Abstract
Depression is highly common throughout the life course and dementia is common in late life. The literature suggests an association between depression and dementia, and growing evidence implies that timing of depression may be important to defining the nature of the association. In particular, earlier-life depression or depressive symptoms consistently have been shown to be associated with a 2-fold or greater increase in risk of dementia. In contrast, studies of late-life depression have been more conflicting but the majority support an association; yet, the nature of this association is unclear (e.g., if depression is a prodrome or consequence or risk factor for dementia). The likely biological mechanisms linking depression to dementia include vascular disease, alterations in glucocorticoid steroids and hippocampal atrophy, increased deposition of β-amyloid plaques, inflammatory changes, and deficits of nerve growth factors. Treatment strategies for depression might intervene on these pathways and in turn may alter risk for dementia. Given the projected increase of dementia in the coming decades, it is critically important that we understand whether treatment for depression alone or combined with other regimens improves cognition. In this review, we summarize and analyze current evidence for late-life and earlier-life depression and their relationship to dementia, discuss the primary underlying mechanisms and implications for treatment.
Introduction
Depression is common across the lifespan with 1 in 5 individuals experiencing a depressive episode during their lifetime.1 Dementia is also very common in late life with the risk doubling every five years after age 65,2 increasing up to 50% among those ≥ 90 years.3,4 There are several ways in which depression and dementia may be related. First, depressive symptoms often occur among patients with dementia. Second, depression may be a reaction to early cognitive deficits. Third, depression can impair cognitive function leading to a “pseudodementia” presentation. Finally, depression may be a risk factor or early symptom of dementia.
Given the current and projected growth of the older segment of the population,5 a better understanding of the link between depression and risk of dementia is important, especially for possible treatment and prevention. However, several challenges to this understanding exist. Major depressive disorder is common among patients with dementia, occurring in up to 20% of patients with Alzheimer's disease (AD) and up to 50% of patients with vascular dementia (VaD)6-8 and, thus, disentangling which came first can be difficult. In addition, although depression and dementia are considered distinct clinical entities, they share some common features, including impairment in attention and working memory, changes in sleep patterns and reduction of social and occupational function.9 In fact, the concept of “pseudodementia” highlights how the distinction between depression and dementia may be blurry.10,11 Thus, the inter-relationship of depression and dementia is complex and when co-occurring the two entities may be indistinguishable, complicating the ability to determine the exact relationship of depression to dementia.
In this article, we review current knowledge on late-life and earlier-life depression and their associated risk of developing dementia, and focus on mechanisms that may underlie the link between depression and dementia. We primarily focus on the evidence suggesting that depression is a prodromal symptom or risk factor for dementia and discuss potential implications for screening and treatment.
Depression and risk of dementia
Most previous studies have examined late-life (e.g., that occur in those age 60 or older) depression or depressive symptoms and risk of dementia, while a few studies have considered earlier-life depression. Given the variable nature of depression onset, the high occurrence of depression in young adulthood and middle age, and the long preclinical period of dementia, studying earlier-life depression may offer an opportunity to determine if depression is a risk factor of dementia, years before the syndrome starts. On the other hand, a significant relationship of late-life depression and dementia may allow for better study of depression as part of the prodromal stage of dementia. As such, studies focusing on both earlier- and late-life depression may provide complementary evidence.
Late-life depression and dementia risk
Sixteen recent studies have examined late-life depression and dementia, 12 were of prospective design, while 2 were cross-sectional (Table 1) and 2 were meta-analyses (not shown in Table 1). Of the 12 prospective studies, 6 found evidence supporting that late-life depression was associated with a 2 to 5-fold increased risk of dementia,12-17 1 found that even 1 additional depressive symptom increased risk,18 3 found evidence supporting an association between late-life depression and dementia but only in specific subgroups,19-21 and 2 found no effect.22,23 Of the 2 longitudinal studies that found no effect, one was underpowered22 and the other measured history of depression with only a self-administered questionnaire which may be susceptible to recall bias.23
Table 1.
Studies of late-life depression and risk of dementia
| Study [country] |
Design | Number and age of study participants | Measure of depression | Dementia diagnosis | Results OR or HR (95% CI) |
|---|---|---|---|---|---|
| Hebert et al. (2000)17 [Canada] |
Prospective nested case-control | Cases, N=105 Controls, N=802 (≥ 65 years) |
Self-administered questionnaire for history | VaD over 5 years | 2.4 (1.2-4.5) |
| Zalsman et al. (2000)25 [Israel] |
Cross-sectional case-control | Cases, N=36 Controls, N=466 (≥ 50 years) |
Previous late-onset DSM-IV | All-cause | 1.9 (0.98-3.8) |
| Geerlings et al. (2000)21 [Netherlands] |
Prospective cohort | N=1,911 (≥ 65 years) non-demented subjects stratified by education | GMS | AD over 4 years | > 8 years of education: 3.7 (1.3-10.8) ≤ 8 years of education: 0.7 (0.2-2.5) |
| Lindsay et al. (2002)23 [Canada] |
Prospective nested case-control | Cases, N=194 (mean=81 years) Controls, N=3,894 (mean=73 years) |
Self-administered questionnaire for history | AD over 5 years | 1.4 (0.8-2.5) |
| Wilson et al. (2002)18 [U.S.] |
Prospective cohort | N=821 (≥ 65 years) non-demented clergy | 10-item CES-D score | AD over 7 years | 1.2 (1.1-1.3) |
| Fuhrer et al. (2003)19 [France] |
Prospective cohort | N=3,777 (≥ 65 years) subjects stratified by gender | 20-item CES-D (French version), repeated 4 visits, cutoff ≥ 16 | All-cause over 8 years | Women: 1.2 (0.7-2.0) Men: 3.5 (1.9-6.5) |
| Andersen et al. (2005)13 [Denmark] |
Prospective cohort | N=3,346 (≥ 65 years) | History assessed by interviewer | AD at baseline, 2 and 5 years | Baseline: 1.7 (1.0-2.7) 2-year: 1.9 (1.0-3.3) 5-year: 1.6 (0.9-2.7) |
| Gatz et al. (2005)14 [Canada] |
