Abstract
A series of 1,3-diaryl-2-propenones 2a–j and analogous 2-benzylidene-1,3-indandiones 3a–j were evaluated against various neoplasms and normal cells. In general, greater cytotoxic potencies and selective toxicity to human malignant cells were observed by the compounds in series 2 rather than 3. In particular, 2i emerged as a lead molecule having an average CC50 figure of 8.6 μM and a selective index value of 18. Various physicochemical features of 2a–j were correlated with the cytotoxic potencies to neoplastic cell lines which provide guidelines for expansion of this series of compounds. The enone 2i induced internucleosomal DNA fragmentation and activated caspase-3 in HL-60 cells suggesting that one of the ways in which the cytotoxicity of the compounds in series 2 is mediated towards some of the cell lines used in this study is by apoptosis. Neurotoxicity in mice was generally lower in series 2 than 3a–j.
Keywords: Apoptosis, Cytotoxicity, Neurotoxicity, Structure-activity relationships, Unsaturated ketones
Introduction
The principal interest of this laboratory is the design, syntheses, and bioevaluations of candidate cytotoxins. These compounds are generally conjugated styryl ketones which have an affinity for thiols but are either inert or far less reactive towards amino and hydroxy groups [1]. Hence, interactions with nucleic acids should be absent thereby avoiding the genotoxic side effects displayed by a number of contemporary anticancer drugs [2]. In addition, a number of α,β-unsaturated ketones such as chalcones are multitargeted ligands [3] which will minimize the likelihood of tumors developing resistance to these compounds. This concept is important when one considers that there are a number of dysregulated processes occurring in neoplastic cells and anti-cancer agents should have pleiotropic capabilities as has been emphasized recently [4, 5]. Naturally in using these compounds there has to be a balance between the chemical reactivity of an enone and a positive therapeutic outcome. For example, if the candidate cytotoxin is highly chemically reactive, the compound may fail to reach its site of action, i. e., it is sequestered to off-target binding sites. Alternatively, if it is somewhat chemically inert, then only a weak biological response will be observed. One may note that different enones have proven to be of value in chemotherapy, e. g., steroids such as prednisone and diuretics exemplified by ethacrynic acid.
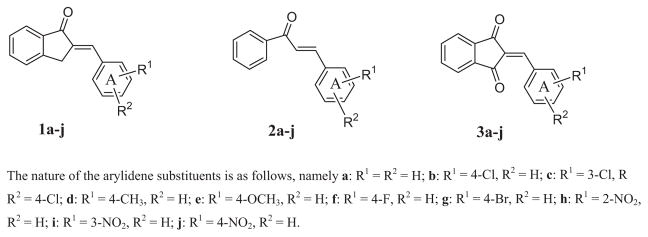
Several years ago, a series of 2-benzylidene-1-indanones 1 were evaluated against human Molt 4/C8 and CEM T-lymphocytes and murine L1210 leukemic cells [6, 7]. The compounds have moderate cytotoxic potencies with approximately half of the compounds possessing IC50 values of less than 100 μM. The decision was made to prepare two series of compounds which are structurally related to 1a–j which may have increased cytotoxic potencies. First, the acyclic chalcones 2a–j have the capacity for greater flexibility than the analogs in series 1. Hence 2a–j may assume one or more conformations which cause increases in antineoplastic potencies. Second, since thiols are considered to interact at the olefinic carbon atom adjacent to ring A, the insertion of a second electron-withdrawing group may increase the electrophilicity of these molecules towards cellular thiols. Such considerations led to the decision to prepare 3a–j. The structures of the compounds in series 1-3 are presented in Fig. 1. Evaluation of series 2 and 3 in the Molt 4/C8, CEM, and L1210 bioassays revealed that the order of potencies was 2 > 3 > 1 and in particular the IC50 figures of 2a–j towards the human cells are almost invariably in the low micromolar range [8].
Figure 1.
The structures of the compounds in series 1–3.
In view of the promising results of the chalcones 2 and the 2-benzylidene-1,3-indandiones 3, further experimentation was planned which would determine whether future development of one or both series of compounds is warranted. First, the most important issue is whether 2a–j and 3a–j display selective toxicity to neoplasms rather than normal cells. Second, an investigation was considered with the view of determining, at least in part, how cytotoxicity is mediated. Third, expansion of either series is dependent on the compounds being well tolerated in mice. In summary therefore, the purpose of this communication is to report on these three investigations, namely probing for selective toxicity, undertaking mode of action studies, and evaluating whether murine toxicity occurs.
