Abstract
Hypophosphatasia (HPP), caused by mutations in the gene ALPL encoding tissue-nonspecific alkaline phosphatase (TNALP), is an inherited systemic skeletal disease characterized by mineralization defects of bones and teeth. The clinical severity of HPP varies widely, from a lethal perinatal form to mild odontohypophosphatasia showing only dental manifestations. HPP model mice (Akp2−/−) phenotypically mimic the severe infantile form of human HPP; they appear normal at birth but die by 2 weeks of age because of growth failure, hypomineralization, and epileptic seizures. In the present study, we investigated the feasibility of fetal gene therapy using the lethal HPP model mice. On day 15 of gestation, the fetuses of HPP model mice underwent transuterine intraperitoneal injection of adeno-associated virus serotype 9 (AAV9) expressing bone-targeted TNALP. Treated and delivered mice showed normal weight gain and seizure-free survival for at least 8 weeks. Vector sequence was detected in systemic organs including bone at 14 days of age. ALP activities in plasma and bone were consistently high. Enhanced mineralization was demonstrated on X-ray images of the chest and forepaw. Our data clearly demonstrate that systemic injection of AAV9 in utero is an effective strategy for the treatment of lethal HPP mice. Fetal gene therapy may be an important choice after prenatal diagnosis of life-threatening HPP.
Sugano and colleagues investigate the feasibility of fetal gene therapy for hypophosphatasia (HPP), which is caused by mutations in tissue-nonspecific alkaline phosphatase (TNALP). Mice that phenotypically mimic the severe infantile form of human HPP underwent transuterine intraperitoneal injection of adeno-associated viral vector type 9 (AAV9) expressing bone-targeted TNALP. Treated mice showed normal weight gain and seizure-free survival for at least 8 weeks after birth, with consistently high levels of ALP activity in plasma and bone.
Introduction
Hypophosphatasia (HPP), caused by a deficiency in tissue-nonspecific alkaline phosphatase (TNALP), is an inherited disease characterized by mineralization defects. HPP is a clinically heterogeneous disease and is classified according to severity and age at diagnosis (Mornet, 2007; Whyte, 2010). Perinatal and infantile forms of HPP are usually severe, and life expectancy is less than 1 year in most cases. The major cause of death is respiratory failure associated with a narrow chest, and pyridoxine-responsive seizures are also observed in some severely affected cases (Nakamura et al., 2010; Whyte, 2010). The childhood and adult forms of HPP show milder phenotypes and odontohypophosphatasia causes premature loss of deciduous teeth without evidence of skeletal disease. Perinatal HPP is more common in Japan than in other countries and is the fifth most common form of fetal-diagnosed skeletal dysplasia (Satoh et al., 2009).
There is no established treatment for HPP, but several experimental approaches have been attempted to treat TNALP knockout mice (Akp2−/−). The phenotype of these mice mimics that of severe infantile HPP; the animals appear normal at birth, but rapidly develop growth failure, epileptic seizures, and hypomineralization, and die by 2 weeks of age. Millán and colleagues reported that Akp2−/− mice can be treated by repeated injection of bone-targeted TNALP with deca-aspartates at the C terminus (TNALP-D10) (Millán et al., 2008). On the basis of these data, new clinical trials of enzyme replacement therapy (ERT) for patients with infantile and childhood HPP have been initiated (http://clinicaltrials.gov/). Another important possibility for the treatment of HPP is gene therapy. We have demonstrated that a single injection of either lentiviral or adeno-associated viral (AAV) vector expressing TNALP-D10 into postnatal HPP mice resulted in prolonged seizure-free survival and phenotypic correction (Yamamoto et al., 2010; Matsumoto et al., 2011). This gene therapy approach is referred to as viral vector-mediated ERT, and it is more practical than classical ERT, which requires repeated injections. Neonatal gene therapy may be an important option for the treatment of severe HPP.
Because of the remarkable progress in prenatal diagnosis with clinical imaging, including echography and computed tomography, as well as molecular testing, the chance of diagnosis of perinatal lethal HPP during the fetal period is increasing. Fetal gene therapy may be among the treatment options for perinatal and severe infantile HPP in the future. In the present study, we evaluated the feasibility of gene therapy during the fetal period. Here, we report that systemic injection of AAV vector in utero is an effective strategy for the treatment of early-onset lethal genetic diseases.
Materials and Methods
Construction and preparation of recombinant adeno-associated viral vector
The AAV vector plasmid carrying cDNA for TNALP-D10 was described previously (Matsumoto et al., 2011). Recombinant AAV serotype 9 (AAV9) vector was generated by the triple transfection method (Salvetti et al., 1998) and purified as described previously (Hermens et al., 1999; Miyake et al., 2011). The titer of each AAV vector was determined by real-time PCR (7500 Fast; Applied Biosystems, Tokyo, Japan). AAV9-EGFP (Miyake et al., 2011) was used to analyze the biodistribution of the vector.
