Abstract
Engineered measles virus (MV) strains deriving from the vaccine lineage represent a promising oncolytic platform and are currently being tested in phase I trials. In this study, we have demonstrated that MV strains genetically engineered to express the human sodium iodide symporter (NIS) have significant antitumor activity against glioma lines and orthotopic xenografts; this compares favorably with the MV strain expressing the human carcinoembryonic antigen, which is currently in clinical testing. Expression of NIS protein in infected cells results in effective concentration of radioactive iodine, which allows for in vivo monitoring of localization of MV-NIS infection by measuring uptake of 123I or 99mTc. In addition, radiovirotherapy with MV-NIS followed by 131I administration resulted in significant increase of MV-NIS antitumor activity as compared with virus alone in both subcutaneous (p=0.0003) and orthotopic (p=0.004) glioblastoma models. In conclusion, MV-NIS-based radiovirotherapy has significant antitumor activity against glioblastoma multiforme and represents a promising candidate for clinical translation.
Opyrchal and colleagues evaluate the efficacy of measles virus (MV) strains engineered to express human sodium iodide symporter (NIS) against glioma lines and xenografts, as well as the impact of the addition of radioactive iodine on the observed antitumor effect. MV-NIS was significantly cytopathic against glioma lines and xenografts in vitro and in vivo, with increased viral proliferation and faster tumor cell killing. The addition of radioactive iodine further improved the efficacy of MV-NIS and prolonged survival in both flank and intracranial mouse models.
Introduction
Glioblastoma is the most common primary brain tumor, and it is associated with a dismal prognosis. Even with the best available treatment, including surgery, radiation, and chemotherapy, the median survival is between 12 and 18 months (Stupp et al., 2005; Grossman et al., 2010). Despite recent advances in the field of oncology, glioblastoma remains an incurable disease, and novel therapeutic approaches are needed. Because it rarely metastasizes, glioblastoma is a good candidate for locoregional delivery of novel therapeutics, including gene and virus therapies.
Virotherapy is a promising novel therapeutic approach in the treatment of malignancies. Measles virus (MV) is an RNA virus belonging to the family of Paramyxoviridae. Derivatives of the Edmonston (Edm) vaccine strain of the MV have a long history of safe administration to patients through vaccination programs (Griffin et al., 2008). MV-Edm vaccine strains enter cells primarily through the CD46 receptor, and the ability of the virus to induce a cytopathic effect, cell-to-cell fusion, and formation of multinucleated aggregates (syncytia) (Dörig et al., 1993; Naniche et al., 1993) is primarily determined by receptor density (Anderson et al., 2004). The MV receptor CD46 is highly expressed in multiple solid tumors, including gliomas, resulting in the ability of MV to preferentially infect tumor cells (Phuong et al., 2003; Anderson et al., 2004; Ulasov et al., 2006).
Our laboratory has previously shown that MV-Edm derivatives engineered to express the human carcinoembryonic antigen (CEA; MV-CEA virus) have significant antitumor activity against glioma cell lines and mouse glioblastoma models (Phuong et al., 2003). These results have led to the initiation of a phase I clinical trial in recurrent glioblastoma patients, which is currently ongoing (Allen et al., 2008a; Msaouel et al., 2009a). Although MV-CEA could allow monitoring of viral replication based on detection of the measurable marker CEA in the blood, it does not allow for localization of viral spread. To address this concern, the MV-Edm virus has been engineered to express the human sodium iodide symporter (NIS) (Dingli et al., 2004). Expression of the NIS protein, a sodium/iodine transporter, can allow noninvasive monitoring of viral infection in vitro and in vivo by using 123I, 124I, 125I, or 99mTc isotopes (Spitzweg et al., 2000; Dingli et al., 2004; Msaouel et al., 2009b).
In addition, MV-NIS represents a promising platform to allow augmentation of a virus-induced cytopathic effect through radiovirotherapy, because the NIS transgene can allow uptake of the β- and γ-emitter 131I by cancer cells infected by the virus (Dingli et al., 2004; Msaouel et al., 2009b). We have previously shown that the activity of oncolytic MV-Edm strains against glioblastoma can be enhanced by external beam radiation therapy (EBRT) (Liu et al., 2007). We therefore hypothesized that MV-NIS activity against glioblastoma could be enhanced by local radiation treatment, accomplished via accumulation of 131I in infected cancer cells expressing the NIS protein. This approach can be especially attractive in glioma treatment, because radiation therapy represents a key therapeutic modality for patients affected by this disease, and use of 131I at recurrence could represent a less toxic alternative to re-irradiation using EBRT. Therefore, we designed this study to evaluate the efficacy of MV-NIS against glioma lines and xenografts, as well as the impact of the addition of 131I on the observed antitumor effect.
