Abstract
Mutations in the Bdnf gene, which produces transcripts with either short or long 3′ untranslated regions (3′UTRs), cause human obesity; however, the precise role of BDNF in the regulation of energy balance remains unknown. Here we show the relationship between long 3′UTR Bdnf mRNA, leptin, neuronal activation and body weight. We found that long 3′UTR Bdnf mRNA was enriched in dendrites of hypothalamic neurons and that insulin and leptin could stimulate its translation in dendrites. Furthermore, mice harboring a truncated long Bdnf 3′UTR developed severe hyperphagic obesity, which was completely rescued by viral expression of long 3′UTR Bdnf mRNA in the hypothalamus. In these animals the ability of leptin to activate hypothalamic neurons and to inhibit food intake was compromised despite normal activation of leptin receptors. These results reveal a novel mechanism linking leptin action to BDNF expression during hypothalamic-mediated regulation of body weight, while also implicating dendritic protein synthesis in this process.
INTRODUCTION
Owing to its high prevalence, costly associated disorders, and lack of effective drugs for treatments, obesity has become a leading health problem1. The biological system controlling energy balance is composed of several organs, including adipose tissues, the pancreas, the gastrointestinal tract, and the brain. Peripheral tissues produce signals reflecting the state of nutrition and fat stores, such as leptin2, insulin3, ghrelin4–7, glucagon-like peptide-1 (8,9), peptide tyrosine tyrosine (10), and certain metabolites (e.g. glucose, fatty acids, and amino acids)11–14. These signals are integrated in several brain regions, including the arcuate nucleus (ARC), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), and paraventricular hypothalamus15,16. These brain regions act to control food intake and energy expenditure in several peripheral tissues16,17. Elucidation of the intricate interaction between neural circuits in these brain regions and factors important for the control of energy balance may provide new strategies for developing effective obesity therapies.
Brain-derived neurotrophic factor (BDNF) is a potent regulator of neuronal development and synaptic function18, and has recently been implicated in the control of energy balance. The first evidence for a role of BDNF in energy balance came from the observation that Bdnf heterozygous mice exhibit hyperphagia and moderate obesity19,20. This finding was confirmed and extended by the severe obesity phenotypes observed in mice expressing the BDNF receptor, TrkB, at ~25% of the normal amount21 and in mice where the Bdnf gene is deleted in neurons expressing Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα)22. Since CaMKIIα is a brain-specific protein23, these observations demonstrate that BDNF acts on neurons of the central nervous system to affect energy balance. More recently, loss of a functional Bdnf allele or a dominant-negative TrkB mutation has been found to cause severe hyperphagia and obesity in children24–26. Furthermore, Bdnf gene variants have been linked to human obesity in large-scale genome-wide association studies27,28. However, the means by which BDNF inhibits food intake remain unclear.
The Bdnf gene in humans and rodents produces two populations of transcripts with either a short (~0.4 kb) or long (~2.9 kb) 3′ untranslated region (3′UTR) due to two alternative polyadenylation sites (Supplementary Fig. 1a)29. Our previous results show that short 3′UTR Bdnf mRNA is restricted to neuronal cell bodies whereas long 3′UTR Bdnf mRNA is also localized to dendrites in cortical and hippocampal neurons30. Numerous mRNA species have been found in neuronal dendrites31, and these dendritic transcripts serve as templates for local translation in response to synaptic activity32. While it has been shown that local protein synthesis in dendrites is required for lasting synaptic plasticity33–36, it is unknown whether local protein synthesis is important for a physiological process like energy homeostasis. Here we report that BDNF translated from long 3′UTR Bdnf mRNA is necessary for leptin-mediated regulation of energy balance.
RESULTS
Truncation of the long Bdnf 3′UTR leads to severe hyperphagic obesity
We previously described a mouse mutant, Bdnfklox/klox, in which long 3′UTR Bdnf mRNA is not generated due to an insertion of three tandem SV40 polyadenylation signals into the genomic sequence encoding the long Bdnf 3′UTR (Supplementary Fig. 1b)30. In these animals the truncation of the long Bdnf 3′UTR led to impairments in dendritic localization of Bdnf mRNA in cortical and hippocampal neurons30. Surprisingly, Bdnfklox/klox mice also developed severe obesity, starting to show higher body weight at 5–6 weeks of age compared to their WT littermates (Fig. 1a, b). By 16 weeks of age, female and male Bdnf mutants were 171% and 90% heavier, respectively, than sex-matched WT mice. Increased weight gain was also observed in male Bdnfklox/+ mice (Fig. 1b) and older female Bdnfklox/+ mice (Supplementary Fig. 2a). Furthermore, these animals exhibited increased linear growth (Fig. 1c). The high body weight of Bdnfklox/klox mice was associated with hyperleptinemia (Supplementary Fig. 2b), greatly enlarged adipose tissues (Supplementary Fig. 2c), and impaired glucose homeostasis (Supplementary Fig. 2d–f). These results show that truncation of the long Bdnf 3′UTR leads to an obesity syndrome.
Figure 1.
Mice lacking long 3′UTR Bdnf mRNA display severe hyperphagic obesity. (a) Body weight curves of female WT (+/+, n=10), heterozygous Bdnfklox/+ (+/k, n=8), and homozygous Bdnfklox/klox (k/k, n=10) mice. There was a significant effect of genotypes on body weight (two-way ANOVA: F(2, 325) = 56.47, P < 0.0001). (b) Body weight curves of male +/+ (n=6), +/k (n=6), and k/k (n=8) mice. There was a significant effect of genotypes on body weight (two-way ANOVA: F(2, 221) = 42.12, P < 0.0001). (c) Body length of +/+ and k/k mice at 5 months of age. (d) Daily food intake of individually housed mice. (e) Normal body weights of pair-fed female k/k mice. Error bars represent the standard error of the mean. ** P < 0.01 by Student’s t test.