Animal procedures and vector injection
All animal procedures were performed in accordance with the guidelines approved by the Nippon Medical School (Tokyo, Japan) Animal Ethics Committee. Akp2−/− mice were obtained by mating Akp2+/– mice with a mixed genetic background of 129/J and C57BL/6J, which were generated by the Millán laboratory (Narisawa et al., 1997; Fedde et al., 1999). AAV9 vector was intraperitoneally injected into fetal or postnatal mice. For fetal vector injection, pregnant dams on day 15 of gestation were anesthetized by intramuscular injection of 0.6 mg of pentobarbital in 100 μl of phosphate-buffered saline (PBS) and inhalation of isoflurane. A midline laparotomy was performed and the uterus was exposed. Each fetus underwent transuterine, intraperitoneal injection of vector (8.3×1010 viral genomes [VG] of AAV9-TNALP-D10 or AAV9-EGFP per gram body weight); a 33-gauge Ito syringe (Ito Corporation, Shizuoka, Japan) was used. The amount of vector for fetal injection was 1.0×1011 Vg/10 μl/ body. After the injections, the peritoneum was filled with 300 μl of PBS and the abdominal muscle layer and the skin layer were closed with 5-0 nylon sutures. After normal delivery, the newborn mice stayed for 1 month with the dam. The genotype of treated fetuses was determined after delivery. For neonatal injection, 2.0×1011 Vg/20 μl/body was intraperitoneally injected into the mice on postnatal day 1.
ALP activity in plasma and organs
Blood samples were collected from the orbital sinus, using heparinized capillaries, on days 14, 28, and 56 after birth. After plasma separation, ALP activity in the plasma was quantified by a colorimetric assay for ALP as described previously (Goseki et al.,1988). ALP activity was described in units (U) defined as the amount of enzyme needed to catalyze the production of 1 μmol of p-nitrophenol formed per minute and calculated as units per milliliter.
Brain, heart, liver, quadriceps, abdominal rectus muscle, and tibial bone were dissected out under deep anesthesia after perfusion with 20 ml of PBS containing heparin (10 U/ml) followed by 20 ml of PBS. Bone marrow and soft tissues were carefully removed from the isolated bone. Each organ was homogenized with 500 μl of distilled H2O, using a Precellys 24 bead-beating homogenizer (Bertin Technologies, Paris, France) and centrifuged at 14,000×g for 5 min. ALP activity of the supernatant was analyzed as described previously and standardized by 1 mg of protein. Protein concentration was determined with a DC protein assay kit (Bio-Rad, Tokyo, Japan).
Histochemical examination of bone
Knee joints were removed under deep anesthesia after perfusion with 20 ml of PBS containing heparin (10 U/ml) and 20 ml of PBS, followed by embedding in super cryoembedding medium (SCEM) compound (Leica Microsystems, Tokyo, Japan) and freezing, without fixation or decalcification. Sections (14 μm thick) were cut by the Kawamoto film method (Leica Microsystems) and washed with 99.5% ethanol and distilled H2O. ALP activity was assayed by incubating the tissue with naphthol AS-MX phosphate (0.1 mg/ml) as a substrate and fast blue BB salt (0.6 mg/ml) as dye in 20 ml of 0.1 M Tris-HCl buffer (pH 8.5) for 15 min at 37°C, as described previously (Sugiyama et al., 2003). After mounting on silane-coated slides (Muto Pure Chemicals, Tokyo, Japan), they were examined under a light microscope (BX60; Olympus, Tokyo, Japan).
For immunostaining, sections (10 μm thick) of the knee joint were cut by the Kawamoto film method (Leica Microsystems) and fixed with 10% formalin. The sections were incubated with 1% skim milk in PBS for 60 min (blocking). After blocking, the sections were incubated with primary antibodies for 60 min at room temperature followed by 48 hr at 4°C. The primary antibodies were rabbit anti-collagen II (diluted 1:100; Abcam, Cambridge, UK) and mouse anti-green fluorescent protein (GFP) (diluted 1:400; Invitrogen, Eugene, OR). After washing with PBS, they were incubated with secondary antibodies, which included goat anti-rabbit IgG conjugated with Alexa 568 (diluted 1:500; Invitrogen) and donkey anti-mouse IgG conjugated with Alexa 488 (diluted 1:500; Invitrogen), at room temperature for 2 hr. After mounting on silane-coated slides (Muto Pure Chemicals), they were examined by fluorescence microscopy (BX60; Olympus).
X-ray analysis
Radiographic images were obtained with a μFX-1000 (Fujifilm, Tokyo, Japan) with an energy of 25 kV and exposure time of 10 sec, and imaged with a Typhoon FLA-7000 scanner (Fujifilm).
