Abstract
The lipidic modification of proteins has recently been shown to be of immense importance, although many of the roles of these modifications remain as yet unidentified. One of such key modifications occurring on several proteins is the covalent addition of a 14-carbon long saturated fatty acid, a process termed myristoylation. Myristoylation can occur during both co-translational protein synthesis and posttranslationally, confers lipophilicity to protein molecules, and controls protein functions. The protein myristoylation process is catalyzed by the enzyme N-myristoyltransferase (NMT), which exists as two isoforms: NMT1 and NMT2. NMT1 is essential for growth and development, during which rapid cellular proliferation is required, in a variety of organisms. NMT1 is also reported to be elevated in many cancerous states, which also involve rapid cellular growth, albeit in an unwanted and uncontrolled manner. The delineation of myristoylation-dependent cellular functions is still in a state of infancy, and many of the roles of the myristoylated proteins remain to be established. The development of cells of the leukocytic lineage represents a phase of rapid growth and development, and we have observed that NMT1 plays a role in this process. The current review outlines the roles of NMT1 in the growth and differentiation of the cells of leukocytic origin. The described studies clearly demonstrate the roles of NMT1 in the regulation of the developmental processes of the leukocytes cells and provide a basis for further research with the aim of unraveling the roles of protein myristoylation in both cellular and physiological context.
Keywords: N-myristoyltransferase, Lipid modification, Myristoylation, Leukocyte development
Introduction
N-myristoyltransferase (NMT) is an ubiquitously distributed enzyme and belongs to the GCN5 acetyltransferase superfamily (Boutin 1997; Resh 1999; Dyda et al. 2000; Farazi et al. 2001). The enzyme catalyses the covalent attachment of the myristoyl group, generally to the N-terminal glycine residue of proteins (Boutin 1997; Resh 1999; Farazi et al. 2001; Selvakumar et al. 2007; Wright et al. 2010; Hannoush and Sun 2010). Myrisitic acid constitutes less than 1% of the total fatty acid pool and is considered a rare fatty acid in cells (Boutin 1997). However, myristoylation constitutes a large subset of the total fatty acylated proteins, and at least 0.5% of eukaryotic proteins are predicted to be myristoylated (Resh 2006; Hannoush and Sun 2010; Wright et al. 2010). This suggests that myristoylation has a special role and cannot be substituted by other lipidic modifications of proteins. The myristoylation moiety was first identified as a N-terminal blocking group in the catalytic subunit of cyclic AMP-dependent protein kinase and calcineurin B (Carr et al. 1982; Aitken et al. 1982). The process of protein myristoylation initially reported to be a co-translational event has now also been shown to occur post-translationally (Wilcox et al. 1987; Zha et al. 2000). N-myristoylation occurs absolutely on an exposed N-terminal glycine and on a general consesus motif of GXXXS/T, where X is any amino acid (Boutin 1997; Resh 1999; Farazi et al. 2001; Wright et al. 2010; Hannoush and Sun 2010). During the co-translational protein myristoylation, the initiator methionine at the N-terminus is removed by methionine aminopeptidase thus allowing the exposure of a glycine residue on an available myristoylation site (Wilcox et al. 1987; Deichaite et al. 1988). Post-translational myristoylation events are initiated when an internal myristoylation site is exposed following a proteolytic cleavage (Utsumi et al. 2003; Sakurai and Utsumi 2006; Vilas et al. 2006; Martin et al. 2011). The process of N-myristoylation follows an ordered Bi Bi reaction mechanism in which myristoyl-CoA first binds to the NMT molecule inducing a conformational change and thus allowing for substrate binding followed by a direct nucleophilic addition–elimination reaction and the sequential release of CoA and the myristoyl-peptide (Farazi et al. 2001; Wright et al. 2010). The enzyme NMT consists of saddle-shaped β-sheet flanked by α helices and exhibits a pseudo two-fold symmetry with regions corresponding to the N- and C-terminal portions of the enzyme. The N-terminal half forms the myristoyl-CoA-binding site, whereas the C-terminal half forms the major portion of the peptide-binding site (Farazi et al. 2001; Wright et al. 2010).
