Abstract
Widespread distribution of gene products at clinically relevant levels throughout the CNS has been challenging. Adeno-associated virus type 9 (AAV9) vector has been reported as a good candidate for intravascular gene delivery, but low levels of preexisting antibody titers against AAV in the blood abrogate cellular transduction within the CNS. In the present study we compared the effectiveness of vascular delivery and cerebrospinal fluid (CSF) delivery of AAV9 in transducing CNS tissue in nonhuman primates. Both delivery routes generated similar distribution patterns, although we observed a more robust level of transduction after CSF delivery. Consistent with previous reports administering AAV9, we found greater astrocytic than neuronal tropism via both routes, although we did find a greater magnitude of CNS transduction after CSF delivery compared with intravascular delivery. Last, we have demonstrated that delivery of AAV9 into the CSF does not shield against AAV antibodies. This has obvious implications when developing and/or implementing any clinical trial studies.
Samaranch and colleagues compare the effectiveness of vascular and cerebrospinal fluid (CSF) delivery of AAV serotype 9 (AAV9) vector in transducing CNS tissue in nonhuman primates. They demonstrate that both delivery routes generate similar overall distribution patterns and result in primarily astrocytic transduction, although the CSF route leads to a greater magnitude of CNS transduction. They also confirm that preexisting anti-AAV antibodies abrogate brain transduction via both routes of administration.
Introduction
Studies by us (Foust et al., 2009) and others (Duque et al., 2009; Fu et al., 2011; Gray et al., 2011) have indicated that adeno-associated virus type 9 (AAV9) displays a remarkable ability to bypass the blood–brain barrier (BBB) and effectively transduce the CNS of rodents and nonhuman primates (NHPs) after intravenous injection of high doses of vector. The clinical potential of this finding has triggered considerable enthusiasm (Manfredsson et al., 2009; Forsayeth and Bankiewicz, 2011), particularly in neonatal metabolic diseases, for which more global CNS delivery is desirable (Forsayeth and Bankiewicz, 2011). Like any new therapeutic strategy, technical challenges exist and must be dealt with for clinical translation to be possible. In our view, there are three key issues to be resolved: (1) circulating anti-AAV antibodies in patients, (2) the requirement for high vector doses, and (3) cellular tropism.
Samulski and colleagues reported that even a quite low (1:200) neutralizing antibody titer in the blood of NHPs completely abrogated glial transduction after intravenous infusion of AAV9 (Gray et al., 2011). Most adults are seropositive for AAV antibodies (Chirmule et al., 1999) as are infants and children (Calcedo et al., 2011), although Wilson and colleagues point out that AAV antibody titers are modest in infants and children, typically less than 1:50, and decline through the first year of life, rising thereafter (Calcedo et al., 2011). Thus, pediatric populations may represent the most fruitful avenue for intravenous gene therapy because infants appear to have the best chance of being truly seronegative (Halbert et al., 2006). However, individuals with anti-AAV titers greater than 1:200 would face significant problems with vascular transduction. Even though 70% of adults are negative for anti-AAV9 antibodies at titers greater than 1:200 (Boutin et al., 2010), this is a significant issue.
The relatively poor transduction of neurons by vascular AAV9 represents another potential limitation, because many neurological diseases may require localized neuronal expression of therapeutic agents. Vascular delivery of AAV9 results in a mixture of neuronal and astrocytic transduction (Gray et al., 2011) that perhaps depends on the age of the animal (Foust et al., 2009). The use of astrocytes to deliver secreted transgene products into the brain has, in our view, considerable potential if the problem of humoral immunity to AAV9 can be avoided, clearly a strong possibility in neonates. In published studies, the amounts of AAV9 required to obtain effective transduction in mouse brain after intravenous injection are on the order of 1013 vector genomes (VG)/kg. For a 70- to 80-kg human, this would be a dose of about 1015 VG, a significant amount of vector. Although daunting, this in itself is not an insuperable problem.