Results
The synthesis of 2a–j and 3a–j has been described previously [8]. All of the compounds in series 2 and 3 were evaluated against HL-60 human promyelocytic leukemic cells as well as HSC-2 and HSC-4 human oral squamous cell carcinoma. The chalcones 2a–j were also assessed against HSC-3 human oral squamous cell carcinoma. In addition, assessment was made using the following normal human cells viz. HGF gingival fibroblasts, HPC pulp cells, and HPLF peridontal ligament fibroblasts. These data are presented in Table 1. A representative compound 2i induced apoptosis in HL-60 cells but its cytotoxicity towards the HSC-2 cell line appears to be by non-apoptotic mechanisms. Doses of 30, 100, and 300 mg/kg of the compounds in series 2 and 3 were injected intraperitoneally into mice and the animals were examined after 0.5 and 4 h for lethality and neurotoxic symptoms.
Table 1.
Evaluation of 2a–j and 3a–j towards some malignant and normal cells.
| Compound | Normal Cells
|
Malignant Cells
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC50 (μM)a
|
HL-60
|
HSC-2
|
HSC-4
|
HSC-3
|
||||||||
| HGF | HPC | HPLF | Ave | CC50 (μM)a | SIb | CC50 (μM)a | SIb | CC50 (μM)a | SIb | CC50 (μM)a | SIb | |
| 2a | 37 | 40 | 52 | 43 | 10 | 4.3 | 15 | 2.9 | 8.9 | 4.8 | 12 | 3.6 |
| 2b | 49 | 78 | 49 | 59 | 9.4 | 6.3 | 21 | 2.8 | 10 | 5.9 | 16 | 3.7 |
| 2c | 39 | 43 | 27 | 36 | 5.2 | 6.9 | 7.6 | 4.7 | 3.1 | 12 | 5.2 | 6.9 |
| 2d | 55 | 72 | 45 | 57 | 12 | 4.8 | 25 | 2.3 | 11 | 5.2 | 12 | 4.8 |
| 2e | 56 | 79 | 70 | 68 | 15 | 4.5 | 20 | 3.4 | 14 | 4.9 | 26 | 2.6 |
| 2f | 46 | 65 | 68 | 60 | 11 | 5.5 | 14 | 4.3 | 11 | 5.5 | 17 | 3.5 |
| 2g | 83 | 174 | 52 | 103 | 5.9 | 18 | 12 | 8.6 | 9.1 | 11 | 13 | 7.9 |
| 2h | 28 | 39 | 33 | 33 | 9.0 | 3.7 | 8.8 | 3.8 | 6.1 | 5.4 | 9.3 | 3.6 |
| 2i | 144 | 84 | 200 | 143 | 7.0 | 20 | 12 | 12 | 5.8 | 25 | 9.4 | 15 |
| 2j | 48 | 137 | 50 | 78 | 11 | 7.1 | 13 | 6.0 | 5.3 | 15 | 11 | 7.1 |
| 3a | 73 | 34 | 148 | 85 | 11 | 7.7 | 40 | 2.1 | 47 | 1.8 | – | – |
| 3b | 56 | 40 | >400 | >165 | 8.7 | >19 | 21 | >7.9 | 65 | >2.5 | – | – |
| 3c | 41 | >400 | >400 | >280 | 156 | >1.8 | >400 | ~ 0.7 | >400 | ~ 0.7 | – | – |
| 3d | 185 | 75 | 282 | 181 | 34 | 5.3 | 43 | 4.2 | 94 | 1.9 | – | – |
| 3e | 138 | 176 | >400 | >238 | 65 | >3.7 | 141 | >1.7 | 194 | >1.2 | – | – |
| 3f | 100 | 41 | 179 | 107 | 20 | 5.4 | 33 | 3.2 | 34 | 3.2 | – | – |
| 3g | 47 | 29 | 386 | 154 | 21 | 7.3 | 26 | 5.9 | 66 | 2.3 | – | – |
| 3h | 31 | 11 | 46 | 29 | 7.8 | 3.7 | 22 | 1.3 | 18 | 1.6 | – | – |
| 3i | 14 | >400 | >400 | >271 | 23 | >12 | 31 | >8.7 | >400 | >0.7 | – | – |
| 3j | 23 | >400 | >400 | >274 | 40 | >6.9 | 74 | >3.7 | 119 | >2.3 | – | – |
| Melphalanc | >200 | >200 | >200 | >200 | 6 | >33 | 35 | >5.7 | 81 | >2.5 | 115 | >1.7 |
The CC50 figure is the concentration of the compound required to kill 50% of the cells. It is the average of two independent experiments which differed by less than 5%;
The letters SI indicate the selectivity index;
The data were previously reported ([28]; copyright (2007) with permission of Elsevier). Solubility problems with melphalan precluded using concentrations higher than 200 μM.
