Abstract
Background
Plasmodium vivax is responsible for a significant proportion of malaria cases worldwide and is increasingly reported as a cause of severe disease. The objective of this study was to characterize severe vivax disease among children hospitalized in intensive care units (ICUs) in the Western Brazilian Amazon, and to identify risk factors associated with disease severity.
Methods and Findings
In this retrospective study, clinical records of 34 children, 0–14 years of age hospitalized in the 11 public pediatric and neonatal ICUs of the Manaus area, were reviewed. P. falciparum monoinfection or P. falciparum/P. vivax mixed infection was diagnosed by microscopy in 10 cases, while P. vivax monoinfection was confirmed in the remaining 24 cases. Two of the 24 patients with P. vivax monoinfection died. Respiratory distress, shock and severe anemia were the most frequent complications associated with P. vivax infection. Ninety-one children hospitalized with P. vivax monoinfections but not requiring ICU were consecutively recruited in a tertiary care hospital for infectious diseases to serve as a reference population (comparators). Male sex (p = 0.039), age less than five years (p = 0.028), parasitemia greater than 500/mm3 (p = 0.018), and the presence of any acute (p = 0.023) or chronic (p = 0.017) co-morbidity were independently associated with ICU admission. At least one of the WHO severity criteria for malaria (formerly validated for P. falciparum) was present in 23/24 (95.8%) of the patients admitted to the ICU and in 17/91 (18.7%) of controls, making these criteria a good predictor of ICU admission (p = 0.001). The only investigated criterion not associated with ICU admission was hyperbilirubinemia (p = 0.513)].
Conclusions
Our study points to the importance of P. vivax-associated severe disease in children, causing 72.5% of the malaria admissions to pediatric ICUs. WHO severity criteria demonstrated good sensitivity in predicting severe P. vivax infection in this small case series.
Introduction
Malaria is one of the most important public health problems in the world, with almost half of the world's population at risk of disease and an estimated 800.000 deaths annually, mainly in children under five years of age [1]. Of the various Plasmodium species affecting humans, Plasmodium vivax was previously considered a relatively benign infection. Recent reports, however, have linked P. vivax to severe disease [2], [3].
Malaria in Brazil has been by and large attributed to P. vivax infection since the 1990's [4]. In 2009, Brazil reported 308,498 cases of malaria (257,571 of P. vivax), predominantly in the Amazon Region, representing 54.9% of all the malaria reported in the Americas [1]. In the last two decades, the city of Manaus (capital of the Amazonas State) has faced successive outbreaks of malaria due to intense immigration, deforestation and unplanned human settlements [5]. Today, with an estimated population of 2 million people, Manaus is one of three cities, which account for 25% of all reported malaria cases in Brazil. High case burdens persist despite a well-structured public health system with universal access to diagnosis (based on thick blood smear) and treatment.
Previously, P. vivax was considered a non-fatal infection. This perception, however, has changed in recent years, and vivax has become recognized as a cause of severe malarial disease. Severe clinical complications of P. vivax infection have been reported in Brazil for the past decade, especially in Manaus [6]–[12]. Despite being a significant source of morbidity, to date, no data on risk factors of severe P. vivax disease have been published in Latin America. The current data on predictors of clinical outcomes originates from regions where P. falciparum and P. vivax are equally prevalent, which could make the clinical tools inappropriate for use in managing vivax monoinfections [13]. This lack of prioritizing predictors of severity in P. vivax is due to the fact that fatalities related to this species remain uncommon. According to the official statistics, between 1998 and 2008, only 234 deaths related to P. vivax infection were reported in the Brazilian Amazon [4].
The first attempt to assemble the available knowledge on severe malaria was conducted at an informal technical meeting convened by the World Health Organization (WHO) in Kuala Lumpur, Malaysia in 1985 [14]. The document focused on P. falciparum infection, since, at that time, P. vivax was not thought to cause severe disease. Throughout the years, no effort has been made to validate or standardize specific severity criteria for vivax infections, and current definitions seem to be heterogeneous. This absence of consistency poses technical problems for literature review, meta-analyses and estimation of risk factors.
Alarmingly, the incidence of severe infection appears to be increasing [15]. Data from Manaus clearly demonstrate this increase in a setting where uniform study criteria are applied across time. The mechanism behind this increase remains poorly understood as are the clinical predictors of severity and outcome. The most robust endpoint for validating a criterion linked to severity is death, but in vivax infections in Brazil the number of deaths is small, which limits prognostic studies. Given this limitation a surrogate is required to replace death. Intensive care unit (ICU) admission could serve as this surrogate marker of severity, in combination with well-validated severity scoring systems that predict mortality in ICU patients. A uniform comparator is urgently needed to help establish management guidelines for vivax infections, especially for children under the age of 14, which accounted for 24.3% of all malaria infections in 2010 in Manaus (SIVEP, Malaria 2010).
This study aimed to characterize all children under 14 years of age admitted with a malaria diagnosis to an ICU in the Manaus area from 2004 to 2009, to assess potential risk factors associated with ICU admission (as proxy of clinical severity), and secondarily to address the applicability of WHO falciparum severity criteria to vivax infection, by comparing these cases to P. vivax hospitalized children not requiring intensive care.
Methods
Ethical considerations
The review of clinical files was approved by the Ethics Review Board (ERB) of the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (approval number 1980), as well as by the ERB of the Health Secretariat of Manaus and the Health Secretariat of the Amazonas State. All data analyzed were anonymized. Since data were obtained exclusively from clinical charts, the ERB gave a waiver of informed consent.
Study Sites and Patient Selection
The selection of cases was performed in the city of Manaus, capital of the Amazonas State, where Anopheles darlingi is the major malaria vector and the annual parasite index (API) was 11.5 cases/1,000 inhabitants, in 2009. The city has universal health assistance for citizens (Sistema Único de Saúde - SUS), and offers five public pediatric ICUs (44 total available beds) and six neonatal ICUs (50 total available beds) in 11 tertiary care pediatric hospitals and maternity wards. The infrastructure of all 11 hospitals is very similar and staff pediatricians are from the same cooperative society, which utilizes a single set of admission criteria and clinical management guidelines for ICU admission (severe compromise of respiratory and/or hemodynamic functions and/or coma). Since every febrile patient in Manaus is subject to a thick blood smear (TBS) and the symptoms of uncomplicated P. vivax and P. falciparum malaria are similar, treatment seeking bias seems unlikely. Since the access to tertiary care hospitals is relatively easy, we assume that all critically ill patients seeking health care were subsequently hospitalized in one of Manaus' ICUs. Patients in private ICUs in Manaus were excluded from this study because the patients are generally from non-malaria endemic areas. Patients infected with P. vivax that required an ICU admission were compared to children of the same age range infected with P. vivax not requiring an ICU admission but concurrently admitted to a tertiary care center for infectious diseases (Fundação de Medicina Tropical Dr. Heitor Vieira Dourado).