The development of obesity in Bdnfklox/klox mice could result from increased energy intake and/or decreased energy expenditure. Effects on energy intake were examined by determining the daily food intake of these animals from 6 to 8 weeks of age. Both female and male Bdnfklox/klox mice exhibited a marked hyperphagia, consuming 69–80% more food than WT mice (Fig. 1d). To determine if decreased energy expenditure also contributes to obesity in these mice, Bdnfklox/klox mice were pair-fed to restrict their daily food intake to WT levels. Each day, female Bdnfklox/klox mice were provided the amount of food consumed by female WT littermates on the previous day, starting at 4 weeks of age when Bdnfklox/klox mice were not obese. Concurrently one group of Bdnfklox/klox mice was not pair-fed and was given ad libitum access to food. As expected, non-pair-fed Bdnfklox/klox mice became severely obese (Fig. 1e). However, body weights of pair-fed Bdnfklox/klox mice were not significantly different from those of WT mice throughout the 12-week pair-feeding period. These results demonstrate that hyperphagia is the sole cause of obesity in Bdnfklox/klox mice. Hyperphagia has also been found to cause moderate obesity in Bdnf heterozygous mice37.
Levels of Bdnf mRNA are reduced in the VMH of Bdnfklox/klox mice
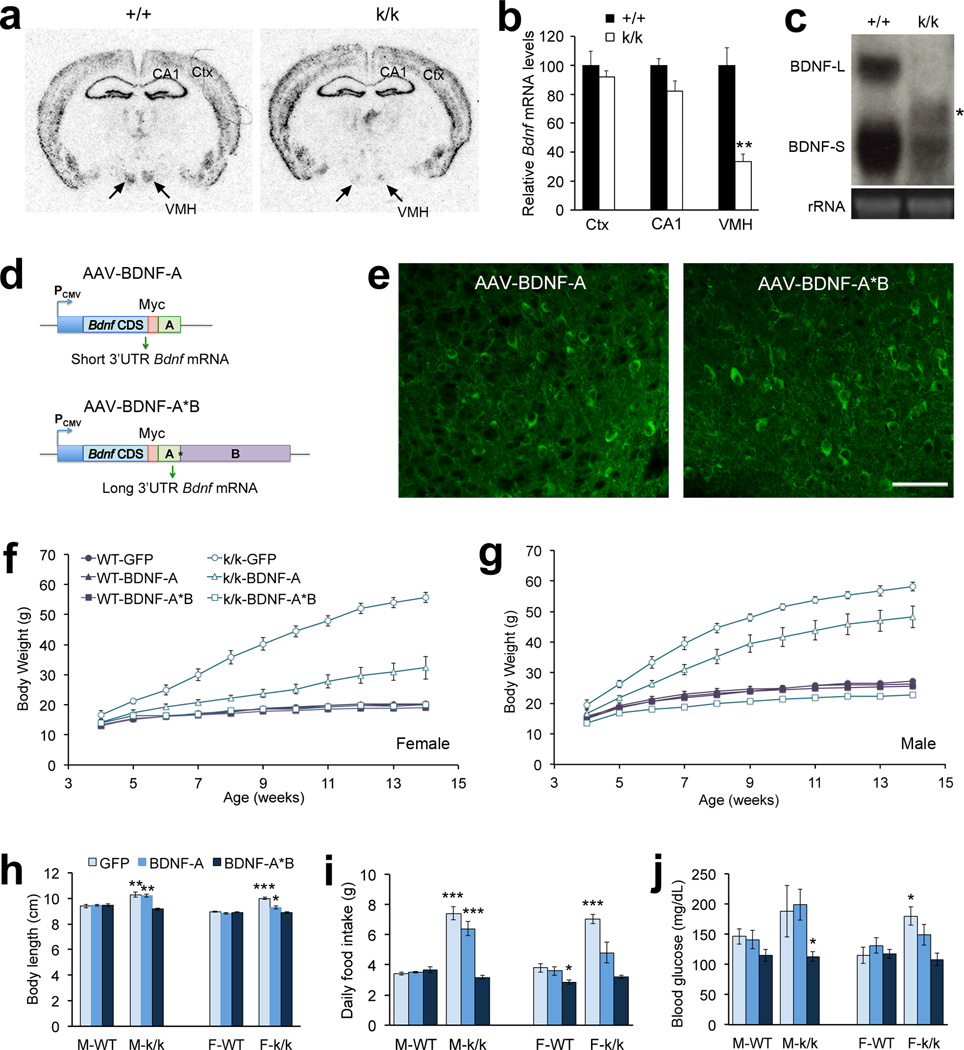
BDNF is expressed in the VMH, and fasting drastically and selectively reduces levels of Bdnf mRNA in this region21,38. Furthermore, deletion of the Bdnf gene in the VMH and DMH using Cre-expressing virus leads to increased weight gain38, whereas BDNF overexpression in these region reduces body weight39. These results highlight the importance of VMH BDNF in the control of energy balance. By employing radioactive in situ hybridization, we found that young Bdnfklox/klox and WT mice had similar amounts of Bdnf mRNA in the cerebral cortex and hippocampal CA1 region (Fig. 2a, b). However, the level of VMH Bdnf mRNA in Bdnfklox/klox mice was only one third of that in WT littermates (Fig. 2b). This reduction in Bdnf mRNA levels was unlikely due to stability issues of the truncated long 3′UTR Bdnf mRNA, because the ratio of this mRNA species to short 3′UTR Bdnf mRNA in the Bdnfklox/klox hypothalamus appeared similar to the ratio of long 3′UTR Bdnf mRNA to short 3′UTR Bdnf mRNA in the WT hypothalamus (Fig. 2c). These results suggest that the long 3′UTR is required for the maintenance of normal levels of Bdnf mRNA in the VMH.
