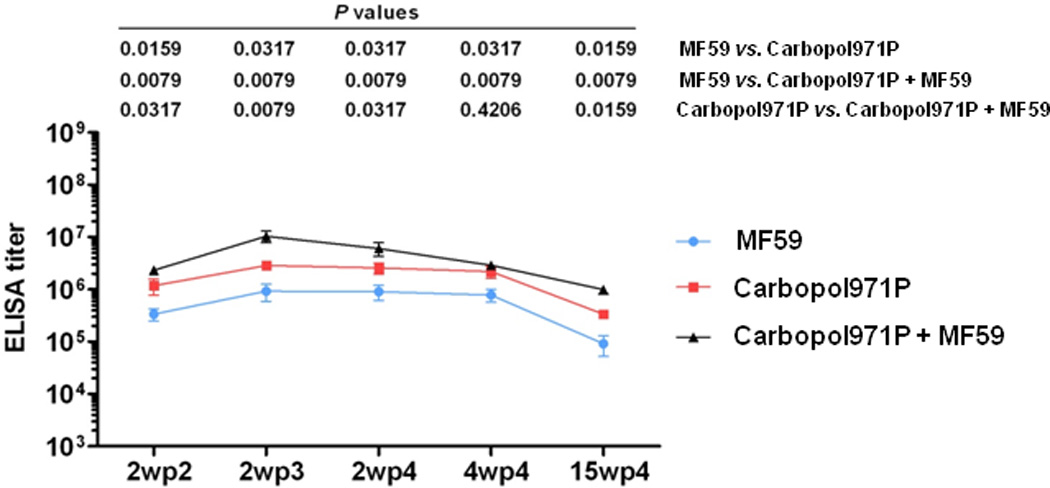
Total binding gp140 protein-specific antibodies as determined by ELISA following immunization with gp140 protein adjuvanted with MF59, Carbopol971P or MF59 plus Carbopol971P. ELISA mean titers ± SD of SF162 gp140 protein-specific antibodies from animals at various time-points following immunization (2wp2, 2wp3, 2wp4, 4wp4 and 15wp4) to determine the durability of the response upon immunization of gp140 protein adjuvanted with MF59, or Carbopol971P or Carbopol971P plus MF59. The p values for the differences between groups at various time points are indicated.
When sera were analyzed to measure the overall strength of binding of the polyclonal antibodies to various antigenic sites on gp140 protein, i.e., avidity, the gp140 protein adjuvanted either with MF59 or Carbopol971P alone elicited antibodies with similar avidities (Figure 4), except at 2wp2 and 15wp4 where gp140 protein adjuvanted with Carbopol971P alone generated higher avidity antibodies [2wp2 (p = 0.0119), 2wp3 (p = 0.2492), 2wp4 (p = 0.3443), 4wp4 (p = 0.9161) and 15wp4 (p = 0.0157)] (Figure 4). However, when gp140 protein was delivered with Carbopol971P plus MF59, the antibodies elicited had significantly higher avidities at almost all time-points in comparison to those from the gp140 protein delivered with MF59 [2wp2 (p = 0.0119), 2wp3 (p = 0.0159), 2wp4 (p = 0.0119), 4wp4 (p = 0.0079) and 15wp4 (p = 0.0119)] (Figure 4) and gp140 protein delivered with Carbopol971P groups [2wp2 (p = 0.6752, non-significant), 2wp3 (p = 0.0459), 2wp4 (p = 0.0079), 4wp4 (p = 0.0079) and 15wp4 (p = 0.0362)] (Figure 4). In all, significantly higher avidity antibodies were generated when gp140 protein was delivered with Carbopol971P plus MF59.
Figure 4.
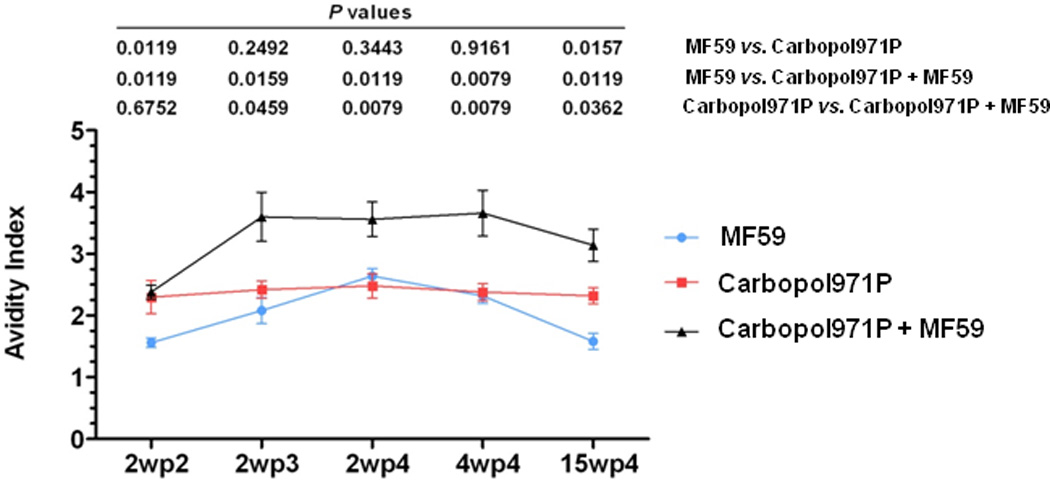
Avidity of gp140 protein-specific antibodies as determined by ELISA following immunization with gp140 protein adjuvanted with MF59, Carbopol971P or MF59 plus Carbopol971P. Avidity indexes (mean ± SD) of SF162 gp140 protein-specific antibodies from animals at various time-points following immunization (2wp2, 2wp3, 2wp4, 4wp4 and 15wp4) to determine the durability of the response upon immunization of gp140 protein adjuvanted with MF59, or Carbopol971P or Carbopol971P plus MF59. The p values for the differences between groups are indicated.
