Abstract
The morning after participating in a dog show, a 2-year-old Pomeranian dog was found dead in a pool of bloody feces. Necropsy revealed hemorrhagic gastroenteritis of the entire gastrointestinal tract, with many Gram-positive bacilli on the surface and in the lumen and crypts of the intestine. Enterotoxin-positive type A Clostridium perfringens were isolated in large numbers. This dramatic case of fatal C. perfringens gastroenteritis highlights the need to better understand the role of this bacterium in enteric disease of dogs.
Résumé
Gastroentérite aiguë mortelle à Clostridium perfringens chez un chien. Le matin après avoir participé à une exposition canine, un chien Poméranien âgé de 2 ans a été trouvé mort dans une mare de fèces sanglantes. La nécropsie a révélé une gastroentérite hémorragique de l’ensemble du tube digestif, avec de nombreux bacilles à Gram positif sur la surface et dans la lumière et les glandes de l’intestin. Une quantité importante du bacille Clostridium perfringens de type A positif pour les entérotoxines a été isolée. Ce cas tragique de gastroentérite mortelle à C. perfringens souligne le besoin de mieux comprendre le rôle de cette bactérie dans la maladie entérique chez les chiens.
(Traduit par Isabelle Vallières)
A 2-year-old female Pomeranian dog was found dead in a pool of bloody feces the morning after it had been at a dog show. The previous day the dog was bright, alert, responsive, eating, and drinking. It had been fed a commercial diet. The dog was in good health; it had no history of vomiting or diarrhea. The dog had not been treated recently and was up-to-date on vaccinations. The owners were concerned about the possibility of poisoning so they submitted the body for necropsy to the Animal Health Laboratory (AHL) in Guelph, Ontario.
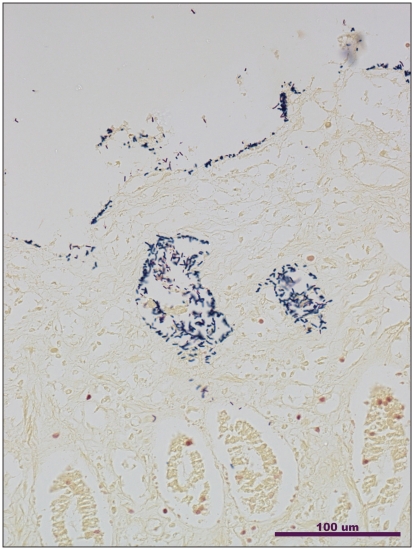
Necropsy revealed acute hemorrhagic gastroenteritis. Externally, the dog was in good condition except that the hair of the hind legs was matted in red fecal material. The serosal and mucosal surfaces of the entire gastrointestinal tract were diffusely hemorrhagic, with watery-red intestinal contents. Histopathology of the stomach, and small and large intestine revealed many Gram-positive clostridia-like bacilli on the surface of the mucosa and in the lumen and crypts of the small and large intestine (Figure 1). The mucosa of the villi in the small intestine appeared necrotic as did the superficial mucosal glands in the stomach. The sub-mucosal vessels of the stomach and the colon were congested. The red pulp of the spleen was markedly congested. There was marked pulmonary congestion. Bacterial culture from the ileum isolated large numbers of C. perfringens on Shahidi-Ferguson Perfringens (SFP)-egg yolk agar plates (Becton Dickinson, Sparks, Maryland, USA) incubated overnight in anaerobic conditions at 37°C. Individual colonies were sub-cultured on blood agar plates (Becton Dickinson) incubated overnight in anaerobic conditions at 37°C. No Campylobacter spp. or Yersinia spp. were isolated from the ileum on selective media. No Salmonella spp. were isolated from the ileum on selective media after the use of an enrichment medium.
Figure 1.
Gram-stained section of the small intestine from the case of hemorrhagic gastroenteritis in a dog showing large numbers of clostridia-like bacilli on the surface of the mucosa and within the crypts (Gram stain, 20×).