Figure 2.
Viral expression of long 3′UTR Bdnf mRNA in the ventromedial hypothalamic region rescues the obesity syndrome in Bdnfklox/klox mice. (a) Representative Bdnf in situ hybridization images of brain sections from +/+ and k/k mice at 5–6 weeks of age. (b) Quantification of in situ hybridization signals in the cerebral cortex (Ctx), CA1 region, and VMH (n=4 mice per genotype). (c) Northern blot analysis of Bdnf mRNA isolated from hypothalami of +/+ and k/k mice. BDNF-L and BDNF-S mark long and short 3′UTR Bdnf mRNA, and the asterisk denotes a new Bdnf mRNA species. Lower panel: 18S rRNA as a loading control. (d) Diagram of AAV constructs expressing either short or long 3′UTR Bdnf mRNA. The Bdnf coding region (CDS) is linked to a Myc tag sequence at its 3' end. (e) Myc immunoreactivity in the ventromedial hypothalamic region of k/k mice injected with either AAV-BDNF-A or AAV-BDNF-A*B viral particles. Scale bar, 50 µm. (f) Body weight curves of female +/+ and k/k mice that received injection of AAV viral particles expressing GFP, short 3′UTR Bdnf mRNA (BDNF-A), or long 3′UTR Bdnf mRNA (BDNF-A*B) (n=6 mice per group). There was a significant effect from genotype and viral injection (two-way ANOVA: F(5, 330) = 57.46, P < 0.0001). (g) Body weight curves of male +/+ and k/k mice that received injection of AAV viral particles expressing GFP, short 3′UTR Bdnf mRNA (BDNF-A), or long 3′UTR Bdnf mRNA (BDNF-A*B) (n=4 mice for the k/k-GFP group and 6 mice for other groups). There was a significant effect from genotype and viral injection (two-way ANOVA: F(5, 308) = 25.14, P < 0.0001). (h–j) Body length, daily food intake, and fasting blood glucose of male +/+ (M-WT) and k/k (M-k/k) mice and female +/+ (F-WT) and k/k (F-k/k) mice injected with AAV-GFP, AAV-BDNF-A, or AAV-BDNF-A*B. Error bars represent the standard error of the mean. * P < 0.05, ** P < 0.01, *** P < 0.001 by Student’s t test.
Viral expression of long 3′UTR Bdnf mRNA in the hypothalamus blunts the obesity phenotype of Bdnfklox/klox mice
Since the level of total VMH Bdnf mRNA in Bdnfklox/klox mice was reduced, it was not clear if these animals developed obesity due to diminished Bdnf expression in the VMH or the lack of long 3′UTR Bdnf mRNA. To distinguish between these two possibilities, we investigated whether viral expression of either short or long 3′UTR Bdnf mRNA in the VMH would rescue the obesity phenotype observed in Bdnfklox/klox mice. We generated two adeno-associated viral (AAV) constructs by linking a Myc-tagged Bdnf coding sequence to either sequence “A” that encodes the short Bdnf 3′UTR (AAV-BDNF-A) or sequence “A*B” that encodes the entire long Bdnf 3′UTR (AAV-BDNF-A*B, where the first polyadenylation signal of the long Bdnf 3′UTR was mutated) (Fig. 2d). These two viruses expressed BDNF well in hypothalamic neurons (Fig. 2e). Because it remains unclear whether BDNF regulates neuronal development or neuronal function to affect body weight, we decided to inject stereotaxically AAV into the VMH of WT and Bdnfklox/klox pups at 2 weeks of age so that overexpressed BDNF has an effect on synaptic development and/or synaptic function. In addition to the VMH, viral infection was also detected in the ARC and DMH. Viral expression of either short or long 3′UTR Bdnf mRNA did not affect body weights of WT mice, as both female and male WT mice infected with either AAV-BDNF-A or AAV-BDNF-A*B had comparable body weights to sex-matched WT mice infected with a control virus, AAV-GFP (P > 0.05 at all ages by two-way ANOVA Bonferroni posttests) (Fig. 2f, g). Viral expression of short 3′UTR Bdnf mRNA significantly reduced body weights of female Bdnfklox/klox mice (female k/k-BDNF-A mice vs. female k/k-GFP mice: P < 0.01 at 7 weeks of age or later by two-way ANOVA Bonferroni posttests) (Fig. 2f). Viral expression of short 3′UTR Bdnf mRNA also reduced body weights of male Bdnfklox/klox mice, but the effect was not statistically significant (P > 0.05 at all ages by two-way ANOVA Bonferroni posttests) (Fig. 2g). Remarkably, viral expression of long 3′UTR Bdnf mRNA completely rescued the obesity phenotype in both female and male Bdnfklox/klox mice (WT-GFP mice vs. k/k-BDNF-A*B mice: P > 0.05 at all ages for both genders by two-way ANOVA Bonferroni posttests) (Fig. 2f, g). It also normalized body length, food intake, and blood glucose levels in Bdnfklox/klox mice (Fig. 2h–j). These results, along with the obesity phenotype of Bdnfklox/klox mice, indicate that long 3′UTR Bdnf mRNA plays more important roles than short 3′UTR Bdnf mRNA in the control of food intake.