Study Design
This study is a retrospective review of clinical and laboratory records from hospital databases. Case files for children 0–14 years old admitted to any of the ICUs with a suspicion of malaria between January 2004 to December 2009 were reviewed for: (1) ICU logbooks (in which demographic data from each admitted patient, a presumptive diagnosis at admission and a final diagnosis at discharge are systematically registered), (2) hospital laboratory logbooks (in which the semi-quantitative results of TBSs performed in each hospital are systematically registered), and (3) SIVEP-Malaria (the National Malaria Information System available on-line, which registers results of TBSs performed in each reporting unit such as the hospitals of interest). Cases were included if there was evidence of malaria diagnosis by microscopy in any of the three information systems searched. The study enrolled only patients after 2004 because of better reliability of the National Malaria Information System (SIVEP-Malaria) and access to the clinical files, avoiding retrieval bias.
As comparators, clinical and laboratory data from children of the same age group admitted between January 2009 and July 2010 to the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado with a diagnosis of malaria were retrieved electronically from the computerized hospital records system (iDoctor®) and reviewed. With the exception of automated full blood count, routinely requested for all these hospitalized children, more specific laboratory tests (such as biochemistry and arterial gas analysis) were requested only if clinical symptoms were suggestive of complications. As part of routine follow-up, all malaria admitted children were reassessed in the Outpatient Clinics seven days after discharge to guarantee adequate clinical and parasitological follow-up.
Diagnosis of Malaria and Quantification of Parasitemia
TBSs are routinely performed for the diagnosis of malaria in Brazil, prepared as recommended by the Walker technique [16] and evaluated by a local microscopist. The results of peripheral parasitemia are given using the following semi-quantitative system: ½+ (200–300 parasites/mm3); 1+ (301–500 parasites/mm3); 2+ (501–10,000 parasites/mm3); 3+ (10,001–100,000 parasites/mm3); and 4+ (>100,001 parasites/mm3). The cut-off to define high parasitemia (in the final analysis) was based on the distribution of semi-quantitative parasitemia results. All positive slides and 10% of negative slides are routinely reviewed in a reference unit with experienced microscopists. In case of divergence, the reviewed result is updated in the on-line system. PCR diagnosis for malaria was not routinely performed in these patients. Only children with a positive TBS for Plasmodium spp. are treated according to the Brazilian Anti-Malarial Treatment Guidelines, which recommends chloroquine (25 mg/kg over 3 days)+primaquine (0.5 mg/kg/day over 7 days) for P. vivax infection and artemether/lumefantrine for 3 days for non-severe P. falciparum infection. Severe P. falciparum infections are treated with parenteral artemether or artesunate for 7 days.
Data collection and definitions
Data for each admitted individual was systematically retrieved from the medical charts by the same member of the study team, and included the following variables: sex, age, malarial species, semi-quantitative parasitemia, duration of disease (in days) prior to the admission to the ICU or ward, outcome, presence of a given acute co-morbidity other than malaria or a chronic disease confirmed through a reliable diagnostic method, and presence of any WHO-defined severe malaria criteria [17]. Briefly, WHO criteria for severe falciparum malaria validated for adults and children include: (1) impaired consciousness or unrousable coma (Blantyre coma scale ≤2 or Glasgow coma scale ≤10); (2) prostration, i.e. generalized weakness such that the patient is unable to walk or sit up without assistance; (3) failure to feed; (4) multiple convulsions (greater than two episodes in 24 h); (5) respiratory distress defined as the presence of deep (acidotic) breathing or retractions; (6) circulatory collapse or shock, systolic blood pressure <50 mmHg (algid malaria) and/or need for vasopressor support; (7) clinical jaundice or total bilirubin >3 mg/dL; (8) hemoglobinuria (blackwater fever); (9) abnormal spontaneous bleeding (disseminated intravascular coagulation); (10) pulmonary edema (radiological); (11) hypoglycemia (blood glucose <40 mg/dL); (12) metabolic acidosis (plasma bicarbonate <15 mmol/L); (13) severe anemia (Hb<5 g/dL); (14) hyperlactatemia (lactate >5 mmol/L); or (15) acute renal failure (serum creatinine >3 mg/dL). Acute respiratory distress syndrome (ARDS) was defined as acute bilateral lung infiltrates in chest X-rays, and a PaO2∶FiO2<200 mmHg. The data retrieved were reviewed by another member of the study team and discrepancies resolved by a third member if necessary.
In all ICUs, collection of venous blood samples for aerobic culture and arterial blood for gas analysis was routinely carried out on admission prior to administration of antibiotics. From all the patients hospitalized in the ICU, the Pediatric Index of Mortality (PIM) score was calculated with data from the first hour of admission, based on the systolic blood pressure, pupillary reactions to bright light, PaO2, FiO2, base excess in arterial blood, need for mechanical ventilation at any time during the first hour in ICU, elective admission, and presence of concomitant diagnoses [18]. Severity was defined in the presence of at least one of the aforementioned WHO severity criteria. As blood gas analysis samples were not systematically collected for patients not admitted to the ICU, hyperlactatemia and metabolic acidosis were not used to classify severity nor assessed as a risk factor associated with ICU admission. The disease was classified as being directly caused by plasmodial infection if no other cause was identified and no concurrent acute or chronic diseases were confirmed.
Statistical Analyses
Data were analyzed using SPSS® version 16.0 for Windows (SPSS Inc.® Chicago, IL, USA). Proportions of fatality were compared by Fisher exact test (corrected by Yates' test if necessary); differences were considered statistically significant for p<0.05. The crude Odds Ratio (OR) with its respective 95% Confidence Interval (95% CI) was determined considering the admission to ICU as the dependent variable. Logistic regression was used for the multivariate analyses and the adjusted Odds Ratios with 95% CI were also calculated. A multivariate logistic regression was performed with admission to the ICU as the outcome, using an automated backward and forward stepwise estimation. All variables that were associated with admission to the ICU at a significance level of p<0.10 in the univariate analysis were included in the multivariate analysis. Statistical significance was considered if p<0.05 in the Hosmer-Lemeshow goodness-of-fit test.