To test the ability of the gp140-specific antibodies to neutralize HIV-1 strains, the standard luciferase-based TZM-bl assay described previously [54, 55], was used with the standard tiered panels [54] of pseudoviruses (Figure 5). When the neutralization of Tier 1A pseudoviruses (Figure 5A) was evaluated, in general, the 2wp4 (p4) sera showed higher titers of virus neutralization than those seen at 2wp3 (p3), suggesting a positive impact of the repeated boosting on virus neutralization titers in this study. Particularly, in the case of neutralization of the “homologous” SF162. LS pseudovirus with an envelope matching the HIV-1 SF162 subtype B vaccine strain, sera from animals vaccinated with gp140 protein adjuvanted with Carbopol971P had ID50 titers higher [(at p3, p = 0.2222) (at p4, p = 0.0079)] than those given gp140 protein with MF59. In addition, animals vaccinated with gp140 protein adjuvanted with Carbopol971P plus MF59 generated higher titers of neutralizing antibodies than those vaccinated with gp140 protein with MF59 alone [(at p3, p = 0.0079) (at p4, p = 0.0079)] or gp140 protein with Carbopol971P alone [(at p3, p = 0.0079) (at p4, p = 0.0079)] (Figure 6). For neutralization of the Tier 1A MW965.26 pseudovirus from a subtype C HIV-1 strain, at 2wp3 (p3), gp140 protein in Carbopol971P plus MF59 generated higher titers of neutralizing antibodies than those vaccinated with gp140 protein with MF59 alone [(at p3, p = 0.0317)] or gp140 protein with Carbopol971P alone [(at p3, p = 0.0317)] (Figure 6). However, the significant difference seems to be lost at the 2wp4 (p4) time-point despite higher ID50 titers (Figure 6). For neutralization of Tier 1B pseudovirus from the subtype B strain, Bal.26, a similar pattern was observed except that the difference between Carbopol971P and Carbopol971P plus MF59 groups, particularly at 2wp4 was non-significant (p > 0.05) (Figure 5B, 6). For the subtype B Tier 1B pseudovirus, SS1196.1, some low neutralizing activity was seen, primarily at 2wp4, with immune sera from animals given gp140 protein adjuvanted with Carbopol971P alone and Carbopol971P plus MF59. No significant neutralizing activity was measured against Tier 2 viruses using any of the immune sera tested here in the TZM-bl assay (Figure 5C). As a negative control, MLV was employed (Figure 5D), and it was found that none of the 2wp3 or 2wp4 sera from the three groups neutralized MLV in a nonspecific manner validating the specificity of Env-mediated viral neutralization in this assay.
Figure 5.
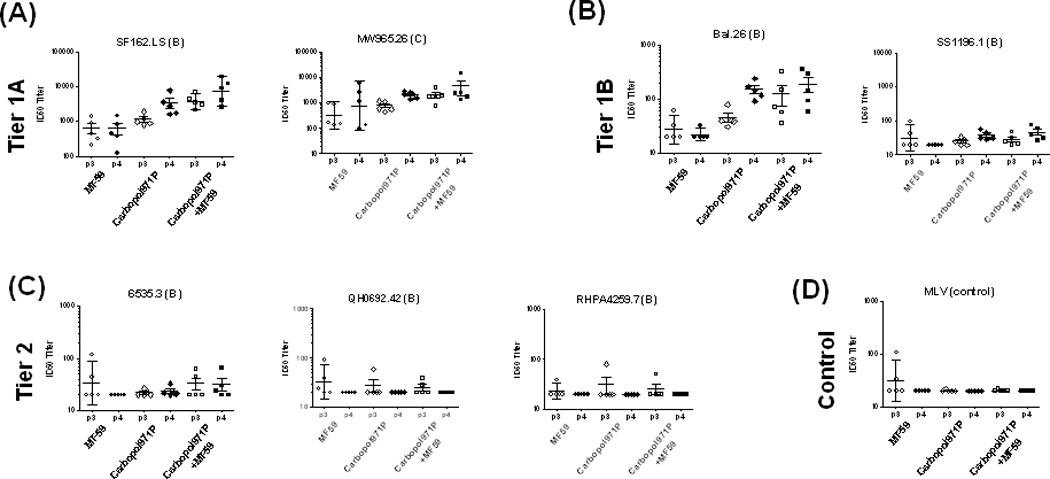
(A–D): Analysis of breadth by neutralization of HIV-1 pseudoviruses in TZM-bl cells by 2wp3 (p3) and 2wp4 (p4) sera from animals immunized with gp140 protein adjuvanted with MF59, Carbopol971P or Carbopol971P plus MF59. ID50 neutralization titers are shown for the following viral isolates: (A) SF162.LS, MW965.26, (B) Bal.26, .SS1196.1, (C) 6535.3, QH0692.42 and RHPA45259.7. The subtypes of the viruses are highlighted in parenthesis. Neutralization of (D) MLV is shown here as a control.