We demonstrated that MV-NIS has significant cytopathic effect against glioma lines in vitro and xenografts in vivo, with increased viral proliferation and faster tumor-cell killing as compared with MV-CEA. The addition of 131I further improved the efficacy of MV-NIS and prolonged survival in both flank and intracranial mouse models. Furthermore, we demonstrated that NIS enables noninvasive tracking of MV-NIS infection and localization in both subcutaneous and orthotopic xenografts. These promising results underline the significant translational potential of the MV-NIS-mediated radiovirotherapy in the treatment of gliomas.
Materials and Methods
Viral construction
Construction of MV-CEA (Peng et al., 2002) and MV-NIS (Dingli et al., 2004) have been previously described (Fig. 1). The viruses were propagated in Vero cells and titrated as previously described (Msaouel et al., 2009b).
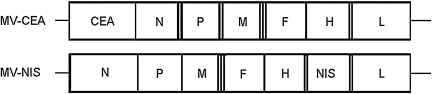
FIG. 1.
Schematic representation of MV-Edm strains. F, fusion protein gene; H, hemagglutinin gene; L, large protein gene; M, matrix protein gene; N, nucleoprotein gene; P, phosphoprotein gene; CEA, human carcinoembryonic antigen gene; NIS, sodium iodide symporter gene.
Comparison of MV-CEA and MV-NIS cytopathic effects in vitro
Cells were plated in six-well plates in duplicate at a density of 5×105 cells/well (U87, U251, GBM6, GBM10, GBM12, GBM39, GBM43, and GBM44). Twenty-four hours after plating, cells were infected at a multiplicity of infection (MOI) of 1.0 or 0.1 with either MV-CEA or MV-NIS in 1 ml of Opti-MEM for 2 hr at 37°C. At the end of the incubation period, the virus was removed and the cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1×penicillin/streptomycin. The number of viable cells in each well was determined using a hemacytometer at 3, 5, 7, and 9 days after infection using the trypan blue exclusion assay. The percentage of surviving cells was calculated by dividing the number of viable cells in the infected well by the number of viable cells in uninfected wells corresponding to the same time point.
Assessment of radioactive iodine uptake in vitro
Cells were plated in six-well plates in duplicate at a density of 5×105 cells/well (U251, GBM12, GBM39, and GBM43). Twenty-four hours after plating, cells were infected at an MOI of 1.0 or 0.1 with MV-NIS in 1 ml of Opti-MEM for 2 hr at 37°C; uninfected wells served as controls. At the end of the incubation period, the virus was removed and the cells were maintained in DMEM containing 10% FBS and 1×penicillin/streptomycin. Forty-eight hours post infection, either 0.9 ml Hanks' balanced salt solution (HBSS) with HEPES (pH 7.3) or 0.8 ml HBSS with HEPES and 0.1 ml of 0.01 mM potassium perchlorate were added per well. Na125I (0.1 μCi/ml, pH 7.3) was then added to each well, and activity was determined in a gamma counter.
Comparative assessment of virotherapy efficacy in orthotopic tumor models
All animal experiments were approved by the Mayo Institutional Animal Care and Use Committee. Glioblastoma orthotopic xenografts were established by implantation of 3×105 primary glioblastoma cells (GBM43 and GBM39) into the right caudate nucleus of 5-week-old BALB/c nude mice, using the small animal stereotactic frame (ASI Instruments, Warren, MI) and a 26-gauge Hamilton syringe. Treatment was initiated 1–2 weeks post implantation by intratumoral injection using the same coordinates as for implantation 1.5×105 TCID50 per dose was administered in 10 μl three times per week for a total of four doses. The following groups were included (10 animals each): UV-inactivated MV-CEA, MV-CEA, or MV-NIS. Mice were observed daily and were euthanized when neurological impairment or greater than 10% weight loss was observed. The experiment was terminated at 120 days post implantation. Brains of euthanized animals were fixed in paraformaldehyde and embedded in paraffin for subsequent analysis.
Assessment of NIS expression in vivo in a flank xenograft glioblastoma model
U251 flank tumors were developed by implantation of 5×106 U251 cells mixed 1:2 with Matrigel (BD Biosciences, Bedford, MA) in BALB/c nude mice. Water for these mice was supplemented with levothyroxine (5 mg/ml) to suppress thyroidal NIS expression. On days 12 and 13 post tumor implantation, mice received an intratumoral dose of 1.5×106 TCID50 MV-NIS or UV-inactivated MV-NIS. Daily tumor imaging was performed 60 min after the mice were injected with 123I (1 mCi) intraperitoneally using a gamma camera (Helix System; Elscint, Haifa, Israel).
Assessment of NIS expression in vivo in an orthotopic glioblastoma model
Orthotopic xenografts were established in female BALB/c nude mice by stereotactic implantation of 3×105 GBM43 cells. Mice were treated orthotopically on days 5, 7, 9, 12, 14, and 16 post implantation with MV-NIS (2.5×106 TCID50 per treatment dose) or UV-inactivated MV-NIS. Mice were imaged twice weekly until day 20 after completion of viral treatment. Images were obtained using a microSPECT/CT imaging system 1 hr after intraperitoneal injection of 1 mCi of 123I (group 1) or 1 mCi of 99Tc (group 2).