Results
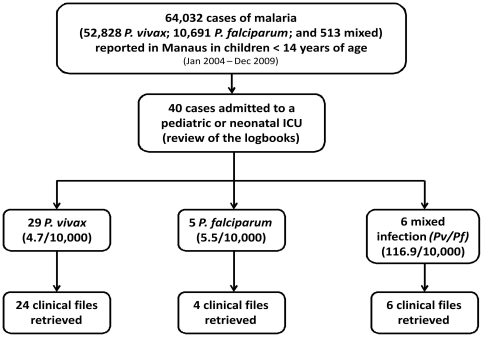
According to the official reporting systems, during the period of 2004–2009, 64,032 children 0–14 years of age were diagnosed with malaria in Manaus (52,828 with vivax; 10,691 with falciparum and 513 with both species). The review of logbooks disclosed that 40 of these children were admitted with malaria to one of the 11 pediatric or neonatal ICUs in Manaus. Thirty-four clinical files were actually retrieved from these 40 cases (29 of which were due to P. vivax infections), as shown in Figure 1, and six cases were excluded because records could not be found. A rough estimation of the risk of ICU admission per species showed similar relative risks for P. vivax (4.7/10,000 cases) and P. falciparum (5.5/10,000 cases), but a much higher relative risk for mixed infections (116.9/10,000 cases).
Figure 1. Flow diagram of patients admitted to ICUs enrolled in the analysis.
Table 1 summarizes the clinical description of the 10 ICU cases in which P. falciparum was diagnosed (four cases as monoinfection, and six cases of P. falciparum/P. vivax mixed infection). Altogether, respiratory distress (7/10) and shock (7/10) were the most common clinical complications in these children and the case fatality rate was 30.0% (3/10) (only two with P. falciparum monoinfection). No other concurrent cause for ICU admission was found in 40.0% (4/10) of the children while in the other six children, acute and/or chronic conditions were probably acting synergistically to produce the complications. Only one concomitant bacterial infection was diagnosed (Pseudomonas aeruginosa) in a child with P. falciparum monoinfection. In all 10 patients, at least one of the WHO criteria for severe malaria was present.
Table 1. Clinical information from 10 children 0–14 years of age admitted to any of the pediatric or neonatal ICUs in Manaus, from January 2004 to December 2009, with parasitological diagnosis of P. falciparum or mixed infection (P. falciparum/P. vivax).
| Patient | Species | Age/gender | Time of disease (days) | Associated acute comorbidity | Associated chronic comorbidity | Respiratory distress | Severe anemia | ARF | Coma | Jaundice | Shock | Metabolic acidosis | Low glucose | PIM score (%) | Outcome |
| 1 | P.f. | 6 y/F | 14 | Rotavirus gastroenteritis*+Sepsis* (Pseudomonas aeruginosa) | Congenital cardiopathy* | + | + | 84.7 | Died | ||||||
| 2 | P.f. | 7 y/F | 5 | + (ARDS) | + | + | 40.0 | Recovered | |||||||
| 3 | P.f. | 10 y/F | 5 | + | + | + | + | + | + | 45.7 | Died | ||||
| 4 | P.f. | 1 mo/M | 7 | + | + | 10.6 | Recovered | ||||||||
| 5 | P.f./P.v. | 7 mo/F | 2 | Rotavirus gastroenteritis | + | + | 7.4 | Recovered | |||||||
| 6 | P.f./P.v. | 13 y/M | 30 | G6PD deficiency* | + | +** | + | 1.3 | Recovered | ||||||
| 7 | P.f./P.v. | 5 mo/F | 48 | Malnutrition* | + | + | + | NA | Died | ||||||
| 8 | P.f./P.v. | 3 y/M | 7 | Rotavirus gastroenteritis | + (ARDS) | + | 15.4 | Recovered | |||||||
| 9 | P.f./P.v. | 9 y/M | 7 | Sickle cell anemia* + Malnutrition | + | + | + | 1.4 | Recovered | ||||||
| 10 | P.f./P.v. | 2 mo/M | 2 | + (ARDS) | + | 13.9 | Recovered |
Y: years; mo: months; d: days; NA: Non-available; ARDS: acute respiratory distress syndrome; ARF: acute renal failure; PIM: Pediatric Index of Mortality.
Malnutrition confirmed by body mass index (BMI) Z-score <−2; rotavirus gastroenteritis confirmed by immunochromatographic rapid test in the stool; sepsis confirmed by two positive blood cultures; G6PD deficiency confirmed by qualitative Brewer's test; congenital cardiopathy confirmed by echocardiogram; sickle cell anemia confirmed by electrophoresis; ARDS defined as acute bilateral lung infiltrates and a PaO2:FiO2<200 mmHg.
Blackwater fever.
Clinical and laboratory data from the 24 children with P. vivax monoinfection admitted to the ICU are presented in tables 2 and 3, respectively. Considering all the patients (admitted or not to the ICU), 46/108 (∼42%) patients presented with semi-quantitative parasitemia ≥2+ (500–10,000 parasites/mm3), and therefore 500 parasites/mm3 (the lower bound of this range) was chosen as the cut-off to define high parasitemia in our analysis, enabling similar number of patients in both categories (low and high parasitemia). Respiratory distress (16/24) and shock (13/24) were also the most common complications associated with this species. Three out of 16 patients with respiratory distress had ARDS and 8/16 presented with simultaneous metabolic acidosis. No other apparent concurrent cause for ICU admission was found in 10/24 (41.6%) children, while in the remaining 14 children there was evidence of the participation of acute and/or chronic conditions in the occurrence of complications. All children were treated with chloroquine (through nasogastric catheter when the child was intubated), primaquine after discharge, and one or more empiric antibiotic regimens. Although, only two patients (one of which with dual infection) presented positive blood cultures during the admission (table 2). Two patients with confirmed glucose-6-phosphate dehydrogenase (G6PD) deficiency experienced severe hemolysis, hemoglobinuria and acute renal failure after the beginning of primaquine (all started with hemoglobinuria during the second or third day of treatment). All the patients who developed shock syndrome required administration of vasoactive amines. With the exception of one patient with a previous diagnosis of idiopathic thrombocytopenic purpura (ITP), who presented with moderate bleeding and was admitted to the ICU as a result, all remaining 23 patients had one or more WHO severity criteria for malaria. All the patients demonstrated a TBS negative for Plasmodium spp. following chloroquine treatment.