Prospective cohort | N=766 (≥ 65 years) | 20-item CES-D, several cutoffs | All-cause over 5 years | Total CES-D: 1.0 (1.0-1.1) CES-D ≥ 16: 2.4 (1.0-5.5) CES-D ≥ 21: 2.8 (0.98-8.1) |
| Cankurtaran et al. (2008)24 [Turkey] |
Cross-sectional | N=1,436 (≥ 65 years) | GDS and clinical evaluation | AD and VaD | AD: 1.4 (1.1-2.3) VaD: 1.2 (1.1-2.3) |
| Irie et al. (2008)20 [U.S., Asian] |
Prospective cohort | N=1,932 (> 70 years) non-demented men | 11-item CES-D, cutoff ≥ 9 | All-cause over 6 years | Depression: 1.6 (0.8-3.0) Depression + APOE4: 7.1 (3.0-16.7) |
| Chen et al. (2008)15 [China/UK] |
Prospective cohort | N=1,254 (China) and N=3,341 (UK) non-demented subjects (≥ 65 years) | GMS and AGECAT, severe depression | All-cause over 1-4 years | China (1-year): 5.1 (1.6-16.3) UK (2-year): 2.1 (1.1-4.1) UK (4-year): 2.5 (1.2-5.2) |
| Becker et al. (2009)22 [U.S.] |
Prospective cohort | N=288 (≥ 70 years) subjects adjudicated cognitively normal | 10-item CES-D, persistently elevated, cutoff > 9 | All-cause over 9 years | 1.3 (0.5-3.7) |
| Saczynski et al. (2010)12 [U.S.] |
Prospective cohort | N=949 (mean=79 years) non-demented subjects | 20-item CES-D, cutoff ≥ 16 | All-cause over 17 years | 1.7 (1.0-2.8) |
| Byers et al. (in press)16 [U.S.] |
Retrospective cohort | N=281,540 (≥ 55 years) veterans without dementia diagnosis | ICD-9, depression and dysthymia | All-cause over 7 years | Depression: 2.2 (CI 2.1-2.3) Dysthymia: 2.0 (CI 1.7-2.3) |
Abbreviations: AD, Alzheimer disease; AGECAT, Automated Geriatric Examination for Computer Assisted Taxonomy123; CES-D, Center for Epidemiological Studies Depression Scale124; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition125; GDS, Geriatric Depression Scale126; GMS, Geriatric Mental State Schedule – Dutch version (for Geerlings et al.),127 Geriatric Mental State Examination (for Chen et al.)128; HR, hazard ratio; ICD-9, International Classification of Diseases, Ninth Revision; OR, odds ratio; VaD, vascular dementia.
The longest longitudinal study covering 17 years of follow-up reported a 70% greater risk of developing dementia with depression,12 suggesting that longer follow-up may allow for better assessment of depression as a risk factor for dementia. However, the follow-up times and statistical significance of other studies were highly variable. For example, one study reported that history of depression increased risk of AD almost 2-fold at baseline and 2-year follow-up, but the association was not observed at 5-year follow-up.13 While another study found significant results at 5-year follow-up,14 and still another found non-significant results at 9-year follow-up.22 These discrepancies might be explained by variations in sample sizes, measures, and frequency and severity of depression. In fact, severity of late-life depression has been found to be important to the risk of dementia, as shown by four longitudinal studies. One study demonstrated that number of depressive symptoms at baseline predicted development of AD during a 7-year period with risk of AD increasing by 20% with each additional depressive symptom.18 While two other studies reported that more severe depressive symptoms14 or a clinical diagnosis of depression15 increased risk almost 3-fold as much as five years later. Finally, one longitudinal study included a separate diagnosis for dysthymia or minor depression. This study reported that severity of depression matters,16 since patients diagnosed with dysthymia and those diagnosed with depression had more than a 2-fold increased risk of dementia diagnosis over 7 years compared to those without dysthymia or depression, and there was a “dose-effect” with depression having a higher risk compared with dysthymia.
Another longitudinal study reported an almost 4-fold increased likelihood of depression associated with dementia over 8 years, but the finding was only observed among men.19 Interestingly, one longitudinal study found that depression was significantly associated with a 7-fold increased risk of dementia over a 6-year period, but only when considered together with apolipoprotein E ε4 status.20 Additionally, a third study demonstrated that among older adults with more than 8 years of education those with depressed mood had nearly a 4-fold increased odds of developing AD during a 4-year period of follow-up compared to those without depressed mood.21 These studies suggest that late-life depression may be an early symptom or subclinical expression of dementia that is more overt in certain groups, and, perhaps, observed at the time of more advanced AD.
The two cross-sectional studies are conflicting on whether late-life depression and dementia are associated. One of these studies reported that individuals with depression were 20% and 40% more likely to be diagnosed with VaD and AD, respectively.24 The other cross-sectional (case-control) study showed a marginal association between depression and all-cause dementia, but the study was underpowered.25 In agreement with the former cross-sectional study, a prospective nested case-control study found a 2-fold increased likelihood of incident VaD in patients with a history of depression,17 suggesting that results may be consistent across AD and VaD.
Two separate meta-analyses concluded that depression significantly increased the risk of dementia by 2-fold.26,27 These meta-analyses included mostly case-control studies from prior to 2000, but the pooled findings suggest that late-life depression is associated with subsequent dementia, even if the effect is not robust across all individual studies.
Current research supports an association between late-life depression and risk of dementia, but inconsistencies across individual studies exist. Discrepancies may be due to methodological differences (e.g., sampling procedures and attrition rates, definition and operationalization of depression), potential cultural differences between the various studies (i.e., U.S., Canada, Israel, Netherlands, France, Denmark, Turkey, China, and UK), or sub-population differences (e.g., veterans, clergy, Japanese American men). Further, because of the timing of many of these studies in late life, they cannot distinguish if depression or depressive symptoms are a prodromal phase of dementia or consequence of the onset of AD or risk factor. In fact, the variability of the duration of follow-up (0-17 years) and unknown frequency or duration of depressive episodes may explain the heterogeneity of findings.
Earlier-life depression and dementia risk
Recent studies investigating the risk of dementia associated with earlier-life depression (typically defined as depression or depressive symptoms occurring before age 60) have included case-control and prospective cohort studies (Table 2). These studies have used both historical measures of depression diagnosis and standard instruments for symptoms or diagnosis. Together these studies suggest that earlier life or early onset of depression is significantly associated with risk of developing dementia.