NMT exists as a single copy gene in lower eukaryotes, whereas in higher eukaryotes, two genes encoding for the two isoforms of NMT have been identified (Giang and Cravatt 1998). The two isoforms NMT1 and NMT2 share about 76% amino acid sequence identity in humans (Giang and Cravatt 1998). The N-terminal glycine myristoylation of various key proteins is necessary for normal cell functioning, and thus NMT is essential for survival and growth in a number of organisms (Duronio et al. 1989; Lodge et al. 1994; Weinberg et al. 1995; Boutin 1997; Wright et al. 2010). In organisms in which a single NMT isoform exists, the targeting of the endogenous NMT functions has been the candidate of choice for the treatment of many human pathogenic states with a focus on developing species-specific NMT inhibitors as anti-fungal, antiparasitic, and antiviral agents (Duronio et al. 1991; Sikorski et al. 1997; Lodge et al. 1998; Georgopapadakou 2002; Price et al. 2003; Gelb et al. 2003; French et al. 2004; Panethymitaki et al. 2006; Bowyer et al. 2007; Brannigan et al. 2010; Frearson et al. 2010). In the higher eukaryotes, the two isoforms NMT1 and NMT2 have overlapping but distinct substrate specificities (Giang and Cravatt 1998; Ducker et al. 2005). The NMT1 isoform is homologous to the NMT from lower eukaryotes and has been shown the rescue myistic acid auxotrophy in yeast (Duronio et al. 1992). With respect to the functional importance of the NMT1 and NMT2 enzymes in vivo, it has been observed that during the embryonic development of mice, NMT2 is not able to rescue N-myristoylation of proteins for the proper development of the embryos in NMT1−/− mice knockouts and the embryos die during early embryogenesis (Yang et al. 2005). This clearly indicates the specific roles played by the individual NMTs and further suggests that the two isoforms cannot compensate for each other’s specific functional roles. The cellular myristoylated protein participates in signal transduction, cellular transformation, and oncogenesis, and hence, myristoylation is extremely important for the full expression of biological function of many proteins (Boutin 1997; Resh 1999). Mristoylation increases protein lipophilicity and controls the functioning of proteins by targeting them to specific localizations, promoting specific protein-protein and protein-lipid interactions and ligand-induced conformational changes (Resh 1999; Farazi et al. 2001; Wright et al. 2010). A detailed list of the myristoylated proteins is available in several excellent reviews (Boutin 1997; Resh 1999; Maurer-Stroh and Eisenhower 2004; Maurer-Stroh et al. 2004; Selvakumar et al. 2007). Many of the myristoylated proteins such as the non-receptor tyrosine kinases, fyn, lyn, and src, are involved in oncogenic processes (Summy and Gallick 2003; Lieu and Kopetz 2010), and the levels of the myristoylated tyrosine kinases, pp60c-src and pp60c-yes, have been observed to be several fold higher in colonic preneoplastic lesions and neoplasms compared with normal colon cells (Bolen et al. 1987; Weber et al. 1992; Termuhlen et al. 1993). Myristoylation has also been linked to several other pathogenic states such as Noonan-like syndrome, which is a rare developmental disorder (Mazzanti et al. 2003; Schubbert et al. 2007; Cordeddu et al. 2009), diabetes (King et al. 1993, 1995) and ischemia-reperfusion injury (Rajala et al. 2002). Other than their direct involvement in these disorders, NMTs also play a role in human immune deficiency (HIV) infections and several other viral diseases that have been discussed in detail elsewhere (Maurer-Stroh and Eisenhower 2004; Selvakumar et al. 2007; Wright et al. 2010; Martin et al. 2011). The focus of the current review is on the roles of NMT in leukocytic differentiation processes and the roles of NMT1 in neutrophil apoptosis.
Expression and localization of NMT in leukocytic cells
NMT expression is elevated in various forms of cancer, and a direct relationship between the increase in NMT expression and activity in colon cancer progression has been reported (Magnuson et al. 1995; Kumar et al. 2011). The inhibition of NMT1 functions in human and murine melanoma cell lines has been shown to reduce proliferation and to induce apoptosis and also to block tumor growth in vivo (Bhandarkar et al. 2008). The short interfering RNA (siRNA)-mediated knockdown of human NMT1 in vivo inhibits cell replication associated with the loss of c-Src activation and its target focal adhesion kinase and causes a reduction of various protein-kinase-regulated pathways (Ducker et al. 2005). In a mouse model, among the two isozymes NMT1 and NMT2, the intratumoral injection of NMT1 siRNA is mainly responsible for the inhibition of tumor growth (Ducker et al. 2005). NMT1 is predominantly a cytoplasmic enzyme; however, its localization has been observed to change in cancerous states. The high expression levels and the differential localization of NMT1 in peripheral blood mononuclear cells (PBMC) and bone marrow cells (BMC) form the basis for screening tools for the detection of colon cancer at an early stage (Shrivastav et al. 2007; Kumar et al. 2011). In rats with colonic tumors, NMT1 expression and activity have been observed to be significantly elevated in PBMC and BMC compared with those in normal rats (Fig. 1).
Fig. 1.