Figure 6.
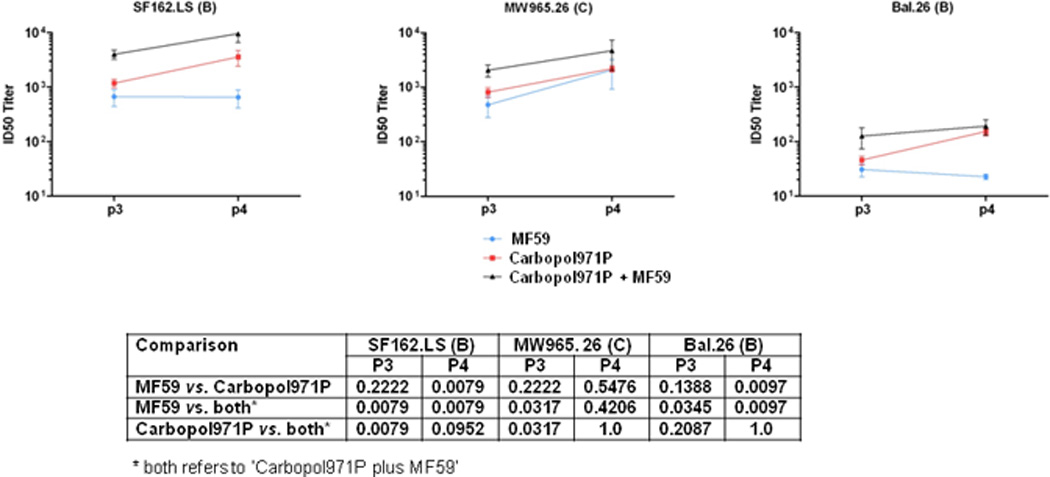
ID50 titers of sera from animals immunized with gp140 protein adjuvanted with MF59, Carbopol971P, or Carbopol971P plus MF59. ID50 neutralization titers in TZM-bl cells, are indicated against the two Tier 1A (SF162.LS and MW965.26) and one Tier 1B (Bal.26) viruses. The subtypes are indicated in parenthesis. P values for the differences between groups, at 2wp3 (p3) and 2wp4 (p4), in MF59, Carbopol971P or Carbopol971P plus MF59 are listed in the table below.
Overall, although neutralizing antibody responses against Tier 2 viruses were not elicited by any of the three formulations, Carbopol971P plus MF59 adjuvant generated higher ‘homologous’ and cross-subtype ‘heterologous’ neutralizing antibodies against Tier 1A viruses. Considering the total binding and neutralizing antibody responses, it was clear that gp140 protein adjuvanted with Carbopol971P plus MF59 elicited better humoral response than either of the adjuvant used alone.
Carbopol 971P plus MF59 formulation was well tolerated without any adverse reaction
Different Carbopols have been used as excipient in a diverse range of pharmaceutical products for humans as well as in veterinary vaccines, but has not yet been used in vaccines for humans. In contrast, MF59 is a well-established, safe and effective adjuvant [19–21, 27, 67–69]. Having observed enhanced humoral responses against HIV-1 gp140 protein by combining Carbopol971P and MF59, it was important to also assess tolerability in animals of the Carbopol971P plus MF59 formulation. To do so, the local reactogenicity of the vaccines was monitored at the sites of injection for signs of edema or erythema and animals were observed for any obvious health problems following vaccination. Although mild forms of edema and erythema were detected in a subset of rabbits at 1–2 h after and 24 h post-vaccination, these signs disappeared at later time-points. In addition, no obvious health problems were reported post-administration of the vaccine containing Carbopol971P and MF59.
Body weights of the animals were also monitored as part of the evaluation. While a transient and modest 2–4% drop in body weight was measured during the first 1–3 days following vaccination, all animals recovered to their pre-vaccination weight and exhibited subsequent normal increase in body mass (Figure 7A and B). In Figure 7B, we specifically looked into differences in body weight before (measured on the day of the immunization) and after (2 and 4 days after immunization) in each animal (#11–15); we found no significant differences (p≥0.05) in body weights before and after immunization. This observation suggested that Carbopol971P and MF59, when administered in combination with HIV-1 gp140 protein, did not cause any serious adverse reactions and therefore appeared to be a well-tolerated adjuvant for vaccine evaluation in small animals such as rabbits. We also performed a similar evaluation post-administration of the vaccine adjuvanted with Carbopol971P alone or MF59 alone, and saw no obvious health problems or any notable effects on body weights post-administration of the vaccines in either group (data not shown).
Figure 7.