Table 2.
Studies of earlier-life depression and risk of dementia
|
Study [country] |
Design | Number and age of study participants | Measure of depression | Dementia diagnosis | Timing of depression | Results OR or HR (95% CI) |
|---|---|---|---|---|---|---|
| Green et al. (2003)32 [U.S.] |
Cross-sectional case-control | Cases, N=1,953 (mean=70 years) Controls, N=2,093 (mean=70 years) |
Respond ‘yes’ to single depression question§; additionally, asked age at first episode | AD, or definite AD by autopsy | First occurred before AD: a)1 year b)> 1 year c)> 25 years |
a)4.6 (2.9-7.3) b)1.4 (1.0-1.9) c)1.7 (1.0-2.8) |
| Dal Forno et al. (2005)29 [U.S.] |
Prospective cohort | N=1,357 (≥ 38 years) non-demented subjects | 20-item CES-D over multiple times, cutoff ≥ 16 | All-cause over 14 years | Depression at a)no lag b)2-year lag c)≥ 4-year lag before onset of dementia symptoms |
a)Women: 1.2 (0.7-2.0) Men: 2.3 (1.6-3.2) b)Women: 1.4 (0.8-2.3) Men: 2.1 (1.4-3.1) c)Women: 1.3 (0.7-2.4) Men: 2.0 (1.2-3.1) |
| Geerlings et al. (2008)28 [Netherlands] |
Prospective cohort | N=486 (≥ 60 years) non-demented subjects | History, age at onset, and current symptoms (20-item CES-D, cutoff ≥ 16) | AD over 10 years | a)Early-onset, age < 60 years b)Late-onset, age ≥ 60 years |
a)3.8 (1.4-10.1) b)2.3 (0.8-6.7) |
| Dotson et al. (2010)30 [U.S.] |
Prospective cohort | N=1,239 (≥ 50 years) non-demented subjects | 20-item CES-D, cutoff ≥ 16 | All-cause up to 51 years | Recurrent depression: 0, 1, or 2+ episodes | 1: 1.9 (1.2-2.9) 2+: 2.1 (1.2-3.5) |
| Barnes et al. (2010)31 [U.S.] |
Prospective cohort | N=13,535 (≥ 40 years at mid-life) non-demented subjects | Question assessing feeling unhappy or depressed measured at mid-life and ICD-9 diagnoses assessed at mid-life and late-life | AD and VaD up to 45 years | a)Mid-life depressive symptoms b)Late-life depression c)Both mid-life and late-life |
a)AD: 1.1 (0.9-1.3) VaD: 1.2 (0.9-1.7) b)AD: 2.1 (1.7-2.6) VaD: 1.5 (1.0-2.1) c)AD: 2.0 (1.5-2.7) VaD: 3.5 (2.4-5.1) |
Single depression question: Aside from normal reaction to bereavement (death in family) or the impact of physical/medical condition, has there been a period of weeks to several months (or longer) when you were unable to perform social and occupational functions normally because of depression?” [For proxy reporting about a relative with AD, the question substituted “your relative was” for “you were.”]
Abbreviations: AD, Alzheimer disease; CES-D, Center for Epidemiological Studies Depression Scale124; HR, hazard ratio; ICD-9, International Classification of Diseases, Ninth Revision; OR, odds ratio; VaD, vascular dementia.
Four of the 5 studies were longitudinal and suggest that early onset of depression, as well as the duration and frequency of depression, were associated with a 2 to 4-fold increased risk of developing dementia. The one longitudinal study that found an almost 4-fold increased risk of AD among those with early-onset depression (prior to age 60 years), but not late-onset, appeared underpowered.28 Although following a large number of subjects over many years can be challenging and costly, the other three longitudinal studies had larger sample sizes and greater follow-up time. Two of these studies used innovative methods and assessed depressive symptoms as time-dependent covariates. The first study used a lag time approach of depression assessment (i.e., depression analyzed at multiple preset time lengths prior to dementia onset) in their analyses in order to better address temporality and direction of the depression-dementia association,29 while the second study measured recurrent depression over many decades.30 The results of the first study suggest that prodromal dementia was not the cause of the association, because the 2-fold increase in risk of dementia associated with depression remained (for men) when the lag between detection of symptoms and diagnosis of dementia was more than 4 years. The second study demonstrated a strong association between the number of depressive episodes (i.e., recurrent depression) and risk of dementia over a median follow-up time of 24 years, suggesting a dose-dependent relationship of cumulative depression episodes to risk of dementia.
One longitudinal study examined the association of earlier-life depression with different subtypes of dementia, including VaD and AD, and compared risk of dementia in those with depressive symptoms at mid- and late-life.31 The authors concluded that mid-life depressive symptoms were associated with risk of AD and VaD, whereas risk of AD was approximately doubled in individuals with depressive symptoms in late life (either alone or in combination with mid-life symptoms) while the risk of VaD was more than tripled in those with both mid-life and late-life depressive symptoms. These findings suggest that depression over the lifetime is associated with greater risk of VaD but risk of AD was more dependent on late-life exposure.
Although the one cross-sectional (case-control) study suggests that history of depression even 25 years prior to AD onset is significantly associated with an almost 2-fold increase in the likelihood of developing AD,32 depression symptoms within the first year before onset of AD were associated with an almost 5-fold increased risk. This finding may be due to assessment of depressive symptoms close in time to dementia diagnosis with an overlap of symptomatology. In addition, despite the study's large sample size and ability to assess multiple years of depression symptoms prior to onset of AD, the results of this study need to be interpreted with caution as their accuracy may be questionable due to recall bias, e.g., informants of patients with AD may be more likely to recall events in line with their preconceptions about potential links.
In sum, earlier-life depression consistently has been studied to be a risk factor (and unlikely prodrome) to dementia. A strength of these studies, unlike the studies of late-life depression, are their relative homogeneity—four out of five are samples from the U.S. population, all measured depressive symptoms with similar questions and cutoffs, and all had substantial follow-up time. However, more longitudinal studies over the lifespan are necessary in order to further understand the relationship of depression and depressive symptoms to risk of developing dementia in late life.
