Abstract
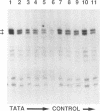
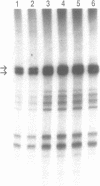
We present a reproducible method for the preparation of nuclear extracts from the yeast Saccharomyces cerevisiae that support efficient RNA polymerase B (II)-dependent transcription. Extracts from both a crude nuclear fraction and Percoll-purified nuclei are highly active for site-specific initiation and transcription of a G-free cassette under the Adenovirus major late promoter. At optimal extract concentrations transcription is at least 5 times more efficient with the yeast extracts than with HeLa whole cell extracts. We show that the transcriptional activity is sensitive to alpha-amanitin and to depletion of factor(s) recognizing the TATA-box of the promoter. The in vitro reaction showed maximal activity after 45 min, was very sensitive to Cl-, but was not affected by high concentrations of potassium. We find that the efficiency of in vitro transcription in nuclear extracts is reproducibly high when spheroplasting is performed with a partially purified beta 1,3-glucanase (lyticase). Therefore a simplified method to isolate the lyticase from the supernatant of Oerskovia xanthineolytica is also presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati B. B., Gasser S. M. Chromosomal ARS and CEN elements bind specifically to the yeast nuclear scaffold. Cell. 1988 Sep 23;54(7):967–978. doi: 10.1016/0092-8674(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Lue N. F., Kornberg R. D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988 Dec;8(12):5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Sharp P. A., Guarente L. Function of a yeast TATA element-binding protein in a mammalian transcription system. Nature. 1988 Jul 7;334(6177):37–42. doi: 10.1038/334037a0. [DOI] [PubMed] [Google Scholar]
- Cavallini B., Huet J., Plassat J. L., Sentenac A., Egly J. M., Chambon P. A yeast activity can substitute for the HeLa cell TATA box factor. Nature. 1988 Jul 7;334(6177):77–80. doi: 10.1038/334077a0. [DOI] [PubMed] [Google Scholar]
- Cooke R., Penon P. In vitro transcription from cauliflower mosaic virus promoters by a cell-free extract from tobacco cells. Plant Mol Biol. 1990 Mar;14(3):391–405. doi: 10.1007/BF00028775. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Griep M. A., McHenry C. S. Glutamate overcomes the salt inhibition of DNA polymerase III holoenzyme. J Biol Chem. 1989 Jul 5;264(19):11294–11301. [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue N. F., Buchman A. R., Kornberg R. D. Activation of yeast RNA polymerase II transcription by a thymidine-rich upstream element in vitro. Proc Natl Acad Sci U S A. 1989 Jan;86(2):486–490. doi: 10.1073/pnas.86.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue N. F., Flanagan P. M., Sugimoto K., Kornberg R. D. Initiation by yeast RNA polymerase II at the adenoviral major late promoter in vitro. Science. 1989 Nov 3;246(4930):661–664. doi: 10.1126/science.2510298. [DOI] [PubMed] [Google Scholar]
- Lue N. F., Kornberg R. D. Accurate initiation at RNA polymerase II promoters in extracts from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8839–8843. doi: 10.1073/pnas.84.24.8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncollin V., Miyamoto N. G., Zheng X. M., Egly J. M. Purification of a factor specific for the upstream element of the adenovirus-2 major late promoter. EMBO J. 1986 Oct;5(10):2577–2584. doi: 10.1002/j.1460-2075.1986.tb04537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Saltzman A. G., Weinmann R. Promoter specificity and modulation of RNA polymerase II transcription. FASEB J. 1989 Apr;3(6):1723–1733. doi: 10.1096/fasebj.3.6.2649403. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- Scott J. H., Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980 May;142(2):414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Molecular mechanisms of transcriptional regulation in yeast. Annu Rev Biochem. 1989;58:1051–1077. doi: 10.1146/annurev.bi.58.070189.005155. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., Kops L. E., Minghetti P. P., O'Malley B. W. Transcription factors from oviduct and HeLa cells are similar. J Biol Chem. 1981 Dec 25;256(24):13055–13059. [PubMed] [Google Scholar]
- Tsuda M., Suzuki Y. Faithful transcription initiation of fibroin gene in a homologous cell-free system reveals an enhancing effect of 5' flanking sequence far upstream. Cell. 1981 Nov;27(1 Pt 2):175–182. doi: 10.1016/0092-8674(81)90371-8. [DOI] [PubMed] [Google Scholar]
- Tyler B. M., Giles N. H. Accurate transcription of cloned Neurospora RNA polymerase II-dependent genes in vitro by homologous soluble extracts. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5450–5454. doi: 10.1073/pnas.82.16.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J. M. Regulatory DNA-binding proteins in yeast: an overview. Yeast. 1990 Jul-Aug;6(4):271–297. doi: 10.1002/yea.320060402. [DOI] [PubMed] [Google Scholar]
- Vrsanská M., Biely P., Krátký Z. Enzymes of the yeast lytic system produced by Arthrobacter GJM-1 bacterium and their role in the lysis of yeast cell walls. Z Allg Mikrobiol. 1977;17(6):465–480. doi: 10.1002/jobm.3630170608. [DOI] [PubMed] [Google Scholar]
- Vrsanská M., Krátký Z., Biely P. Lysis of intact yeast cells and isolated cell walls by an inducible enzyme system of Arthrobacter GJM-1. Z Allg Mikrobiol. 1977;17(5):391–402. doi: 10.1002/jobm.3630170509. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Woontner M., Jaehning J. A. Accurate initiation by RNA polymerase II in a whole cell extract from Saccharomyces cerevisiae. J Biol Chem. 1990 Jun 5;265(16):8979–8982. [PubMed] [Google Scholar]