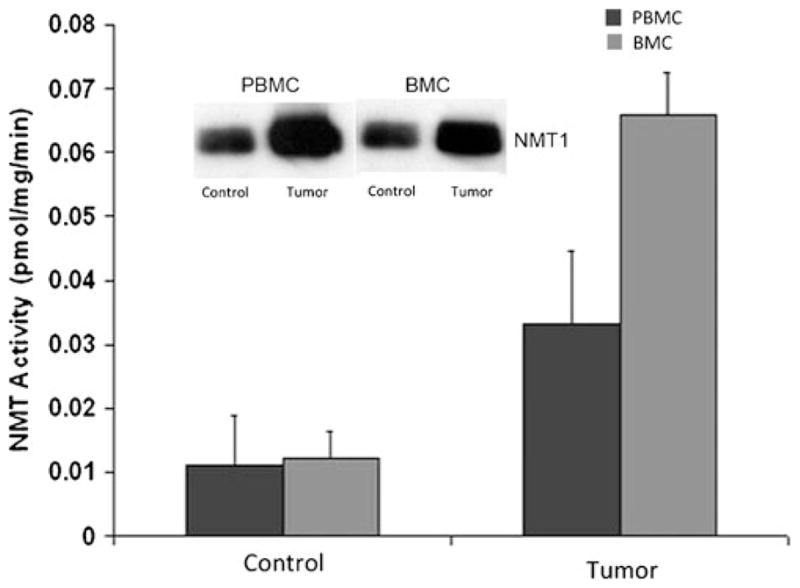
N-myristoyltransferase (NMT) activity in peripheral blood mononuclear cells (PBMC) and bone marrow cells (BMC) of control (Control) and colorectal-tumor-bearing (Tumor) rats. Isolated peripheral blood mononuclear cells from peripheral blood of control or tumor-bearing rats were assessed for NMT activity by using cAMP-dependent protein-kinase-derived peptide substrate. Inset Western blot analysis of protein extracts from PBMC and BMC of control and colorectal tumor-bearing rats [adapted from Shrivastav et al. 2007]
NMT activity is observed to be three-fold higher in PBMC from rats with colonic cancer than in controls, whereas it is elevated five-fold in BMC from rats with colonic cancer compared with a normal group (Fig. 1). In addition to the elevated expression and activity profile, the localization of NMT1 is also altered in PBMC and BMC in the cancerous state. Rare or no positivity is observed for NMT1 in a control group (Fig. 2a), whereas strong staining of more than 50% mononuclear cells is observed in cancerous tissue (Fig. 2b). Intense NMT expression is observed in PBMC from highly invasive tumors. The immunohistochemical staining of PBMC shows negative to rare weak positivity for NMT1 in healthy controls, and the percentage of positive staining is less than 20% (Fig. 2c, d). However, strong NMT staining in more than 80% cells is seen in monocytes, lymphocytes, and neutrophils in blood smears of patients with colonic cancer (Fig. 2e, f).
Fig. 2.
Immunohistochemical analysis of PBMC of control and tumor-bearing hosts. Smears of peripheral blood cells were incubated with anti-NMT antibody (brown positive staining). a PBMC (mostly lymphocytes) from control rat are devoid of NMT staining. b Intense NMT expression (arrows) in the PBMC of colorectal-tumor-bearing rats. c Lack of staining of lymphocytes (arrow) in a control. d Little staining of monocytes (arrows) in peripheral blood smear of a control. e Peripheral blood smear of patient with colon cancer; note positive staining of macrophages (arrows). f Peripheral blood smear of colon cancer patient showing positive staining of neutrophil (fat arrows), lymphocyte (thin long arrow), and macrophages (arrow) [adapted from Shrivastav et al. 2007]. Magnification: 50× oil (a–d), 100× oil (e, f)
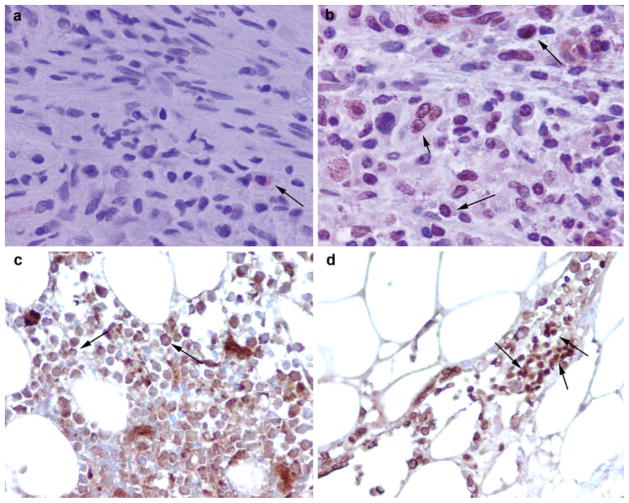
In contrast to the PBMC, a differential localization of NMT1 is found in the bone marrow mononuclear cells. In the normal bone marrow specimens, NMT1 remains cytoplasmic (Fig. 3a, c), whereas NMT1 shifts to a nucleolar (and cytoplasmic) location in BMC from tumor-bearing rats and human cancer patients, respectively (Fig. 3b, d).
Fig. 3.
Immunohistochemical analysis of bone marrow of control and tumor-bearing hosts. a Cytoplasmic staining of NMT (arrow) in BMC from control rats. b Nuclear localization of NMT in BMC (short arrow) from a tumor-bearing rat (long arrows little cytoplasmic staining). c Mostly cytoplasmic NMT staining in bone marrow (BM) of control (arrows). d Intense nuclear (and some cytoplasmic) staining for NMT observed in the BM of a colon cancer patient (arrows) [adapted from Shrivastav et al. 2007]. Magnification: 20× dry (a, b), 10× dry (c, d)
The intense staining and differential localization of NMT1 in mononuclear cells from highly proliferative (cancerous) tissues suggests a role of NMT1 in the differentiation of these cells.