Potential mechanisms
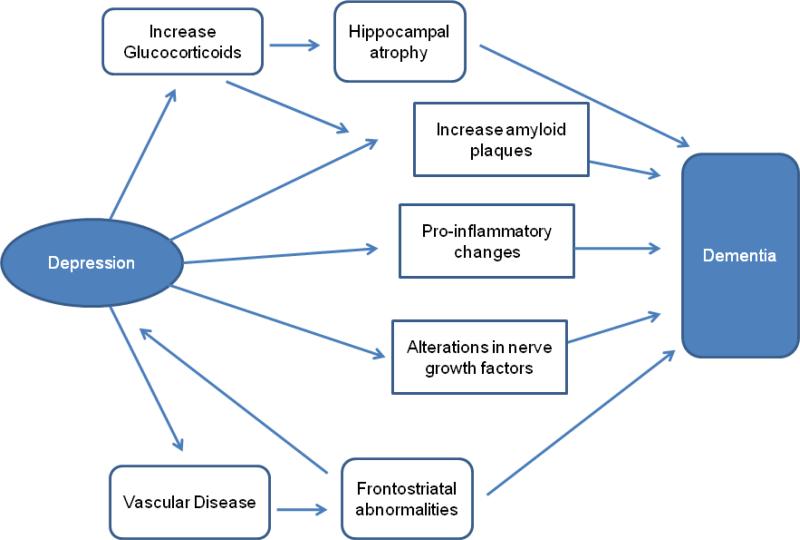
The most prominent mechanisms that may link depression and dementia are presented in Figure 1. Each hypothesized pathway represents a mechanistic link between depression-related processes and dementia-specific neuropathology including: 1) vascular disease; 2) alterations in glucocorticoid steroids and hippocampal atrophy; 3) increased deposition of β-amyloid plaques; 4) inflammatory changes; and 5) deficits of nerve growth factors or neurotrophins.
Figure 1.
Proposed predominant pathways linking depression as a risk factor to the onset of dementia
Vascular Disease
Vascular disease has the strongest evidence linking depression and dementia. This link is largely grounded in the “vascular depression hypothesis”,33,34 which postulates that cerebrovascular disease predisposes, precipitates, or perpetuates some geriatric depressive syndromes.35,36 A number of studies have reported that vascular lesions as well as structural brain changes may contribute to depression in late life.37-40 However, whether vascular disease or vascular lesions contribute to depression or result from depression is debatable, as each condition is associated with an increased risk of developing the other.41
Figure 1 shows that the pathway from vascular disease to depression to dementia is probably not sequential. Prior depression is related to subsequent vascular disease through multiple proposed mechanisms, including behavioral conditions (e.g., smoking, inactivity), hypothalamic-pituitary-adrenal (HPA) axis dysregulation and elevated cortisol related to the metabolic syndrome, disruption of normal endothelial function and development of hypertension, and pro-inflammatory cytokines.42 In particular, depression increases risk for first-ever myocardial infarction and stroke.43 Additionally, there is strong evidence that vascular disease promotes development of depression. Risk for depression is substantially increased post-MI and post-stroke.41 In particular, MRI studies have shown robust associations between ischemic brain lesions and depression or depressive symptoms in older adults.44,45 Longitudinal studies provide evidence that large cortical white matter lesions and severe subcortical white matter grade are significant risk factors for developing depressive symptoms.45 These white matter changes have been found to predate and predict late-life depression.45,46
In further support of the “vascular-depression-dementia hypothesis,” it has been determined that vascular disease contributes to the clinical manifestation of dementia symptoms.47,48 Ischemic damage, largely in the frontostriatal brain regions, may lead to significant cognitive deficits.49 Finally, the ischemic damage to frontostriatal brain regions may explain the executive function, psychomotor slowing and resistance to treatment common in late-life depression.42,49,50 This suggests that ischemic structural changes in the brain are a common etiologic factor of both the depression and the related cognitive impairment.
Cortisol-Hippocampal Pathway
Increased cortisol production leading to atrophy of the hippocampus is another of the primary proposed links of depression and dementia. In this pathway, depression and depressive symptoms activate the HPA axis and increase glucocorticoid production, which may in turn damage the hippocampus and results in a down-regulation of glucocorticoid receptors (Figure 1). The net effect is impaired negative feedback to the HPA axis and chronic elevation of adrenal glucocorticoids (or “glucocorticoid cascade”51); ultimately resulting in a vicious cycle with hippocampus atrophy and cognitive deficits.42,52 Impairment in glucocorticoid metabolism (e.g., increased cortisol bioavailability) has been documented in patients with depression53 and patients with dementia.54 Additionally, atrophy of the hippocampus is one of the early brain changes in AD,55 and reduced hippocampal volume has been found in patients with depression.56-58
Research investigating the cortisol-hippocampal link has mainly come from animal studies involving response to stress and suggest that high-stress conditions or exogenous glucocorticoids can cause hippocampal neuronal damage59 and memory impairment60 and that hippocampal damage progresses over a lifetime of stress or glucocorticoid excess.61 Because stressful life events are highly related to depressive episodes in humans62-64 and the HPA axis is hyperactive in depression,65,66 a similar cycle may exist in humans.
Some studies have found that hippocampal volume loss increases the risk of cognitive decline67,68 and even dementia69 in adults with depression, but these findings are conflicting.28,70 In one study, history of depression was not associated with hippocampal volumes at baseline and these volumes did not mediate the increased risk for AD associated with prior depression28 implying that the effect of depression is not through hippocampal atrophy. In contrast, reductions in hippocampal volume have been more consistently found among individuals with recurrent depression and longer duration of illness,58,71,72 suggesting support for the cortisol-hippocampal pathway.
However, even if it is confirmed that the relationship between depression and hippocampal atrophy exists, there is inconsistent evidence that this association is mediated by increased cortisol levels.56,58 Additionally, in other studies, hippocampal atrophy was associated with inflammatory changes and deficits of nerve growth factors in patients with depression and other psychiatric disorders.73-75 This suggests that mechanisms other than or in addition to elevated cortisol levels may be important, but further research is necessary to elucidate pathways.