Assessment of combination therapy with MV-NIS and 131I in vivo in flank and orthotopic glioblastoma xenografts
U251 tumors were developed by implantation of 1×107 U251 cells mixed 1:2 with Matrigel (BD Biosciences) in the flank of BALB/c nude mice. Seven or eight mice were randomized into four groups for treatment with either UV-inactivated virus, 131I, MV-NIS, or MV-NIS in combination with 131I. The UV group was treated with UV-inactivated virus intratumorally on days 12 and 13 post tumor implantation. The 131I group received 1 mCi of 131I intraperitoneally on day 16 post tumor implantation. The MV-NIS group was treated with MV-NIS on days 12 and 13 post tumor implantation (2×106 TCID50 per dose). The MV-NIS/131I group was treated with MV-NIS on days 12 and 13 (2×106 TCID50 per dose in 0.1 ml volume) and received 1 mCi of 131I intraperitoneally on day 3 after the last viral treatment. Mice were euthanized when any tumor dimension exceeded 2.0 cm or ulceration was noted.
Orthotopic xenografts were established in BALB/c nude mice by stereotactic implantation of 3×105 GBM43 cells. Mice were treated orthotopically (131I administered intraperitoneally) with either UV-inactivated virus, MN-NIS, 131I, or MV-NIS/131I. MV-NIS was administered intratumorally using the same stereotactic coordinates used for tumor cell implantation on days 8, 13, and 15 following implantation (0.6×106 TCID50 per dose in 10 μl). 131I (1 mCi) was administered intraperitoneally on day 18 post implantation. Mice were followed daily and were euthanized when signs of CNS-related symptoms or greater than 10% body weight loss was observed.
Histology and in situ hybridization for MV-NIS N (nucleoprotein) mRNA
BALB/c nude mice had GBM43 tumors implanted as described above. After NIS expression was confirmed via 131I imaging, the mice were killed and paraffin-embedded brains were analyzed. Serial sections (12 μm) were used for either hematoxylin and eosin (H&E) staining or in situ hybridization (ISH) for MV-NIS N mRNA as previously described (Allen et al., 2008b). A custom-designed digoxigenin-labeled oligonucleotide probe (GeneDetect) was used for ISH. The samples were treated with proteinase K (20 mg/ml) for 20 min at 37°C, washed twice with phosphate-buffered saline/glycine, and prehybridized for 2 hr at 39.4°C. The sections were then washed and incubated overnight with the digoxigenin-labeled oligonucleotide probe (250 ng) at 39.4°C in a humidified chamber. The next day the slides were washed in saline-sodium citrate/dithiothreitol followed by the detection step performed using anti-digoxigenin-AP, Fab fragments (Roche Diagnostics, Indianapolis, IN) at 4°C in a humidified chamber, followed by serial washes, nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate detection, and counterstaining with nuclear fast red (Sigma, St. Louis, MO).
Results
The MV-CEA (Peng et al., 2002), MV-NIS (Dingli et al., 2004), and MV-Edm strains were used in these comparative experiments (Fig. 1).
MV-NIS infects glioblastoma cell lines more effectively than MV-CEA
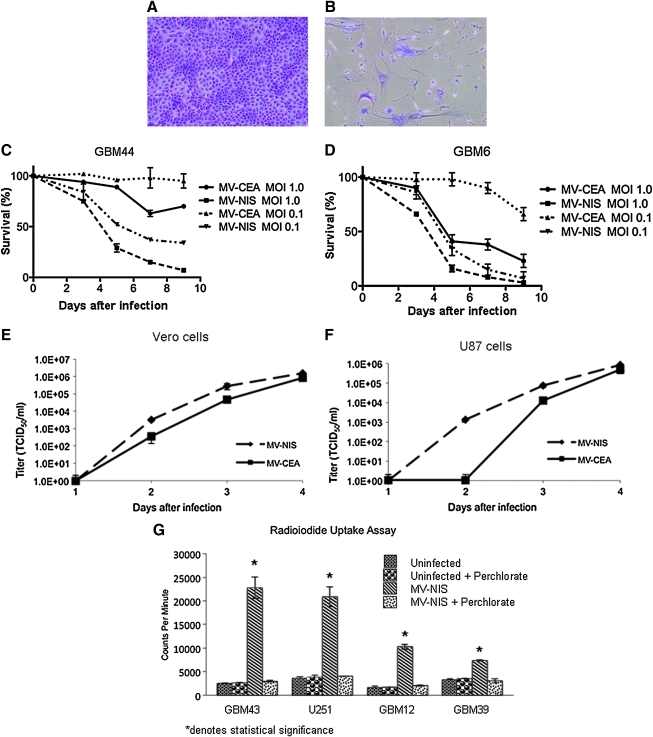
The infectivity and cytopathic effect of MV-NIS were tested in several glioblastoma cell lines, both established and primary [derived from glioblastoma (GBM) patients and maintained as xenografts]. MV-NIS demonstrated significant cytopathic activity in all tested glioma cell lines (U87, U251, GBM6, GBM10, GBM12, GBM39, GBM43, and GBM44) (Fig. 2A and B). When compared with MV-CEA, MV-NIS had enhanced cytopathic activity in vitro, even at very low MOI (MOI of 0.1; Fig. 2C and D), and increased replication in one-step viral growth curves performed in Vero and U87 glioma cells (Fig. 2E and F).