Discussion
Three aspects of the biodata presented in Table 1 will be discussed, namely, first, the cytotoxicity towards malignant cells, second, the selective toxicity to neoplasms and third, the identification of lead molecules based on cytotoxic potencies and preferential lethality for malignant cells.
First, the evaluation of the cytocidal effects of each compound in series 2 and 3 is presented in Table 1. The percentage of compounds having CC50 figures below 10 μM towards HL-60, HSC-2, and HSC-4 cells in series 2 and 3 are 43 and 7, respectively, suggesting that in general greater cytotoxic potencies reside in series 2. This viewpoint was confirmed by considering the average CC50 values towards these three cell lines which are presented in Table 2. In particular, the greater than 17-fold difference in average potency between series 2 and 3 towards HSC-4 cells is noteworthy. In terms of identifying a lead compound, 2c has the lowest CC50 values towards each of the four malignant cell lines. In fact, this enone has 1.2, 4.6, 26, and 22 times the potency of melphalan in the HL-60, HSC-2, HSC-4, and HSC-3 screens, respectively.
Table 2.
A summary of the cytotoxicity and selectivity of 2a–j and 3a–j.
| Series | Average CC50 values (μM)
|
Average SI figures
|
||||||
|---|---|---|---|---|---|---|---|---|
| HL-60 | HSC-2 | HSC-4 | HSC-3 | HL-60 | HSC-2 | HSC-4 | HSC-3 | |
| 2 | 9.6 | 14.8 | 8.4 | 13.1 | 8.1 | 5.1 | 9.5 | 5.9 |
| 3 | 38.7 | >83.1 | >144 | – | >7.3 | >3.9 | >1.8 | – |
A physicochemical evaluation of the cytotoxicity data was confined to the more active series of compounds, namely 2a–j. In this case, linear and semilogarithmic plots were made between the Hammett σ, Taft σ*, Hansch π, and MR constants of the substituents in ring A with the CC50 figures in the HL-60, HSC-2, HSC-4, and HSC-3 bioassays. In this way, correlations may emerge between the electronic, hydrophobic, and steric parameters of the aryl substituents and potencies which would be of considerable value in providing guidelines for further development of these compounds. A negative correlation was noted between the σ/σ* alues and the CC50 figures generated in the HSC-2, HSC-4, and HSC-3 bioassays (p < 0.05). A negative trend towards significance was noted in the HL-60 screen (p < 0.1). In addition, a negative trend to significance was observed between the CC50 values and both the π constants in the HL-60 test and the MR figures in the HSC-4 screen (p < 0.1). No other correlations were noted (p > 0.1). The conclusion drawn is that in developing these compounds further, substituents should be placed in ring A which are highly electron-withdrawing.
Second, the issue of whether the compounds in series 2 and 3 display preferential toxicity towards neoplasms rather than normal cells was addressed. In cancer patients a malignancy is surrounded by a variety of normal cells. Hence, in order to simulate clinical conditions, selectivity was determined as the quotient of the average CC50 values for the three normal cell lines and the CC50 figure against a neoplastic cell line. This process yielded selectivity index (SI) values which are presented in Table 1. The results obtained reveal that SI figures of greater than 1 in 96% of the determinations made. Substantial increases in the selective toxicity for malignant cells were found in 44% of the evaluations made in which SI values of 5 or more were noted. However, in order to identify lead molecules, SI figures of 10 or more were set as the criterion and achieved by the following compounds (cell line in parentheses), namely 2c (HSC-4), 2g (HL-60, HSC-4), 2i (HL-60, HSC-2, HSC-4, HSC-3), 2j (HSC-4), 3b (HL-60) and 3i (HL-60), i. e., in 14% of the evaluations.