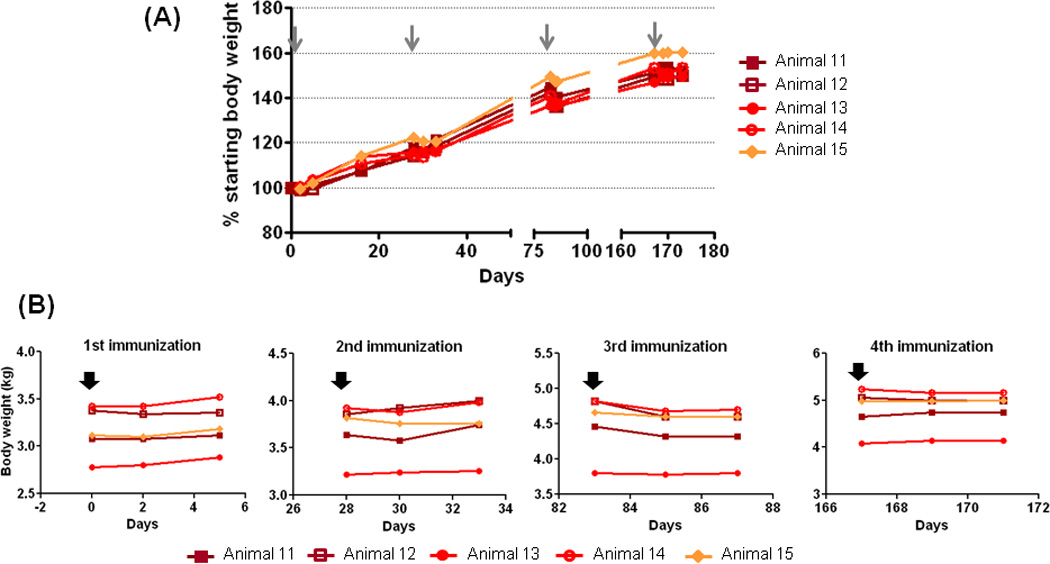
Body weights of animals following immunization. (A) Changes in percentage (%) body weight of animals immunized with gp140 protein adjuvanted with Carbopol971P plus MF59 to highlight no adverse reactivity or no obvious health problems (NOHP) upon use of Carbopol971P plus MF59 during vaccination. The grey arrows highlight the four immunization time-points. Data for the other two groups, using MF59 only or Carbopol971P only, are similar and show no adverse reactivity and NOHP (not shown). (B) Changes in body weight (in kg) in five animals (#11, 12, 13, 14, 15) monitored before (indicated by black arrow) immunization with gp140 protein adjuvanted with Carbopol971P plus MF59 and two time-points after the immunization. Although subtle drops in body weights were observed, particularly after 3rd immunization, the differences were not statistically significant (p≥0.05).
DISCUSSION
The relatively low immunogenicity of soluble recombinant HIV-1 envelope glycoproteins can be attributed to multiple factors including, but not limited to, extensive glycosylation, the variability and flexibility of accessible variable-loop regions, and conformational masking of key neutralizing epitopes.[70, 71]. Therefore, to surmount some of the challenges surrounding the elicitation of a potent and protective immune response to the HIV-1 Env via vaccination, the use of safe and effective adjuvant formulations will be required. Here, the ability of a specific Carbopol, Carbopol971P, to adjuvant gp140 proteins when administered either alone or with Novartis’ proprietary adjuvant, MF59, was evaluated.
While other Carbopols have been used in veterinary vaccines such as the killed PCV (Porcine Circovirus) 1–2 chimeric vaccine (Suvaxyn® PCV2 One Dose™), and more recently, in pre-clinical studies using influenza HA and HIV-1 gp140 proteins [38, 39], Carbopol971P was chosen due to its low specific viscosity and lower amount of cross-linking. These properties might allow use at a lower percentage in suspension (0.1–0.2%, w/v) and this particular Carbopol might be more amenable to parenteral administration. The Carbopol971P suspension was formulated in a very specific manner, generating a low percentage suspension and maintaining the inherent low pH, to allow surface adsorption of antigen to potentially aid antigen adsorption and delivery. This appears to be the first example of using Carbopol971P as an adjuvant with a viral antigen, in this case HIV-1 gp140 protein, in a vaccine setting. It is also the first study showing improvement of anti-HIV antibody responses when Carbopol971P and MF59 are used in combination, as adjuvants.
Overall, gp140 protein-Carbopol971P complexes were generated in a manner that was designed to potentiate antigen delivery and adjuvanticity. In vitro, the gp140 protein was shown to physically interact with the Carbopol971P, most likely by surface adsorption mediated by electrostatic interactions; moreover, gp140 protein was stable in low pH conditions in the presence of Carbopol971 as analyzed by Western blot analysis and ELISA (Figure 2). In the Western blot (Figure 2A), we observed a relative decrease in intensity of gp140-bands detected over time i.e., gp140 incubated with Carbopol971P for less time (0,1h) showed higher intensity bands than those incubated for greater length of time with Carbopol971P (>2hrs). It is possible that over time, (increased) interaction of polyanionic carbomer with basic amino acids in the V3-loop region perturbed binding of a subset of V3-specific antibodies in the polyclonal sera (that is predominantly V3-specific) and thus effected full detection. This hypothesis was directly supported by decreased binding of gp140 incubated with Carbopol971P to anti-V3 mAb F425-B4e8 in comparison to gp140 alone (Figure 2B). In addition, we did not observe any degradation or cleaved fragments in those gp140s with lower-intensity bands (Figure 2A) and the ligand-binding analysis of gp140 protein after 3 h incubation in Carbopol971P (Figure 2B) clearly showed that the antigen was stable. Therefore, taken together, we believe that this was not a reflection of instability of the protein in Carbopol.