Table 2. Detailed clinical information from 24 children 0–14 years old, admitted to any of the pediatric or neonatal ICUs in Manaus, from 2004 to 2009, with parasitological diagnosis of P. vivax.
| Patient | Age/gender | Time of disease (days) | Associated acute comorbidity | Associated chronic comorbidity | Respiratory distress | Severe anemia | Coma | Jaundice | Shock | Metabolic acidosis | Low glucose | Blackwater fever (ARF) | PIM score (%) | Outcome |
| 1 | 6 y/M | 7 | + | + | 1.9 | Recovered | ||||||||
| 2 | 1 mo/M | 7 | + | + | + | + | 24.8 | Recovered | ||||||
| 3 | 8 y/F | 3 | Viral encephalitis* | + | + | 45.9 | Died | |||||||
| 4 | 1 y/F | 4 | Gastroenteritis | Malnutrition* | + | + | + | 28.1 | Recovered | |||||
| 5 | 3 y/M | 4 | Rotavirus gastroenteritis* | Cystic fibrosis* | + | 1.6 | Recovered | |||||||
| 6 | 1 mo/M | 3 | + | + | + | + | 9.1 | Recovered | ||||||
| 7 | 1 mo/M | 1 | Gastroenteritis and drug-induced hepatitis | + | + | + | + | 21.1 | Recovered | |||||
| 8 | 1 y/M | 2 | Neurological sequelae | + (ARDS) | + | + | + | + | 40.5 | Recovered | ||||
| 9 | 2 y/F | 25 | + (ARDS) | 9.9 | Recovered | |||||||||
| 10 | 11 y/M | 7 | Malnutrition | + | + | + | + | 24.9 | Recovered | |||||
| 11 | 7 mo/F | 26 | Rotavirus gastroenteritis+Sepsis (Acinetobacter sp.+Klebsiella sp.)* | + (ARDS) | + | 11.4 | Recovered | |||||||
| 12 | 8 y/M | 10 | G6PD deficiency* | + | + | 4.0 | Recovered | |||||||
| 13 | 3 y/M | 23 | + | + | + | 11.3 | Recovered | |||||||
| 14 | 3 y/M | 7 | G6PD deficiency | + | + | + | 2.4 | Recovered | ||||||
| 15 | 2 y/M | 2 | + | + | 8.4 | Recovered | ||||||||
| 16 | 2 y/M | 4 | Bacterial pneumonia with lung empyema* | + | + | + | 12.6 | Recovered | ||||||
| 17 | 8 mo/F | 15 | + | + | 4.1 | Recovered | ||||||||
| 18 | 2 mo/F | 10 | Urosepsis (Klebsiella sp.)* | Neurological sequelae | + | + | 7.5 | Recovered | ||||||
| 19 | 3 y/M | 7 | + | + | + | 55.7 | Died | |||||||
| 20 | 1 mo/M | 8 | Rotavirus gastroenteritis | Malnutrition | + | + | 8.7 | Recovered | ||||||
| 21 | 2 y/F | 3 | ITP* | 2.0 | Recovered | |||||||||
| 22 | 1 y/M | 31 | + | + | + | 36.6 | Recovered | |||||||
| 23 | 5 mo/M | 6 | Rotavirus gastroenteritis | Malnutrition | + | + | 2.8 | Recovered | ||||||
| 24 | 18 d/M | 5 | + | 1.2 | Recovered |
y years; mo: months; d: days; NA: Non-available; ARDS: Acute Respiratory Distress Syndrome; ARF: Acute Renal Failure; PIM: Pediatric Index of Mortality; ITP: Immune Trombocytopenic Purpura.
Viral encephalitis confirmed by autopsy; diagnosis of malnutrition confirmed by body mass index (BMI) Z-score <−2; rotavirus gastroenteritis confirmed by immunochromatographic rapid test in the stool; cystic fibrosis suggested by lung biopsy; sepsis confirmed by two positive blood cultures; G6PD deficiency confirmed by qualitative Brewer's test; lung empyema confirmed by computed tomography; urosepsis confirmed by both positive urine and blood culture; ITP confirmed by ASH criteria; ARDS defined as acute bilateral lung infiltrates and a PaO2∶FiO2<200 mmHg.
Table 3. Detailed laboratorial information from 24 children 0–14 years old, at the moment of admission to any of the pediatric or neonatal ICUs in Manaus, from 2004 to 2009, with parasitological diagnosis of P. vivax.
| Patient | Hb (g/dL) | Leukocyte/mm3 | Platelets/mm3 | AST (mg/dL) | ALT (mg/dL) | Creatinin (mg/dL) | Total bilirubin (mg/dL) | Glucose (mg/dL) |
| 1 | 9.1 | 18,600 | 49,000 | 286 | 253 | 0.7 | 0.9 | 90 |
| 2 | 2.8 | 25,600 | 34,000 | 160 | 111 | 0.6 | 4.3 | 55 |
| 3 | 8.5 | 6,400 | 161,000 | 23 | 15 | 0.6 | 0.6 | 70 |
| 4 | 8.5 | 19,300 | 180,000 | 73 | 22 | 1.9 | 0.3 | 304 |
| 5 | 10.4 | 9,000 | 378,000 | 20 | 30 | 1.0 | 1.0 | 199 |
| 6 | 2.5 | 28,600 | 13,000 | 83 | 48 | 0.6 | 8.3 | 59 |
| 7 | 5.0 | 22,500 | 16,000 | 961 | 1400 | 2.7 | 1.0 | 64 |
| 8 | 9.5 | 13,700 | 113,000 | 15 | 23 | 0.8 | 1.0 | 28 |
| 9 | 8.3 | 30,100 | 207,000 | 17 | 19 | 0.5 | 1.0 | 64 |
| 10 | 8.6 | 12,700 | 129,000 | 23 | 27 | 0.8 | 6.7 | 88 |
| 11 | 7.9 | 57,700 | 93,000 | 34 | 45 | 1.1 | 1.0 | 67 |
| 12 | 5.0 | 12,100 | 170,000 | 222 | 53 | 7.5 | 2.7 | 42 |
| 13 | 5.9 | 10,100 | 174,000 | 56 | 10 | 0.4 | 1.0 | 63 |
| 14 | 4.2 | 18,700 | 303,000 | 29 | 26 | 7.1 | 1.4 | 39 |
| 15 | 8.8 | 4,600 | 78,000 | 30 | 28 | 0.4 | 1.0 | 38 |
| 16 | 6.5 | 16,600 | 611,000 | 25 | 15 | 0.9 | 1.0 | 75 |
| 17 | 9.1 | 16,800 | 196,000 | 22 | 29 | 0.4 | 1.0 | 90 |
| 18 | 5.0 | 31,500 | 76,000 | 34 | 43 | 0.4 | 1.0 | 67 |
| 19 | 7.4 | 8,500 | 190,000 | 133 | 46 | 0.3 | 2.8 | 98 |
| 20 | 3.3 | 31,600 | 19,000 | 30 | 45 | 0.8 | 1.6 | 70 |
| 21 | 6.2 | 29,600 | 6,000 | 20 | 17 | 0.9 | 1.0 | 88 |
| 22 | 8.7 | 19,600 | 104,000 | 159 | 64 | 0.7 | 1.0 | 52 |
| 23 | 7.3 | 11,200 | 69,000 | 34 | 50 | 1.0 | 1.0 | 70 |
| 24 | 7.4 | 2,100 | 15,000 | 45 | 23 | 1.0 | 24.2 | 101 |
Hb: hemoglobin; ALT: alanine aminotransferase; AST: aspartate aminotrasferase.