With these challenges in mind, we undertook the present study to determine how two distinct delivery routes affected the transduction patterns of AAV9. More specifically, we asked whether vector administration to the CNS via the infusion of AAV9-GFP into the internal carotid artery (ICA) or cisterna magna (CM) of NHPs provided any improvement over simple intravenous injection that requires large infusion volumes and is distributed throughout the whole body. We found that ICA delivery of AAV9-GFP resulted in transgene expression and distribution similar to intravenous administration, whereas CM infusion led to dramatically stronger expression of the transgene throughout the cortex and cerebellum. Both delivery routes resulted in mainly astrocytic transduction. In addition, we also confirmed that animals with a significant preexisting anti-AAV antibody titer abrogated brain transduction, even when CM injection was used to deliver the vectors. Further studies are required to determine how serious a problem this is.
Materials and Methods
Animals and tissue collection
Six male (2.0–4.5 kg) and two female (2.8 and 3.4 kg) cynomolgus macaques (Macaca fascicularis) and two female rhesus macaques (Macaca mulatta, 5.2 and 8.9 kg) were included in this study (Table 1). Daily health observations and periodic body weight measurements were recorded in order to assess the welfare of the animals. Veterinarians reported no abnormal symptoms throughout the study. All procedures were approved by the Institutional Animal Care and Use Committee at Valley Biosystems (Sacramento, CA).
Table 1.
Study Summary of Dose, Volume, and Blood Total Anti-AAV Antibody Titer
| |
Internal carotid artery (AAV9-GFP) |
Cisterna magna (AAV9-GFP) |
Cisterna magna (AAV9-hAADC) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NHP 1 | NHP 2 | NHP 3 | NHP 1 | NHP 2 | NHP 1 | NHP 2 | NHP 3 | NHP 4 | NHP 5 | |
| Animal weight (kg) | 2.0 | 2.2 | 3.4 | 2.3 | 3.0 | 2.8 | 5.2 | 1.9 | 4.5 | 8.9 |
| Dose (VG×1013) | 3.0 | 3.0 | 3.0 | 1.8 | 1.8 | 3.0 | 3.0 | 1.0 | 0.3 | 0.3 |
| Volume (ml) | 11 | 21 | 40 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Antibody titer | Neg | 1:200 | Neg | Neg | Neg | Neg | 1:400 | 1:200 | Neg | 1:400 |
AAV9, adeno-asociated virus serotype 9; GFP, green fluorescent protein; hAADC, human aromatic l-amino acid decarboxylase cDNA; Neg, seronegative; NHP, nonhuman primate; VG, vector genomes.
Briefly, animals were anesthetized with a mixture of ketamine (Ketaset, 7 mg/kg) and dexmedetomidine hydrochloride (Dexdomitor, 0.015 mg/kg) for both delivery strategies. After ICA surgery, all animals received an intramuscular dose of atipamezole hydrochloride to reverse anesthesia (Antisedan, 0.15 mg/kg). Twenty-one days after AAV9 administration, all animals were deeply anesthetized and perfused transcardially with phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA) in PBS.
Brains and organs were collected and processed for histological analysis. Briefly, brains were sliced coronally into 6-mm blocks, postfixed in 4% PFA–PBS overnight, and cryoprotected in 30% (w/v) sucrose the next day. For histological staining, a sliding microtome (Thermo Scientific Microm HM 450; Thermo Fisher Scientific, Waltham, MA) was used to cut brain blocks into 40-μm serial sections and a cryostat (Thermo Scientific Microm HM 500M; Thermo Fisher Scientific) was used to cut the organs into 20-μm sections. Sections were then placed in cryoprotectant solution until use.
Vectors
Two reporter transgenes were delivered by the AAV9 vector: a self-complementary DNA sequence for green fluorescent protein (GFP) under the control of a chicken β-actin hybrid promoter (Foust et al., 2009) and a single-stranded human aromatic l-amino acid decarboxylase cDNA (hAADC) sequence under the control of a cytomegalovirus promoter (Children's Hospital of Philadelphia, Philadelphia, PA). Vectors were diluted on the day of surgery and the animals were randomly assigned into various treatment groups (Table 1).