With regard to ligand-binding, we observed no difference in binding of gp140 protein, either alone or following incubation with Carbopol971P, to ligands directed to CD4-binding site (CD4IgG2 and mAb b12) (Figure 2B). However, we did observe differences in binding of the gp140 protein (alone or with Carbopol971P) to CD4i-site specific mAb 17b in presence of sCD4 and to anti-V3 F425-B4e8. Upon non-linear regression analysis and assessment for significant differences in their EC50s, we found that while the EC50s for 17b binding in presence of sCD4 to gp140 (0.15 µg/ml) and gp140-Carbopol971P (0.22 µg/ml) differed, the difference was not statistically significant (p>0.05). On the contrary, the gp140 protein bound the mAb F425-B4e8 with statistically significant (p = 0.0002) >4-fold lower EC50 (0.14 µg/ml) than gp140-Carbopol971P (0.6 µg/ml).
Upon vaccination of rabbits, gp140 proteins adjuvanted with Carbopol971P plus MF59 generated higher antibody responses in comparison to proteins adjuvanted with Carbopol971P or MF59 alone. The improved humoral responses observed upon exploratory use of Carbopol971P, particularly in the manner described, warrants further investigation and exploration in future studies. Most licensed vaccines and adjuvant formulations in current use have been developed empirically. But recent advances in our understanding of innate immune receptors, target cells, and signaling pathways is now providing opportunities for the rational design of novel adjuvants. Until 2009, before Food and Drug Administration (FDA)’s approval of GlaxoSmithKline’s HPV-16/18 vaccine, Cervarix® that contains AS04 [72, 73], alum was the only adjuvant in licensed vaccines in the United States. However in Europe, besides alum, several adjuvants have been part of licensed vaccines - MF59, as a component of influenza vaccine, FLUAD®, approved in 1997 for the elderly [74–76]; AS04, as a component of HBV vaccine, Fendrix® [77] and HPV vaccine, Cervarix® [78]; and AS03, as a component of pandemic influenza vaccines, Prepandrix® and Pandemrix® [79, 80]. Although it is not entirely clear how these adjuvants work, their abilities in effectively stimulating innate immune responses is undeniable. Several recent studies have identified ways in which alum may stimulate the innate immune response by activation of inflammasome [14] or independent of the activation of inflammasome [81, 82]. Similarly, studies involving MF59 have shown that it can serve as an antigen delivery system [10, 83] and also as an immuno-stimulator by increasing recruitment of immune cells into the injection site, enhancing monocyte differentiation into dendritic cells (DCs), augmenting antigen uptake and facilitating migration of DCs into tissue-draining lymph nodes to prime adaptive immune responses [10, 84]. As for Carbopol971P or Carbopol971P plus MF59 formulations, the mechanism of immune activation has not yet been studied. In general, polyanions of different chemical compositions have been shown to potentiate humoral or cellular immunity [85–89]. Specifically, reports on linear polyanions such as dextran sulfate indicate that they can stimulate B-cells towards antibody production by substituting for the absence of T-cell help i.e., removing the need for cellular interaction for B-cell activation [90–92]. We observed a significant increase in antibody avidity following immunization with gp140 in Carbopol971P plus MF59 (Figure 4). We speculate that the improved antibody avidity may be the result of a combined effect of direct B-cell activation and antigen delivery by the anionic polymer coupled with MF59’s ability to enhance antibody responsiveness [93] by improved antigen presentation and increased recruitment and activation of antigen presenting cells [11, 84]. Besides improved antibody production, Carbopols have also been associated with T-cell responses typified by high levels of both Th1 and Th2 cytokines [38]. Overall, this indicates that Carbopols have immuno-stimulatory effects in addition to direct B-cell activation, possibly serving as an antigen delivery system. Recently, this hypothesis has been supported by studies of inactivated influenza antigens in which the use of Carbopol974P as a carrier resulted in improved kinetics and levels of IgG titers in a dose-dependent manner [94].
Because safety is a major concern when considering new adjuvant formulations for vaccine applications, the local reactogenicity and tolerability of Carbopol971P in vaccinated rabbits was assessed in this study. Except for a very modest and transient weight loss in some animals, and short-lived edema or erythema that resolved within 24 h, no serious reactogenicity or obvious health problems were seen following intramuscular administration of Carbopol971P formulations in rabbits. In addition, since Carbopols have been shown to be effective toward improving immune responses in various animal species [38, 39, 65, 95, 96], it provides a useful tool for pre-clinical studies leading to clinical applications. Therefore, Carbopol971P, in combination with MF59, as vaccine adjuvant warrants further investigation to evaluate its full potential.
HIGHLIGHTS.
First report of use of Carbopol (here Carbopol971P) plus MF59 as vaccine adjuvant.
First report of use of Carbopol971P as vaccine adjuvant to HIV gp140.
Novel formulation of Carbopol971P to aid HIV gp140 adsorption for antigen delivery.
Improved antibody-responses to HIV gp140 using Carbopol971P plus MF59 as adjuvant.
ACKNOWLEDGEMENTS
We thank Drs. Rino Rappuoli, Global Head of Vaccines Research (Novartis Vaccines & Diagnostics, NVD), and Christian Mandl, Head of US Vaccines Research (NVD), for their support and encouragement. We also thank Dr. James Robinson for providing 17b monoclonal antibody and Dr. Lisa Cavacini for providing F425-B4e8 monoclonal antibody; Mark Wininger, Karen Matsuoka and Frank Situ for their assistance with cell cultures in generating the HIV-1 SF162 gp140 proteins. We thank Dr. Deborah Novicki, Global Head of Toxicology (NVD), for careful and expert review of the manuscript. This work was supported by the NIAID-NIH HIV Vaccine Research and Design (HIVRAD) grant # 5P01 AI066287 and NIAID NIH Primate Core Immunology Laboratory for AIDS Vaccine Research and Development. Grant # HHSN27201100016C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
AKD, BB, YS, KH and SWB are employees at Novartis Vaccines & Diagnostics (NVD). SWB is a shareholder of NVD. This does not alter our adherence to all the Vaccine policies on sharing data and materials.