Long 3′UTR Bdnf mRNA is localized to dendrites of hypothalamic neurons
Long 3′UTR Bdnf mRNA is targeted to the dendrites of hippocampal neurons where it plays an important role in regulating spine morphology and synaptic plasticity30. To investigate if long 3′UTR Bdnf mRNA is also targeted to the dendrites of hypothalamic neurons, we performed fluorescent in situ hybridization (FISH) on cultured rat hypothalamic neurons using RNA probes derived from the Bdnf coding region or a 1.9 kb cDNA fragment corresponding to the 3′ end of the long Bdnf 3′UTR (Fig. 3a). The coding region probe recognizes both populations of Bdnf mRNA whereas the 3′UTR probe detects long 3′UTR Bdnf mRNA. We found that the ratio of the FISH signal in the initial 50 µm segment of dendrites to the somatic FISH signal from the 3′UTR probe was ~4-fold higher than that from the coding region probe (Fig. 3b). Thus, long 3′UTR Bdnf mRNA is preferentially targeted to the dendrites of hypothalamic neurons.
Figure 3.
Insulin stimulates local translation of transcripts containing the long Bdnf 3′UTR in the dendrites of hypothalamic neurons. (a) Fluorescent in situ hybridization showing the distribution of Bdnf mRNA in cell bodies and dendrites of cultured rat hypothalamic neurons. MAP2 immunostaining marked cell bodies and dendrites. Scale bar, 50 µm. (b) Ratio of the FISH signal in dendrites to that in cell bodies. (c–h) Co-expression of BDNF and the insulin receptor in the DMH and VMH of BdnfLacZ/+ mice. Arrows denote neurons expressing both BDNF and the insulin receptor. Scale bar, 50 µm. (i) Representative images of hypothalamic neurons expressing myr-d1GFP-nls-A*B treated with either vehicle or insulin. MAP2 immunohistochemistry was used to reveal cell bodies and dendrites of cultured neurons. Scale bar, 100 µm. (j) Stimulatory effects of insulin on dendritic translation of myr-d1GFP-nls-A*B mRNA. Dendritic GFP fluorescence was measured at 150–200 µm away from the soma [n=38, 57, 21, and 42 neurons for the vehicle, insulin, rapamycin (Rap), and Rap + insulin treatments, respectively]. Error bars indicate standard errors of the mean. *** P < 0.001 by Student’s t test.
Insulin stimulates local BDNF synthesis in hypothalamic neurons via the mTOR pathway
Our previous results indicate that the long Bdnf 3′UTR is sufficient to direct dendritic localization and local translation of mRNA in cultured hippocampal neurons30. On the basis of this observation, we reasoned that factors important for the control of energy balance, such as insulin and leptin, might regulate translation of long 3′UTR Bdnf mRNA in the dendrites of hypothalamic neurons. If insulin directly stimulates local BDNF synthesis, BDNF neurons should also express the receptor for insulin in the hypothalamus. We performed immunohistochemistry against insulin receptor β (IRβ) and β-galactosidase on brain sections of BdnfLacZ/+ knockin mice in which the LacZ coding sequence replaces the Bdnf coding region, and found that the majority of BDNF neurons also express IRβ in the DMH and VMH (Fig. 3c–h).
To test the possibility that insulin regulates local BDNF synthesis in hypothalamic neurons, we generated two local protein synthesis reporter constructs by attaching the myr-d1GFP-nls coding sequence to sequence “A” (myr-d1GFP-nls-A) or sequence “A*B” (myr-d1GFP-nls-A*B). The membrane-anchoring myristoylation peptide (myr), the nuclear localization sequence (nls), and the short half-life of the destabilized d1GFP protein impede GFP synthesized in cell bodies from diffusing to distal dendrites, so that GFP in distal dendrites better reflects local protein synthesis40. Application of insulin to hypothalamic cultures increased the amount of GFP in distal dendrites of neurons expressing myr-d1GFP-nls-A*B, but not in neurons expressing myr-d1GFP-nls-A (Fig. 3i; Supplementary Fig. 3a), indicating that insulin stimulates local protein synthesis through the long Bdnf 3′UTR. Importantly, the stimulation was specific to dendritic protein synthesis, as insulin did not increase levels of GFP in cell bodies (Supplementary Fig. 3b). One important regulator of local protein synthesis is the mammalian target of rapamycin (mTOR), which can be activated by phosphoinositol-3 kinase (PI3K)32. As insulin strongly activates PI3K41, we examined whether insulin stimulated local translation of transcripts containing the long Bdnf 3′UTR via the mTOR pathway. Pretreatment of neuronal cultures with an mTOR specific inhibitor, rapamycin, abolished the stimulating effect of insulin on dendritic protein synthesis (Fig. 3j). These results indicate that insulin can directly stimulate local BDNF synthesis from long 3′UTR Bdnf mRNA in the dendrites of hypothalamic neurons via an mTOR-dependent mechanism.
Leptin activates hypothalamic BDNF-expressing neurons through network activity
Unlike the insulin receptor, we did not detect co-expression of BDNF and the leptin receptor (LepR) in the DMH or VMH. Although alternative mRNA splicing produces multiple isoforms of the LepR, the long leptin receptor form (LepRb) is the one that mediates leptin’s physiological action42,43. It has been shown that after leptin administration the immunoreactivity for phosphorylated signal transducer and activator of transcription protein 3 (pSTAT3) sensitively and reliably identifies neurons that express LepRb44. Double immunohistochemistry against pSTAT3 and β-galactosidase on brain sections of leptin-treated BdnfLacZ/+ mice revealed that there was little co-expression of pSTAT3 and BDNF in the DMH (Fig. 4a) or VMH (Fig. 4b, c). To further examine if BDNF and LepRb are co-expressed in the hypothalamus, we introduced the LepR-Cre allele to Bdnfklox/+ mice. In these mice, the IRES-Cre sequence was inserted into the 3′UTR for LepRb45, and BDNF neurons start to express β-galactosidase once the floxed Bdnf allele is deleted by Cre-mediated recombination46. In LepR-Cre;Bdnfklox/+ mice, we found many neurons expressing β-galactosidase in the dentate gyrus (Fig. 4d), which is consistent with previous observations that these neurons express BDNF as well as LepRb46,47. However, very few β-galactosidase-expressing neurons were detected in the VMH (Fig. 4e) and DMH (Fig. 4f), confirming lack of co-expression between BDNF and LepRb in these two brain regions. These results suggest that it is unlikely that leptin would directly stimulate local BDNF synthesis in these two hypothalamic regions.