FIG. 2.
Increased cytopathic effect and replication of MV-NIS as compared with MV-CEA in primary and established glioma lines in vitro. GBM43 cells 5 days after either mock infection (A) or MV-NIS infection at MOI of 1.0 (B) (crystal violet stain) are shown. Infected cells demonstrate the characteristic cytopathic effect with formation of syncytia. Cytopathic effect of MV-CEA and MV-NIS on GBM44 cells (C) and GBM6 cells (D) at different MOIs, was determined by trypan blue exclusion assay. MV-NIS infection led to faster elimination of tumor-cell monolayers at comparable MOIs. One-step viral growth curves demonstrate increased replication of MV-NIS as compared with MV-CEA in Vero cells (E) and U87 glioma cells (F)±(G) In vitro 125I uptake by glioblastoma cell lines infected by MV-NIS at an MOI of 1.0. Cells infected with MV-NIS effectively accumulated 125I. Uptake was significant (above 7,000 CPM) even in the glioma line (GBM39) with the lowest ability to concentrate iodine. Uninfected controls did not concentrate 125I. Infected cells exposed to KClO4, a competitive inhibitor of iodine uptake by NIS, were unable to concentrate iodine, similar to the uninfected controls.
MV-NIS infection results in increased uptake of 125I in vitro
We also assessed the ability of MV-NIS-infected glioma cells to concentrate radioactive iodine. As Fig. 2G illustrates, glioblastoma cell lines (U251, GBM12, GBM39, and GBM43) infected with MV-NIS in vitro were able to effectively concentrate radioactive iodine (125I). This effect was abrogated with the addition of potassium perchlorate (KClO4), which specifically blocks the NIS transporter, thus demonstrating that 125I uptake in these lines was due to functional NIS protein expression (Fig. 2G).
MV-NIS shows increased antitumor activity as compared with MV-CEA in orthotopic glioblastoma xenograft models
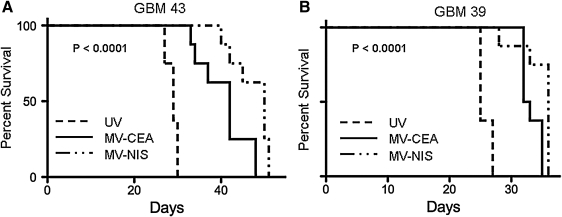
These in vitro results suggested that MV-NIS could be a superior oncolytic agent compared with MV-CEA against glioblastoma. This hypothesis was tested in the orthotopic GBM39 and GBM43 GBM models. These models are derived from Mayo Clinic GBM patients, kept as xenografts, and maintain the molecular characteristics and invasiveness of the primary tumor they derive from (Giannini et al., 2005; Carlson et al., 2011). Treatment was initiated at 1 week (GBM43) or 2 weeks (GBM39) after intracranial implantation of primary glioblastoma cell lines. Mice received a total of four treatments of the virus (1.5×105 TCID50 per dose). As seen in Fig. 3, the groups treated with MV derivatives had a statistically significant prolongation of survival as compared with the corresponding group treated with UV-inactivated virus (p<0.001) in both glioma models. When the two treatment groups were compared, the MV-NIS-treated group had a modest but statistically significant prolongation of survival when compared with the MV-CEA-treated group (p=0.009 in the GBM39 model, p=0.012 in the GBM43 model).
FIG. 3.
Oncolytic activity of MV-NIS as compared with MV-CEA in orthotopic xenograft glioblastoma model. (A) GBM43 cells. (B) GBM39 cells. Mice (n=8 per group) received four treatments with MV-NIS or MV-CEA for a total dose of 6×105 TCID50 control animals received UV inactivated virus. Mice treated with MV-NIS had a statistically significant prolongation of survival as compared with UV-inactivated control (p<0.001 in both models), and a modest but significant prolongation of survival as compared with MV-CEA-treated mice (A, p=0.012; B, p=0.009).