Amyloid plaque formation
Like hippocampal atrophy, amyloid plaques in the brain are a diagnostic feature of AD. Both β-amyloid and tau protein accumulate in AD and are the main constituents of neuritic plaques and neurofibrillary tangles, respectively.76 Interestingly, studies have shown that both plaques and tangles accumulate in higher numbers in the hippocampus of AD patients with depression as compared with AD patients without depression.77,78 Further, the literature supports the role of β-amyloid as primary in promoting the cascade of events that leads to neuronal death and AD.79,80 Thus, a possible mechanism linking depression to AD involves amyloid plaque formation; however, the association is controversial and not well understood.
One hypothesized pathway linking depression and β-amyloid may occur due to increased β-amyloid production initiated by a stress response associated with depression and glucocorticoids.11,52 This has primarily been suggested by animal models of AD, where administration of stress-level glucocorticoids promotes β-amyloid formation by increasing steady-state levels of amyloid precursor protein (APP) and the β-APP cleaving enzyme.81 Additionally, depression may influence β-amyloid accumulation through direct interaction with the amyloidogenic processing early in AD, a process that may be linked to the serotonergic system.52,82,83
Finally, not all, but some recent work has shown that plasma β-amyloid concentrations in patients with depression are related to cognitive impairment. A type of amyloid-associated depression [depression with a high ratio of plasma β-amyloid peptide 40 (Aβ40) to Aβ42] has been linked with impairment in memory, visuospatial abilities, and executive function.84 Although more research is necessary in this area, amyloid-associated depression may represent a preclinical or prodromal depression of AD or a path to AD.
Inflammatory changes
Research suggests that chronic inflammation has a central role in the pathophysiology of both depression and dementia.73,85,86 Figure 1 suggests two potential pathways that involve inflammatory changes within the central nervous system (CNS). First, the increase in cytokines found in depression may lead to a decrease in anti-inflammatory and immunosuppressant regulation, an increase in pro-inflammatory changes within the CNS, and ultimately cognitive deficits and dementia.87,88 Second, pro-inflammatory cytokines interfere with serotonin metabolism, and reduce both synaptic plasticity and hippocampal neurogenesis.11,73
The inflammatory process is potentially intricately linked with multiple neurodegenerative pathways for depression and pro-inflammatory cytokines. In in vitro and in vivo studies, β-amyloid activates the microglia to release pro-inflammatory cytokines,89 and microglia activation has been found in patients with mild cognitive impairment.90 Thus, chronic inflammation may explain why a history of depression can promote development of dementia; however, more studies are necessary to elucidate and confirm this link, as the role of microglial cells and inflammation has been recently challenged.91
Nerve growth factors
Another proposed link between depression and dementia is a decrease in the levels and activities of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) (Figure 1).11 This neurotrophin is part of a class of growth factors that are necessary for maintenance of neuronal health and modulation of synaptic plasticity.92 Impairment in BDNF signaling has been detected in stress-induced animal models of depression93 and individuals with depression,75,94 as well as exhibited in AD patients.95,96 In fact, studies of both depressed and AD patients have demonstrated decreased mRNA levels of BDNF in the hippocampus;52,75 however, there is conflicting data on this.97,98 Given these findings and the key role BDNF plays in regulating hippocampal plasticity, BDNF is presumed to be important to the integrity of the hippocampus and to the maintenance of cognition. Other important neurotrophic factors include transforming growth factor-β1 (TGF-β1), insulin-like growth factor-1 (IGF-1), and vascular endothelial-derived growth factor (VEGF).99
Treatment and management implications
Currently, there are 35.6 million individuals with dementia worldwide and the associated costs in 2010 were more than 600 billion (U.S.) dollars.100 Dementia is projected to increase to 65.7 million by 2030 with associated costs expected to rise 85%.100 Thus, delaying dementia onset could prevent millions of cases and save billions of dollars.
Although research to date has not resolved whether depression is a prodrome or risk factor for dementia, the link between depression and risk of dementia as a whole suggests a strong association. If a patient presents with depression or depressive symptoms, particularly in late life, then screening for cognitive deficits should become part of the patient's management or clinical care. In addition, studies on earlier-life depression suggest that older adults with a history of previous depression should be closely monitored for both recurrent depression and cognitive decline. Some studies have shown that treatment of depression in elderly patients (i.e., pharmacological, behavioral, or other modalities) improves cognition, leading to improved memory and other cognitive performance101-104 and may reduce pathophysiological alterations related to dementia,71,105-107 but other studies have shown that cognitive deficit either persists or still ensues after successful treatment for depression.108-110 When considering the risk of developing AD more than doubles in adults with co-occurring depression and mild cognitive impairment (MCI) than MCI alone,111 monitoring and reducing cognitive decline in depressed individuals with executive as well as memory impairment is imperative.
An alternative strategy to improving the course of cognitive decline with depression-interventions is an integrated treatment approach. A recent pilot study in this area has shown promising results, where addition of a cholinesterase inhibitor following antidepressant medication treatment in elderly depressed, cognitively impaired patients showed significant improvement in memory.112 Another approach may be to combine antidepressants with behavioral interventions that may protect against cognitive decline (e.g., exercise, cardiovascular risk modification). A combined treatment approach of antidepressants, cholinesterase inhibitors, vitamins, and diet, lifestyle and exercise modifications has been found to protract cognitive decline over 24 months and improve memory and frontal lobe functions.113 Although testing the efficacy and effectiveness of such interventions is in its infancy, these integrated strategies hold great promise. Thus, future studies should continue to examine the implication for depression modification in clinical trials, focusing on whether simultaneous or subsequent interventions have additive or multiplicative effects on cognition.
Of a related topic, the efficacy and effectiveness of treating depression and reducing depressive symptoms in patients with AD is controversial. Although experts recommend treating depression in AD,114 findings from controlled pharmacological trials are less certain. Some studies have shown that antidepressant treatment reduces depressive symptoms,115 while most have shown no difference between placebo and standard antidepressant medications in patients with depression and AD.116-118 This suggests potentially a different neuropathology of depressive symptoms in the context of AD. For example, there has been consistent evidence that an increased loss of norepinephrine-producing neurons in the locus coeruleus is associated with co-occurring depression and AD,119-121 implicating the noradrenergic system as another pathway linking depression to dementia and another possible target of intervention. Thus, more work is necessary in this area, as it is essential to reduce neuropsychiatric disturbances in AD patients and improve their quality of life.