REFERENCES
- 1.Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. Aids. 2008;22(3):339–348. doi: 10.1097/QAD.0b013e3282f3ca57. [DOI] [PubMed] [Google Scholar]
- 2.Burke B, Gomez-Roman VR, Lian Y, Sun Y, Kan E, Ulmer J, et al. Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits. Virology. 2009;387(1):147–156. doi: 10.1016/j.virol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spearman P, Lally MA, Elizaga M, Montefiori D, Tomaras GD, McElrath MJ, et al. A trimeric, V2-deleted HIV-1 envelope glycoprotein vaccine elicits potent neutralizing antibodies but limited breadth of neutralization in human volunteers. J Infect Dis. 2011;203(8):1165–1173. doi: 10.1093/infdis/jiq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert P, Wang M, Wrin T, Petropoulos C, Gurwith M, Sinangil F, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis. 2010;202(4):595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194(12):1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4(9):e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 8.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 9.Dey AK, Srivastava IK. Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev Vaccines. 10(2):227–251. doi: 10.1586/erv.10.142. [DOI] [PubMed] [Google Scholar]
- 10.Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 11.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 12.Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011;108(27):11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27(25–26):3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 16.O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6(5):699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- 17.O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011;10(4):447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 18.Ott G. The adjuvant MF59: a ten year perspective. New York, NY, USA: Humana Press; 2000. [Google Scholar]
- 19.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 20.Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26(26):3209–3222. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 21.Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines. 2003;2(2):197–203. doi: 10.1586/14760584.2.2.197. [DOI] [PubMed] [Google Scholar]
- 22.Vesikari T, Groth N, Karvonen A, Borkowski A, Pellegrini M. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine. 2009;27(45):6291–6295. doi: 10.1016/j.vaccine.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O'Hagan DT, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J. 2009;28(7):563–571. doi: 10.1097/INF.0b013e31819d6394. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 2008;197(5):667–675. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 25.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A. 2009;106(10):3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One. 2009;4(2):e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361(25):2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 28.Fragapane E, Gasparini R, Schioppa F, Laghi-Pasini F, Montomoli E, Banzhoff A. A heterologous MF59-adjuvanted H5N1 prepandemic influenza booster vaccine induces a robust, cross-reactive immune response in adults and the elderly. Clin Vaccine Immunol. 2010;17(11):1817–1819. doi: 10.1128/CVI.00461-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3(85) doi: 10.1126/scitranslmed.3002336. 85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keefer MC, Graham BS, McElrath MJ, Matthews TJ, Stablein DM, Corey L, et al. Safety and immunogenicity of Env 2–3, a human immunodeficiency virus type 1 candidate vaccine, in combination with a novel adjuvant, MTP-PE/MF59. NIAID AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1996;12(8):683–693. doi: 10.1089/aid.1996.12.683. [DOI] [PubMed] [Google Scholar]
- 31.Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28(38):6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singla AK, Chawla M, Singh A. Potential applications of carbomer in oral mucoadhesive controlled drug delivery system: a review. Drug Dev Ind Pharm. 2000;26(9):913–924. doi: 10.1081/ddc-100101318. [DOI] [PubMed] [Google Scholar]
- 34.Rabiskova M, Sedlakova M, Vitkova M, Kuna M. [Carbomers and their use in pharmaceutical technology] Ceska Slov Farm. 2004;53(6):300–303. [PubMed] [Google Scholar]
- 35.Mumford JA, Wilson H, Hannant D, Jessett DM. Antigenicity and immunogenicity of equine influenza vaccines containing a Carbomer adjuvant. Epidemiol Infect. 1994;112(2):421–437. doi: 10.1017/s0950268800057848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gualandi GL, Losio NM, Muratori G, Foni E. The ability by different preparations of porcine parvovirus to enhance humoral immunity in swine and guinea pigs. Microbiologica. 1988;11(4):363–369. [PubMed] [Google Scholar]
- 37.Tollersrud T, Norstebo PE, Engvik JP, Andersen SR, Reitan LJ, Lund A. Antibody responses in sheep vaccinated against Staphylococcus aureus mastitis: a comparison of two experimental vaccines containing different adjuvants. Vet Res Commun. 2002;26(8):587–600. doi: 10.1023/a:1020960402112. [DOI] [PubMed] [Google Scholar]