MV-NIS efficiently infects glioblastoma xenografts and facilitates noninvasive imaging of tumor infection
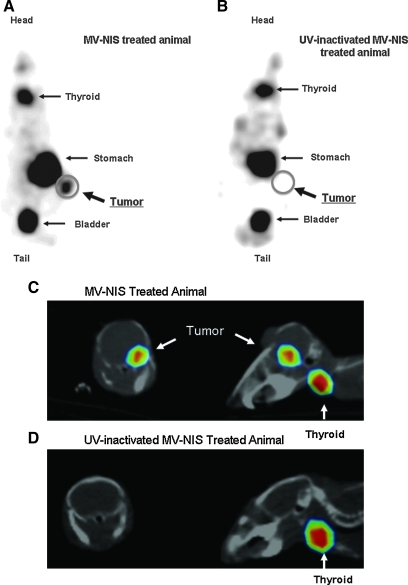
To evaluate expression of the NIS protein after viral infection in vivo, both mouse flank xenografts and orthotopic GBM models were used. Mice with U251 flank tumors were treated with two intratumoral injections (1.5×106 TCID50) of MV-NIS with UV-inactivated MV-NIS treated animals serving as control. NIS expression was monitored daily 1 hr after injection of 1 mCi of 123I with a gamma-camera. Significant iodine uptake was demonstrated within the tumor (Fig. 4A). In contrast, there was no 123I uptake in the tumors of UV-inactivated MV-NIS-treated animals (Fig. 4B). The signal, as expected, was also seen in thyroid and stomach, which normally express the NIS transporter protein (Josefsson et al., 2002), as well as bladder due to excretion of the radioisotope in urine. Peak signal was observed at 3 days after the last intratumoral treatment.
FIG. 4.
In vivo imaging of glioblastoma xenografts infected with MV-NIS. (A) Mice with U251 subcutaneous tumor xenografts received two intratumoral treatments of 1.5×106 TCID50 of the MV-NIS. Expression of the NIS protein in the tumor was confirmed by 123I uptake. A representative image is shown at 3 days following the viral treatment. (B) No tracer uptake was observed in tumors of UV-inactivated MV-NIS-treated animals. (C) GBM43 xenografts were established orthotopically by stereotactic implantation of 3×105 cells. Five days later, mice were treated with six intratumoral injections of the MV-NIS with UV-inactivated virus serving as control for a total dose of 1.5×107 TCID50. Gamma camera imaging was performed at multiple time points following administration of 123I or 99mTc. A representative image of MV-NIS-treated tumor at 20 days following viral administration is shown. (D) No tracer accumulation was observed in orthotopic tumors of UV-inactivated MV-NIS-treated animals.
To evaluate NIS expression in an intracerebral GBM43 model, 5 days following orthotopic implantation of GBM43 cells, treatment was initiated with MV-NIS or UV-inactivated MV-NIS for a total of six treatments (2.5×106 TCID50 per dose). Mice were imaged twice weekly 1 hr after intraperitoneal injection of 1 mCi of 123I or 99mTc. There was increased tumor uptake of radioisotope in MV-NIS-treated mice, which peaked at 3 days and persisted up to 20 days following viral administration (Fig. 4C). In contrast, there was no tracer uptake in the tumors of UV-inactivated MV-NIS-treated animals (Fig. 4D). Therefore, in vivo infection with MV-NIS results in expression of NIS protein in tumor tissue and concentration of 123I or 99mTc, which could be used for assessment and localization of viral infection, and could serve as the basis for therapeutic application of 131I.
Combined radiovirotherapy with MV-NIS and 131I results in improved antitumor efficacy as compared with virotherapy alone in both subcutaneous and orthotopic mouse glioblastoma models
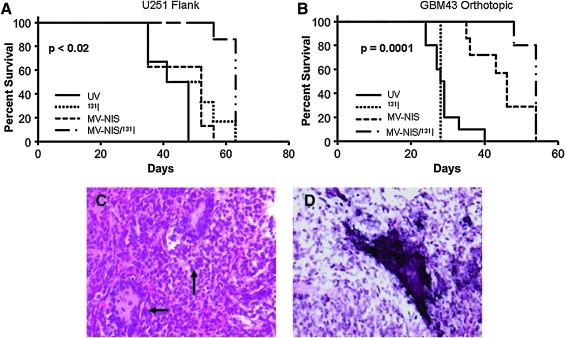
To evaluate the added benefit of 131I to MV-NIS treatment in vivo, we tested the efficacy of this combination radiovirotherapy approach in both subcutaneous and orthotopic glioblastoma models. After establishment of U251 flank tumors, mice were treated with MV-NIS or UV-inactivated control on days 12 and 13 post implantation (2×106 TCID50 per dose). Low doses of MV-NIS were chosen to facilitate the demonstration of additional benefit derived from the radioisotope treatment. The combination therapy group received 1 mCi of 131I intraperitoneally 3 days after the last viral treatment, coinciding with maximal NIS expression as determined by the 123I imaging study in the subcutaneous xenograft model. As Fig. 5A demonstrates, treatment of tumors with only two MV-NIS injections did not improve the survival of the animals. Furthermore, there was no significant difference in survival between 131I-treated animals and UV-inactivated virus-treated controls (p=0.26). The addition of 131I, however, resulted in considerable improvement in survival of the animals compared with MV-NIS or UV-inactivated MV-NIS-treated groups (p=0.011). Comparison of the 131I/MV-NIS treated group with the MV-NIS treated group showed a significant survival advantage for the combination treated animals (p=0.0003). Mice treated with 131I did exhibit late gastrointestinal toxicity and failure to thrive, however, likely related to the radioisotope treatment, which was manifested as weight loss around day 60.