Role of NMT1 in the monocytic differentiation process
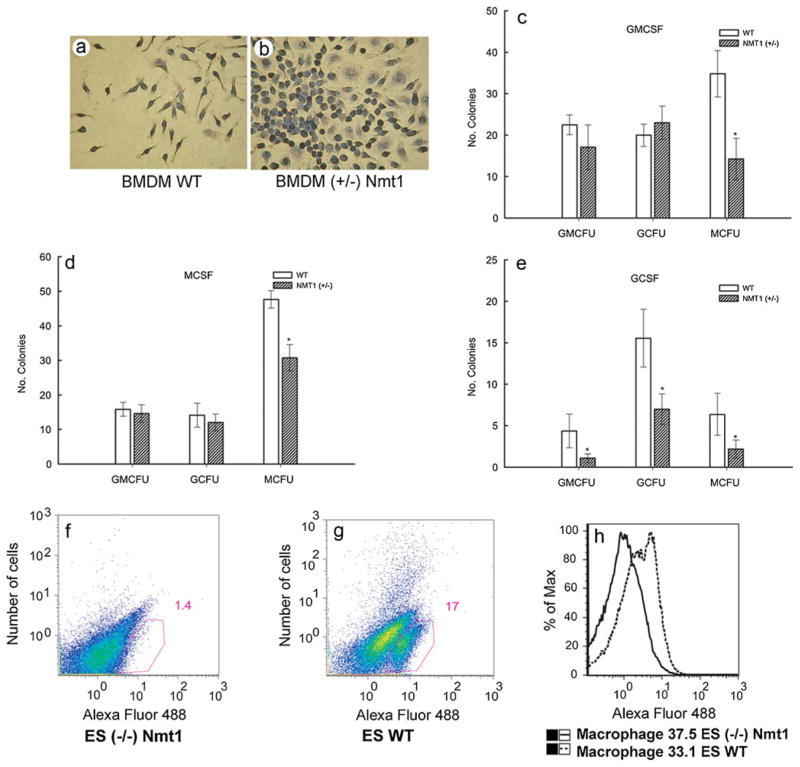
The BMC taken from wild-type (WT) and heterozygous (+/−) Nmt1-deficient mice and cultured in the presence of mouse macrophage colony-stimulating factor (mMCSF) for differentiation into monocytes/macrophages do not develop in a similar fashion (Shrivastav et al. 2008). The Wright-Giemsa-stained bone-marrow-derived macrophages (BMDM) from WT mice (BMDM WT) are markedly different from those of heterozygous (+/−) Nmt1-deficient mice (Fig. 4a, b, respectively). Additionally, the total number of BMDM obtained from heterozygous (+/−) Nmt1-deficient mouse are almost one-fourth those of BMDM WT (Shrivastav et al. 2008). Furthermore, more abundant cytoplasm with cytoplasmic projections and the presence of few cytoplasmic granules are observed in BMDM WT, whereas BMDM from heterozygous (+/−) Nmt1-deficient mice lack these features (Fig. 4a, b, respectively). The colony-forming ability of the bone marrow from WT and heterozygous (+/−) Nmt1-deficient mice have been studied in order to understand the role of NMT1 in myelopoesis (Shrivastav et al. 2008). When the BMC obtained from WT and heterozygous (+/−) Nmt1-deficient mice are cultured in the presence or absence of GM-CSF (Fig. 4c), M-CSF (Fig. 4d), or G-CSF (Fig. 4e), the BMC of WT mice consistently provide a higher number of total CFU (Fig. 4c–e). The differential number of M-CFU also remains significantly higher in the BMC of WT mice compared with that of Nmt1 (+/−) mice, irrespective of being incubated in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), or granulocyte colony-stimulating factor (G-CSF; Fig. 4c–e). However, in the BMC of WT and heterozygous (+/−) mice upon incubation with G-MCF or M-CSF, the number of GM-CFU and G-CFU are comparable (Fig. 4c, d, respectively). On the other hand, significantly higher GM-CFU and G-CFU were observed in the BMC of WT mice compared with those of Nmt1 (+/−) mice when incubated in the presence of G-CSF (Fig. 4e). The results indicate a partially diminished ability of the myeloid lineage in Nmt1 (+/−) mice to differentiate (Shrivastav et al. 2008). When embryonic stem (ES) cells isolated from WT and homozygous (−/−) Nmt1-deficient mice are cultured on a feeder-independent system in the presence of M-CSF, and when the macrophage population is determined by the expression of the F4/80 surface marker by flow cytometry, the expression of AlexaFluor-488-conjugated F4/80 is observed in 1.4% of the homozygous (−/−) Nmt1-deficient ES cells as compared with 17.0% of the WT ES cells (Fig. 4f, g, respectively). However, no appreciable difference can be noted in the mean channel intensity of the antigen detected by F4/80 (Fig. 4h), which further validates that NMT1 is essential for proper monocytic differentiation (Shrivastav et al. 2008).
Fig. 4.