- 38.Krashias G, Simon AK, Wegmann F, Kok WL, Ho LP, Stevens D, et al. Potent adaptive immune responses induced against HIV-1 gp140 and influenza virus HA by a polyanionic carbomer. Vaccine. 2010;28(13):2482–2489. doi: 10.1016/j.vaccine.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 39.Cranage MP, Fraser CA, Cope A, McKay PF, Seaman MS, Cole T, et al. Antibody responses after intravaginal immunisation with trimeric HIV-1 CN54 clade C gp140 in Carbopol gel are augmented by systemic priming or boosting with an adjuvanted formulation. Vaccine. 2011;29(7):1421–1430. doi: 10.1016/j.vaccine.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava IK, Stamatatos L, Kan E, Vajdy M, Lian Y, Hilt S, et al. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J Virol. 2003;77(20):11244–11259. doi: 10.1128/JVI.77.20.11244-11259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava IK, Stamatatos L, Legg H, Kan E, Fong A, Coates SR, et al. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J Virol. 2002;76(6):2835–2847. doi: 10.1128/JVI.76.6.2835-2847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dey AK, David KB, Klasse PJ, Moore JP. Specific amino acids in the N-terminus of the gp41 ectodomain contribute to the stabilization of a soluble, cleaved gp140 envelope glycoprotein from human immunodeficiency virus type 1. Virology. 2007;360(1):199–208. doi: 10.1016/j.virol.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 44.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70(2):1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75(22):10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker CE, Deterding LJ, Hager-Braun C, Binley JM, Schulke N, Katinger H, et al. Fine definition of the epitope on the gp41 glycoprotein of human immunodeficiency virus type 1 for the neutralizing monoclonal antibody 2F5. J Virol. 2001;75(22):10906–10911. doi: 10.1128/JVI.75.22.10906-10911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allaway GP, Davis-Bruno KL, Beaudry GA, Garcia EB, Wong EL, Ryder AM, et al. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 1995;11(5):533–539. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 48.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, et al. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67(7):3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavacini L, Duval M, Song L, Sangster R, Xiang SH, Sodroski J, et al. Conformational changes in env oligomer induced by an antibody dependent on the V3 loop base. Aids. 2003;17(5):685–689. doi: 10.1097/00002030-200303280-00006. [DOI] [PubMed] [Google Scholar]
- 50.Dey AK, Khati M, Tang M, Wyatt R, Lea SM, James W. An aptamer that neutralizes R5 strains of human immunodeficiency virus type 1 blocks gp120-CCR5 interaction. J Virol. 2005;79(21):13806–13810. doi: 10.1128/JVI.79.21.13806-13810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore JP, Jarrett RF. Sensitive ELISA for the gp120 and gp160 surface glycoproteins of HIV-1. AIDS Res Hum Retroviruses. 1988;4(5):369–379. doi: 10.1089/aid.1988.4.369. [DOI] [PubMed] [Google Scholar]
- 52.Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70(3):1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore JP, Wallace LA, Follett EA, McKeating JA. An enzyme-linked immunosorbent assay for antibodies to the envelope glycoproteins of divergent strains of HIV-1. Aids. 1989;3(3):155–163. doi: 10.1097/00002030-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Humana Press; 2009. [DOI] [PubMed] [Google Scholar]
- 56.Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22(19):2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 57.Otten GR, Schaefer M, Doe B, Liu H, Srivastava I, Megede J, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J Virol. 2005;79(13):8189–8200. doi: 10.1128/JVI.79.13.8189-8200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez-Roman VR, Florese RH, Peng B, Montefiori DC, Kalyanaraman VS, Venzon D, et al. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr. 2006;43(3):270–277. doi: 10.1097/01.qai.0000230318.40170.60. [DOI] [PubMed] [Google Scholar]
- 59.Xu R, Srivastava IK, Greer CE, Zarkikh I, Kraft Z, Kuller L, et al. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res Hum Retroviruses. 2006;22(10):1022–1030. doi: 10.1089/aid.2006.22.1022. [DOI] [PubMed] [Google Scholar]
- 60.Shu Y, Winfrey S, Yang ZY, Xu L, Rao SS, Srivastava I, et al. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25(8):1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnett SW, Srivastava IK, Ulmer JB, Donnelly JJ, Rappuoli R. Development of V2-deleted trimeric envelope vaccine candidates from human immunodeficiency virus type 1 (HIV-1) subtypes B and C. Microbes Infect. 2005;7(14):1386–1391. doi: 10.1016/j.micinf.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 62.Lian Y, Srivastava I, Gomez-Roman VR, Zur Megede J, Sun Y, Kan E, et al. Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain. J Virol. 2005;79(21):13338–13349. doi: 10.1128/JVI.79.21.13338-13349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnett SW, Lu S, Srivastava I, Cherpelis S, Gettie A, Blanchard J, et al. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J Virol. 2001;75(12):5526–5540. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nandi A, Sun Y, Hartog K, Zambonelli C, Dubey A, Matsuoka K, et al. Selection and evaluation of HIV-1 Subtype C Early Transmitted Virus gp140 immunogens. 2011 Manuscript under preparation. [Google Scholar]
- 65.Donnelly L, Curran RM, Tregoning JS, McKay PF, Cole T, Morrow RJ, et al. Intravaginal immunization using the recombinant HIV-1 clade-C trimeric envelope glycoprotein CN54gp140 formulated within lyophilized solid dosage forms. Vaccine. 2011;29(27):4512–4520. doi: 10.1016/j.vaccine.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cranage MP, Fraser CA, Stevens Z, Huting J, Chang M, Jeffs SA, et al. Repeated vaginal administration of trimeric HIV-1 clade C gp140 induces serum and mucosal antibody responses. Mucosal Immunol. 2010;3(1):57–68. doi: 10.1038/mi.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Sahly H. MF59™ as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines. 2010;9(10):1135–1141. doi: 10.1586/erv.10.111. [DOI] [PubMed] [Google Scholar]