FIG. 5.
Therapeutic effect of radiovirotherapy in glioblastoma xenograft mouse models. (A) Mice (n=8 per group) with established right flank U251 tumors received two injections with MV-NIS or matched UV-inactivated control (4×106 TCID50 total dose). Three days later, they were treated with 1 mCi of 131I through intraperitoneal injection or PBS only. Mice in the radiovirotherapy group had significantly longer survival (p=0.0003) over mice treated with MV-NIS or 131I alone. (B) Mice (n=8 to 10 per group) with established GBM43 orthotopic brain tumors received four treatments starting 8 days post implantation with MV-NIS or matched UV-inactivated control (1.8×106 TCID50 total dose). The radiovirotherapy group received 131I intraperitoneally 3 days after the last viral treatment, with the timing determined based on imaging studies. MV-NIS treatment extended the survival of the animals (p=0.0004) as compared with the UV-MV-treated control group, and addition of 131I significantly extended survival of the animals as compared with the MV-NIS-only treated group (p=0.039). (C) Sections from brains from GBM43-implanted mice were stained with H&E: extensive syncytia formation was observed (arrow). Original magnification, 200×. (D) In situ hybridization for MV N mRNA is positive, confirming active viral replication in tumors.
The potential additional benefit of 131I administration following MV-NIS infection was also tested in an orthotopic GBM model (Fig. 5 B). Mice were inoculated intracranially with GBM43 cells and then treated with MV-NIS or with UV-inactivated control on days 8, 11, and 13 after cell inoculation (6×105 TCID50 per injection). The combination (MV-NIS plus 131I) therapy group received 1 mCi of 131I intraperitoneally, at 3 days following the last viral dose, timing was determined based on the imaging study. Mice were then followed and observed for any CNS symptoms and overall health status. Although the MV-NIS treatment group had superior survival compared with the UV-inactivated control (p=0.0004), the addition of 131I further improved the efficacy of the MV-NIS treatment as compared with MV-NIS alone (p=0.004), indicating a synergistic effect of radiovirotherapy. There was no significant difference between 131I-treated animals and UV-inactivated virus-treated controls (p=0.46). Similar to the subcutaneous model, mice treated with 131I did exhibit gastrointestinal toxicity, manifested as weight loss after day 55, with no neurological symptoms.
MV-NIS efficiently infects glioblastoma orthotopic brain xenografts
To further document MV-NIS infection in vivo, animals implanted with GBM43 cells and treated with MV-NIS were euthanized following demonstration of MV-NIS expression by imaging. H&E staining revealed visible syncytia in tumor samples from the MV-NIS-treated group, confirming viral infection and cytopathic effect (Fig. 5C). In situ hybridization for MV N mRNA was also performed, which was positive in the MV-NIS-treated group, further confirming active replication of the virus (Fig. 5D).
Discussion
Despite its initial short-term response to radiation and chemotherapy, glioblastoma tumors invariably progress (Wallner et al., 1989; Stupp et al., 2005; Grossman et al., 2010). They rarely metastasize, however, which makes them good candidates for locoregional delivery of virotherapy agents. Our laboratory has demonstrated in vitro and in vivo antitumor activity of MV strains against glioma lines and xenografts, which has led to initiation of a phase I clinical trial of MV-CEA in recurrent GBM patients (Phuong et al., 2003; Allen et al., 2008).
Radiotherapy has been the mainstay of treatment for glioblastoma for many years (Shapiro et al., 1989; Galanis and Buckner, 2000; Simpson and Galanis, 2006). Our laboratory has previously demonstrated synergistic activity when combining MV therapy with EBRT. Irradiation of glioma cells is associated with increased replication of MV (Liu et al., 2007), and resulted in augmentation of the antitumor effect in vitro and in vivo through an increase in viral replication, activation of the extrinsic caspase pathway, and an increase in apoptosis (Liu et al., 2007).