The C. perfringens isolates were type A, genotyped using a real-time polymerase chain reaction (PCR) assay based on the method of Albini et al (1) with the addition of primers and probes for the netB, tpeL, and atypical cpb2 toxin genes. Five of six colonies from the same plate were positive for the atypical β2 (cpb2) toxin gene and the enterotoxin (cpe) gene, but were negative for the cpb, etx, itx, and consensus β2 (cpb2) toxin genes. The other isolate was Type A, positive for the alpha (cpa) toxin gene only. Immuno-histochemistry (IHC) using rabbit polyclonal antibody for CPB2 toxin on intestinal sections was negative (figure not shown). Based on these findings a diagnosis of acute hemorrhagic gastroenteritis due to C. perfringens type A infection was made.
Clostridium perfringens is separated into 5 types (A through E) based on the production of alpha, beta, epsilon, and iota toxins; β2, enterotoxin and several other important toxins have also been identified and may be produced by any type (2). Type A C. perfringens is a normal inhabitant of the environment and gastrointestinal tract of many animals including dogs (2), since it can be cultured from more than 80% of both diarrheic and non-diarrheic dogs (3). Clostridium perfringens type A has been implicated in a wide range of enteric diseases including human food poisoning, abomasitis and enteritis in ruminants, and necrotic enteritis of broiler chickens (2). In recent years, additional toxins have been recognized in C. perfringens type A, including NetB (3) and TpeL (4), so that the full range of toxins associated with this type is probably under-recognized.
Clostridium perfringens type A-associated diarrhea and enteric disease in dogs is not well-characterized, but may range in severity from mild and self-limiting to the fatal acute hemorrhagic diarrhea seen in the current case (5). Acute hemorrhagic gastroenteritis associated with C. perfringens type A infection in dogs has been described previously, and is characterized by severe inflammation of the gastrointestinal tract, and hemorrhage and rapid death similar to the current case (6,7). The presence of large numbers of clostridia-like bacilli, identified as C. perfringens, adhering to mucosal surfaces (Figure 1) is a striking finding common to these cases (6,7). Isolates from previous case reports were not genotyped for the range of toxin genes tested for in the present study, and the current study reports for the first time the presence of the cpe and atypical cpb2 genes in isolates from a case of fatal infection.
Understanding of the role of C. perfringens in diarrheal illness in dogs is incomplete, although less severe diarrheal illness attributed to C. perfringens has been described (5). No gold standard for the diagnosis of diarrhea associated with C. perfringens exists, so veterinarians must carefully consider the clinical signs and molecular diagnostic evidence, and the absence of other pathogens, before making a diagnosis (5). In the present case, clinical signs, the presence of large numbers of clostridia-like bacilli on the surface of the mucosa in the small and large intestines, the necrotizing inflammation typical of severe C. perfringens enteritis seen in numerous other species (5), the heavy growth of C. perfringens from the ileum and the lack of isolation of other pathogens from the gastrointestinal tract all support a causative role for C. perfringens. The sudden death caused by this infection was so striking that the dog was initially assumed to have been poisoned at the dog show. What precipitated the infection is not clear. There was no history of unusual feeding. In other species, there is an association between colostrum- or food-associated trypsin-inhibition (which prevents trypsin breaking down secreted toxin) and severe C. perfringens enteritis (2), but such a possibility of food-associated infection is purely speculative.