AAV delivery
Internal carotid artery delivery
A 50-ml syringe was connected to intravenous tubing with a 0.5-ml dead volume. The syringe was mounted onto the pump and transferred to the surgery table. To expose the right ICA, a 1-cm incision was made in the skin followed by careful dissection of muscle and underlying tissue adjacent to the ICA. A 27-gauge needle was then attached to tubing and primed with the vector. The needle was inserted into the ICA and vector was infused at 4 ml/min.
Cisterna magna delivery
After induction of anesthesia, the animal's head was placed in a stereotactic frame and flexed in a prone position, and the back of the neck was cleaned with povidone-iodine and alcohol. A 3-ml syringe attached to a 1-inch 23-gauge needle was mounted onto the micromanipulator, and the needle was manually guided into the CM. Penetration was verified by aspiration of a small volume of cerebrospinal fluid (CSF) into the syringe, which was then secured to the micromanipulator. The three-way stopcock on the syringe was then adjusted to allow infusion of 2 ml of vector at 0.5 ml/min with a pump (3500 Medfusion; Strategic Applications, Libertyville, IL). After completion of the infusion, the line was flushed with 0.2 ml of saline. Last, a small volume of CSF was aspirated to verify that the needle was still in the CM. After verification, the needle was slowly removed.
Immunohistochemistry
Chromogenic and immunofluorescence staining were performed on 40-μm coronal sections throughout four alternate 6-mm blocks from the prefrontal cortex to the cerebellum. Liver and spleen blocks were also collected and processed for histology.
Chromogenic GFP and AADC staining was performed as described previously (Hadaczek et al., 2009). Briefly, sections were washed in PBS, blocked for endogenous peroxidase activity in 1% H2O2–30% ethanol in PBS, and rinsed in PBST (PBS plus 0.1% Tween 20). Sections were then incubated in Background Sniper blocking solution (BS966G; Biocare Medical, Concord, CA) and incubated for up to 24 hr at 4°C with primary antibody against GFP (rabbit anti-GFP, diluted 1:3000; Millipore, Bedford, MA) or AADC (rabbit anti-DDC, diluted 1:1000; Millipore) in Da Vinci Green diluent (PD900; Biocare Medical). The next day, after being washed in PBST, sections were incubated in Rabbit Mach 3 Polymer HRP (RP531L; Biocare Medical) for GFP staining or Rabbit Mach 2 Polymer HRP for AADC staining at ambient temperature and color was developed with 3,3′-diaminobenzidine (DAB) (DAB peroxidase substrate kit, SK-4100; Vector Laboratories, Burlingame, CA). Sections were then mounted on slides, dehydrated, and coverslipped with Shandon-Mount (cat. no. 1900333; Thermo Fisher Scientific).
To determine the phenotype of GFP-positive cells, double immunofluorescence staining was performed. Brain sections were washed with PBST, blocked for 60 min in 20% normal horse serum (NHS; Jackson ImmunoResearch, West Grove, PA), and then incubated overnight at 4°C with anti-GFP (rabbit or mouse, diluted 1:200; Millipore) together with antibodies specific for either microglia (anti-Iba1, rabbit polyclonal, diluted 1:500; Biocare Medical), neurons (anti-NeuN, mouse monoclonal, diluted 1:300; Millipore), cortical interneurons (anti-glutamic acid decarboxylase-67 [GAD67], mouse monoclonal, diluted 1:300; Millipore), fibrous astrocytes (anti-glial–fibrillary acidic protein [GFAP], mouse monoclonal, diluted 1:500; Dako, Carpinteria, CA), protoplasmic astrocytes (anti-S100, rabbit polyclonal, diluted 1:300; Millipore), or oligodendrocytes (anti-Olig2, rabbit polyclonal, diluted 1:500; Millipore). All antibodies were diluted in Da Vinci Green diluent (PD900; Biocare Medical). After incubation with primary antibodies, sections were washed in PBST; incubated with a cocktail of corresponding secondary antibodies (FITC-conjugated anti-rabbit [diluted 1:200; Jackson ImmunoResearch] or Alexa 488-conjugated anti-mouse [diluted 1:500; Molecular Probes/Invitrogen, Carlsbad, CA] and Alexa 555-conjugated anti-mouse [diluted 1:500; Molecular Probes/Invitrogen] or Alexa 555-conjugated anti-rabbit [diluted 1:500; Molecular Probes/Invitrogen]) in PBST for 2 hr at room temperature; washed in PBS; and wet-mounted on frosted slides. Sections were coverslipped with a 4′,6-diamidino-2-phenylindole (DAPI)-containing hard-set media to identify all nuclei (VECTASHIELD H-1200; Vector Laboratories).