Differentiation of BM or embryonic stem (ES) cells. Morphology of Wright-Giemsa-stained bone-marrow-derived macrophages (BMDM) from (a) WT C57BL/6 mice and (b) heterozygous (+/−) Nmt1-deficient mice. Magnification: 20× dry. Colony-forming ability of BM from heterozygous (+/−) Nmt1-deficient mice or WT mice in the presence of mouse-derived (c) granulocyte macrophage colony-stimulating factor (GMCSF), (d) macrophage colony-stimulating factor (MCSF), and (e) granulocyte colony-stimulating factor (GCSF). Fluorescence-activated cell sorting analysis of (f) homozygous (−/−) Nmt1-deficient ES cells and (g) WT mouse ES cells cultured in presence of mouse MCSF and stained with AlexaFluor-488-conjugated anti-F4/80 antibody. Mean channel intensity (h) of the F4/80-expressing cell populations in homozygous (−/−) Nmt1-deficient and WT mouse ES cells [adapted from Shrivastav et al. 2008]
NMT in activated macrophages and neutrophils and its roles in neutrophil longevity
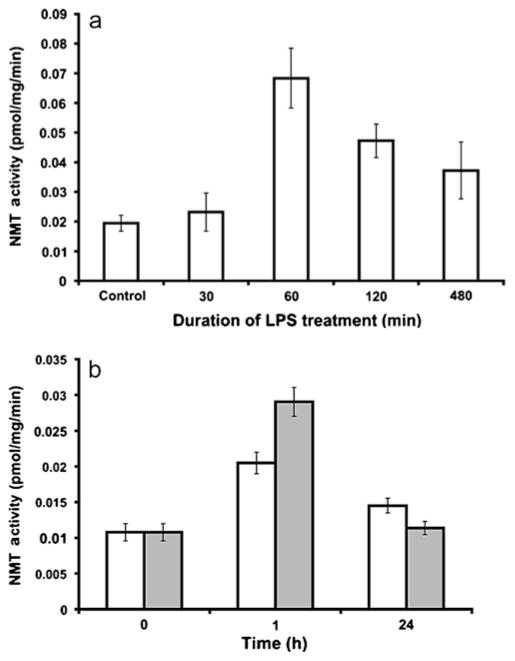
Upon in vitro activation with lipo-polysaccharides (LPS; 10 μg/ml LPS) derived from Escherichia coli, peripheral blood neutrophils show an initial increase in NMT activity (up to ~1 h), which then declines with time (Fig. 5a). To investigate this phenomenon further, the U937 promonocytic cell line has been selected as a model in vitro system, as it can differentiate along the monocyte/macrophage cell lineage by induction with phorbol myristate acetate (PMA). When U937 promonocytic cells are differentiated into monocytes/macrophages by PMA, and when NMT activity is assessed following treatment with E. coli LPS, the findings are similar to those observed in neutrophils (Shrivastav et al. 2010). At both the low and high concentrations of LPS used in the study (Fig. 5b), NMT activity increases during a short-term treatment with LPS; however, prolonged treatment with LPS does not have a significant effect on NMT activity (Fig. 5b).
Fig. 5.
NMT activity in phagocytic cells. a Neutrophils isolated from peripheral blood of control animals. b Phorbol myristate acetate (PMA)-induced differentiated U937 cells treated with lipopolysac-charides (LPS) for the indicated times (clear bar 1 μg/ml LPS, shaded bar 10 μg/ml LPS) [adapted from Shrivastav et al. 2010]
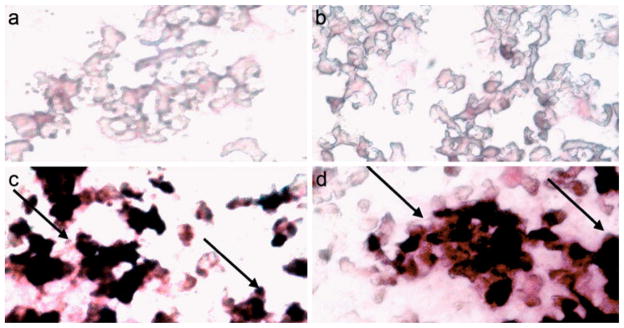
The immunohistochemical analysis of LPS-treated neutrophils shows increased expression of NMT1, and intense NMT1 staining has been noticed in neutrophils incubated with LPS, whereas only light staining is detected in control neutrophils (Fig. 6).
Fig. 6.
NMT1 staining in neutrophils. a No staining in neutrophils stained with secondary antibody. b Light NMT1 staining in control neutrophils. c Intense NMT1 staining (arrows) in neutrophils incubated (30 min) with LPS. d Intense NMT1 staining (arrows) in neutrophils stained with NMT1 at 30 min after a 5-min LPS exposure [adapted from Shrivastav et al. 2010]. Magnification: 40× (a–e)
Since the NMT activity showed a similar profile upon LPS induction, both in the neutrophils and in the PMA-induced U937 cells (Fig. 5a, b), further effects of LPS treatment on NMT1 expression profile have been analyzed in PMA-induced U937 cells. Short-term (1 h) but not long-term treatment with both low and high concentrations of LPS enhances NMT1 expression (Shrivastav et al. 2010). The expression of NMT inhibitor protein 71 is not altered upon short- and long-term LPS treatment suggesting that LPS activates NMT in a time-dependent manner (Shrivastav et al. 2010). Through the suppression of constitutive apoptosis, activated neutrophils live longer. NMT1 and NMT2 have reported roles in cell survival and embryogenesis (Wilcox et al. 1987; Duronio et al. 1989; Lodge et al. 1994; Farazi et al. 2001; Ntwasa et al. 2001; Yang et al. 2005). When probed for their roles in neutrophil longevity, the knockdown of either NMT1 or NMT2, both in the presence or absence of LPS, induces ~30% cell death compared with the control (Shrivastav et al. 2010). Our data show the regulation of neutrophil lifespan by the increased activity of NMT and the expression of NMT1 in activated neutrophils. The reduced lifespan following NMT1 knockdown provides novel insights into the role of NMT1 in regulating neutrophil lifespan.