Table 4 summarizes the results of the univariate and multivariate logistic regression evaluating risk factors associated with ICU admission. Males [OR = 5.90 (95% CI = 1.28–34.25); (p = 0.039)], age <5 years [OR = 6.36 (95% CI = 2.67–13.63); (p = 0.028)], peripheral parasitemia greater than 500/mm3 [OR = 4.94 (95% CI = 1.31–18.63); (p = 0.018)], the presence of acute [OR = 8.13 (95% CI = 1.33–49.81); (p = 0.023)] and/or chronic co-morbidities [OR = 6.02 (95% CI = 1.38–26.35); (p = 0.017)] were all independently associated with the risk of developing life-threatening P. vivax monoinfection requiring admission to an ICU. None of the children not admitted to the ICU developed severe disease after their discharge, as reviewed in their electronic files from follow-up visits in the outpatient clinics.
Table 4. Univariate and multivariate (logistic regression) analysis of factors associated to the admission in the ICUs of children 0–14 years old with P. vivax infection, in Manaus, from 2004 to 2009.
| Cases admitted to the ICU n/N (%) | Patients not requiring admission to the ICU n/N (%) | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | p-value | |
| Male sex | 17/24 (70.8) | 53/91 (58.2) | 1.91 (0.92–5.71) | 5.90 (1.28–34.25) | 0.039 |
| Age (<5 years) | 20/24 (83.3) | 25/91 (27.5) | 4.64 (2.05–7.65) | 6.36 (2.67–13.63) | 0.028 |
| Peripheral parasitemia (>500 parasites/mm3) | 14/19 (73.6) | 32/89 (36.0) | 4.99 (1.65–15.12) | 4.94 (1.31–18.63) | 0.018 |
| Time of acute disease prior to treatment (>7 days) | 8/24 (33.3) | 22/91 (24.2) | 1.57 (0.60–4.16) | 3.39 (0.81–14.10) | 0.093 |
| Presence of any chronic disease | 10/24 (41.7) | 9/91 (9.9) | 6.50 (2.25–18.86) | 6.02 (1.38–26.35) | 0.017 |
| Presence of any other acute co-morbidity | 9/24 (37.5) | 7/91 (7.7) | 7.20 (2.33–22.30) | 8.13 (1.33–49.81) | 0.023 |
Table 5 compares ICU admitted P. vivax monoinfections with hospitalized P. vivax patients not requiring ICU admission. Of all variables assessed for both groups, the odds of being admitted to the ICU increased in patients fulfilling at least one WHO severity criterion, particularly in those with severe anemia or coma. The mean of the PIM, a validated score that parallels bad prognosis in pediatric ICUs, was higher in children with shock and coma. P. falciparum or P. falciparum/P. vivax infected children (30.0%) died more frequently than those infected with P. vivax monoinfection (8.3%), but this difference was not significant (Yate's corrected Fisher's exact test; p = 0.138). P. falciparum monoinfection showed higher fatality (2/4; 50.0%) when compared to P. vivax monoinfection (2/24; 8.6%), but this difference likewise was not significant (p = 0.086).
Table 5. Descriptive data and univariate analysis of P. vivax monoinfection cases admitted to the ICU versus patients not requiring ICU admission, according to the WHO type of severity.
| Cases admitted to the ICU n/N (%) | Patients not requiring ICU admission n/N (%) | Odds ratio (95% CI) | p-value | PIM Score (%) of cases (Mean ± SD) | |
| WHO criteria for malaria severity | 23/24 (95.8) | 17/91 (18.7) | 100.11 (12.60–793.60) | <0.001 | 16.2±15.6 |
| Respiratory distress | 16/24 (66.7) | 0/91 (0.0) | - | - | 18.4±15.7 |
| Shock (algid malaria) | 13/24 (54.2) | 0/91 (0.0) | - | - | 23.3±17.0 |
| Metabolic acidosis | 10/24 (41.6) | - | - | - | 16.0±12.3 |
| Severe anemia | 7/24 (29.2) | 5/91 (5.5) | 7.08 (2.00–24.97) | 0.003 | 11.0±8.5 |
| Coma | 5/24 (20.8) | 2/91 (2.2) | 11.71 (2.11–64.94) | <0.001 | 37.2±18.1 |
| Hyperbilirrubinemia | 4/24 (16.7) | 11/91 (12.1) | 1.45 (0.42–5.05) | 0.513 | 15.0±11.8 |
| Hypoglycemia | 3/24 (12.5) | 0/91 (0.0) | - | - | 17.1±20.4 |
| Acute renal failure (ARF) due to hemoglobinuria | 2/24 (8.3) | 0/91 (0.0) | - | - | 3.2±1.1 |
| Death | 2/24 (8.3) | 0/91 (0.0) | - | - | 50.8±6.9 |
Discussion
This study showed that in Manaus most of the malaria-associated hospitalizations in ICUs were related to P. vivax during the period of study, confirming the public health impact of this infection [19]–[20]. Male sex, age less than five years, parasitemia greater than 500/mm3, and the presence of any acute or chronic co-morbidity were independently associated with ICU admission. At least one of the WHO severity criteria for malaria (formerly validated for P. falciparum infection only) was present in most of the patients admitted to the ICU. WHO severity criteria may also serve as predictors of severity for P. vivax patients. Further studies, however, are needed to confirm this finding considering the small number of cases presented in our series.