Biodistribution of AAV vector
Biodistribution of AAV9 vector was determined by real-time PCR of genomic DNA of Akp2+/+ mice after intraperitoneal injection of AAV9-EGFP. Heart, liver, quadriceps, abdominal rectus muscle, tibial bone, brain, and gonads were removed under deep anesthesia after perfusion with 20 ml of PBS containing heparin (10 U/ml) followed by 20 ml of PBS at 14 days of age. Each tissue of the mice was homogenized with a Precellys 24 bead-beating homogenizer (Bertin Technologies) followed by DNA extraction with a Gentra Puregene kit (Qiagen Sciences, Germantown, MD). Real-time PCR was performed with primers designed to amplify part of the cytomegalovirus (CMV) promoter of AAV9 (sense, 5′-GACGTCAATAATGACGTATG-3′; antisense, 5′-GGTAATAGCGATGACTAATACG-3′). Reaction was carried out with 100 ng of template, a 0.2-μmol/liter concentration of each primer, SYBR Premix Ex Taq (Perfect Real Time; Takara Bio, Tokyo, Japan) and ROX reference dye II (Takara Bio). The amplification conditions were 95°C for 10 sec, followed by 40 cycles of 95°C for 15 sec and 60°C for 34 sec. Dissociation was performed at 95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec. GFP expression in the tissues of mice treated with AAV9-EGFP was visualized by immunohistochemical staining with anti-EGFP antibody (Medical & Biological Laboratories, Aichi, Japan) and the avidin/biotinylated enzyme complex method using diaminobenzidine dihydrochloride (DAB) as described previously (Iwamoto et al., 2009).
Statistical analyses
Differences between groups were tested for statistical significance by Student t test. In all analyses, p<0.05 was taken to indicate statistical significance. The survival rate was analyzed by the Kaplan–Meier method, and differences in survival rate were assessed by the Wilcoxon test.
Results
Fetal injection of AAV9-TNALP-D10 prolongs survival and improves development of Akp2−/− mice
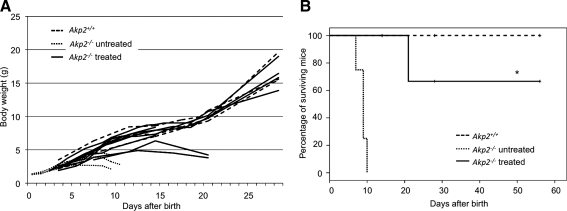
Fetal mice were obtained by mating Akp2+/– heterozygous mice, which are healthy and have a normal life span. AAV9-TNALP-D10 (1×1011 VG/10 μl) was injected intraperitoneally into a total of 88 fetuses on embryonic day 15 from 11 pregnant dams. Among the treated mice, 47 (53%) were live-born, whereas 41 (47%) did not live longer than half a day because they were eaten by the dam. Genotyping of live neonates showed that 9 of 47 were Akp2−/−. Nontreated Akp2−/− mice were born with a normal appearance but showed growth failure with epileptic seizures and did not live longer than 11 days. In contrast, the weight and growth rate of treated Akp2−/− mice (seven of nine) were indistinguishable from those of wild-type littermates (16.3±1.8 g [n=4] vs. 16.3±1.9 g [n=4] on day 28 [Fig. 1A], 19.3±2.8 g [n=3] vs. 21.7±1.7 g [n=3] on day 56) and the life span was significantly extended up to at least 56 days (the time of sacrifice for analysis) (Fig. 1B). Seizures were not observed in these surviving mice throughout the experimental period. Two of the Akp2−/− fetuses failed to respond to treatment. They showed severe weight loss and convulsions, and died on postnatal day 21.
FIG. 1.
Therapeutic effects of fetal gene therapy. (A) Growth curves of Akp+/+ mice (n=4, dashed lines), untreated Akp−/− mice (n=4, dotted lines), and treated Akp−/− mice (n=9, continuous lines). (B) Survival curves of Akp+/+ mice (n=8, dashed line), untreated Akp−/− mice (n=4, dotted line), and treated Akp−/− mice (n=9, continuous line). Statistical analysis revealed that the proportion of surviving treated Akp−/− mice was significantly elevated compared with that of untreated Akp−/− mice (*p<0.001).