Figure 4.
Leptin activates hypothalamic BDNF-expressing neurons through network activity. (a–c) Confocal images showing lack of co-localization of BDNF and pSTAT3 in the DMH, dorsomedial VMH (VMHdm), and ventrolateral VMH (VMHvl) of BdnfLacZ/+ mice injected with leptin. Scale bar, 50 µm. (d–f) Microscopic images showing β-galactosidase immunoreactivity in the dentate gyrus (DG), VMH, and DMH in LepR-Cre/+;Bdnfklox/+ mice. Arrows denote β-galactosidase-expressing neurons. Scale bar, 100 µm. (g, h) Confocal images showing some colocalization of BDNF and c-Fos in the VMH and DMH of leptin-injected BdnfLacZ/+ mice. Arrows denote representative BDNF-expressing neurons that are positive for c-Fos immunoreactivity. Scale bar, 50 µm. (l) KCl stimulation of dendritic local translation of myr-d1GFP-nls-A*B mRNA in hypothalamic neurons (n=16, 25, and 20 neurons for vehicle, vehicle + KCl, and Rap + KCl, respectively). Error bars represent standard errors of the mean. * P < 0.05, ** P < 0.01, *** P < 0.001 by Student’s t test; n.s., not significantly different (P > 0.05).
We then asked if leptin could stimulate local BDNF synthesis in vivo via network activity. Leptin administration has been shown to induce STAT3 activation and c-Fos expression in distinct populations of neurons in the DMH and VMH48. By using c-Fos expression as a marker for neuronal activation, we found that leptin administration activated many BDNF-expressing neurons in the DMH (Fig. 4g) and VMH (Fig. 4h). Quantification revealed that 32% (46/142) in the DMH and 33% (54/163) in the VMH of BDNF neurons expressed c-Fos. Finally, we employed KCl-induced neuronal depolarization to determine if neuronal activity is sufficient to stimulate local BDNF synthesis in cultured hypothalamic neurons using the in vitro reporter assay described earlier. We found that KCl treatment increased GFP synthesis in dendrites of cultured hypothalamic neurons expressing GFP transcripts containing the long Bdnf 3′UTR, and this stimulation was independent of mTOR (Fig. 4i). Taken together, our results indicate that leptin can activate BDNF-expressing hypothalamic neurons through activated neural circuits, which in turn stimulates dendritic BDNF synthesis.
Mice lacking long 3′UTR Bdnf mRNA fail to respond to leptin administration
Because long 3′UTR Bdnf mRNA is required for the control of energy balance (Fig. 1, 2) and leptin can stimulate local translation of transcripts containing the long Bdnf 3′UTR indirectly through neuronal activation (Fig. 4), we reasoned that Bdnfklox/klox mice might not respond to leptin. To test this hypothesis, we intraperitoneally injected young and lean Bdnfklox/klox and WT mice with leptin three times over a 24-h period. The leptin administration significantly reduced food intake by 26% over the 24-h period in WT mice (Fig. 5a). The same treatment, however, did not affect food intake in Bdnfklox/klox mice (Fig. 5a), although young WT and Bdnfklox/klox mice at 5–6 weeks of age had comparable serum leptin levels (female: 0.34 ± 0.03 ng/ml for WT and 0.33 ± 0.03 ng/ml for Bdnfklox/klox, P=0.859, n=8 mice per genotype; male: 0.44 ± 0.05 ng/ml for WT and 0.76 ± 0.16 ng/ml for Bdnfklox/klox, P=0.076, n=8 mice per genotype). These observations suggest that local BDNF synthesis is required for the anorexigenic effect of leptin.
Figure 5.
Leptin normally activates the LepRb in Bdnfklox/klox mice. (a) Food intake response of young and lean Bdnfklox/klox (k/k) mice to leptin administration. (b–d) Co-expression of TrkB and pSTAT3 in the ARC of leptin-injected TrkBLacZ/+ mice. White arrows denote representative neurons that contain both TrkB and pSTAT3. White arrowheads indicate representative neurons that express TrkB but are negative for pSTAT3. Yellow arrowheads indicate the TrkB+ processes of tanycytes that align the ventral 3rd ventricle. Scale bar, 50 µm. (e) Representative images of basal or leptin-induced pSTAT3 immunoreactivity in the ARC and DMH of +/+ or k/k mice. Scale bar, 50 µm. (f) Quantification of pSTAT3-positive cells in the ARC, VMH, and DMH of +/+ and k/k mice (n=3 mice per group). Error bars represent standard errors of the mean. Comparison between the vehicle and leptin groups for each genotype was analyzed using Student’s t test: * P<0.05 and ** P<0.01.