The NIS is a plasma membrane glycoprotein, most commonly studied in connection with the thyroid gland, where NIS mediates the active transfer of iodine into the thyroid follicular cells as a crucial first step in thyroid hormone biosynthesis (Carrasco, 1993; Dadachova and Carrasco, 2004). NIS expression from thyroid tissue allows the use of 131I for treatment of thyroid cancer metastasis (Dadachova and Carrasco, 2004), including brain metastases (Hjiyiannakis et al., 1996). In proof-of-principle experiments, Cho and colleagues demonstrated that stable transduction of the F98 glioma lines with the NIS gene with a retroviral vector allowed tumor imaging using 99mTc or 123I scintigraphy. Use of radioactive iodine prolonged animal survival in this model (Cho et al., 2002; Cho, 2004). Despite these initial proof-of-principle studies, no follow-up work had been performed exploiting the concept of NIS-gene delivery as a therapeutic modality in the treatment of gliomas. The MV-Edm derivative expressing NIS (MV-NIS) virus allows us to capitalize on the potential of this novel therapeutic gene, the NIS gene, and NIS-mediated radiovirotherapy in glioma treatment, thus further augmenting the activity of this promising oncolytic platform by 131I administration.
131I-based therapeutics, usually in the form of 131I-labeled monoclonal antibodies, have shown promise in the treatment of recurrent gliomas. In a phase II trial in recurrent glioma patients, intracavitary administration of 100 mCi of 131I-m81C6, a murine monoclonal antibody targeting tenascin, in combination with chemotherapy was associated with superior median survival as compared with historic controls (Reardon et al., 2006). In another trial, resection cavity administration of 131I-labeled TM-601 (a synthetic version of chlorotoxin) in 18 adult recurrent glioma patients (17 with GBM) was well tolerated (Mamelak et al., 2006) with some preliminary evidence of activity. We therefore hypothesized that radiovirotherapy with MV-NIS and radioactive 131I would be more effective than either agent alone in glioma treatment.
In this study, we have demonstrated that MV-NIS has significant cytopathic activity against glioblastoma cells in vitro and antitumor effect in vivo, in both settings antitumor activity was improved as compared with MV-CEA. This increase in cytopathic effect is most likely due to more effective viral replication, as demonstrated in Fig. 2. A potential explanation for the improved antitumor efficacy of MV-NIS pertains to the position of transgene [position 1 for MV-CEA versus position 6 for MV-NIS (Fig. 1)]. MV genome is a single-stranded nonsegmented RNA of negative polarity. During transcription, mono- and bicistronic messenger RNAs are produced with a transcriptional gradient from the N to L gene, i.e., transcription is less efficient as it progresses from the 3′ to the 5′ end of the viral genome (Griffin and Bellini, 1996). An MV strain encoding the transgene downstream from the N, P, M, F, and H genes, such as MV-NIS, would therefore be expected to have a proliferation advantage, resulting in superior antitumor activity, as compared with MV-CEA (encoding the CEA transgene in position 1), because transcription of its genome results in higher copy numbers of MV structural genes. This is supported by the 1 to 2 log higher titers of MV-NIS as compared with MV-CEA obtained in one-step viral growth curves in both glioma and Vero cells, and the in vivo efficacy data in GBM39 and GBM43 (Fig. 2).
In vitro expression of NIS protein and significant incorporation of iodine by infected cells were observed following MV-NIS infection. This effect was blocked by the specific NIS inhibitor, potassium perchlorate. Furthermore, NIS expression in tumor cells allowed for monitoring and localization of the viral replication in vivo. NIS expression was detected both in subcutaneous and in orthotopically implanted glioma xenografts following intratumoral administration of MV-NIS. These findings support the promise of this method in the monitoring of MV-NIS infection in future glioblastoma trials.
Detectable levels of NIS expression in vivo allowed us to proceed with testing of the radiovirotherapy combination. We showed that addition of radioactive iodine therapy to treatment with MV-NIS improved survival in both flank and intracranial orthotopic murine glioblastoma models (p<0.05). This result can be attributed to improved viral replication and cytopathic effect of MV-NIS on glioblastoma cells exposed to radiation (Liu et al., 2007) in combination with the 131I radiation treatment effect. Our study is the first to demonstrate the therapeutic potential of MV-NIS-based radiovirotherapy in a glioblastoma model. Delivering targeted radiation treatment directly to glioblastoma tumors has the potential advantage of achieving high radiation doses in the tumor, minimizing the side effects of external radiation treatment to normal tissue, and resulting in therapeutic benefit even in patients who have previously failed EBRT. It is of note that single-agent administration of MV derivatives such as MV-CEA in recurrent GBM patients has not resulted into any dose-limiting toxicity to date (Galanis et al., 2008). Although formal toxicology studies would be required prior to clinical translation, given the safety track record of both MV-virotherapy and dosimetry-based radioisotope administration (Mamelak et al., 2006; Reardon et al., 2006), we expect that the combination treatment will be well tolerated.