ALP activities are elevated in treated fetal mice
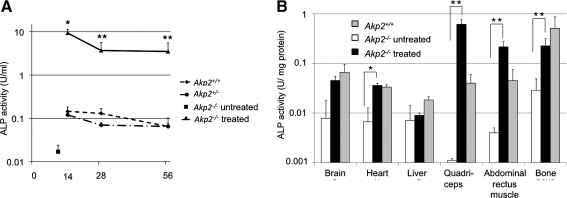
To measure the levels of TNALP-D10 expression in the bloodstream and tissues after fetal gene therapy, ALP activity was analyzed by colorimetric assay. Plasma ALP activities of in utero-treated mice were markedly increased and, significantly, remained more than 10 times higher than those in wild-type mice (Akp2+/+) during the observation period (9.3±2.5 vs. 0.15±0.085 U/ml [n=3] on day 14, p<0.01; 3.7±2.0 vs. 0.13±0.037 U/ml [n=3] on day 28, p<0.05; and 3.5±1.5 vs. 0.065±0.020 U/ml [n=3] on day 56, p<0.05) (Fig. 2A). Despite the supraphysiological levels of ALP activity, no gross deformities of the bones or abnormal calcification were observed on X-ray images (data not shown).
FIG. 2.
ALP activity in the plasma and tissues. (A) Concentration of plasma ALP activity of Akp+/+ mice (n=4, solid diamonds), Akp+/– mice (n=4, solid circles), and treated Akp−/− mice (n=3, solid triangles) at 14, 28, and 56 days after birth. Solid square, ALP activity in the plasma of untreated Akp−/− mice (n=4) 10 days after birth. (B) ALP activity in the tissues of Akp+/+ mice (day 14; n=2 or 3, gray columns), untreated Akp−/− mice (day 10; n=3, white columns), and treated Akp−/− mice (day 14; n=3, black columns). Data are presented as means and SD. A Student t test was employed for comparisons between the two groups (*p<0.01, **p<0.05).
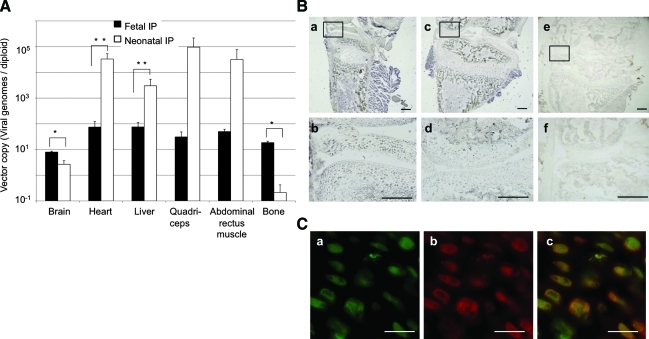
ALP activities in the tissues of treated Akp2−/− mice were measured 14 days after birth (20 days after vector injection). ALP activity was elevated in heart, quadriceps, abdominal rectus muscle, and, surprisingly, in bone (Fig. 2B), which was not observed in our previous study with neonatal injection of AAV vector.
Elevated ALP activities are detected in bone after fetal gene therapy
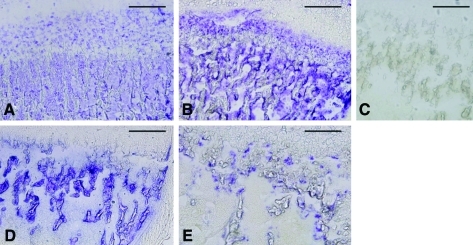
The cartilage zone of the proximal tibia was analyzed histochemically for ALP activity, using the azo dye technique. In the epiphysis of Akp2+/+ mice, strong ALP activity was observed in hypertrophic chondrocytes and on the surface of endosteal bones on day 14 (Fig. 3A) and day 56 (Fig. 3B). ALP staining of the bone of Akp2+/+ mice appeared to strengthen with age. In the corresponding area of the tibial bone of untreated Akp−/− mice on day 10, growth of the trabecula was significantly inhibited and no ALP signal was detected (Fig. 3C). After fetal gene therapy, strong ALP signals were detected mainly on the surface of endosteal bones on day 14, although the density of the trabecula was still less than that of Akp2+/+ mice (Fig. 3D). Faint scattered ALP signals were also observed in the cartilage zone of treated fetal Akp2−/− mice on day 56 (Fig. 3E).
FIG. 3.