Anti-AAV9 antibody titer in NHPs
The titer of antibodies directed against AAV9 capsid was determined by ELISA on blood samples before vector administration. Briefly, a 2×1010 VG/ml solution of empty AAV9 capsids in 1 mM carbonate buffer was distributed into a 96-well titer plate and incubated overnight at 4°C. The next day, the plate was washed and blocked with a 5% nonfat milk solution in PBST. Sera were diluted over a range from 1:50 to 1:6400 and incubated at room temperature for 1 hr. Wells were then washed with PBST and incubated with a horseradish peroxidase (HRP)-conjugated anti-monkey secondary antibody (Sigma-Aldrich, St. Louis, MO) at room temperature for 1 hr. The wells were washed with again in PBST and then developed with 3,3′,5,5′-tetramethylbenzidine (TMB). The reaction was stopped by the addition of hydrochloric acid and absorbance was read at 450 nm on a plate reader (Bevan et al., 2011).
Results
AAV9-GFP distribution throughout the CNS
We determined the extent of gene transfer within the CNS of NHPs by delivering AAV9 in two ways. Volumes of AAV9-GFP, ranging from 11 to 40 ml, were delivered into NHPs via the ICA (n=3). No adverse effects were detected within the vasculature and/or parenchymal tissue. In a second cohort of NHPs, a dose of 2 ml of vector was delivered into the CM, also without apparent adverse effects (n=2).
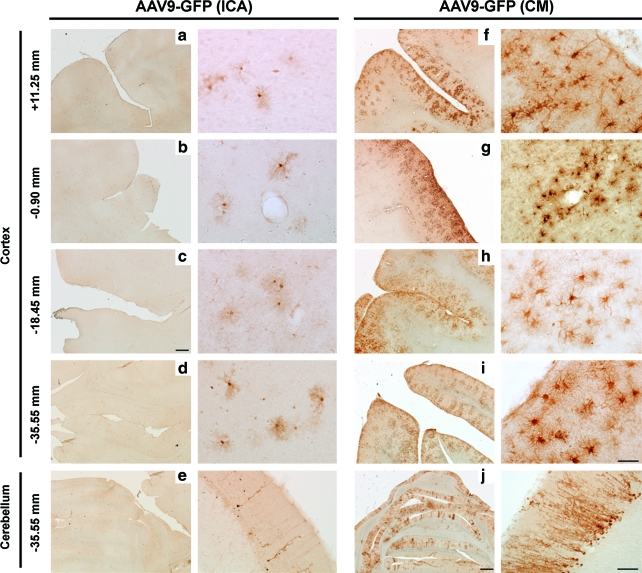
After ICA vector delivery, only two of three animals showed GFP expression in the CNS. In these two animals, GFP expression was detected throughout the brain. The most prominent level of transduction was found in the animal that received the higher infusion volume (40 ml; Table 1), demonstrating broader transgene distribution throughout the CNS, extending through several cortical regions (Fig. 1a–e). Scattered GFP-expressing cells were found from the prefrontal to occipital cortex, and in the cerebellum.