In summary, we have demonstrated that MV-NIS is a more effective oncolytic agent against gliomas than MV-CEA both in vitro and in vivo. In addition, NIS expression can be used to noninvasively monitor viral infection. The expression of NIS can also be used to further increase the efficacy of the virotherapy via 131I administration. MV-NIS therefore represents an attractive candidate as a virotherapy agent against glioblastoma, and clinical translation is warranted.
Acknowledgments
This work was supported by R01 CA 154348 and P50 CA 108961.
Author Disclosure Statement
The authors have nothing to disclose.
References
- Allen C. Paraskevakou G. Liu C., et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin. Biol. Ther. 2008a;8:213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C. Parakevakou G. Iankov I., et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 2008b;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.D. Nakamura T. Russell S.J. Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Carlson B.L. Pokorny J.L. Schroeder M.A. Sarkaria J.N. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr. Protoc. Pharmacol. 2011 doi: 10.1002/0471141755.ph1416s52. Chapter 14, Unit 14.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N. Iodide transport in the thyroid gland. Biochim. Biophys. Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- Cho J.Y. A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: imaging and therapy. Curr. Gene Ther. 2004;2:393–402. doi: 10.2174/1566523023347599. [DOI] [PubMed] [Google Scholar]
- Cho J.Y. Shen D.H. Yang W., et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002;9:1139–1145. doi: 10.1038/sj.gt.3301787. [DOI] [PubMed] [Google Scholar]
- Dadachova E. Carrasco N. The Na/I symporter (NIS): imaging and therapeutic applications. Semin. Nucl. Med. 2004;34:23–31. doi: 10.1053/j.semnuclmed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Dingli D. Peng K.W. Harvey M.E., et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Dörig R.E. Marcil A. Chopra A. Richardson C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Galanis E. Buckner J. Chemotherapy for high-grade gliomas. Br. J. Cancer. 2000;82:1371–1380. doi: 10.1054/bjoc.1999.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E. O’Neill B.P. Piepegras D., et al. Phase 1 intratumoral and resection cavity administration of a measles virus derivative expressing the human carcionembroyonic antigen (CEA) in patients with recurrent glioblastoma multiforme. Neuro-Oncology. 2008;10:819. (Abstract MA-15) [Google Scholar]
- Giannini C. Sarkaria J.N. Saito A., et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.E. Bellini W.J. Measles virus. In: Fields B.N., editor; Knipe D.M., editor; Howley P.M., editor. Fields' Virology. Lippincott–Raven Publishers; Philadelphia, PA: 1996. pp. 1267–1312. [Google Scholar]
- Griffen D.E. Pan C.H. Moss W.J. Measles vaccines. Front. Biosci. 2008;13:1352–1370. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- Grossman S.A. Ye X. Piantadosi S., et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjiyiannakis P. Jefferies S. Harmer C.L. Brain metastases in patients with differentiated thyroid carcinoma. Clin. Oncol. (R. Coll. Radiol.) 1996;8:327–330. doi: 10.1016/s0936-6555(05)80722-8. [DOI] [PubMed] [Google Scholar]
- Josefsson M. Grunditz T. Ohlsson T. Ekblad E. Sodium/iodide-symporter: distribution in different mammals and role in entero-thyroid circulation of iodide. Acta Physiol. Scand. 2002;175:129–137. doi: 10.1046/j.1365-201X.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- Liu C. Sarkaria J.N. Petell C.A., et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin. Cancer Res. 2007;13:7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- Mamelak A.N. Rosenfeld S. Bucholz R., et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 2006;24:3644–3650. doi: 10.1200/JCO.2005.05.4569. [DOI] [PubMed] [Google Scholar]
- Msaouel P. Dispenzieri A. Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr. Opin. Mol. Ther. 2009a;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- Msaouel P. Iankov I.D. Allen C., et al. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol. Ther. 2009b;17:2041–2048. doi: 10.1038/mt.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D. Varior-Krishnan G. Cervoni F., et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K.W. Facteau S. Wegman T., et al. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Phuong L.K. Allen C. Peng K.W., et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- Reardon D.A. Akabani G. Coleman R.E., et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J. Clin. Oncol. 2006;24:115–122. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- Shapiro W.R. Green S.B. Burger P.C., et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J. Neurosurg. 1989;71:1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- Simpson L. Galanis E. Recurrent glioblastoma multiforme: advances in treatment and promising drug candidates. Expert Rev. Anticancer Ther. 2006;6:1593–1607. doi: 10.1586/14737140.6.11.1593. [DOI] [PubMed] [Google Scholar]
- Spitzweg C. O'Connor M.K. Bergert E.R., et al. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- Stupp R. Mason W.P. van den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Ulasov I.V. Tyler M.A. Zheng S., et al. CD46 represents a target for adenoviral gene therapy of malignant glioma. Hum. Gene Ther. 2006;17:556–564. doi: 10.1089/hum.2006.17.556. [DOI] [PubMed] [Google Scholar]
- Wallner K.E. Galicich J.H. Krol G., et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]