Leptin receptor activation is normal in the hypothalamus of Bdnfklox/klox mice
Leptin resistance in young lean Bdnfklox/klox mice might result from impaired leptin signaling in TrkB-expressing hypothalamic neurons. To address this possibility, we first examined co-expression of TrkB and LepRb in the ARC, VMH, and DMH by using immunohistochemistry against β-galactosidase and pSTAT3 on brain sections of leptin-injected TrkBLacZ/+ mice. We detected many TrkB-expressing neurons in the ARC (Fig. 5b) and DMH (Supplementary Fig. 4a), a small percentage of which also contained pSTAT3 immunoreactivity (Fig. 5b–d; Supplementary Fig. 4a–c). In the VMH, few TrkB-expressing neurons were detected and there was little co-expression of TrkB and pSTAT3 (Supplementary Fig. 4d–f). The low co-expression of TrkB and LepRb suggests that deficits in BDNF-to-TrkB signaling are unlikely to impair leptin signaling in LepRb-expressing hypothalamic neurons in Bdnfklox/klox mice. To test this prediction, we examined the ability of leptin to activate STAT3, a major signaling component in the leptin pathway, in hypothalami of young Bdnfklox/klox mice (Fig. 5e; Supplementary Fig. 4g–j). Cell counting revealed that STAT3 activation in the ARC, VMH, and DMH of Bdnfklox/klox mice was not significantly different from WT littermates (Fig. 5f). Furthermore, Bdnfklox/klox mice had normal levels of mRNAs for suppressor of cytokine signaling 3 (SOCS3), pro-opiomelanocortin (POMC), neuropeptide Y (NPY), and agouti-related protein (AgRP) in the ARC (Supplementary Fig. 5), which are all directly regulated by LepRb signaling49. Thus, lack of long 3′UTR Bdnf mRNA does not impair the leptin receptor activation.
Leptin-induced neuronal activation is impaired in Bdnfklox/klox mice
It has been shown that leptin induces STAT3 activation and c-Fos expression in distinct neuronal populations of several hypothalamic nuclei48. We observed a similar phenomenon in the DMH and VMH (Fig. 4a–c, g, h). This observation suggests that LepRb-expressing neurons send inputs to non-LepRb-expressing neurons and subsequently induce c-Fos expression in these cells. If the BDNF protein translated from long 3′UTR Bdnf mRNA is required for this information flow, the truncation of the long 3′UTR may lead to leptin resistance in Bdnfklox/klox mice. To test this possibility, we examined c-Fos induction in young and lean WT and Bdnfklox/klox mice after leptin administration (Fig. 6a; Supplementary Fig. 6). Cell counting revealed that c-Fos induction was abolished in the DMH and significantly impaired in the ARC and VMH (Fig. 6b). These results indicate that BDNF derived from long 3′UTR Bdnf mRNA controls the information flow from leptin-sensing neurons to non-LepRb-expressing neurons, likely by regulating the formation or function of neuronal connections. In support of this argument, the projection of anorexgenic POMC neurons, the majority of which express LepRb49, into the DMH was significantly reduced in Bdnfklox/klox mice (Supplementary Fig. 7).
Figure 6.
Leptin-induced neuronal activation is impaired in Bdnfklox/klox mice. (a) Representative images of c-Fos immunoreactivity in the ARC and DMH of +/+ and k/k mice under basal or leptin-stimulated condition. Scale bar, 100 µm. (b) Counts of cells expressing c-Fos in the ARC, VMH, and DMH (n= 3 or 4 mice per group). Student’s t test: * P < 0.05, ** P < 0.01, and *** P < 0.001 when compared with the vehicle group of the same genotype; # P < 0.05, ## P < 0.01, and ### P < 0.001 when the two leptin groups of different genotypes were compared. Error bars represent standard errors of the mean. (c) Localization of c-Fos and TrkB in the DMH of a leptin-injected TrkBLacZ/+ mouse. Arrows denote representative cells expressing both c-Fos and TrkB, whereas arrowheads denote representative cells expressing TrkB but not c-Fos. Scale bar, 50 µm.
To determine if the impairment in leptin-induced c-Fos expression is restricted to TrkB-expressing neurons, we examined co-localization of c-Fos and β-galactosidase in brain sections of leptin-administered TrkBLacZ/+ mice. We focused our study on the DMH as a result of our finding that leptin-induced c-Fos expression in the DMH was completely abolished in Bdnfklox/klox mice (Fig. 6b). We found that only 20% (23/113) of c-Fos expression was induced in TrkB-expressing DMH neurons (Fig. 6c). This result indicates that information flow to both TrkB- and non-TrkB-expressing neurons is impaired in the absence of BDNF synthesis from long 3′UTR Bdnf mRNA.
Discussion
Local protein synthesis offers a mechanism to selectively and quickly strengthen or weaken individual synapses in response to neuronal activity. It is required for long-lasting synaptic plasticity in the hippocampus33–35. In this study we showed that Bdnfklox/klox mice lacking the dendritically targeted long 3′UTR Bdnf mRNA developed hyperphagic obesity. We further showed that both leptin and insulin could stimulate local translation of long 3′UTR Bdnf mRNA in the dendrites of hypothalamic neurons. These observations implicate for the first time dendritic local protein synthesis in the control of feeding behavior. This finding suggests that mutations in the Bdnf 3′UTR and proteins important for controlling dendritic localization and translation of Bdnf mRNA may increase susceptibility to obesity.
Our results indicate that the lack of long 3′UTR Bdnf mRNA rather than the reduced level of VMH Bdnf mRNA is the main cause of the obesity syndrome observed in Bdnfklox/klox mice. First, the obesity syndrome in Bdnfklox/klox mice is not identical to the one observed in conditional mutant mice where the Bdnf gene is deleted in CaMKIIα-expressing neurons22. For example, the conditional mutant showed severe hyperglycemia22, whereas blood glucose levels were only modestly elevated in female Bdnfklox/klox mice and normal in male Bdnfklox/klox mice. Furthermore, Bdnfklox/klox mice developed more severe obesity than the Bdnf conditional mutant mice, which is likely due to incomplete Bdnf deletion in the hypothalamus of the conditional mutant22. Second, obese Bdnf+/− mice are only approximately 20% heavier than WT mice at 4 months of age, although the level of VMH Bdnf mRNA is drastically reduced in these heterozygous mice20. Lastly and most importantly, we showed that viral expression of long 3′UTR Bdnf mRNA in the ventromedial hypothalamic region completely rescued hyperphagic obesity in Bdnfklox/klox mice, whereas viral expression of short 3′UTR Bdnf mRNA only partially rescued obesity in female Bdnfklox/klox mice and did not have significant effects on body weight in male Bdnfklox/klox mice. Given that overexpression usually diminishes the specificity of protein action, it is possible that BDNF derived from the endogenous short 3′UTR Bdnf mRNA in the ventromedial hypothalamic region would have a minimal effect on body weight, even in females. Therefore, we conclude that BDNF synthesized from long 3′UTR Bdnf mRNA has more important roles than BDNF derived from short 3′UTR Bdnf mRNA in the control of energy balance.