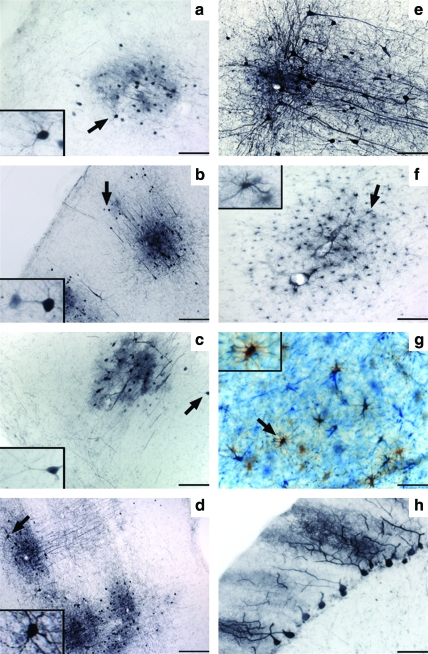
FIG. 1.
Stronger AAV9-GFP transduction in cortex and cerebellum after cisterna magna (CM) injection compared with internal carotid artery (ICA) delivery. Immunostained sections against GFP revealed similar prefrontal to occipital distribution between ICA (a–d) and CM (f–i), although images also show a more robust level of transduction after CM delivery (f–i), especially in the Purkinje cells of the cerebellum (e and j). Images in the right-hand columns for both ICA and CM are higher magnification pictures of images in the left-hand columns. Scale bars: (a–j) left column: 500 μm; (a–d, f–i) right column: 50 μm; (e and j) right column: 200 μm. Color images available online at www.liebertonline.com/hum
CM delivery of AAV9-GFP resulted in a distribution of gene transfer throughout the brain, similar to ICA administration. After both delivery strategies, GFP-positive fibers and cells were found throughout the prefrontal to occipital cortex and cerebellum. However, the number and intensity of GFP-positive cells were much greater after CM infusion than ICA delivery (Fig. 1f–j). We found that GFP-positive cells surrounded blood vessels, although after ICA delivery this was not always directly proximal to identifiable blood vessels. The largest number of transduced cells was found in the cerebellum. After both ICA and CM infusions, GFP signal in Purkinje cells was remarkably strong. However, the number of GFP-positive Purkinje neurons was greater after CM than ICA infusion (Fig. 1j and e, respectively).
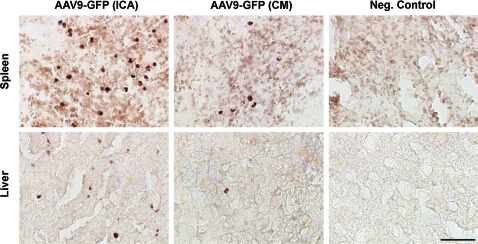
Although AAV9 vector was delivered into the right ICA, there was no evidence of hemispheric preference in GFP expression. As expected, we found many GFP-positive cells in the spleen and liver, suggesting widespread peripheral organ transduction after ICA infusion (Fig. 2). Sparse GFP expression was observed in the spleen and liver of NHPs that received CM infusion, suggesting a low amount of peripheral vector exposure (Fig. 2).
FIG. 2.
