Abstract
COPI, a coatomer protein complex of secretory vesicles, is involved in Golgi and endoplasmic reticulum traffic and in early endosome maturation. The loss of COPI results in the fragmentation of Golgi, accumulation of immature autophagosomes, inhibition of autophagy, and cell death. Since COPI is required by all cells, it would appear an unlikely target for cancer treatment. However, our recent function-based genomic screen unexpectedly identified a specific COPI subunit, ζ1, as a cancer-specific target. The existing cancer drugs kill only proliferating but not growth-arrested tumor cells, but the depletion of ζ1 induces cell death in both dividing and nondividing tumor cells, while sparing normal cells. The mechanism of this remarkable tumor selectivity turned out to be surprising and heretofore unprecedented.
Keywords: autophagy, cancer chemotherapy, cancer targets, COPI, COPZ1, COPZ2, function-based genomics, intracellular traffic, microRNA
Identifying novel tumor-specific drug targets is a key direction in cancer treatment research. Targeted drugs are effective against those tumors that depend on the function of the gene targeted by the drug, e.g., trastuzumab (Herceptin) works in breast cancers that overexpress its target protein HER2/NEU. Almost all of the current cancer drugs act on molecular targets that are involved in cell proliferation. These drugs kill proliferating tumor cells but are ineffective against growth-arrested cells, which include dormant tumor cells and tumor stem cells that will eventually cause the tumor to recur. We have embarked on a comprehensive study, aimed at identifying presently unknown potential targets for new cancer drugs. Our approach was based on the selection of growth-inhibitory genetic suppressor elements (GSEs), short cDNA fragments that block the function of their cognate gene by directing the synthesis of dominant negative protein fragments or antisense RNA molecules. The goal of this study has been to identify genes, the inhibition of which will inhibit the growth of tumor but not normal cells.
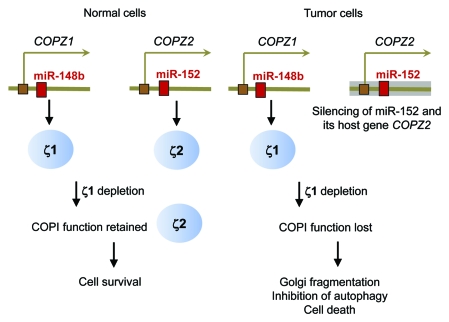
One of the targets identified through this approach is COPZ1, a gene that encodes ζ1, one of the two isoforms of the ζ component of COPI; the second isoform being ζ2, encoded by the COPZ2 gene. Growth-inhibitory GSEs targeting COPZ1 were selected in cell lines derived from different types of cancer but not in normal fibroblasts. COPZ1 knockdown with different synthetic siRNAs inhibits cell proliferation in different tumor cell types, whereas COPZ2 siRNAs have no effect. In contrast to tumor cells, hTERT-immortalized but otherwise normal fibroblasts or normal prostate epithelial cells are killed only when COPZ1 and COPZ2 are knocked down together, but not individually. To elucidate the mechanism of this tumor specificity, we measured the RNA levels of COPI proteins in different types of normal and tumor cells and found a striking pattern: while most of the COPI components were expressed at comparable levels in normal and tumor cells, one gene, COPZ2, was drastically downregulated in the majority of tumors. Hence, normal cells express two isoforms of the ζ subunit of COPI, ζ1 and ζ2, whereas tumor cells have only ζ1, due to the silencing of the COPZ2 gene encoding ζ2. This suggested that normal cells survive the loss of ζ1 because ζ2 substitutes for it in the COPI complex, whereas tumor cells have no such fallback (Fig. 1). We confirmed this interpretation by demonstrating that reintroducing COPZ2 into tumor cells allowed them to survive COPZ1 knockdown. Hence, tumor cells are dependent on COPZ1 not because it’s an oncogene (COPZ1 has no known oncogenic properties), but because it becomes essential for tumors due to the silencing of its paralogous gene COPZ2, encoding the other ζ isoform. We term this new type of tumor dependence “isoform addiction,” as opposed to the widely used concept of “oncogene addiction,” which is often assumed to underlie all cases of tumor dependence on an individual protein.
Figure 1.
Mechanism of tumor dependence on COPZ1.
Surprisingly, the restoration of COPZ2 expression in tumor cells did not inhibit their growth in vitro or in vivo, indicating that COPZ2 is not a tumor suppressor. Why then is COPZ2 silenced in many different tumors? We noticed that the first intron of the COPZ2 gene encodes a microRNA, miR-152, which was reported to be silenced in some cancers and to have some tumor-suppressive activities. (The COPZ1 gene also hosts a microRNA, miR-148b, which is homologous to miR-152; Fig. 1). When we expressed miR-152 in different tumor cell lines, their growth was inhibited either in vitro or in vivo. Hence, miR-152 is indeed a tumor suppressor, in agreement with the recent paradigm that microRNAs are key regulators of carcinogenesis and other phenotypic transitions. miR-152 and its host gene COPZ2 are transcribed from the same promoter, and we have found that they were silenced in parallel in different tumor cell lines. Hence, COPZ2 shutdown in tumor cells is a consequence of the silencing of tumor-suppressive miR-152, leading in its turn to tumor dependence on the COPZ1 gene (Fig. 1).
What happens to tumor cells when COPZ1 is inhibited? As in the previous studies on the depletion of other COPI subunits, we observed the disruption of the Golgi, accumulation of immature autophagosomes, and cell death. We do not know at present whether the inhibition of autophagy is responsible for the cell death, either directly, or through the failure to eliminate the Golgi fragments. Time-lapse video microscopy showed that cells died through apoptosis, either directly or after first rounding up and remaining rounded for 10–20 h. In contrast to apoptosis induced by most of the anticancer drugs, which typically occurs after abnormal mitosis (mitotic catastrophe), COPZ1-depleted cells enter apoptosis from interphase, without entering mitosis first. This suggested to us that the loss of COPZ1 could be lethal to nondividing cells, which are resistant to conventional drugs. Indeed, we have found that both dividing and nondividing tumor cells are killed by COPZ1 siRNA, whereas a control growth-inhibitory siRNA, which targets a gene involved in cell cycle progression, has no effect on nondividing cells. The failure of chemotherapy to destroy nondividing tumor cells is a general cause of tumor relapse after the initial remission, but drugs that kill nondividing cells would typically damage normal tissues. The unique tumor selectivity of COPZ1-targeting agents (such as siRNAs or small molecules that can potentially be developed in the future), coupled with their ability to destroy both proliferating and nondividing tumor cells, offers a potential new solution to this general problem in cancer chemotherapy.
Acknowledgments
Supported by NIH grants R33 CA95996 and R01 AG028687 (I.B.R.) and grant W81XWH-08-1-0070 from the Department of Defense Prostate Cancer Research Program (M.S.).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/17659