Concluding remarks
Rapid cellular growth is a hallmark of many physiological processes, which include embryonic developmental stages, the repair of tissue damage, and the turnover of cells with a short half-life and a pathogenic state of tumor growth. NMT is a key enzyme essential for growth and development. This makes NMT an attractive candidate for drug-based therapies, because the targeting of NMT functions causes developmental defects and lethality in a variety of organisms (Farazi et al. 2001; Ntwasa et al. 2001; Yang et al. 2005; Wright et al. 2010). The rapid growth of cells is also reflected in the cells of the immune system; however, no direct evidence has been presented for the involvement of NMT in these processes. The findings from our studies show that NMT plays a role in the regulation of development and differentiation of leukocytic cells. However, with our current knowledge of myristoylation-dependent leukocytic functions, we are still far from understanding the precise roles of this lipidic modification in modulating immune behavior. To comprehend the mechanisms of the myristoylation-dependent regulation of proteins involved in immune and signaling pathways, further studies are definitely warranted so as to tailor therapeutic targeting of these pathways in various pathogenic states.
Contributor Information
Sujeet Kumar, Department of Pathology and Laboratory Medicine, College of Medicine, University of Saskatchewan, Saskatoon, SK S7N 0W8, Canada, Cancer Research Unit, Saskatchewan Cancer Agency, 20 Campus Drive, Saskatoon, SK S7N 4H4, Canada.
Baljit Singh, Department of Veterinary Biomedical Sciences, University of Saskatchewan, Western College of Veterinary Medicine, 52 Campus Drive, Saskatoon, SK S7N 5B4, Canada.
Jonathan R. Dimmock, Drug Design and Discovery Research Group, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, SK S7N 5C9, Canada
Rajendra K. Sharma, Department of Pathology and Laboratory Medicine, College of Medicine, University of Saskatchewan, Saskatoon, SK S7N 0W8, Canada, Cancer Research Unit, Saskatchewan Cancer Agency, 20 Campus Drive, Saskatoon, SK S7N 4H4, Canada
References
- Aitken A, Cohen P, Santikarn S, Williams DH, Calder AG, Smith A, Klee CB. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982;150:314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Bhandarkar SS, Bromberg J, Carrillo C, Selvakumar P, Sharma RK, Perry BN, Govindarajan B, Fried L, Sohn A, Reddy K, Arbiser JL. Tris (dibenzylideneacetone) dipalladium, a N-myristoyltransferase-1 inhibitor, is effective against melanoma growth in vitro and in vivo. Clin Cancer Res. 2008;14:5743–5748. doi: 10.1158/1078-0432.CCR-08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen JB, Veillette A, Schwartz AM, DeSeau V, Rosen N. Activation of pp 60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci USA. 1987;84:2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin JA. Myristoylation. Cell Signal. 1997;9:15–35. doi: 10.1016/s0898-6568(96)00100-3. [DOI] [PubMed] [Google Scholar]
- Bowyer PW, Gunaratne RS, Grainger M, Withers-Martinez C, Wickramsinghe SR, Tate EW, Leatherbarrow RJ, Brown KA, Holder AA, Smith DF. Molecules incorporating a benzothiazole core scaffold inhibit the N-myristoyltransferase of Plasmodium falciparum. Biochem J. 2007;408:173–180. doi: 10.1042/BJ20070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannigan JA, Smith BA, Yu Z, Brzozowski AM, Hodgkinson MR, Maroof A, Price HP, Meier F, Leatherbarrow RJ, Tate EW, Smith DF, Wilkinson AJ. N-myristoyltransferase from Leishmania donovani: structural and functional characterisation of a potential drug target for visceral leishmaniasis. J Mol Biol. 2010;396:985–999. doi: 10.1016/j.jmb.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr SA, Biemann K, Shoji S, Parmelee DC, Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci USA. 1982;79:6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, Fodale V, Cecchetti S, Cardinale A, Martin J, Schackwitz W, Lipzen A, Zampino G, Mazzanti L, Digilio MC, Martinelli S, Flex E, Lepri F, Bartholdi D, Kutsche K, Ferrero GB, Anichini C, Selicorni A, Rossi C, Tenconi R, Zenker M, Merlo D, Dallapiccola B, Iyengar R, Bazzicalupo P, Gelb BD, Tartaglia M. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichaite I, Casson LP, Ling HP, Resh MD. In vitro synthesis of pp 60v-src: myristylation in a cell-free system. Mol Cell Biol. 1988;8:4295–4301. doi: 10.1128/mcb.8.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker CE, Upson JJ, French KJ, Smith CD. Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis. Mol Cancer Res. 2005;3:463–476. doi: 10.1158/1541-7786.MCR-05-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio RJ, Towler DA, Heuckeroth RO, Gordon JI. Disruption of the yeast N-myristoyl transferase gene causes recessive lethality. Science. 1989;243:796–800. doi: 10.1126/science.2644694. [DOI] [PubMed] [Google Scholar]