Histochemical staining for ALP activity. ALP activity is shown as purple staining. The pictures show the tibial bones of 14-day-old (A) and 56-day-old (B) Akp+/+ mice, a 10-day-old untreated Akp−/− mouse (C), and 14-day-old (D) and 56-day-old (E) treated Akp−/− mice. Scale bars: 250 μm. Color images available online at www.liebertonline.com/hum
X-ray images reveal skeletal phenotypic correction after fetal gene therapy
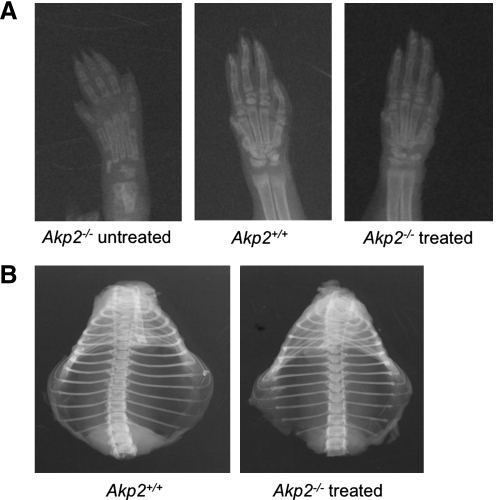
Mineralization of treated fetal Akp2−/− mice was evaluated by X-ray examination. The radiographic changes in Akp2−/− mice became apparent during the first 7 to 10 days of life, although the severity of the mineralization defects was highly variable. X-ray images of the most severely affected cases on day 10 showed a lack of secondary ossification centers and reduced numbers of carpal bones compared with Akp2+/+ mice, indicating ossification incompetence in HPP mice. In contrast, apparent ossification centers were detected in all treated mice on day 10 (n=3; Fig. 4A). No differences in skeletal structure or mineralization, including the size of the thoracic cage, were observed between Akp2+/+ and treated Akp2−/− mice on day 56 (n=3; Fig. 4B).
FIG. 4.
X-ray images. (A) Forepaw of 10-day-old untreated Akp−/− mouse, Akp+/+ mouse, and treated Akp−/− mouse. (B) Thoracic cage of 56-day-old Akp+/+ mouse and treated Akp−/− mouse.
Chondrocytes are transduced by fetal injection of AAV9
To confirm whether the elevated ALP activity in the tissues (especially bones) was derived from other tissues via the bloodstream or was expressed in the original tissues, the biodistribution of AAV9 vector was determined by real-time PCR on genomic DNA. AAV9-EGFP, which does not have special affinity for bone, was used to confirm vector biodistribution. DNA was isolated from each tissue of Akp2+/+ mice treated by fetal or neonatal intraperitoneal injection of AAV9-EGFP. After neonatal intraperitoneal injection, high copy numbers of AAV9 were observed in the heart and liver, whereas transduction into bone was low. This distribution pattern was similar to that obtained after neonatal intravenous injection of AAV8 vector (Matsumoto et al., 2011). On the other hand, after fetal intraperitoneal injection, AAV9 vector was widely distributed to all organs examined except the gonads. Importantly, AAV sequence was significantly elevated in brain and bone compared with neonatal intraperitoneal injection (p<0.01; Fig. 5A). No vector sequence was detected in the gonads of the pups (data not shown). AAV9-mediated transduction of bone tissue in utero was confirmed by histochemical staining (Fig. 5B and C). EGFP expression detected by DAB staining was observed, especially in chondrocytes in the growth plate cartilage. The cells doubly positive for EGFP and collagen II in the treated mice confirm that chondrocytes are susceptible to AAV9 transduction, at least during the fetal period.
FIG. 5.
Biodistribution of AAV9-EGFP. (A) Vector copy number in each tissue of Akp+/+ mice after fetal (n=3, solid column) or neonatal (n=3, open column) IP injection of AAV9-EGFP (real-time PCR). Data are presented as means and SD. A Student t test was employed for comparisons between the two groups (*p<0.01, **p<0.05). (B) DAB staining of tibial bone after fetal IP injection (a and b) and neonatal IP injection (c and d). Negative controls (e and f) were mice without vector injection. (b), (d), and (f) are high-resolution photographs of the frames in (a), (c), and (e), respectively. Scale bars: 250 μm. (C) Immunostaining of tibial bone with anti-GFP (a) and anti-collagen type II (b); (c) merging of anti-GFP and anti-collagen type II. Scale bars: 50 μm. Color images available online at www.liebertonline.com/hum
Discussion
Perinatal HPP is the most severe form of HPP, with an autosomal recessive mode of inheritance, and is more common in Japan than in other countries (Satoh et al., 2009). We have determined the prevalence of c.1559delT in ALPL, a common mutation resulting in perinatal HPP among the Japanese, and the carrier frequency was 1 in 480 (Watanabe et al., 2010). It is possible that some perinatal HPP patients are passed over as stillborn babies, with the cause of death unknown. On the other hand, with developments in perinatal care, the chance of diagnosis of perinatal lethal HPP during the fetal period is increasing. This indicates the need for fetal gene therapy, which was advocated in the report entitled “Prenatal Gene Transfer: Scientific, Medical, and Ethical Issues” by the Recombinant DNA Advisory Committee (1999).