Since dendritic mRNAs are packaged into transport granules and are translationally repressed in the cytoplasm and during dendritic transport32, translation of long 3′UTR Bdnf mRNA should mainly occur in dendrites in response to stimulation. If this inference is correct, the obesity phenotype in Bdnfklox/klox mice should result from lack of dendritic local BDNF synthesis. It is possible that the long 3′UTR controls BDNF synthesis in the cytoplasm in response to stimulation, and loss of this specific control in Bdnfklox/klox mice also contributes to the development of obesity. Further studies employing new techniques are needed to address the relative contribution of somatic vs. dendritic translation of long 3′UTR Bdnf mRNA in the control of energy balance.
Our results show that lack of long 3′UTR Bdnf mRNA leads to leptin resistance, a primary risk factor for obesity, without affecting LepRb signaling. This leptin resistance is likely due to defective neural circuits, as leptin-induced c-Fos expression was impaired or abolished in the ARC, DMH and VMH of Bdnfklox/klox mice while leptin activated LepRb normally in these brain regions. Leptin induces LepRb signaling and c-Fos expression in distinct populations of DMH neurons48; however, it remains unknown whether LepRb-expressing neurons within or outside the DMH innervate the DMH neurons that express c-Fos in response to leptin administration. If POMC neurons in the ARC induce c-Fos expression in their target neurons within the DMH, our results may provide one mechanism underlying leptin resistance in young Bdnfklox/klox mice. We detected significantly fewer POMC fibers within the DMH in these animals. This impairment in axonal projection and innervation could diminish the ability of POMC neurons to activate DMH neurons in response to leptin. Similar projection deficits from LepRb-expressing neurons may occur in other brain regions. This projection defect would indicate that BDNF controls energy balance in part by regulating the formation and/or maintenance of hypothalamic connections. In light of low co-expression of TrkB and LepRb in the adult ARC, we were surprised to observe the impairment in the projection of POMC neurons to the DMH in Bdnfklox/klox mice. Further studies are needed to investigate whether TrkB is more widely expressed in the developing ARC.
On the basis of our results, we propose that leptin and BDNF have a linked role in the control of energy balance (Supplementary Fig. 8). Leptin can stimulate translation of long 3′UTR Bdnf mRNA in neuronal dendrites through neuronal activity. BDNF derived from this form of transcripts in turn is required for leptin-induced neuronal activity in several hypothalamic areas, likely by regulating the formation, maintenance, and/or function of neuronal connections. When BDNF signaling is compromised, neural circuits in these hypothalamic areas are dysfunctional, leading to leptin resistance and obesity.
METHODS
Animals
Bdnfklox/klox, BdnfLacZ/+ and TrkBLacZ/+ mouse strains were on the C57BL6/J genetic background. All animal procedures were approved by the Georgetown University Animal Care and Use Committee.
In situ hybridization
Fluorescent in situ hybridization of cultured neurons was performed using digoxigenin-labeled riboprobes and the TSA Plus Fluorescein System (PerkinElmer, Waltham, MA) as previously described30. Radioactive in situ hybridization of brain sections was performed using 35S-labeled riboprobes as previously described21.
Viral BDNF overexpression
To generate BDNF-expressing AAV constructs, we first sequentially subcloned the CMV promoter, the mouse Bdnf coding sequence that is extended at its 3′ end with a sequence encoding the Myc tag (gccGAACAAAAACTCATCTCAGAAGAGGATCTGaatagctag, where the Myc-encoding sequence is shown in capital), and the mouse genomic sequence encoding the short Bdnf 3′UTR (A) or the long Bdnf 3′UTR (A*B) into pBluescript II KS (−). The whole CMV-Bdnf-Myc-A or CMV-Bdnf-Myc-A*B fragment could be released from the plasmids using NotI restriction enzyme. We digested pAAV-MCS vector (Stratagene, Cedar Creek, TX, USA) with NotI to remove a 1.7-kb fragment and subcloned the two NotI fragments, CMV-Bdnf-Myc-A (1.8 kb) and CMV-Bdnf-Myc-A*B (4.2 kb), into pAAV-MCS to generate pAAV-BDNF-A and pAAV-BDNF-A*B, respectively. In this way, the construct expressing long 3′UTR Bdnf mRNA was still within the AAV packaging capacity. Virus titers were determined by quantitative PCR, and 1µl of viral preparation (~1 × 108 viral particles) was stereotaxically injected to each VMH of mice at P14 using the following coordinates: anteroposterior, −1.2 mm; mediolateral, ±0.5 mm; dorsoventral, −5.9 mm.
In vitro local protein synthesis assays
Hypothalamic neurons were isolated and cultured according to a previously described procedure50 with some modifications. Culture medium was replaced with fresh medium omitting glucose and insulin at 6 day in vitro (DIV). Cells were transfected using Lipofectamine 2000 (Invitrogen Corporation, Carlsbad, CA) at 7 DIV, and treated with insulin (50 nM for 2 h; Sigma-Aldrich) or KCl (50 mM for 30 min) at 8 DIV with or without the rapamycin (25 µM for 30 min; Calbiochem, San Diego, CA) pretreatment. Cultures were fixed and stained using antibodies against MAP2. GFP intensity in cell bodies and dendrites was quantified using NIH ImageJ software.