- 68.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nitayaphan S, Khamboonruang C, Sirisophana N, Morgan P, Chiu J, Duliege AM, et al. A phase I/II trial of HIV SF2 gp120/MF59 vaccine in seronegative thais. AFRIMS-RIHES Vaccine Evaluation Group. Armed Forces Research Institute of Medical Sciences and the Research Institute for Health Sciences. Vaccine. 2000;18(15):1448–1455. doi: 10.1016/s0264-410x(99)00421-1. [DOI] [PubMed] [Google Scholar]
- 70.Grundner C, Pancera M, Kang JM, Koch M, Sodroski J, Wyatt R. Factors limiting the immunogenicity of HIV-1 gp120 envelope glycoproteins. Virology. 2004;330(1):233–248. doi: 10.1016/j.virol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 71.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320(5877):760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 72.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 73.McKeage K, Romanowski B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix(R)): a review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs. 2011;71(4):465–488. doi: 10.2165/11206820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 74.De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, et al. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17(23–24):3094–3101. doi: 10.1016/s0264-410x(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 75.Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, et al. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17(2):99–104. doi: 10.1016/s0264-410x(98)00185-6. [DOI] [PubMed] [Google Scholar]
- 76.Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19(17–19):2673–2680. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 77.Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin Biol Ther. 2008;8(2):235–247. doi: 10.1517/14712598.8.2.235. [DOI] [PubMed] [Google Scholar]
- 78.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8(12):1663–1679. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 79.Jones T. GSK's novel split-virus adjuvanted vaccines for the prevention of the H5N1 strain of avian influenza infection. Curr Opin Mol Ther. 2009;11(3):337–345. [PubMed] [Google Scholar]
- 80.Carter NJ, Plosker GL. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs. 2008;22(5):279–292. doi: 10.2165/00063030-200822050-00001. [DOI] [PubMed] [Google Scholar]
- 81.Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34(4):514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 82.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17(4):479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 83.Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186(1):18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 84.Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008;105(30):10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Braun W, Nakano M. Antibody formation: stimulation by polyadenylic and polycytidylic acids. Science. 1967;157(3790):819–821. doi: 10.1126/science.157.3790.819. [DOI] [PubMed] [Google Scholar]
- 86.Winchurch R, Braun W. Antibody formation: premature initiation by endotoxin or synthetic polynucleotides in newborn mice. Nature. 1969;223(5208):843–844. doi: 10.1038/223843a0. [DOI] [PubMed] [Google Scholar]
- 87.Turner W, Chan SP, Chirigos MA. Stimulation of humoral and cellular antibody formation in mice by poly Ir:Cr. Proc Soc Exp Biol Med. 1970;133(1):334–338. doi: 10.3181/00379727-133-34469. [DOI] [PubMed] [Google Scholar]
- 88.Diamantstein T, Wagner B, Beyse I, Odenwald MV, Schulz G. Stimulation of humoral antibody formation by polyanions. II. The influence of sulfate esters of polymers on the immune response in mice. Eur J Immunol. 1971;1(5):340–343. doi: 10.1002/eji.1830010507. [DOI] [PubMed] [Google Scholar]
- 89.Diamantstein T, Wagner B, L'Age-Stehr J, Beyse I, Odenwald MV, Schultz G. Stimulation of humoral antibody formation by polyanions. 3. Restoration of the immune response to sheep red blood cells by polyanions in thymectomized and lethally irradiated mice protected with bone marrow cells. Eur J Immunol. 1971;1(4):302–304. doi: 10.1002/eji.1830010418. [DOI] [PubMed] [Google Scholar]
- 90.Diamantstein T, Ruhl H, Vogt W, Bochert G. Stimulation of B-cells by dextran sulphate in vitro. Immunology. 1973;25(4):743–747. [PMC free article] [PubMed] [Google Scholar]
- 91.Wetzel GD, Kettman JR. Activation of murine B cells. II. Dextran sulfate removes the requirement for cellular interaction during lipopolysaccharide-induced mitogenesis. Cell Immunol. 1981;61(1):176–189. doi: 10.1016/0008-8749(81)90364-6. [DOI] [PubMed] [Google Scholar]
- 92.Wetzel GD, Kettman JR. Activation of murine B lymphocytes. III. Stimulation of B lymphocyte clonal growth with lipopolysaccharide and dextran sulfate. J Immunol. 1981;126(2):723–728. [PubMed] [Google Scholar]
- 93.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2(15) doi: 10.1126/scitranslmed.3000624. 15ra5. [DOI] [PubMed] [Google Scholar]
- 94.Coucke D, Schotsaert M, Libert C, Pringels E, Vervaet C, Foreman P, et al. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine. 2009;27(8):1279–1286. doi: 10.1016/j.vaccine.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 95.Lee SH, Lillehoj HS, Jang SI, Lee KW, Yancey RJ, Dominowski P. The effects of a novel adjuvant complex/Eimeria profilin vaccine on the intestinal host immune response against live E. acervulina challenge infection. Vaccine. 2010;28(39):6498–6504. doi: 10.1016/j.vaccine.2010.06.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garg NK, Mangal S, Khambete H, Sharma PK, Tyagi RK. Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat Drug Deliv Formul. 2010;4(2):114–128. doi: 10.2174/187221110791185015. [DOI] [PubMed] [Google Scholar]