Presence of AAV9-GFP in peripheral organs. Vascular delivery (i.e., internal carotid artery [ICA]) of AAV9-GFP resulted in higher levels of transduction in the spleen and liver compared with cisterna magna (CM) infusions. Organs obtained from NHPs used in a separate study were included as control tissues, and these tested negative against the GFP transgene. Scale bar: 50 μm. Color images available online at www.liebertonline.com/hum
AAV9-GFP tropism
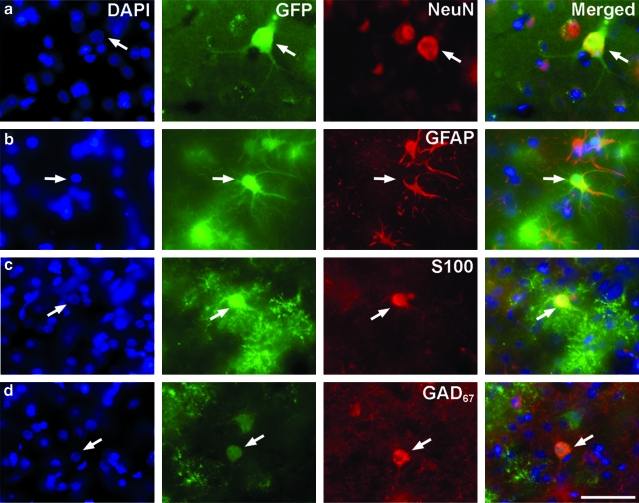
To determine the tropism of cellular transduction after AAV9 delivery, we immunostained multiple brain sections for transgene and cell-specific phenotypic markers. The intensity of GFP fluorescence was weaker after ICA delivery compared with the signal seen in animals that received AAV9-GFP via the CM. In the cortex, where the most robust GFP expression was detected, we found GFP-positive cells that colocalized with neuronal marker (NeuN) and had the morphology of pyramidal neurons (Fig. 3a). However, most of the cortical GFP-positive cells were costained for GFAP or S100, suggesting that most transgene expression after AAV9-GFP delivery into the ICA or CM occurs in fibrous and protoplasmic astrocytes, respectively (Fig. 3b and c). After CM, but not ICA, delivery, there were a few cortical GFP-positive cells that also expressed GAD67, a marker of GABAergic interneurons in the cortex (Fig. 3d). This pattern was consistent throughout the cortex and cerebellum, where we demonstrated the transduction of Purkinje cells via both administration routes. Even though in brain sections Iba1 and Olig2 clearly identified microglia and oligodendrocytes, respectively, we could not find GFP-expressing cells that colocalized with either the Iba1 or Olig2 marker, suggesting that neither microglial cells nor oligodendrocytes were transduced.
FIG. 3.
Cell-specific expression after AAV9-GFP infusion. Brain sections were stained for GFP and either a neuronal (NeuN), astrocytic (GFAP, S100), or cortical interneuron (GAD67) marker. Both ICA and CM infusion resulted in the transduction of neurons (a) as well as fibrous and protoplasmic astrocytes (b and c). Interestingly, some GFP-positive cortical interneurons (d) could also be seen but only after CM delivery. Sections were counterstained with DAPI, a nuclei marker. Arrows depict examples of GFP-positive cells expressing the various markers. Scale bar: 50 μm. Color images available online at www.liebertonline.com/hum
Effect of circulating antibodies
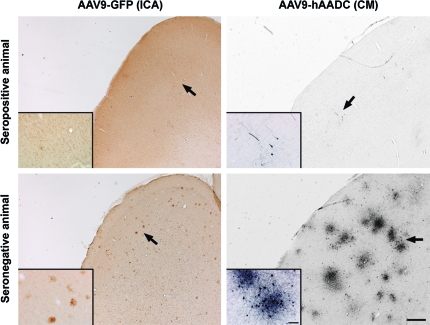
It has been shown previously that preexisting anti-AAV9 immunity in NHP blood (antibody titer ≥1:4) completely eliminated cellular transduction when vector was delivered intravenously (Gray et al., 2011). Although we have never observed significant titers of anti-AAV antibodies in NHP CSF, we were interested in whether preexisting antibodies interfered with transduction either by the ICA or CM route. Thus, we assayed animals for preexisting AAV antibodies (Table 1). Analysis of serum revealed a strong presence of antibodies against AAV in one of the animals that received an ICA infusion. The rest of them were seronegative (antibody titer ≤1:50) for AAV antibodies. Interestingly, almost no GFP-positive cells were found within the brain of the NHP that was seropositive at baseline (Fig. 4). Both animals that received AAV9-GFP in the CM were also seronegative (≤1:50). However, in a separate experiment, in which we infused a range of AAV9-hAADC doses into the CM of five NHPs (Table 1), three of the five animals exhibited a high anti-AAV antibody level (>1:200). After 3 weeks, all animals were killed and we examined AADC expression throughout the CNS. The three seropositive NHPs exhibited almost no exogenous AADC expression (Fig. 4). Conversely, the other two animals, which were seronegative (<1:50), showed neuronal and astrocytic AAV9-hAADC transduction throughout the cortex and the cerebellum in both hemispheres (Fig. 5), in addition to normal endogenous AADC expression in areas such as the caudate-putamen.