Conclusions
This Review highlights the current research on depression and risk of dementia. These findings emphasize the importance of closely monitoring depression for development of dementia. Further, there is a need for study of potential mechanisms linking depression to AD and VaD in order to target intervention, prevention, and health care needs. In particular, future research should include a life course approach to the study of depression and dementia. Earlier-life depression has been shown to be an important risk factor for dementia, but whether it is a true risk factor for development of dementia or whether a third factor causes depression and dementia is still unknown. In addition, the nature of the association between late-life depression and dementia is unclear. Therefore, a life course approach to the study of depression and risk of developing dementia would be highly informative.
In sum, the population is rapidly aging and the risk of dementia is projected to quadruple by 2050,122 thus intervening on a modifiable risk factor like depression and studying its effect on reducing the risk of dementia is imperative. The underlying pathway linking depression to dementia is multi-factorial and probably not mutually exclusive, suggesting a combined regimen as the most promising treatment approach.
Key Points.
Depression is common throughout the life course, while dementia is very common in late life.
Late-life depression or depressive symptoms may be associated with dementia, but inconsistencies across studies exist.
Current research supports that earlier-life depression or depressive symptoms are associated with dementia; however, more studies are needed examining depression over the life course.
The most likely links are the following hypothesized mechanisms: 1) vascular disease; 2) alterations in glucocorticoid steroids and hippocampal atrophy; 3) increased deposition of β-amyloid plaques; 4) inflammatory changes; and 5) deficits of nerve growth factors or neurotrophins.
A patient presenting with early- or late-life depression or depressive symptoms, especially if chronic, should be screened and monitored for cognitive deficits over the long term.
It is critically important to determine if treatment of depression alone or combined with other regimens would delay or prevent dementia.
ACKNOWLEDGMENTS
Study funding: This work was supported by the National Institute of Mental Health (K01 MH079093 [ALB] and R01 MH086498 [KY]) and the National Institute on Aging (K24 AG031155 [KY]).
Footnotes
Amy L. Byers is Assistant Professor of Psychiatry, University of California, San Francisco and Mental Health Research PI, San Francisco VA Medical Center
Kristine Yaffe is Professor of Psychiatry, Neurology and Epidemiology, University of California, San Francisco and Chief of Geriatric Psychiatry and Director of the Memory Disorders Clinic, San Francisco VA Medical Center
Review Criteria
Recent literature on late-life and earlier-life depression and risk of developing dementia (i.e., clinical diagnosis based on standard criteria and a control group for comparison) were examined for this Review. Studies were reviewed if they were published in 2000 or later, if they measured late-life or earlier-life depression and late-life dementia, and if they were published in English. Articles were selected on the basis of the authors’ personal knowledge and searches of the PubMed database using each of the terms “depression”, “depressive symptoms”, and “major depression” in combination with the terms “dementia” or “Alzheimer's disease” or “vascular dementia.” Reference lists of the identified papers were examined for further leads. The final selection was based on relevance, as judged by the authors.
Conflicts of interest: Dr. Byers reports no financial relationships with commercial interests. Dr. Yaffe reports having served as a consultant to Novartis, Inc. for reasons not related to the current project, and she serves on DSMB committees for Pfizer, Medivation, Inc., and CITAD trial for the NIMH. The authors have no competing interests, including specific financial interests or relationships or affiliations relevant to the subject of this manuscript.
References
- 1.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 3.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, et al. Mild cognitive impairment, dementia and subtypes among oldest old women. Arch. Neurol. doi: 10.1001/archneurol.2011.82. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Census Bureau US. An Older and More Diverse Nation by Midcentury. U.S. Census Bureau Newsroom [online] 2008 http://www.census.gov/newsroom/releases/archives/population/cb08-123.html.
- 6.Park JH, et al. Depression in vascular dementia is quantitatively and qualitatively different from depression in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2007;23:67–73. doi: 10.1159/000097039. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, Bannister C, Solis M, Oyebode F, Wilcock G. The prevalence, associations and symptoms of depression amongst dementia sufferers. J. Affect. Disord. 1996;36:135–144. doi: 10.1016/0165-0327(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 8.Ballard C, et al. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J. Affect. Disord. 2000;59:97–106. doi: 10.1016/s0165-0327(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 9.Steffens DC, Potter GG. Geriatric depression and cognitive impairment. Psychol. Med. 2008;38:163–175. doi: 10.1017/S003329170700102X. [DOI] [PubMed] [Google Scholar]
- 10.Korczyn AD, Halperin I. Depression and dementia. J. Neurol. Sci. 2009;283:139–142. doi: 10.1016/j.jns.2009.02.346. [DOI] [PubMed] [Google Scholar]
- 11.Caraci F, Copani A, Nicoletti F, Drago F. Depression and Alzheimer's disease: neurobiological links and common pharmacological targets. Eur. J. Pharmacol. 2010;626:64–71. doi: 10.1016/j.ejphar.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Saczynski JS, et al. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen K, Lolk A, Kragh-Sørensen P, Petersen NE, Green A. Depression and the risk of Alzheimer disease. Epidemiology. 2005;16:233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- 14.Gatz JL, Tyas SL, St John P, Montgomery P. Do depressive symptoms predict Alzheimer's disease and dementia? J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:744–747. doi: 10.1093/gerona/60.6.744. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, et al. Severity of depression and risk for subsequent dementia: cohort studies in China and the UK. Br. J. Psychiatry. 2008;193:373–377. doi: 10.1192/bjp.bp.107.044974. [DOI] [PubMed] [Google Scholar]
