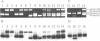Abstract
Rice alpha-amylases are encoded by a multigene family that has previously been classified into 5 hybridization groups. DNA sequence and Southern blot analysis identified three genes (RAmy1A, RAmy1B and RAmy1C) in Group 1 with DNA sequence identity of at least 90%. Hybridization Group 2 is represented by only one gene, RAmy3D, which is identical to a previously characterized cDNA, pOS137. RAmy3D is physically linked to the sole representative of Group 5, RAmy3E. The identity between these two genes is 81.4% in the coding region but less than 50% in the 5' and 3' flanking regions. Northern blot analysis and RNA-PCR were used to detect the expression of alpha-amylase genes in various tissues. Alpha-amylase mRNA was abundant in germinating seeds and callus. Some genes were also expressed at much lower levels in roots, young leaves and immature seeds. RAmy1A and RAmy3E were expressed in all tissues while RAmy3D was expressed in all tissues except the immature seeds. RAmy1B was weakly expressed only in callus. RAmy1A transcript was most abundant in the germinating seeds, while RAmy3D and RAmy3E transcripts were most abundant in callus and immature seeds, respectively.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulcombe D C, Huttly A K, Martienssen R A, Barker R F, Jarvis M G. A novel wheat alpha-amylase gene (alpha-Amy3). Mol Gen Genet. 1987 Aug;209(1):33–40. doi: 10.1007/BF00329833. [DOI] [PubMed] [Google Scholar]
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Chelly J., Concordet J. P., Kaplan J. C., Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. A., Benz C. C., Liu E. Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene. 1989 Sep;4(9):1153–1157. [PubMed] [Google Scholar]
- Hewlett I. K., Ruta M., Cristiano K., Hawthorne C. A., Epstein J. S. Co-amplification of multiple regions of the HIV-1 genome by the polymerase chain reaction: potential use in multiple diagnosis. Oncogene. 1989 Sep;4(9):1149–1151. [PubMed] [Google Scholar]
- Huang J. K., Swegle M., Dandekar A. M., Muthukrishnan S. Expression and regulation of alpha-amylase gene family in barley aleurones. J Mol Appl Genet. 1984;2(6):579–588. [PubMed] [Google Scholar]
- Huang N., Sutliff T. D., Litts J. C., Rodriguez R. L. Classification and characterization of the rice alpha-amylase multigene family. Plant Mol Biol. 1990 May;14(5):655–668. doi: 10.1007/BF00016499. [DOI] [PubMed] [Google Scholar]
- Huttly A. K., Martienssen R. A., Baulcombe D. C. Sequence heterogeneity and differential expression of the alpha-Amy2 gene family in wheat. Mol Gen Genet. 1988 Oct;214(2):232–240. doi: 10.1007/BF00337716. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursheed B., Rogers J. C. Barley alpha-amylase genes. Quantitative comparison of steady-state mRNA levels from individual members of the two different families expressed in aleurone cells. J Biol Chem. 1988 Dec 15;263(35):18953–18960. [PubMed] [Google Scholar]
- Muthukrishnan S., Gill B. S., Swegle M., Chandra G. R. Structural genes for alpha-amylases are located on barley chromosomes 1 and 6. J Biol Chem. 1984 Nov 25;259(22):13637–13639. [PubMed] [Google Scholar]
- O'Neill S. D., Kumagai M. H., Majumdar A., Huang N., Sutliff T. D., Rodriguez R. L. The alpha-amylase genes in Oryza sativa: characterization of cDNA clones and mRNA expression during seed germination. Mol Gen Genet. 1990 Apr;221(2):235–244. doi: 10.1007/BF00261726. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittier R. F., Dean D. A., Rogers J. C. Nucleotide sequence analysis of alpha-amylase and thiol protease genes that are hormonally regulated in barley aleurone cells. Nucleic Acids Res. 1987 Mar 25;15(6):2515–2535. doi: 10.1093/nar/15.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]