A significant male sex predominance among children admitted to the ICU in our study contrasts with data from Indonesia and India, where the female sex was associated with severity in vivax infection [3], [21]. One possible explanation of this difference is the heterogeneous age range of infected children in these continents.
Similar to what was observed by Tjitra and colleagues in Papua, Indonesia [3], respiratory distress, present in two-thirds of patients in our series, was the most frequent complication among patients admitted to the ICU with P. vivax infection, and had a particularly poor prognosis. In P. falciparum malaria, respiratory distress arises secondary to either primary pulmonary pathology mediated by sequestration of parasitized erythrocytes in the lung microvasculature or from the drive to compensate metabolic acidosis. For P. vivax malaria, however, the pathophysiology behind respiratory distress is not well understood. Studies performed in uncomplicated vivax patients suggest that progressive alveolar-capillary dysfunction occurs after treatment, and this inflammatory response is proportional to initial parasite burden in P. vivax [22]. More recently, ex vivo adhesion of P. vivax-parasitized red blood cells to human lung endothelial cells was demonstrated for the first time [23], suggesting that cytoadhesion could play a role in the pathogenesis of this disease. Although, in a patient who died of ARDS, histopathological analysis did not show any evidence of cythoadhesion [24]. Patient outcomes in this series support the hypothesis of a primary pulmonary lesion driving the respiratory clinical manifestations, complicated by the presence of concomitant metabolic acidosis or severe anemia. Half of the 16 patients with respiratory distress had concomitant metabolic acidosis, but only 2 had severe anemia simultaneously. Pneumonia is another confounding diagnosis of respiratory distress, and in all the children in the present report antibiotics were prescribed empirically.
The occurrence of severe anemia also paralleled data from other endemic areas [25], and was consistent with previous data published from Manaus [11], [26]. Further corroborating data from Indonesia [27], we observed that 5 out of 6 children under 3 months of age presented severe anemia, confirming P. vivax as a cause of severe morbidity in the early infancy. Severe anemia can also be seen, not triggered by the parasite itself, in patients with G6PD deficiency, as a complication of the use of primaquine for the radical cure of hypnozoites, as observed in three patients described here (two with pure P. vivax and one with mixed infection). If followed by acute renal failure (ARF), it constitutes the syndromic presentation of blackwater fever [28]. Acute renal failure was mostly related to acute hemolysis in patients with G6PD deficiency. The possibility of concomitant P. malariae infection, a species known to cause acute or delayed renal complications seems highly improbable, as this species is not detected in Manaus [33]. G6PD deficiency seems to be frequent in Brazilian malaria-endemic areas, where it is not routinely screened before the prescription of antimalarials. The prevalence of the A− variant among males has been estimated at 3% [29], and a number of hospitalizations due to this complication have been reported [30]. If G6PD deficiency is not ruled out in epidemiological studies focusing on vivax-related anemia, this complication triggered by the parasite per se could be falsely overestimated.
Circulatory collapse (algid malaria) associated with P. vivax infection was both frequent and severe, requiring the use of vasopressors, with or without a confirmed positive blood culture for aerobes. Even for P. falciparum malaria, the etiology of this complication is uncertain and the potential role of septic shock as a concomitant entity has been proposed [31]. In such cases therefore, blood cultures are mandatory to rule out bacterial co-infections, which may be related to severity and consequently to ICU hospitalization.
The presence of hyperbilirubinemia is becoming recognized recently as an inappropriate criterion of severe disease if found in isolation [32]. In our data this laboratory finding was not predictive of ICU hospitalization [OR = 1,45 (CI = 0,42–5,05), p = 0,513]. Jaundice detected on a routine physical exam, however, could still be used as a warning sign of severity, given that in 3/4 cases jaundice was accompanied by another severity criterion. As serology for the common viral hepatitis in this region was not requested, it is difficult to ascertain whether such episodes were primarily caused by P. vivax per se or resulted from a concomitant liver infection.
Coma was also reported in our series, but in 3 out of 5 children with coma, additional explanations other than cerebral malaria could be found, such as viral encephalitis in one, and decompensation of previous neurological sequelae in two. In one patient hypoglycemia could also be a superimposed factor. Cerebral vivax malaria actually seems to be more common in children [34], but the possibility of other infectious diseases of the central nervous system has to be taken into consideration given the low prevalence of cerebral malaria in vivax infection [35].
It is noteworthy that acute or chronic co-morbidities were detected in 58% (14/24) of the patients with P. vivax. This was also observed in an almost identical proportion (6/10) of P. falciparum patients, which leads to the conclusion that the presence of co-morbidities may contribute to or facilitate the appearance of complications in all malaria. Importantly, this aspect seems to have been specially underreported in prospective studies on vivax severity [3]. Recent data suggests that P.vivax-triggered anemia could be related to parvovirus B19 infection [36]. In our series, rotavirus infection was found among ICU-admitted P. vivax (4 cases) and P. falciparum (2 cases) children. Despite the lack of local data on rotavirus infection, the worldwide-recognized increased severity in those less than 5 years of age could in part explain the younger age of the ICU hospitalized children. Furthermore, in our children, septic shock with confirmed bacteremia was observed in three cases. In Mozambique, the systematic performance of blood cultures on hospital admissions showed that a positive culture for aerobes in children with malaria was associated with an increased risk of death [37]. Therefore, a predisposition to malarial infection in the setting of other bacterial infections may apply to all Plasmodium species. The finding of four malnourished children among vivax cases admitted to the ICU does not seem negligible, and should be taken into consideration as a potential confounding factor when severity frequencies are compared across the world [38]. Although P. vivax can surely independently cause severe or even life threatening episodes in adults and children, the relative importance of co-morbidities or other chronic existing conditions in accelerating the progression to a severe disease cannot be disregarded.
Despite the WHO recommendation to treat patients with severe P. vivax malaria as patients with severe P. falciparum/P. vivax malaria, due to the possibility of submicroscopic parasitemia of the first species, the Brazilian Guidelines for Antimalarial Treatment have only recently changed. During the study period of the present study, chloroquine was prescribed to all patients. Resistance to chloroquine was described for the first time in Latin America in Manaus [39], and more recent data suggest that in vivo resistance in this locality is estimated at 10% [40]. However the resistance does not seem to be disseminated throughout the country [41], and the first line treatment for P. vivax is still chloroquine. Ecological studies suggest that severity in vivax infection could be related to chloroquine-resistance [42]. These and previous data, however, show a good response to chloroquine in severe patients, and suggest that in the Brazilian Amazon this association is not well understood [11].