- 16.Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am. J. Geriatr. Psychiatry. doi: 10.1097/JGP.0b013e31822001c1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hébert R, et al. Vascular dementia: incidence and risk factors in the Canadian study of health and aging. Stroke. 2000;31:1487–1493. doi: 10.1161/01.str.31.7.1487. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrer R, Dufouil C, Dartigues JF. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J. Am. Geriatr. Soc. 2003;51:1055–1063. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- 20.Irie F, et al. Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia Aging Study. Arch. Gen. Psychiatry. 2008;65:906–912. doi: 10.1001/archpsyc.65.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geerlings MI, et al. Depressive symptoms and risk of Alzheimer's disease in more highly educated older people. J. Am. Geriatr. Soc. 2000;48:1092–1097. doi: 10.1111/j.1532-5415.2000.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 22.Becker JT, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am. J. Geriatr. Psychiatry. 2009;17:653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay J, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 24.Cankurtaran M, et al. Risk factors and type of dementia: vascular or Alzheimer? Arch. Gerontol. Geriatr. 2008;47:25–34. doi: 10.1016/j.archger.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Zalsman G, et al. Increased risk for dementia in elderly psychiatric inpatients with late-onset major depression. J. Nerv. Ment. Dis. 2000;188:242–243. doi: 10.1097/00005053-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust. N. Z. J. Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 27.Ownby RL, et al. Depression and risk for Alzheimer Disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 29.Dal Forno G, et al. Depressive symptoms, sex, and risk for Alzheimer's disease. Ann. Neurol. 2005;57:381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 30.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes DE, et al. Mid-life versus late-life depressive symptoms and risk of dementia: differential effects for Alzheimer's disease and vascular dementia.. The Alzheimer's Association 2010 International Conference on Alzheimer's Disease; Honolulu, HI. July 10-15, 2010. [Google Scholar]
- 32.Green RC, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch. Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos GS, et al. Vascular depression” hypothesis. Arch. Gen. Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan KRR, Hays JC, Blazer DG. MRI-defined vascular depression. Am. J. Psychiatry. 1997;154:497–500. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 35.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 36.Alexopoulos GS. Vascular disease, depression, and dementia. J. Am. Geriatr. Soc. 2003;51:1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- 37.Camus V, Kraehenbuhl H, Preisig M, Bula CJ, Waeber G. Geriatric depression and vascular diseases: what are the links? J. Affect. Disord. 2004;81:1–16. doi: 10.1016/j.jad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Rao R. Cerebrovascular disease and late life depression: an age old association revisited. Int. J. Geriatr. Psychiatry. 2000;15:419–433. doi: 10.1002/(sici)1099-1166(200005)15:5<419::aid-gps140>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.de Groot JC, et al. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch. Gen. Psychiatry. 2000;57:1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 40.Thomas AJ, Perry R, Barber R, Kalaria RN, O'Brien JT. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann. N. Y. Acad. Sci. 2002;977:333–339. doi: 10.1111/j.1749-6632.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 41.Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J. Affect. Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- 42.Butters MA, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebetrau M, Steen B, Skoog I. Depression as a risk factor for the incidence of first-ever stroke in 85-year-olds. Stroke. 2008;39:1960–1965. doi: 10.1161/STROKEAHA.107.490797. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J. Neurol. Neurosurg. Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 45.Steffens DC, et al. Cerebrovascular disease and evolution of depressive symptoms in the Cardiovascular Health Study. Stroke. 2002;33:1636–1644. doi: 10.1161/01.str.0000018405.59799.d5. [DOI] [PubMed] [Google Scholar]
- 46.Teodorczuk A, et al. White matter changes and late-life depressive symptoms: longitudinal study. Br. J. Psychiatry. 2007;191:212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 47.Flicker L. Vascular factors in geriatric psychiatry: time to take a serious look. Curr. Opin. Psychiatry. 2008;21:551–554. doi: 10.1097/YCO.0b013e32830ef736. [DOI] [PubMed] [Google Scholar]
- 48.Flicker L. Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clin. Geriatr. Med. 2010;26:17–27. doi: 10.1016/j.cger.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol. Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Sheline YI, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am. J. Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 52.Sierksma AS, van den Hove DL, Steinbusch HW, Prickaerts J. Major depression, cognitive dysfunction and Alzheimer's disease: is there a link? Eur. J. Pharmacol. 2010;626:72–82. doi: 10.1016/j.ejphar.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress. Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 54.Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer's disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van de Pol LA, et al. Hippocampal atrophy in Alzheimer disease: age matters. Neurology. 2006;66:236–238. doi: 10.1212/01.wnl.0000194240.47892.4d. [DOI] [PubMed] [Google Scholar]
- 56.O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am. J. Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 57.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 58.Colla M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J. Psychiatr. Res. 2007;41:553–560. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Cereseto M, et al. Chronic treatment with high doses of corticosterone decreases cytoskeletal proteins in the rat hippocampus. Eur. J. Neurosci. 2006;24:3354–3364. doi: 10.1111/j.1460-9568.2006.05232.x. [DOI] [PubMed] [Google Scholar]
- 60.Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn. Mem. 2008;15:271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: synaptic plasticity and memory. Stress. 2006;9:1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- 62.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 63.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 64.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. S. T. K. E. 2004;225:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 65.Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids. Mood, memory, and mechanisms. Ann. N. Y. Acad. Sci. 2009;1179:19–40. doi: 10.1111/j.1749-6632.2009.04980.x. [DOI] [PubMed] [Google Scholar]
- 66.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Hickie I, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br. J. Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 68.Steffens DC, McQuoid DR, Payne ME, Potter GG. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am. J. Geriatr. Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steffens DC, et al. Hippocampal volume and incident dementia in geriatric depression. Am. J. Geriatr. Psychiatry. 2002;10:62–71. [PubMed] [Google Scholar]
- 70.Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 71.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 72.MacQueen GM, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maes M, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 74.Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol. Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 75.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 76.Morishima-Kawashima M, Ihara Y. Alzheimer's disease: beta-Amyloid protein and tau. J. Neurosci. Res. 2002;70:392–401. doi: 10.1002/jnr.10355. [DOI] [PubMed] [Google Scholar]
- 77.Rapp MA, et al. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am. J. Geriatr. Psychiatry. 2008;16:168–174. doi: 10.1097/JGP.0b013e31816029ec. [DOI] [PubMed] [Google Scholar]