- Duronio RJ, Rudnick DA, Johnson RL, Johnson DR, Gordon JI. Myristic acid auxotrophy caused by mutation of S. cerevisiae myristoyl-CoA:protein N-myristoyltransferase. J Cell Biol. 1991;113:1313–1330. doi: 10.1083/jcb.113.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio RJ, Reed SI, Gordon JI. Mutations of human myristoyl-CoA:protein N-myristoyltransferase cause temperature-sensitive myristic acid auxotrophy in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:4129–4133. doi: 10.1073/pnas.89.9.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyda F, Klein DC, Hickman AB. GCN5-related N-acetyltransferases: a structural overview. Annu Rev Biophys Biomol Struct. 2000;29:81–103. doi: 10.1146/annurev.biophys.29.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J Biol Chem. 2001;276:39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- Frearson JA, Brand S, McElroy SP, Cleghorn LA, Smid O, Stojanovski L, Price HP, Guther ML, Torrie LS, Robinson DA, Hallyburton I, Mpamhanga CP, Brannigan JA, Wilkinson AJ, Hodgkinson M, Hui R, Qiu W, Raimi OG, Aalten DM, van Brenk R, Gilbert IH, Read KD, Fairlamb AH, Ferguson MA, Smith DF, Wyatt PG. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464:728–732. doi: 10.1038/nature08893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Schrecengost RS, Copper JE, Xia Z, Smith CD. Cyclohexyl-octahydro-pyrrolo[1,2-a]pyrazine-based inhibitors of human N-myristoyltransferase-1. J Pharmacol Exp Ther. 2004;309:340–347. doi: 10.1124/jpet.103.061572. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Van Voorhis WC, Buckner FS, Yokoyama K, Eastman R, Carpenter EP, Panethymitaki C, Brown KA, Smith DF. Protein farnesyl and N-myristoyl transferases: piggy-back medicinal chemistry targets for the development of antitrypanosomatid and antimalarial therapeutics. Mol Biochem Parasitol. 2003;126:155–163. doi: 10.1016/s0166-6851(02)00282-7. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou NH. Antifungals targeted to protein modification: focus on protein N-myristoyltransferase. Expert Opin Invest Drugs. 2002;11:1117–1125. doi: 10.1517/13543784.11.8.1117. [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. A second mammalian N-myristoyltransferase. J Biol Chem. 1998;273:6595–6598. doi: 10.1074/jbc.273.12.6595. [DOI] [PubMed] [Google Scholar]
- Hannoush RN, Sun J. The chemical tool box for monitoring protein fatty acylation and prenylation. Nat Chem Biol. 2010;6:498–506. doi: 10.1038/nchembio.388. [DOI] [PubMed] [Google Scholar]
- King MJ, Pugazhenthi S, Khandelwal RL, Sharma RK. Elevated N-myristoyl transferase activity is reversed by sodium orthovanadate in streptozotocin-induced diabetic rat. Biochim Biophy Acta. 1993;1165:259–262. doi: 10.1016/0005-2760(93)90134-u. [DOI] [PubMed] [Google Scholar]
- King MJ, Pugazhenthi S, Khandelwal RL, Sharma RK. In vivo modulation of N-myristoyltransferase activity by orthovanadate. Mol Cell Biochem. 1995;153:151–155. doi: 10.1007/BF01075931. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dimmock JR, Sharma RK. The potential use of N-myristoyltransferase as a biomarker in the early diagnosis of colon cancer. Cancers. 2011;3:1372–1382. doi: 10.3390/cancers3011372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu C, Kopetz S. The src family of protein tyrosine kinases: a new and promising target for colorectal cancer therapy. Clin Colorectal Cancer. 2010;9:89–94. doi: 10.3816/CCC.2010.n.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge JK, Jackson-Machelski E, Toffaletti DL, Perfect JR, Gordon JI. Targeted gene replacement demonstrates that myristoyl-CoA:protein N-myristoyltransferase is essential for viability of Cryptococcus neoformans. Proc Natl Acad Sci USA. 1994;91:12008–12012. doi: 10.1073/pnas.91.25.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge JK, Jackson-Machelski E, Higgins M, McWherter CA, Sikorski JA, Devadas B, Gordon JI. Genetic and biochemical studies establish that the fungicidal effect of a fully depeptidized inhibitor of Cryptococcus neoformans myristoyl-CoA:protein N-myristoyltransferase (Nmt) is Nmt-dependent. J Biol Chem. 1998;273:12482–12491. doi: 10.1074/jbc.273.20.12482. [DOI] [PubMed] [Google Scholar]
- Magnuson BA, Raju RV, Moyana TN, Sharma RK. Increased N-myristoyltransferase activity observed in rat and human colonic tumors. J Natl Cancer Inst. 1995;87:1630–1635. doi: 10.1093/jnci/87.21.1630. [DOI] [PubMed] [Google Scholar]
- Martin DD, Beauchamp E, Berthiaume LG. Post-translational myristoylation: fat matters in cellular life and death. Biochimie. 2011;93:18–31. doi: 10.1016/j.biochi.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Eisenhower F. Myristoylation of viral and bacterial proteins. Trends Microbiol. 2004;12:178–185. doi: 10.1016/j.tim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Gouda M, Novatchkova M, Schleiffer A, Schneider G, Sirota FL, Wildpaner M, Hayashi N, Eisenhaber F. MYRbase: analysis of genome-wide glycine myristoylation enlarges the functional spectrum of eukaryotic myristoylated proteins. Genome Biol. 2004;5:R21. doi: 10.1186/gb-2004-5-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti L, Cacciari E, Cicognani A, Bergamaschi R, Scarano E, Forabosco A. Noonan-like syndrome with loose anagen hair: a new syndrome? Am J Med Genet A. 2003;118A:279–286. doi: 10.1002/ajmg.a.10923. [DOI] [PubMed] [Google Scholar]