Several advantages of fetal gene therapy have been proposed for the treatment of genetic diseases. Immunological tolerance to the viral vector or transgenic proteins would be induced by fetal gene delivery, and it can achieve long-term effective expression of the transgene (Lipshutz et al., 2000; Waddington et al., 2003). Another advantage of fetal gene therapy is that gene delivery to tissues that are difficult to penetrate, such as the central nervous system, may be possible not only because of immaturity of the blood–brain barrier but also because of the abundance of stem cell populations in the fetus (Lipshutz et al., 2000). Furthermore, fetal gene therapy requires smaller amounts of vector compared with gene therapy in infants or adults, because of the small size of the fetus.
Enzyme replacement therapy for HPP, using a mineral-targeting recombinant form of TNALP, has been shown to be effective for the prevention of all the skeletal and dental abnormalities of HPP (Millán et al., 2008; McKee et al., 2011; Yadav et al., 2011). Those data indicate that expression of TNALP in cells lacking TNALP activity is not absolutely required for the treatment of HPP. Furthermore, mounting evidence indicates that a continuous supply of soluble TNALP from the circulation might be sufficient to improve mineralization. Indeed, our previous studies showed that systemic injection of lentiviral vector or AAV8 vector harboring either mineral-targeting TNALP or soluble nontargeted TNALP into neonatal mice resulted in sustained expression of TNALP in plasma and successful treatment of HPP, although transduction into bone was low (Yamamoto et al., 2010; Matsumoto et al., 2011). Our present study demonstrates high ALP activity in the bones of treated mice. Real-time PCR and immunohistochemical analysis indicate that AAV9 was directly transferred into chondrocytes after intraperitoneal injection during the fetal period. Therefore, ALP activity in the bones of treated fetal mice should be derived from the circulation and the resident chondrocytes. This approach would seem to be more effective than previous therapeutic strategies based on enzyme replacement by injection of either protein or vector in the neonatal period. Fetal injection of AAV9 vector represents a potential breakthrough for gene delivery into bone cells to treat systemic skeletal diseases, such as osteogenesis imperfecta and mucopolysaccharidosis.
It is interesting that chondrocytes were efficiently transduced with AAV vector after fetal gene transfer. Roybal and colleagues also reported that prenatal systemic administration of lentiviral vector resulted in efficient gene transfer into chondrocytes (Roybal et al., 2011). Therefore, chondrocytes or their stem cells appear to be preferentially susceptible to viral infection in utero. There are several possible explanations for the developmentally dependent distribution patterns of viral vector. Stem cells and progenitor cells of chondrocytes exist at higher frequency in fetal bones. These cells are more accessible to viral vectors than differentiated chondrocytes and provide a large pool of genetically modified chondrocytes. The structure of blood vessels is developmentally regulated (Herbert and Stainier, 2011) and unstable immature vessels at the early prenatal period should be more permeable to viral vectors. Changes in blood flow patterns in developing bones may also affect exposure of chondrocytes to viral vector in the circulation (Schachtner et al., 1999; Mescher, 2009). In this study, two of the Akp2−/− fetuses failed to respond to treatment. These two newborn mice showed failure to thrive and developed seizures after birth. The reason for this failure is not clear. One possibility is that sufficient amounts of vector were not delivered to the fetuses because of technical failure. It is sometimes difficult to perform intraperitoneal injection without leakage through the semitransparent uterine wall.
Perinatal lethal and infantile forms of HPP are often associated with epileptic seizures. One of the vitamin B6 forms, pyridoxal-5′-phosphate (PLP), is the cofactor of numerous enzymes, including enzymes synthesizing neurotransmitters such as γ-aminobutyric acid (GABA), dopamine, and serotonin (5-HT), which TNALP regulates via PLP in the neuropil (Negyessy et al., 2011). TNALP is present widely throughout the human neocortex (Negyessy et al., 2011). Consequently, deficiency of TNALP is thought to lead to epileptic seizures in patients with HPP. Clinically, vitamin B6 is usually administered to patients with HPP with epileptic seizures, but the efficacy has not yet been validated. Here, we chose AAV9, which has advantages regarding efficient transduction into the CNS; in addition, the blood–brain barrier of the fetus or neonate is immature, which may permit vectors to pass through (Foust et al., 2009; Miyake et al., 2011). AAV9 vector-mediated replacement of TNALP within the brain would normalize the levels of neurotransmitters, including GABA, 5-HT, and dopamine, which are also PLP dependent (Dolphin et al., 1986; Hartvig et al., 1995), and would have efficacy in controlling seizures. Moreover, normalizing TNALP levels in the brain from the fetal period was estimated to lead to sufficient development of the neocortex.