The fatality rate among P. vivax patients hospitalized in the ICU (8.3%) was not insignificant, even in the Brazilian scenario where diagnosis and treatment of malaria are free and universally available. These numbers could be far larger, however, if a reasonably established health system with tertiary care facilities is not in place. Our small sample was unable to show that P. falciparum children died more frequently than those infected with P. vivax. Actually data from other settings point to a similar fatality rate between the two species [21], [43]. From our data it is possible to detect a high risk of ICU admission among children with mixed infections, however this finding needs to be confirmed in other similar settings in Latin America. PIM score predicted mortality relatively well and its use should be emphasized in future similar pediatric studies.
An important limitation of this study is its retrospective design, and all analyzed information is based on the available registration in clinical charts. Besides that, the absence of PCR confirmation of malarial infections is an additional limitation. Species-specific PCR increases the diagnostic accuracy and can detect submicroscopic infections that may have been disregarded only after standard microscopy. It is possible therefore, that some of our assumed P. vivax monoinfections may in fact be mixed cases. Although possible, this seems unlikely, as a preliminary analysis from our laboratory shows that only 5% of the positive TBSs for P. vivax identify mixed infections, based on real-time PCR (data not shown). Another limitation is the lack of information on total number of patients hospitalized due to malaria in Manaus in the period.
In the present study we showed that, in absolute numbers, P. vivax infection was responsible for most of the ICU admissions of children 0–14 years old due to severe malaria (despite of similar proportional numbers when compared to P. falciparum infections reported), and that the clinical complications were very similar to those seen in P. falciparum in this same endemic area. Despite the fact that peripheral parasitemia greater than 500/mm3 was associated with ICU hospitalization, the positive predictive value of this cut-off for predicting need for ICU admission is extremely low, given the tiny fraction of malaria cases requiring ICU in the general population. Severe vivax patients should also trigger the routine screening of additional infectious diseases in the daily practice in the tropics. From the public health perspective, decision-makers from national malaria programs should re-orientate clinical management algorithms in order to improve treatment, diminish the burden of hospitalization, decrease costs and reduce malaria-associated mortality.
From the basic science perspective, pathogenesis studies in severe vivax should carefully rule out other diseases, which may alter the immune response [44]. Definitely respiratory distress, shock and anemia are major complications associated with P. vivax infection, for which mechanisms are poorly understood even in the case of severe P. falciparum. More studies are needed to evaluate the applicability of these findings in other age groups. Due to the fact that studies on the clinical aspects and biology of P. vivax have been neglected during most of the 20th century, the large knowledge gaps regarding this parasite [45] may pose challenges for future malaria eradication plans [46].
Acknowledgments
We thank all the directors of the ICUs for their support with data collection, the Health Secretariat of Manaus (SEMSA) and the Health Secretariat of the Amazonas State (SUSAM).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by: PRONEX Malaria Network, funded by the Brazilian Ministry of Science and Technology (MCT), National Council for Scientific and Technological Development (CNPq), Brazilian Ministry of Health (DECIT/SCTIE/MS) and the Research Support Foundation from Amazonas (FAPEAM); Fundació Cellex; and the National Institute of Science and Technology for Innovation in Neglected Diseases (INCT-IDN). Dr. Lacerda is a level 2 productivity fellow from CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO) World malaria report 2008. 2008. Geneva.
- 2.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, et al. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, et al. Multidrug-Resistant Plasmodium vivax Associated with Severe and Fatal Malaria: A Prospective Study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, et al. Malaria in Brazil: an overview. Malar J. 2010;9:115. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saraiva MG, Amorim RD, Moura MA, Martinez-Espinosa FE, Barbosa MG. Urban expansion and spatial distribution of malaria in the municipality of Manaus, State of Amazonas. Rev Soc Bras Med Trop. 2009;42:515–522. doi: 10.1590/s0037-86822009000500008. [DOI] [PubMed] [Google Scholar]
- 6.Lacerda MV, Alexandre MA, Santos PD, Arcanjo AR, Alecrim WD, et al. Idiopathic thrombocytopenic purpura due to vivax malaria in the Brazilian Amazon. Acta Trop. 2004;90:187–190. doi: 10.1016/j.actatropica.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Lomar AV, Vidal JE, Lomar FP, Barbas CV, Matos GJ, et al. Acute respiratory distress syndrome due to vivax malaria: case report and literature review. Braz J Infect Dis. 2005;9:425–430. doi: 10.1590/s1413-86702005000500011. [DOI] [PubMed] [Google Scholar]
- 8.Lacerda MV, Hipolito JR, Passos LN. Chronic Plasmodium vivax infection in a patient with splenomegaly and severe thrombocytopenia. Rev Soc Bras Med Trop. 2008;41:522–523. doi: 10.1590/s0037-86822008000500021. [DOI] [PubMed] [Google Scholar]
- 9.Lacerda MVG, Oliveira SL, Alecrim MGC. Splenic hematoma in a patient with Plasmodium vivax malaria. Rev Soc Bras Med Trop. 2007;40:96–97. doi: 10.1590/s0037-86822007000100023. [DOI] [PubMed] [Google Scholar]
- 10.Siqueira AM, Alexandre MAA, Mourão MPG, Santos VS, Nagahashi-Marie SK, et al. Case Report: Severe Rhabdomyolysis from Vivax Malaria in the Brazilian Amazon. Am J Trop Med Hyg. 2010;83:271–273. doi: 10.4269/ajtmh.2010.10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandre MA, Ferreira CO, Siqueira AM, Magalhaes BL, Mourao MPG, et al. Severe Plasmodium vivax Malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–1614. doi: 10.3201/eid1610.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade BB, Reis-Filho A, Souza-Neto SM, Clarencio J, Camargo LM, et al. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010;9:13. doi: 10.1186/1475-2875-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maitland K, Williams TN, Bennett S, Newbold CI, Peto TE, et al. The interaction between Plasmodium falciparum and P. vivax in children on Espiritu Santo island, Vanuatu. Trans R Soc Trop Med Hyg. 1996;90:614–620. doi: 10.1016/s0035-9203(96)90406-x. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Severe and complicated malaria. World Health Organization Malaria Action Programme. Trans R Soc Trop Med Hyg. 1986;80(Suppl):3–50. [PubMed] [Google Scholar]
- 15.Santos-Ciminera PD, Roberts DR, Alecrim MG, Costa MR, Quinnan GV., Jr Malaria diagnosis and hospitalization trends, Brazil. Emerg Infect Dis. 2007;13:1597–1600. doi: 10.3201/eid1310.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Microscopic diagnosis of Malaria. 2006. 4th edition ed. Geneva.