FIG. 4.
Lack of AAV9-GFP cortical transduction in NHPs with preexisting anti-AAV antibodies. Brain sections from seropositive (antibody titer >1:200) and seronegative (antibody titer <1:50) NHPs show differences in transduction levels. The presence of preexisting anti-AAV antibodies produces an almost complete abolition of the transgene transduction signal. Insets: Higher magnification images of transduced cells (arrows). Scale bars: low magnification, 500 μm; high magnification, 100 μm. Color images available online at www.liebertonline.com/hum
FIG. 5.
Widespread transduction throughout the brain after cisterna magna (CM) infusion of AAV9-hAADC. Representative images of transduced cortical neurons grouped in clusters through the prefrontal (a), temporal (b), parietal (c), and occipital cortex (d). Arrows indicate magnifications of regions of interest (a–d, and f and g). Most of them were located proximal to blood vessels, suggesting perivascular transport (e). Astrocytic transduction in the cortical white matter (f), cerebellum (double staining; AADC in brown and GFAP in blue) (g), and transduction of Purkinje neurons also in the cerebellum (h). Scale bars: (a, c, e, f, and h) 100 μm; (b and d) 200 μm; (g) 50 μm. Color images available online at www.liebertonline.com/hum
Discussion
Vascular delivery of AAV9 to the brain has obvious clinical potential (Foust and Kaspar, 2009; Manfredsson et al., 2009; Forsayeth and Bankiewicz, 2011). However, several challenges stand in the way of bringing this approach into the clinic. The first, and most obvious, is the large quantity of vector required to achieve significant transduction of brain cells (Foust et al., 2009; Gray et al., 2011). The second is the issue of circulating anti-AAV antibodies in humans (Halbert et al., 2006; Boutin et al., 2010; Calcedo et al., 2011). With these considerations in mind, we explored two alternative approaches to global brain delivery of AAV9: (1) ICA infusion, designed to favor the circulatory system of the brain, and (2) injection via the CM, designed to shield AAV9 from anti-AAV antibodies, reduce peripheral organ exposure, and potentially conserve vector.
ICA infusion was accomplished by delivering 3×1013 VG of AAV9-GFP in three different volumes. This experiment was complicated by the fact that the NHP that received a moderate volume (21 ml) had an anti-AAV titer of 1:200. This animal showed no evidence of GFP expression in the CNS and only a few GFP-expressing cells in spleen, possibly macrophages, and accords with a prior study showing that an antibody titer in this range also blocks transduction of tissues (Gray et al., 2011). The two other ICA NHPs evinced transduction of brain cells, and the difference in volume infused did not seem to be important. The intent of ICA delivery was to favor brain transduction over peripheral tissues, with an expectation of greater transduction in the ipsilateral brain hemisphere due to a possible first-pass effect; but this was not the case. Peripheral tissues such as liver and spleen showed only modest GFP expression, in contrast to other reports of ICA and intravenous administration, where much stronger expression was seen (Gray et al., 2011). We conclude from these results that ICA delivery confers little if any benefit over intravenous injection and requires a more invasive, and thus less desirable, intervention to achieve the same end. However, it should be noted that our study confirms the remarkable astrocytic specificity seen after vascular delivery. In fact, this specificity does not depend on the route of delivery. Both ICA and CM infusions generated virtually identical patterns of transduction, that is, mostly astrocytes and few neurons. However, CM infusion was much more efficient than ICA infusion in the transduction of brain and eliminated significant transduction of liver and spleen.