A noteworthy observation is the fact that 2i has SI figures of 20, 12, 25, and 15 against HL-60, HSC-2, HSC-4, and HSC-3 cells, respectively. It is intriguing to observe that while this compound, which possesses a 3-nitro substituent, has similar cytotoxic potencies as the 2-nitro (2h), and 4-nitro (2j) structural isomers, the average SI values of 2h, 2i, and 2j against these four cell lines are markedly divergent, namely 4.1, 18, and 8.8, respectively. Thus optimal selectivity was noted in the sequence of meta- > para- > ortho-substitution. Although potency differences are found among 3h–j, the average SI values of 2.2, >7.1, and >4.3, respectively, also reveal a meta- > para- > ortho-relationships in terms of selective toxicity to malignant cells. The π and MR values of the ortho-, meta-, and para-nitro aryl substituents are identical [9] while the σ* and σ constants are similar being 0.97 [10], 0.71 [9], and 0.78 [9], respectively. However, in view of the importance of the torsion angle θ between an aryl ring and an adjacent unsaturated group in affecting biological potencies [11], models of 2h–j and 3h–j were made and the θ values determined. The θ figures for 2h–j are −39.7, −27.3, and −26.5°, respectively, and in the case of 3h–j, the torsion angles are −53.8, −48.1, and −46.5°, respectively. While no straightforward correlation between θ angles and SI figures emerge, the possibility exists that the 3-nitro and 4-nitro structural isomers possessing smaller θ angles than 2h and 3h give rise to greater selectivity. It is conceivable therefore that the position of the nitro groups in ring A produces different conformations of the arylidene aryl ring which affects its alignment at one or more receptors.
The SI values in Table 1 reveal that in general greater selective toxicity is found in series 2 than with the 1,3-indan-diones 3. This observation is summarized in Table 2. Hence, attempts at finding correlations between various structural features and selectivity were confined to series 2. Linear and semilogarithmic plots were made between the σ/σ*, π, and MR values of the groups in ring A with the SI figures. However, only a positive trend towards significance was observed between the σ/σ* constants and CC50 figures in the HSC-4 screen (p < 0.1). No other correlations were noted (p > 0.1).
Third, in order to detect lead compounds which have potent tumor-specific toxicity, the biodata presented in Table 1 was examined for those compounds which achieve PL10 status. This criterion refers to a promising lead compound which has an average CC50 value of 10 μM or less and a mean SI figure of 10 or more. The information in Table 1 indicates that both 2g and 2i achieve PL10 status. The average CC50 values and mean SI figures regarding the four malignant cell lines for 2g are 10 and 11 μM, respectively, and in the case of 2i, the relevant figures are 8.6 and 18 μM, respectively. Clearly, both molecules, and 2i in particular, are important leads for further development.
The PL10 figures afford a general picture of compounds which are markedly cytotoxic having preferential lethality for tumors in contrast to normal cells. However, the PL10 concept may also be applied to those compounds which have favorable toxicity and selective indices to a particular malignant cell line. In other words, a compound may not display significant cytotoxicity or selectivity in general but it may be a very useful compound against a specific tumor. Hence, the term PL10D (where D indicates a distinct cell line) is used in relation to the CC50 and SI values of the compounds in series 2 and 3 generated against each cell line. The following compounds achieved PL10D status (the specific bioassay is indicated in parentheses) viz. 2c (HSC-4), 2g (HL-60, HSC-4), 2i (HL-60, HSC-2, HSC-4, HSC-3), 2j (HSC-4), 3b (HL-60), and 3i (HL-60). In general, 2c, g, i, j have groups in ring A which are the most electron-withdrawing and have the largest MR values in series 2 which may contribute to their favorable bioactivities.
One may summarize the biodata generated for series 2 and 3 in regard to potency and selective toxicity as follows. (1) In general, greater potencies are found in series 2 than 3 which may have been due to the acyclic nature of chalcones which permits a greater variety of conformations to take place than is possible with the more rigid 1,3-indandiones. This property of the compounds in series 2 may also contribute to the greater toxicity displayed towards neoplastic cells, i. e., this conformational flexibility permits greater interactions at binding sites and/or causes a larger number of interactions with cellular thiols than occurs with 3a–j. (2) In the case of 2a–j, potency towards the three HSC cell lines rose as the magnitude of the electron-withdrawing properties of the substituents in ring A increased. In other words, a decrease in the atomic charges on the olefinic carbon atom adjacent to ring A led to an increase in the chemical reactivity of the chalcones 2 towards cellular thiols. This observation is consistent with the hypothesis that an important mechanism of action of series 2 is by thiol alkylation. Thus, in the future even greater cytotoxic potency may be achieved in chalcones by placing multiple strongly electron-withdrawing groups in ring A as well as the introduction of these substituents in the aryl ring adjacent to the carbonyl group. (3) Some trends to significance were noted in series 2 that potency was raised as both the lipophilicity and size of the groups in ring A increased. This result may be due to increasing the facility of the molecules to be transported across cell membranes while the increase in the size of the groups in ring A may assist the binding of the compounds to receptors in neoplastic cells.