Safety and ethical problems are major concerns of fetal gene therapy. The safety of gene delivery into the immature tissues has not yet been established, and it will be necessary to accumulate much more evidence from animal trials to evaluate the risks and benefits of fetal gene therapy before use in humans. A high incidence of liver tumorigenesis in mice was reported after fetal injection of lentiviral vector (Themis et al., 2005) and neonatal injection of AAV vector (Donsante et al., 2007). As the fetal vasculature may be more permeable than that in adults, germline transmission is a serious ethical problem of fetal gene transfer. In mouse experiments, vector sequence was occasionally detectable in the gonads of treated fetal animals, but gene transfer into spermatozoa or in the offspring has not been found (Tenenbaum et al., 2003; Waddington et al., 2003). We also examined germline tissue, and our data also confirmed the lack of transduction into the germline of treated mice. This may be because fetal gene therapy requires only small amounts of vector. However, evidence of low-efficiency germline transmission into the sperm cells of sheep (Porada et al., 2005) and gonadal cells of rhesus monkeys (Lee et al., 2005) was reported after fetal gene transfer. The timing of fetal gene therapy should also be considered because of the difference in development of the animal model and humans. It has been reported that day 16–17 of gestation for mice corresponds approximately to 15–20 weeks of gestation for humans (Larson et al., 1997). However, the developmental stages of each organ should be quite different between mice and humans. If fetal gene therapy becomes applicable to humans in the future, the timing of treatment must be carefully determined by systemic experiments using large animal models including nonhuman primates.
In conclusion, we demonstrate that lethal murine HPP can be treated by fetal gene therapy. A single injection of AAV vector expressing bone-targeted TNALP in utero resulted in long-term expression and systemic replacement of TNALP. ALP activity was detected in various systemic organs including bone. Treated animals showed good weight gain, normal mineralization, and seizure-free survival until at least 8 weeks of age, when they were killed for analysis. Although long-term follow-up is necessary for further evaluation of the efficacy and safety of fetal gene therapy, this is the first report of successful gene therapy in utero for lethal murine HPP.
Acknowledgments
The authors thank Dr. James Wilson (University of Pennsylvania) for providing AAV packaging plasmid p5E18-VD2/9. This work was supported in part by grants from the Ministry of Health, Labor, and Welfare of Japan and the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and by grant DE12889 from the National Institutes of Health (Bethesda, MD).
Author Disclosure Statement
J.L. Millán is a consultant for Enobia Pharma. The other authors have no competing financial interests.
References
- Dolphin D. Poulson R. Avramovi O. Vitamin B6 pyridoxal phosphate: Chemical, biochemical and medical aspects. John Wiley & Sons; New York: 1986. [Google Scholar]
- Donsante A. Miller D.G. Li Y., et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Fedde K.N. Blair L. Silverstein J., et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D. Nurre E. Montgomery C.L., et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes in CNS. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goseki M. Oida S. Sasaki S. Detection of minor immunological differences among human “universal-type” alkaline phosphatases. J. Cell. Biochem. 1988;38:155–163. doi: 10.1002/jcb.240380303. [DOI] [PubMed] [Google Scholar]
- Hartvig P. Lindner K.J. Bjurling P, et al. Pyridoxine effect on synthesis rate of serotonin in the monkey brain measured with positron emission tomography. J. Neural Transm. Gen. Sect. 1995;102:91–97. doi: 10.1007/BF01276505. [DOI] [PubMed] [Google Scholar]
- Herbert S.P. Stainier D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell. Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens W.T. ter Brake O. Dijkhuizen P.A., et al. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum. Gene Ther. 1999;10:1885–1891. doi: 10.1089/10430349950017563. [DOI] [PubMed] [Google Scholar]
- Iwamoto N. Watanabe A. Yamamoto M., et al. Global diffuse distribution in the brain and efficient gene delivery to the dorsal root ganglia by intrathecal injection of adeno-associated viral vector serotype 1. J. Gene Med. 2009;11:498–505. doi: 10.1002/jgm.1325. [DOI] [PubMed] [Google Scholar]
- Larson J.E. Morrow S.L. Happel L., et al. Reversal of cystic fibrosis phenotype in mice by gene therapy in utero. Lancet. 1997;349:619–620. doi: 10.1016/S0140-6736(05)61567-X. [DOI] [PubMed] [Google Scholar]
- Lee C.C. Jimenez D.F. Kohn D.B. Tarantal A.F. Fetal gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta) Hum. Gene Ther. 2005;16:417–425. doi: 10.1089/hum.2005.16.417. [DOI] [PubMed] [Google Scholar]