Leptin treatment of mice
Mice were maintained on a low-fat diet (Purina diet 5001) to limit the development of obesity. To activate STAT3, we intraperitoneally injected mice at 6 weeks of age with either saline or murine leptin (R&D Systems, Minneapolis, MN) at 5 µg/g of body weight, and perfused the animals 45 min later. Averaged body weight was 18.2 ± 0.7 and 23.3 ± 0.7 g for +/+ and k/k mice, respectively. To induce c-Fos, we handled mice daily at 5 weeks of age for one week, and then intraperitoneally injected mice with either saline or leptin at 5 µg/g of body weight and perfused the animals 2 hr later. The averaged body weight was 18.6 ± 0.8 and 20.6 ± 1.3 g (P=0.22) for +/+ and k/k mice, respectively. For food intake assays, mice were singly housed one week before leptin administration, switched to the standard chow (Purina diet 5058), and injected intraperitoneally with saline daily for a week to reduce their stress response to the injection procedure. Leptin at 3 µg/g of body weight or saline was injected intraperitoneally at 12:00, 18:00 and 1:30. Pre-weighed mouse chow was given to each mouse immediately after the first leptin injection. Food intake was measured 24 h after the first leptin injection. Average body weight for each group was as follows: 23.2 ± 0.7 g for the +/+ saline group (12 mice), 23.1 ± 0.8 g for the +/+ leptin group (12 mice), 25.6 ± 1.5 g for the k/k saline group (9 mice), and 25.9 ± 1.1 g for the k/k leptin group (8 mice).
Immunohistochemistry
Immunohistochemistry was performed as previously described30,51. For pSTAT3 immunohistochemistry, brain sections were sequentially pretreated in 1% NaOH + 1% H2O2 for 20 min, 0.3% glycine for 10 min, and 0.03% SDS for 10 min as previously described44. The primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA; rabbit anti-pSTAT3, 1:2,000), Calbiochem (San Diego, CA; rabbit anti-cFOS, 1:10,000), Promega Corporation (Madison, WI; mouse anti-β-galactosidase, 1:300), and Santa Cruz Biotechnology (Santa Cruz, CA; rabbit anti-MAP2, 1:200).
Counting of immunoreactive neurons
Every fourth brain section from each animal was processed for pSTAT3 or c-Fos immunohistochemistry. Hypothalamic areas corresponding to the ARC, VMH, or DMH were outlined, and immunoreactive neurons were counted using Stereo Investigator software (MicroBrightField Inc, Williston, VT). The total number of pSTAT3- or c-Fos-positive cells for each animal was calculated by multiplying the counted number by 4.
Statistical analysis
All data are expressed as mean ± SEM. Data was analyzed using unpaired Student’s t-tests or two-way ANOVA.
Additional methods
Additional methodology is described in the Supplementary Methods online.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the grants from the National Institutes of Health to BX (R01 DK089237, R21 DK081008, and R01 NS050596), EGW (F30 DK084717), and FV (F31 NS060453) and from the American Diabetes Association to BX (7-07-RA-183).
Footnotes
Author Contributions:
GYL performed experiments addressing the obesity syndrome, local protein synthesis, viral rescue of obesity, co-expression of BDNF and TrkB with the leptin receptor, STAT3 activation, and c-Fos induction. JJA analyzed Bdnf mRNA subcellular localization and gene expression. KG characterized the obesity phenotype of Bdnfklox/klox mice. EGW created viral constructs and helped GYL in stereotaxic AAV microinjection. FV generated the reporter constructs for local BDNF synthesis. KRJ provided Bdnfklox/klox mouse strain. BX supervised the project. BX and GYL wrote the manuscript.
COMPETING INTERESTS STATEMENT
BX, JJA, and EGW are co-inventors on a patent application that has been filed by Georgetown University related to the technology that is described in this paper.
REFERENCES
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Woods SC, Lotter EC, McKay LD, Porte D., Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 6.Shintani M, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 7.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology. 2001;142:4163–4169. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 8.Tang-Christensen M, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 9.Turton MD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 10.Batterham RL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 11.Pocai A, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006;116:1081–1091. doi: 10.1172/JCI26640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 13.Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 14.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 15.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 16.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 17.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 18.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons WE, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 23.Lin CR, et al. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987;84:5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han JC, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo GS, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 26.Gray J, et al. Hyperphagia, Severe Obesity, Impaired Cognitive Function, and Hyperactivity Associated With Functional Loss of One Copy of the Brain-Derived Neurotrophic Factor (BDNF) Gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 28.Hotta K, et al. Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J Hum Genet. 2009;54:727–731. doi: 10.1038/jhg.2009.106. [DOI] [PubMed] [Google Scholar]
- 29.Timmusk T, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 30.An JJ, et al. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- 32.Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 33.Miller S, et al. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 34.Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 35.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 36.Martin KC, et al. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 37.Coppola V, Tessarollo L. Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport. 2004;15:2665–2668. doi: 10.1097/00001756-200412030-00022. [DOI] [PubMed] [Google Scholar]
- 38.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao L, et al. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15:447–454. doi: 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MW, Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 42.Chua SC, Jr., et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 43.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 44.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 45.DeFalco J, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 46.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott MM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubschle T, et al. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21:2413–2424. doi: 10.1523/JNEUROSCI.21-07-02413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annual review of physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 50.Joseph-Bravo P, Perez-Martinez L, Lezama L, Morales-Chapa C, Charli JL. An improved method for the expression of TRH in serum-supplemented primary cultures of fetal hypothalamic cells. Brain Res Brain Res Protoc. 2002;9:93–104. doi: 10.1016/s1385-299x(01)00140-4. [DOI] [PubMed] [Google Scholar]
- 51.Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice. J Neurochem. 2008;105:369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.