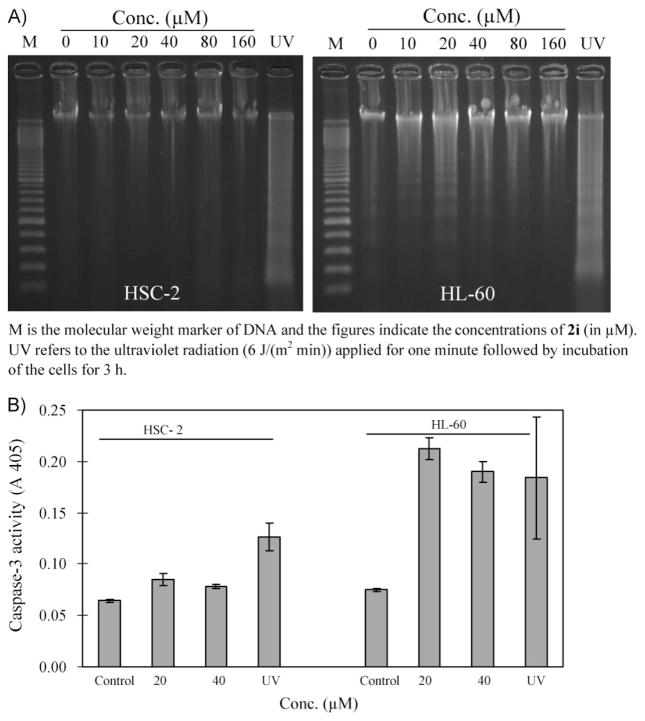
An investigation was undertaken to determine the manner in which a lead compound 2i exerts its lethal effects on malignant cells. Cytotoxic compounds can cause death to neoplasms by a variety of biochemical mechanisms including the induction of apoptosis. In order to explore this possibility, the effect of 2i on the ability to cause DNA fragmentation in HSC-2 and HL-60 cells was investigated. The result portrayed in Fig. 2A reveals that this process occurs in HL-60 cells. Apoptosis can also be caused by activation of caspases and Fig. 2B reveals that 2i causes the activation of caspase-3 in HL-60 cells. However, in HSC-2 cells this compound does not induce DNA fragmentation and activation of caspase-3 is only marginal. Hence, one may conclude that 2i causes cell death in HL-60 cells, at least in part, by apoptosis whereas the toxicity of 2i to HSC-2 cells is mediated by non-apoptotic mechanisms.
Figure 2.
A. Evaluation of 2i on the induction of DNA fragmentation in HSC-2 and HL-60 cells after 6 h of incubation. B. The effect of 2i on the induction of caspase-3 in HSC-2 and HL-60 cells after 6 h of incubation.
An important property of candidate antineoplastics is their mammalian toxicity. A number of anticancer agents are lethal at low doses, e. g., the LD50 figure of melphalan is 4.0 mg/kg in mice [12]. In addition, a number of cytotoxins which contain the 3-aryl-2-propenoyl group (ARCH = CHCO) have neurotoxic properties [13, 14]. Hence, all of the compounds in series 2 and 3 were examined for both lethality and neurotoxicity in a short-term assay in rodents. Doses of 30, 100, and 300 mg/kg of 2a–j and 3a–j were administered intraperitoneally to mice and the animals examined after 0.5 and 4 h. No mortalities were noted. Utilization of the rotorod procedure revealed that after 0.5 h, 2g, j and 3a, c, e, f, g, i, j demonstrated neurotoxicity (specific details of the doses are presented in the experimental section). After 4 h, 2a, d, i and 3c, e, i displayed neurological deficit. Five chalcones, namely 2b, c, e, f, h do not display neurotoxic symptoms whereas only three 1,3-indandiones viz. 3b, d, h are free from this side effect. Furthermore in general neurotoxicity was observed at lower doses with various compounds in series 3 than with the chalcones 2. Doses of 30–50 mg/kg of three representative compounds 2a, f, and 3h were given orally to rats and over the time frame of 0.25–4 h no neurotoxicity was noted. In the case of 2f, a large dose of 500 mg/kg was administered per os and the rats were observed at various time periods up to and including 24 h. No neurological deficit was seen at any time period. One may conclude, therefore, that both series 2 and 3 are well tolerated in rodents in general. However, the chalcones 2 have fewer neurological problems than the analogues in series 3.