- 78.Rapp MA, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch. Gen. Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 79.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 80.Leinonen V, et al. Amyloid and tau proteins in cortical brain biopsy and Alzheimer's disease. Ann. Neurol. 2010;68:446–453. doi: 10.1002/ana.22100. [DOI] [PubMed] [Google Scholar]
- 81.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J. Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho S, Hu Y. Activation of 5-HT4 receptors inhibits secretion of beta-amyloid peptides and increases neuronal survival. Exp. Neurol. 2007;203:274–278. doi: 10.1016/j.expneurol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 83.Lezoualc'h F. 5-HT4 receptor and Alzheimer's disease: the amyloid connection. Exp. Neurol. 2007;205:325–329. doi: 10.1016/j.expneurol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Sun X, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch. Gen. Psychiatry. 2008;65:542–550. doi: 10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem. Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 86.Rojo LE, Fernández JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer's disease. Arch. Med. Res. 2008;39:1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yaffe K, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 89.Maccioni RB, Rojo LE, Fernández JA, Kuljis RO. The role of neuroimmunomodulation in Alzheimer's disease. Ann. N. Y. Acad. Sci. 2009;1153:240–246. doi: 10.1111/j.1749-6632.2008.03972.x. [DOI] [PubMed] [Google Scholar]
- 90.Okello A, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fumagalli F, et al. Neurotrophic factors in neurodegenerative disorders: potential for therapy. CNS Drugs. 2008;22:1005–1019. doi: 10.2165/0023210-200822120-00004. [DOI] [PubMed] [Google Scholar]
- 93.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 95.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 96.Cotman CW. The role of neurotrophins in brain aging: a perspective in honor of Regino Perez-Polo. Neurochem. Res. 2005;30:877–881. doi: 10.1007/s11064-005-6960-y. [DOI] [PubMed] [Google Scholar]
- 97.Benjamin S, et al. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. Am. J. Geriatr. Psychiatry. 2010;18:323–331. doi: 10.1097/JGP.0b013e3181cabd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jessen F, et al. No association of the Val66Met polymorphism of the brain-derived neurotrophic factor with hippocampal volume in major depression. Psychiatr. Genet. 2009;19:99–101. doi: 10.1097/YPG.0b013e32832080ce. [DOI] [PubMed] [Google Scholar]
- 99.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 100.Alzheimer's Disease International World Alzheimer Report 2010: The Global Economic Impact of Dementia. Alzheimer's Association – World Alzheimer's Day [online] 2010 http://www.alz.org/news_and_events_world_alzheimers_day.asp.
- 101.Herrera-Guzman I, et al. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J. Psychiatr. Res. 2009;43:855–863. doi: 10.1016/j.jpsychires.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 102.Mowla A, Mosavinasab M, Pani A. Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment? A double-blind, placebo-controlled, clinical trial. J. Clin. Psychopharmacol. 2007;27:67–70. doi: 10.1097/JCP.0b013e31802e0002. [DOI] [PubMed] [Google Scholar]
- 103.Doraiswamy PM, et al. Does antidepressant therapy improve cognition in elderly depressed patients? Gerontol. A Biol. Sci. Med. Sci. 2003;58:M1137–1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- 104.Areán PA, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am. J. Psychiatry. 2010;167:1391–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hashioka S, McGeer PL, Monji A, Kanba S. Anti-inflammatory effects of antidepressants: possibilities for preventives against Alzheimer's disease. Cent. Nerv. Syst. Agents Med. Chem. 2009;9:12–19. doi: 10.2174/187152409787601897. [DOI] [PubMed] [Google Scholar]
- 106.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 107.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 108.Nebes RD, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J. Psychiatr. Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 109.Bhalla RK, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am. J. Geriatr. Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 110.Devanand DP, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int. J. Geriatr. Psychiatry. 2003;18:123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]
- 111.Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch. Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 112.Pelton GH, et al. Randomized double-blind placebo-controlled donepezil augmentation in antidepressant-treated elderly patients with depression and cognitive impairment: a pilot study. Int. J. Geriatr. Psychiatry. 2008;23:670–676. doi: 10.1002/gps.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bragin V, et al. Integrated treatment approach improves cognitive function in demented and clinically depressed patients. Am. J. Alzheimers Dis. Other Demen. 2005;20:21–26. doi: 10.1177/153331750502000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lyketsos CG, Lee HB. Diagnosis and treatment of depression in Alzheimer's disease. A practical update for the clinician. Dement. Geriatr. Cogn. Disord. 2004;17:55–64. doi: 10.1159/000074277. [DOI] [PubMed] [Google Scholar]
- 115.Lyketsos CG, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch. Gen. Psychiatry. 2003;60:737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 116.Bains J, Birks JS, Dening TR. The efficacy of antidepressants in the treatment of depression in dementia. Cochrane Database Syst Rev. 2002;4:CD003944. doi: 10.1002/14651858.CD003944. [DOI] [PubMed] [Google Scholar]
- 117.Weintraub D, et al. Sertraline for the treatment of depression in Alzheimer disease: week-24 outcomes. Am. J. Geriatr. Psychiatry. 2010;18:332–340. doi: 10.1097/JGP.0b013e3181cc0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosenberg PB, et al. Sertraline for the treatment of depression in Alzheimer disease. Am. J. Geriatr Psychiatry. 2010;18:136–145. doi: 10.1097/JGP.0b013e3181c796eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zweig RM, et al. The neuropathology of aminergic nuclei in Alzheimer's disease. Ann. Neurol. 1988;24:233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- 120.Zubenko GS. Biological correlates of clinical heterogeneity in primary dementia. Neuropsychopharmacology. 1992;6:77–93. [PubMed] [Google Scholar]
- 121.Förstl H, et al. Clinical and neuropathological correlates of depression in Alzheimer's disease. Psychol. Med. 1992;22:877–884. doi: 10.1017/s0033291700038459. [DOI] [PubMed] [Google Scholar]
- 122.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 123.Copeland JRM, Dewey ME, Griffiths-Jones HM. Dementia and depression in elderly persons: AGECAT compared with DSMII and pervasive illness. Int. J. Geriatr. Psychiatry. 1990;5:47–51. [Google Scholar]
- 124.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psycho. Meas. 1977;1:385–401. [Google Scholar]
- 125.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 126.Yesavage JA, et al. Development and validation of a geriatric depression screening scale. A preliminary report. J. Psychiatr. Res. 1982-1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 127.Hooijer C, et al. A standardized interview for the elderly (GMS): reliability studies comparing the Dutch language version with the original. Int. J. Geriatr. Psychiatry. 1991;6:71–79. [Google Scholar]
- 128.Copeland JR, et al. The Geriatric Mental State Examination in the 21st century. Int. J. Geriatr. Psychiatry. 2002;17:729–732. doi: 10.1002/gps.667. [DOI] [PubMed] [Google Scholar]