- Ntwasa M, Aapies S, Schiffmann DA, Gay NJ. Drosophila embryos lacking N-myristoyltransferase have multiple developmental defects. Exp Cell Res. 2001;262:134–144. doi: 10.1006/excr.2000.5086. [DOI] [PubMed] [Google Scholar]
- Panethymitaki C, Bowyer PW, Price HP, Leatherbarrow RJ, Brown KA, Smith DF. Characterisation and selective inhibition of myristoyl CoA:protein N-myristoyl transferase from Trypano-soma brucei and Leishmania major. Biochem J. 2006;396:277–285. doi: 10.1042/BJ20051886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price HP, Menon MR, Panethymitaki C, Goulding D, McKean PG, Smith DF. Myristoyl-CoA:protein N-myristoyltransferase, an essential enzyme and potential drug target in kinetoplastid parasites. J Biol Chem. 2003;278:7206–7214. doi: 10.1074/jbc.M211391200. [DOI] [PubMed] [Google Scholar]
- Rajala RVS, Kakkar R, Kanthan R, Radhi JM, Wang X, Wang R, Datla RSS, Sharma RK. Altered expression and localization of N-myristoyltransferase in experimentally induced rat model of ischemia-reperfusion. J Cell Biochem. 2002;86:509–519. doi: 10.1002/jcb.10248. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochem Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Resh MD. Trafficking and signaling by fatty-acylated and prenylated protein. Nat Chem Biol. 2006;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- Sakurai N, Utsumi T. Posttranslational N-myristoylation is required for the anti-apoptotic activity of human tGelsolin, the C-terminal caspase cleavage product of human gelsolin. J Biol Chem. 2006;281:14288–14295. doi: 10.1074/jbc.M510338200. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Selvakumar P, Shrivastav A, Dimmock JR, Sharma RK. N-myristoyltransferase: a novel molecular target for cancer. Prog Lipid Res. 2007;46:1–36. doi: 10.1016/j.plipres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Shrivastav A, Varma S, Saxena A, Decoteau J, Sharma RK. N-myristoyltransferase: a potential novel diagnostic marker for colon cancer. J Transl Med. 2007;5:58. doi: 10.1186/1479-5876-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav A, Varma S, Lawman Z, Yang SH, Ritchie SA, Bonham K, Singh SM, Saxena A, Sharma RK. Requirement of N-myristoyltransferase 1 in the development of monocytic lineage. J Immunol. 2008;180:1019–1028. doi: 10.4049/jimmunol.180.2.1019. [DOI] [PubMed] [Google Scholar]
- Shrivastav A, Suri SS, Mohr R, Janardhan KS, Sharma RK, Singh B. Expression and activity of N-myristoyltransferase in lung inflammation of cattle and its role in neutrophil apoptosis. Vet Res. 2010;41:9. doi: 10.1051/vetres/2009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski JA, Devadas B, Zupec ME, Freeman SK, Brown DL, Lu HF, Nagarajan S, Mehta PP, Wade AC, Kishore NS, Bryant ML, Getman DP, McWherter CA, Gordon JI. Selective peptidic and peptidomimetic inhibitors of Candida albicans myristoyl-CoA:protein N-myristoyltransferase: a new approach to antifungal therapy. Biopolymers. 1997;43:43–71. doi: 10.1002/(SICI)1097-0282(1997)43:1<43::AID-BIP5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Termuhlen PM, Curley SA, Talamonti MS, Saboorian MH, Gallick GE. Site-specific differences in pp 60c-src activity in human colorectal metastases. J Surg Res. 1993;54:293–298. doi: 10.1006/jsre.1993.1046. [DOI] [PubMed] [Google Scholar]
- Utsumi T, Sakurai N, Nakano K, Ishisaka R. C-terminal 15 kDa fragment of cytoskeletal actin is posttranslationally N-myristoylated upon caspase-mediated cleavage and targeted to mitochondria. FEBS Lett. 2003;539:37–44. doi: 10.1016/s0014-5793(03)00180-7. [DOI] [PubMed] [Google Scholar]
- Vilas GL, Corvi MM, Plummer GJ, Seime AM, Lambkin GR, Berthiaume LG. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc Natl Acad Sci USA. 2006;103:6542–6547. doi: 10.1073/pnas.0600824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TK, Steele G, Summerhayes IC. Differential pp 60c-src activity in well and poorly differentiated human colon carcinomas and cell lines. J Clin Invest. 1992;90:815–821. doi: 10.1172/JCI115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA, McWherter CA, Freeman SK, Wood DC, Gordon JI, Lee SC. Genetic studies reveal that myristoylCoA:protein N-myristoyltransferase is an essential enzyme in Candida albicans. Mol Microbiol. 1995;16:241–250. doi: 10.1111/j.1365-2958.1995.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Wilcox C, Hu JS, Olson EN. Acylation of proteins with myristic acid occurs cotranslationally. Science. 1987;238:1275–1278. doi: 10.1126/science.3685978. [DOI] [PubMed] [Google Scholar]
- Wright MH, Heal WP, Mann DJ, Tate EW. Protein myristoylation in health and disease. J Chem Biol. 2010;3:19–35. doi: 10.1007/s12154-009-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Shrivastav A, Kosinski C, Sharma RK, Chen MH, Berthiaume LG, Peters LL, Chuang PT, Young SG, Bergo MO. N-myristoyltransferase 1 is essential in early mouse development. J Biol Chem. 2005;280:18990–18995. doi: 10.1074/jbc.M412917200. [DOI] [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targetting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]