Conclusions
This study revealed that most of the 1,3-diaryl-2-propenones 2 and 2-benzylidene-1,3-indandiones 3 possess cytotoxic properties to several malignant cell lines and also display preferential toxicity to various neoplasms rather than normal cells. In particular, 2g and 2i are impressive PL10 lead molecules. The enone 2i causes apoptosis in HL-60 cells and by other means in HSC-2 cells. Doses up to and including 300 mg/kg of compounds in both series did not prove to be lethal to mice when observed over a 4 h time period. In terms of future development, the emphasis will be on series 2 which, in general, are more potent cytotoxins, have greater selective toxicity for malignant cells, and display less neurotoxicity than the analogs 3a–j. The correlations noted between various physicochemical constants in series 2 and potency enable guidelines for analog development to be made.
Thus, this study outlining the selective toxicity of chalcones for neoplasms compared to non-malignant cells, coupled to the cytotoxic potencies of certain members of series 2, affords further evidence that chalcones are a cluster of small organic molecules that have very good potential for development as anticancer agents. Currently there is considerable interest in chalcones, not only as antineoplastics [3, 15–17] but also for their antiangiogenic properties [18, 19]. Chalcones exert their cytotoxicity in different ways such as by blocking the cell cycle [19] and acting as antimitotic agents [20, 21]. In addition, these enones are often well tolerated by mammals and are generally easy to synthesize. Hence, further work in this area is required with a view to increasing the chemical arsenal in the warfare against cancer.
Experimental
Chemistry
The preparation of the compounds in series 2 and 3 has previously been reported [8].
Quantitative structure-activity relationships
The σ, π, and molar refractivity (MR) values were obtained from the literature [9] while the σ* figure was culled from an appropriate reference [10]. The MR value of hydrogen is 1.03 not 0.00. Hence, 1.03 was added to the MR constants of the monosubsti-tuted compounds and the MR value of the unsubstituted analog is 2.06. Linear (l) and semilogarithmic (sl) plots were constructed between different physicochemical constants of the aryl substituents in series 2 and the CC50 values generated in the HL-60, HSC-2, HSC-3, and HSC-4 bioassays using a commercial software package [22]. The Pearson’s coefficients and p values are as follows, namely σ/σ*: HL-60: −0.570, 0.085 (l), −0.516, 0.127 (sl); HSC-2: −0.754, 0.012 (l), −0.766, 0.0l0 (sl); HSC-3: −0.695, 0.026 (l), −0.680, 0.030 (sl); HSC-4: −0.871, 0.001 (l), −0.804, 0.005 (sl);π: HL-60: −0.484, 0.156 (l), −0.554, 0.096 (sl); HSC-2: 0.004, 0.991 (l), −0.095, 0.793 (sl); HSC-3: −0.232, 0.518 (l), −0.330, 0.351 (sl); HSC-4: −0.112, 0.758 (l), −0.212, 0.556 (sl); MR: HL-60: −0.409, 0.241 (l), −0.503, 0.138 (sl); HSC-2: −0.310, 0.384 (l), 0.398, 0.255 (sl); HSC-3: −0.263, 0.463 (l), −0.416, 0.232 (sl); HSC-4: −0.439, 0.204 (l), −0.529, 0.116 (sl).
Linear and semilogarithmic plots were also constructed between different physicochemical constants in ring A of 2a–j and the selectivity index (SI) figures obtained. The Pearson’s coefficients and p values generated are as follows, namely σ/σ*: HL-60: 0.261, 0.466 (l), 0.262, 0.465 (sl); HSC-2: 0.454, 0.188 (l), 0.524, 0.120 (sl); HSC-3: 0.476, 0.164 (l), 0.522, 0.121 (sl); HSC-4: 0.563, 0.090 (l), 0.606, 0.063 (sl); π: HL-60: 0.050, .890 (l), 0.147, 0.686 (sl); HSC-2: −0.163, 0.653 (l), −0.136, 0.709 (sl); HSC-3: −0.076, 0.835 (l), 0.073, 0.841 (sl); HSC-4: −0.153, 0.673 (l), −0.011, 0.977 (sl); MR: HL-60: 0.342, 0.333 (l), 0.376, 0.285 (sl); HSC-2: 0.338, 0.340 (l), 0.382, 0.276 (sl); HSC-3: 0.372, 0.289 (l), 0.436, 0.208 (sl); HSC-4: 0.405, 0.245 (l), 0.509, 0.133 (sl). Models of 2h-j and 3h-j were built using Sybyl 8.0 software. Powell minimization methods were applied to a gradient value of 0.05 using the Tripos force field.