- 17.WHO. Guidelines for the treatment of malaria. 2010 [Google Scholar]
- 18.Shann F, Pearson G, Slater A, Wilkinson K. Paediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med. 1997;23:201–207. doi: 10.1007/s001340050317. [DOI] [PubMed] [Google Scholar]
- 19.Khoo KL, Tan WL, Eng P, Ong YY. Malaria requiring intensive care. Ann Acad Med Singapore. 1998;27:353–357. [PubMed] [Google Scholar]
- 20.Koh KH, Chew PH, Kiyu A. A retrospective study of malaria infections in an intensive care unit of a general hospital in Malaysia. Singapore Med J. 2004;45:28–36. [PubMed] [Google Scholar]
- 21.Kochar DK, Tanwar GS, Khatri PC, Kochar SK, Sengar GS, et al. Clinical features of children hospitalized with malaria-a study from Bikaner, northwest India. Am J Trop Med Hyg. 2010;83:981–989. doi: 10.4269/ajtmh.2010.09-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anstey NM, Handojo T, Pain MC, Kenangalem E, Tjitra E, et al. Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis. 2007;195:589–596. doi: 10.1086/510756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, et al. On the Cytoadhesion of Plasmodium vivax-Infected Erythrocytes. J Infect Dis. 2010;202:638–647. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 24.Valecha N, Pinto RG, Turner GD, Kumar A, Rodrigues S, et al. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg. 2009;81:758–762. doi: 10.4269/ajtmh.2009.09-0348. [DOI] [PubMed] [Google Scholar]
- 25.Mueller I, Namuigi P, Kundi J, Ivivi R, Tandrapah T, et al. Epidemic malaria in the highlands of Papua New Guinea. Am J Trop Med Hyg. 2005;72:554–560. [PubMed] [Google Scholar]
- 26.Caicedo O, Ramirez O, Mourao MP, Ziadec J, Perez P, et al. Comparative hematologic analysis of uncomplicated malaria in uniquely different regions of unstable transmission in Brazil and Colombia. Am J Trop Med Hyg. 2009;80:146–151. [PubMed] [Google Scholar]
- 27.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Hasanuddin A, et al. Vivax Malaria: A Major Cause of Morbidity in Early Infancy. Clin Infect Dis. 2009 doi: 10.1086/599041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgoine KL, Bancone G, Nosten F. The reality of using primaquine. Malar J. 2010;9:376. doi: 10.1186/1475-2875-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santana MS, Lacerda MVG, Barbosa MGV, Alecrim WD, Alecrim MGC. Glucose-6-phosphate dehydrogenase deficiency in an endemic area for malaria in Manaus: a cross-sectional survey in the Brazilian Amazon. PLoS ONE. 2009;4:e5259. doi: 10.1371/journal.pone.0005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos Junior WM, Sardinha JF, Costa MR, Santana MS, Alecrim MG, et al. Clinical aspects of hemolysis in patients with P. vivax malaria treated with primaquine, in the Brazilian Amazon. Braz J Infect Dis. 2010;14:410–412. doi: 10.1590/s1413-86702010000400017. [DOI] [PubMed] [Google Scholar]
- 31.Lacerda MV, Mourao MP, Santos PJ, Alecrim MG. Algid malaria: a syndromic diagnosis. Rev Soc Bras Med Trop. 2009;42:79–81. doi: 10.1590/s0037-86822009000100017. [DOI] [PubMed] [Google Scholar]
- 32.Anstey NM, Price RN. Improving case definitions for severe malaria. PLoS Med. 2007;4:e267. doi: 10.1371/journal.pmed.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa MR, Vieira PP, Ferreira Cde O, Lacerda MV, Alecrim WD, et al. Molecular diagnosing of malaria in a tertiary care center in the Brazilian Amazon region. Rev Soc Bras Med Trop. 2008;41:381–385. doi: 10.1590/s0037-86822008000400011. [DOI] [PubMed] [Google Scholar]
- 34.Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, et al. Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–232. doi: 10.4269/ajtmh.2002.67.230. [DOI] [PubMed] [Google Scholar]
- 35.Lampah DA, Yeo TW, Hardianto SO, Tjitra E, Kenangalem E, et al. Coma associated with microscopy-diagnosed Plasmodium vivax: a prospective study in Papua, Indonesia. PLoS Negl Trop Dis. 2011;5:e1032. doi: 10.1371/journal.pntd.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wildig J, Michon P, Siba P, Mellombo M, Ura A, et al. Parvovirus B19 infection contributes to severe anemia in young children in Papua New Guinea. J Infect Dis. 2006;194:146–153. doi: 10.1086/505082. [DOI] [PubMed] [Google Scholar]
- 37.Bassat Q, Guinovart C, Sigauque B, Mandomando I, Aide P, et al. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Health. 2009;14:1011–1019. doi: 10.1111/j.1365-3156.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 38.Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am J Trop Med Hyg. 2004;71:55–63. [PubMed] [Google Scholar]
- 39.Alecrim MGC, Alecrim W, Macêdo V. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev Soc Bras Med Trop. 1999;32:67–68. doi: 10.1590/s0037-86821999000100013. [DOI] [PubMed] [Google Scholar]
- 40.Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, et al. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis. 2007;13:1125–1126. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orjuela-Sanchez P, da Silva NS, da Silva-Nunes M, Ferreira MU. Recurrent parasitemias and population dynamics of Plasmodium vivax polymorphisms in rural Amazonia. Am J Trop Med Hyg. 2009;81:961–968. doi: 10.4269/ajtmh.2009.09-0337. [DOI] [PubMed] [Google Scholar]
- 42.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–435. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 43.Barcus MJ, Basri H, Picarima H, Manyakori C, Sekartuti, et al. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern indonesian papua. Am J Trop Med Hyg. 2007;77:984–991. [PubMed] [Google Scholar]
- 44.Bassat Q, Alonso PL. Defying malaria: Fathoming severe Plasmodium vivax disease. Nat Med. 2011;17:48–49. doi: 10.1038/nm0111-48. [DOI] [PubMed] [Google Scholar]
- 45.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 46.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]