On the basis of previously published data that showed that the presence of antibodies in the bloodstream against AAV neutralized transduction of tissues by intravenous AAV9 (Gray et al., 2011), we investigated whether CM delivery offered any protection from circulating AAV antibodies. Previous work (Scallan et al., 2006) has suggested that even low titers of anti-AAV antibodies in the peripheral circulation absorb and neutralize the transduction capacity of significant amounts of intravenous AAV2. This is no doubt due to the multiplicity of conserved epitopes on AAV particles with consequent high avidity and clearance from the circulation. In this respect, the concept that neutralizing antibodies have some unique status in the circulation is probably incorrect, although such antibodies are undoubtedly useful in cell transduction assays. The totality of all circulating anti-AAV antibodies rather than the subset of neutralizing antibodies is likely to be of greater importance when one considers clearance of AAV particles from the circulation. This is not to say, however, that neutralizing antibodies do not play a more significant role outside of the circulation, for example, brain parenchyma, where clearance mechanisms are likely to play a less prominent role and neutralizing antibodies a reciprocally more important one (Peden et al., 2004; Sanftner et al., 2004). AAV9 carrying the cDNA for hAADC was infused at three vector doses into the CM. Regardless of dose, animals with antibody titers greater than 1:50 failed to show any significant level of transduction. Thus, CM delivery confers little if any immunological protection on AAV9. In fact, it is striking that such an apparently low antibody titer was so efficient at blocking transduction. We speculate that it is possible to overcome such modest titers by increasing the dose of vector. In this study, we used quite modest ranges of vector dose, and perhaps increasing the dose of AAV9 by an order of magnitude in animals with moderate (e.g., 1:200) antibody titers might prove effective. Nevertheless, the issue of antibody titer is still a salient problem. Anti-AAV antibodies are common in primates, both human (Boutin et al., 2010; Calcedo et al., 2011) and nonhuman (present study). The lowest titers are usually found in infants and young children (Calcedo et al., 2011), and this augurs well for the use of AAV9 to treat certain inherited neurological disorders. Of course, the astrocytic specificity of AAV9 in the brain imposes further constraints on the kinds of therapy that can be explored. We stress, however, that we are at the beginning of a new path for CNS delivery of AAV and expect considerable innovation in tackling the significant issue raised in this and other studies.
Acknowledgment
This study was supported by a grant to K.S.B. from NIH-NINDS (1R01NS073940-01).
Author Disclosure Statement
No competing financial interests exist.
References
- Bevan A.K. Duque S. Foust K.D., et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Calcedo R. Morizono H. Wang L., et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N. Propert K. Magosin S., et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Duque S. Joussemet B. Riviere C., et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth J.R. Bankiewicz K.S. AAV9: Over the fence and into the woods. Mol. Ther. 2011;19:1006–1007. doi: 10.1038/mt.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D. Kaspar B.K. Over the barrier and through the blood: To CNS delivery we go. Cell Cycle. 2009;8:4017–4018. doi: 10.4161/cc.8.24.10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D. Nurre E. Montgomery C.L., et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H. Dirosario J. Killedar S., et al. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood–brain barrier gene delivery. Mol. Ther. 2011;19:1025–1033. doi: 10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.J. Matagne V. Bachaboina L., et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: A comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P. Forsayeth J. Mirek H., et al. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: Vector trafficking and immune response. Hum. Gene Ther. 2009;20:225–237. doi: 10.1089/hum.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L. Miller A.D. McNamara S., et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson F.P. Rising A.C. Mandel R.J. AAV9: A potential blood–brain barrier buster. Mol. Ther. 2009;17:403–405. doi: 10.1038/mt.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden C.S. Burger C. Muzyczka N., et al. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanftner L.M. Suzuki B.M. Doroudchi M.M., et al. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol. Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Scallan C.D. Jiang H. Liu T., et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]