Cytotoxicity assays
The bioevaluation of 2a–j, 3a–j, and melphalan using HL-60, HSC-2, HSC-4, HSC-3, HGF, HPC, and HPLF cells was undertaken by a literature procedure [23]. In brief, different concentrations of a compound were added to a cell line and incubated for 24 h at 37°C in a 5% CO2 incubator. Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method and the CC50 value was determined from a dose-response curve [24].
Effect of 2i on DNA fragmentation and activation of caspase-3 in HSC-2 and HL-60 cells
The assays for evaluating compounds for DNA fragmentation and for caspase-3 activation in malignant cells have been presented previously [25].
Evaluation of 2a–j and 3a–j for mortalities and neurotoxicity
The examination of the tolerability of mice to the compounds in series 2 and 3 in regard to mortality and neurotoxicity was conducted by the National Institute of Neurological Disorders and Stroke, USA, according to their protocols [26]. Doses of 30, 100, and 300 mg/kg of each compound were administered intraperitoneally to mice and the animals were examined at the end of 0.5 and 4 h. No deaths were observed.
Neurotoxicity was measured by the rotorod procedure [27]. The number of animals evaluated 0.5 h after administration of doses of 30, 100, and 300 mg/kg of each compound are 4, 8, and 4 mice, respectively. The number of mice examined 4 h after administration of the compounds in series 2 and 3 are 2, 4, and 2, respectively. For example, in the case of 2a, the number of animals displaying neurotoxicity using doses of 30, 100, and 300 mg/kg are 0/4, 0/8, 0/4 (after 0.5 h) and 0/2, 0/4, 1/2 (after 4 h). No neurotoxicity was observed with 2b, c, e, f, h and 3b, d, h. In the remaining cases, some neurotoxicity was noted at specific doses and time periods which are as follows. Neurological deficit was displayed 0.5 h after administration of the following compounds (dose in mg/kg, number of mice displaying neurotoxicity/number of mice evaluated, in parentheses) viz. 2g (300, 1/4), 2j (300, 1/4), 3a (300, 1/4), 3c (100, 1/8; 300, 1/4), 3e (100, 1/8), 3f (300, 1/4), 3g (30, 2/4; 100, 2/8; 300, 1/4), 3i (30, 2/4), and 3j (100, 2/8; 300, 1/4). After 4 h, neurotoxicity was caused by the following compounds, namely 2a (300, 1/2), 2d (300, 1/2), 2i (100, 1/4; 300, 1/2), 3c (100, 1/4), 3e (300, 1/2), and 3i (30, 1/2; 300, 1/2). In addition, two mice did not show neurological deficit 0.25 and 1 h after administration of 100 mg/kg of 2a.
The following compounds were administered orally to four rats (dose in mg/kg in parentheses), namely 2a (30), 2f (50), and 3h (50). The animals were examined after 0.25, 0.5, 1, 2, and 4 h. In addition, a dose of 500 mg/kg of 2f was administered per os to eight rats that were evaluated after 0.25, 0.5, 1, 2, 4, 6, 7.25 and 24 h. At all time periods, no mortalities or neurotoxicity were observed.