Type A strains isolated in the present study were cpe and atypical cpb2 toxin gene positive. Clostridium perfringens entero-toxin (CPE) production is associated with sporulation and is released upon lysis of vegetative cells, acting as a cytotoxic enterotoxin that causes tissue damage (8). Experimental canine models have shown CPE to induce fluid accumulation and diarrhea (9); however, the method by which CPE causes diarrhea is unclear (8). The presence of CPE in the feces of dogs is associated with diarrhea, but a small percentage of dogs without diarrhea also have CPE in their feces (8,10). The presence of the cpe gene has been associated with diarrhea in dogs, but the strongest association with diarrhea is shown when the toxin itself (and not just the cpe gene) is detected (10). The present study detected the gene for cpe but testing for CPE was not done. The established association of cpe with canine diarrhea and its presence in most isolates suggests that it may have played a role in the disease. This needs to be investigated further. Since both cpe and cpb2 are commonly found on a plasmid (11) and since C. perfringens is known to carry multiple virulence-associated plasmids at a time, it is not possible to rule out the presence of other plasmid(s) carrying other as-yet-unknown virulence gene(s) contributing to the disease in this dog. The cpb2 gene, in both the consensus and atypical variants, has been detected in C. perfringens type A isolates from healthy and diarrheic dogs in previous studies, and in combination with cpe (12–14). Jost et al (12) found a high rate of the atypical allele of cpb2 in cpb2-positive C. perfringens type A canine isolates. Atypical cpb2 may not be expressed in vivo (11), as was the case in this dog. Previous studies (13,14) examining the presence of cpb2 in isolates of C. perfringens type A from diarrheic dogs have had small sample sizes, lacked control groups of healthy dog isolates (13) and have not differentiated between atypical and consensus alleles of cpb2. As a result there is no current evidence to support the role of cpb2-toxigenic C. perfringens type A strains in canine diarrhea (2).
Further research involving the characterization and genotyping of C. perfringens type A isolates from similar clinical cases would be helpful in elucidating the role that C. perfringens type A virulence factors play in the pathogenesis of this apparently rare but serious disease. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Albini A, Brodard I, Jaussi A, et al. Real-time multiplex PCR assays for reliable detection of Clostridium perfringens toxin genes in animal isolates. Vet Microbiol. 2008;127:179–185. doi: 10.1016/j.vetmic.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keyburn AL, Boyce JD, Vaz P, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers G, Bruce HL, Hunter DB, et al. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. J Clin Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks SL, Kather EJ. Clostridium perfringens- and Clostridium difficile-associated diarrhea. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3rd ed. St Louis, Missouri: Saunders Elsevier; 2006. pp. 363–366. [Google Scholar]
- 6.Prescott JF, Johnson JA, Patterson JM, Bulmer WS. Haemorrhagic gastroenteritis in the dog associated with Clostridium welchii. Vet Rec. 1978;103:116–117. doi: 10.1136/vr.103.6.116. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki J, Goryo M, Asahina M, Makara M, Shisido S, Okada K. Hemorrhagic enteritis associated with Clostridium perfringens Type A in a dog. J Vet Med Sci. 1999;61:175–177. doi: 10.1292/jvms.61.175. [DOI] [PubMed] [Google Scholar]
- 8.Weese JS, Staempfli HR, Prescott JF, Kruth SA, Greenwood SJ, Weese HE. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med. 2001;15:374–378. [PubMed] [Google Scholar]
- 9.Bartlett ML, Walker HW, Ziprin R. Use of dogs as an assay for Clostridium perfringens enterotoxin. Appl Microbiol. 1972;23:193–197. doi: 10.1128/am.23.1.196-197.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks SL, Kather EJ, Kass PH, Melli AC. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J Vet Intern Med. 2002;16:533–540. doi: 10.1892/0891-6640(2002)016<0533:gapcop>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Fisher DJ, Miyamoto K, Harrison B, Akimoto S, Sarker MR, McClane BA. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol Microbiol. 2005;56:747–762. doi: 10.1111/j.1365-2958.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- 12.Jost BH, Billington SJ, Trinh HT, Bueschel DM, Songer JG. Atypical cpb2 genes encoding Beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect Immun. 2005;73:652–656. doi: 10.1128/IAI.73.1.652-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiede S, Goethe R, Amtsberg G. Prevalence of β2 toxin gene of Clostridium perfringens type A from diarrhoeic dogs. Vet Rec. 2001;149:273–274. doi: 10.1136/vr.149.9.273. [DOI] [PubMed] [Google Scholar]
- 14.Zerbini L, Ossiprandi MC. Molecular typing of Clostridium perfringens strains isolated from dogs by toxin gene amplification. Ann Fac Med Vet Di Parma. 2009;29:115–128. [Google Scholar]