- Lipshutz G.S. Flebbe-Rehwaldt L. Gaensler K.M. Reexpression following readministration of an adenoviral vector in adult mice after initial in utero adenoviral administration. Mol. Ther. 2000;2:374–380. doi: 10.1006/mthe.2000.0136. [DOI] [PubMed] [Google Scholar]
- Matsumoto T. Miyake K. Yamamoto S., et al. Rescue of severe infantile hypophosphatasia mice by AAV mediated sustained expression of soluble alkaline phosphatase. Hum. Gene Ther. 2011;22:1355–1364. doi: 10.1089/hum.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee M.D. Nakano Y. Masica D.L., et al. Enzyme replacement prevents dental defects in a mouse model of hypophosphatasia. J. Dental Res. 2011;90:470–476. doi: 10.1177/0022034510393517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher A.L. Junqueira's Basic Histology. 12th. McGraw Hill; New York: 2009. Bone; pp. 121–139. [Google Scholar]
- Millán J.L. Narisawa S. Lemire I., et al. Enzyme replacement therapy for murine hypophosphatasia. J. Bone Miner. Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N. Miyake K. Yamamoto M., et al. Global gene transfer into the CNS after neonatal systemic delivery of single-stranded AAV vectors. Brain Res. 2011;1389:19–26. doi: 10.1016/j.brainres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Mornet E. Hypophosphatasia. Orphanet. J. Rare Dis. 2007;2:40. doi: 10.1186/1750-1172-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Utsunomiya A. Okada S. Hara K., et al. Clinical characteristics of perinatal lethal hypophosphatasia: A report of 6 cases. Clin. Pediatr. Endocrinol. 2010;19:7–13. doi: 10.1297/cpe.19.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S. Frohlander N. Millán J.L. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Negyessy L. Xiao J. Kantor O., et al. Layer-specific activity of tissue non-specific alkaline phosphatase in the human neocortex. Neuroscience. 2011;172:406–418. doi: 10.1016/j.neuroscience.2010.10.049. [DOI] [PubMed] [Google Scholar]
- Porada C.D. Park P.J. Tellez J., et al. Male germ-line cells are at risk following direct-injection retroviral-mediated gene transfer in utero. Mol. Ther. 2005;12:754–762. doi: 10.1016/j.ymthe.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Recombinant DNA Advisory Committee, National Institutes of Health. Prenatal gene transfer: Scientific, medical, and ethical issues. 1999. http://oba.od.nih.gov/oba/rac/gtpcreport.pdf. [Dec;2011 ]. http://oba.od.nih.gov/oba/rac/gtpcreport.pdf
- Roybal J.L. Endo M. Zoltick P.W. Flake A.W. Early gestational gene transfer of IL-10 by systemic administration of lentiviral vector can prevent arthritis in a murine model. Gene Ther. 2011;18:719–726. doi: 10.1038/gt.2011.23. [DOI] [PubMed] [Google Scholar]
- Salvetti A. Oreve S. Chadeuf G., et al. Factors influencing recombinant adeno-associated virus production. Hum. Gene Ther. 1998;9:695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]
- Satoh N. Murotsuki A. Sawai H. [The birth prevalence rates for skeletal dysplasia in the registration system of the Japan Forum of Fetal Skeletal Dysplasia] [in Japanese] J. Jap. Perinat. Neonat. Med. 2009;45:1005–1007. [Google Scholar]
- Schachtner S.K. Buck C.A. Bergelson J.M. Baldwin H.S. Temporally regulated expression patterns following in utero adenovirus-mediated gene transfer. Gene Ther. 1999;6:1249–1257. doi: 10.1038/sj.gt.3300939. [DOI] [PubMed] [Google Scholar]
- Sugiyama O. Orimo H. Suzuki S., et al. Bone formation following transplantation of genetically modified primary bone marrow stromal cells. J. Orthop. Res. 2003;21:630–637. doi: 10.1016/S0736-0266(02)00260-7. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L. Lehtonen E. Monahan P.E. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr. Gene Ther. 2003;3:545–565. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- Themis M. Waddington S.N. Schmidt M., et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 2005;12:763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- Waddington S.N. Buckley S.M. Nivsarkar M., et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood. 2003;101:1359–1366. doi: 10.1182/blood-2002-03-0779. [DOI] [PubMed] [Google Scholar]
- Watanabe A. Karasugi T. Sawai H., et al. Prevalence of c.1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasia in Japanese and effects of the mutation on heterozygous carriers. J. Hum. Genet. 2010;56:166–168. doi: 10.1038/jhg.2010.161. [DOI] [PubMed] [Google Scholar]
- Whyte M.P. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann. N.Y. Acad. Sci. 2010;1192:190–200. doi: 10.1111/j.1749-6632.2010.05387.x. [DOI] [PubMed] [Google Scholar]
- Yadav M.C. Lemire I. Leonard P., et al. Dose response of bone-targeted enzyme replacement for murine hypophosphatasia. Bone. 2011;49:250–256. doi: 10.1016/j.bone.2011.03.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S. Orimo H. Matsumoto T., et al. Prolonged survival and phenotypic correction of Akp2−/− hypophosphatasia mice by lentiviral gene therapy. J. Bone Miner. Res. 2010;26:135–142. doi: 10.1002/jbmr.201. [DOI] [PMC free article] [PubMed] [Google Scholar]