The animals were housed, fed, and handled according to the directives of the National Research Council publication “Guide for the Care and Use of Laboratory Animals”. Euthanasia was performed according to the procedures of the Institute of Laboratory Resources.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for a grant to J. R. Dimmock and the Ministry of Education, Science, Sports and Culture of Japan for a Grant-in-Aid (No. 19592156) to H. Sakagami. The National Institute of Neurological Disorders and Stroke, USA kindly undertook the in-vivo experimentation with mice and rats. Erin Watson is thanked for assisting with some of the literature retrieval.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Pati HN, Das U, Sharma RK, Dimmock JR. Mini Rev Med Chem. 2007;7:131–139. doi: 10.2174/138955707779802642. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Moore MJ. In: Principles of Medical Pharmacology. 7. Kalant H, Grant DM, Mitchell J, editors. Elsevier; Toronto: 2007. p. 778. [Google Scholar]
- 3.Dimmock JR, Elias DW, Beazely MA, Kandepu NM. Curr Med Chem. 1999;6:1125–1149. [PubMed] [Google Scholar]
- 4.Galanski M, Keppler BK. Anti-Cancer Agents Med Chem. 2007;7:55–73. doi: 10.2174/187152007779314017. [DOI] [PubMed] [Google Scholar]
- 5.Frantz S. Nature. 2005;437:942–943. doi: 10.1038/437942a. [DOI] [PubMed] [Google Scholar]
- 6.Dimmock JR, Kandepu NM, Nazarali AJ, Kowalchuk TP, et al. J Med Chem. 1999;42:1358–1366. doi: 10.1021/jm9806695. [DOI] [PubMed] [Google Scholar]
- 7.Dimmock JR, Zello GA, Oloo EO, Quail JW, et al. J Med Chem. 2002;45:3103–3111. doi: 10.1021/jm010559p. [DOI] [PubMed] [Google Scholar]
- 8.Pati HN, Das U, De Clercq E, et al. J Enzym Inhib Med Chem. 2007;22:37–42. doi: 10.1080/14756360600958057. [DOI] [PubMed] [Google Scholar]
- 9.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley and Sons; New York: 1979. p. 49. [Google Scholar]
- 10.Taft RW., Jr . In: Steric Effects in Organic Chemistry. Newman MS, editor. John Wiley and Sons; New York: 1956. p. 591. [Google Scholar]
- 11.Pandeya SN, Dimmock JR. An Introduction to Drug Design. New Age International (P) Limited; New Delhi: 1997. pp. 73–74. [Google Scholar]
- 12.Quinn FR, Milne GWA. Fundam Appl Toxicol. 1986;6:270–277. doi: 10.1016/0272-0590(86)90240-x. [DOI] [PubMed] [Google Scholar]
- 13.Dimmock JR, Gunda SGR, Vashishtha SC, et al. J Enzym Inhib Med Chem. 2004;19:303–313. doi: 10.1080/14756360409162442. [DOI] [PubMed] [Google Scholar]
- 14.Dimmock JR, Jha A, Zello GA, Allen TM, et al. Pharmazie. 2003;58:227–232. [PubMed] [Google Scholar]
- 15.Katson AM, Hadjipavlou D. Curr Med Chem. 2009;16:1062–1081. [Google Scholar]
- 16.Go ML, Wu X, Liu XL. Curr Med Chem. 2005;12:481–499. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- 17.Bandgar BP, Gawande SS, Bodade RG, Totre JV, Khobragade CN. Bioorg Med Chem. 2010;18:1364–1370. doi: 10.1016/j.bmc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 18.Mojzis JV, Varinska L, Mojzisova G, Kostova I, Mirossay L. Pharmacol Res. 2008;57:259–265. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Boumendjel A, Ronot X, Boutonnat J. Curr Drug Targets. 2009;10:363–371. doi: 10.2174/138945009787846416. [DOI] [PubMed] [Google Scholar]
- 20.Ducki S. Curr Med Chem. 2009;9:336–347. doi: 10.2174/1871520610909030336. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence NJ, McGown AT. Curr Pharm Des. 2005;11:1679–1693. doi: 10.2174/1381612053764733. [DOI] [PubMed] [Google Scholar]
- 22.Statistical Package for Social Sciences. SPSS for Windows, Release 14.0.0. SPSS Inc; Chicago: 2005. [Google Scholar]
- 23.Motohashi N, Wakabayashi H, Kurihara T, Fukushima H, et al. Phytother Res. 2004;18:212–223. doi: 10.1002/ptr.1426. [DOI] [PubMed] [Google Scholar]
- 24.Motohashi N, Wakabayashi H, Kurihara T, Yuko T, et al. Phytother Res. 2003;17:348–352. doi: 10.1002/ptr.1144. [DOI] [PubMed] [Google Scholar]
- 25.Sekine T, Takahashi J, Nishishiro M, Arai A, et al. Anticancer Res. 2007;27:133–144. [PubMed] [Google Scholar]
- 26.Stables JP, Kupferberg HJ. In: Molecular and Cellular Targets for Antiepileptic Drugs. Vanzini G, Tanganelli P, Avoli M, editors. John Libbey and Company Ltd; London: 1997. pp. 191–198. [Google Scholar]
- 27.Dunham MS, Miya TA. J Am Pharm Assoc Sci Ed. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 28.Das U, Kawase M, Sakagami H, Ideo A, et al. Bioorg Med Chem. 2007;15:3373–3380. doi: 10.1016/j.bmc.2007.03.022. [DOI] [PubMed] [Google Scholar]