Abstract
Background
Schizophrenia is associated with functional decoupling between cortical regions, but we do not know whether and where this occurs in low-frequency electromagnetic oscillations. The goal of this study was to use magnetoencephalography (MEG) to identify brain regions that exhibit abnormal resting-state connectivity in the alpha frequency range in patients with schizophrenia and investigate associations between functional connectivity and clinical symptoms in stable outpatient participants.
Method
Thirty patients with schizophrenia and fifteen healthy comparison participants were scanned in resting-state MEG (eyes closed). Functional connectivity MEGI (fcMEGI) data were reconstructed globally in the alpha range, quantified by the mean imaginary coherence between a voxel and the rest of the brain.
Results
In patients, decreased connectivity was observed in left pre-frontal cortex (PFC) and right superior temporal cortex while increased connectivity was observed in left extrastriate cortex and the right inferior PFC. Functional connectivity of left inferior parietal cortex was negatively related to positive symptoms. Low left PFC connectivity was associated with negative symptoms. Functional connectivity of midline PFC was negatively correlated with depressed symptoms. Functional connectivity of right PFC was associated with other (cognitive) symptoms.
Conclusions
This study demonstrates direct functional disconnection in schizophrenia between specific cortical fields within low-frequency resting-state oscillations. Impaired alpha coupling in frontal, parietal, and temporal regions is associated with clinical symptoms in these stable outpatients. Our findings indicate that this level of functional disconnection between cortical regions is an important treatment target in schizophrenia.
Keywords: magnetoencephalography, functional connectivity, resting-state, schizophrenia, neuroimaging, symptoms
Introduction
There is a growing recognition in psychiatry research that it is critical to move beyond a receptor-based molecular neuropharmacology approach to psychiatric illness, and to engage in neural systems-based paradigms for treatment development. A promising approach is to develop a deeper understanding of networks of oscillatory patterns that emerge from specific neural circuits in mental illness, their function and dysfunction, and their response to interventions (From Discovery to Cure, NIMH, August 2010). Indeed, emerging research indicates that neural rhythms are impoverished in schizophrenia, for example, and that they play a key role not only in symptoms but also in deficits of cognition and sensory processing (1-3).
In recent years, evidence has begun to accumulate that a core feature of schizophrenia may be “disconnectivity” between cortical regions—a provocative and ambiguous term. Functional disconnectivity is often referred to as reduced statistical dependence between neurophysiological time series of separate brain regions. Recent studies that have examined such neural interactions using fMRI have provided evidence for “disconnection” in schizophrenia, with aberrant, diminished neural interactions (primarily between temporal and pre-frontal cortical fields) observed across a range of cognitive and affective tasks (4-10). The disconnectivity hypothesis has also been tested by examining cortical oscillations in high temporal fidelity electroencephalogram (EEG) recordings, and by estimating correlations, coherence and phase synchronization in oscillatory activity between electrode sites during cognitively demanding tasks (11-13). However, due to the spatial restrictions of EEG (e.g. artifacts due to volume conduction, reference electrode placement), it is unclear which specific brain regions and cortical fields contribute to changes in electrode coherence recorded in these patients during behavior.
Functional neuroimaging studies during behavior can also be confounded by a number of methodological factors, including subject compliance during performance of the task; the exclusion of participants who are unable to perform a demanding behavior; and the averaging across multiple trials to produce an adequate signal for analyses (14). Furthermore, differences in experimental design across studies (task, scan parameters) can make it difficult to generalize behaviorally-based findings in order to establish fundamental properties of atypical neural system function specific to patients with schizophrenia. Instead, an emerging focus in the imaging literature has been on “resting-state” experimental designs, which are not dependent on subject compliance or on task-specific factors (14-16). Both resting-state fMRI studies (17-23) and spontaneous EEG recordings (24-27) have identified changes in functional connectivity in patients with schizophrenia. These studies support the hypothesis that alterations in interactions between cortical regions are persistent even in the absence of behavior.
It is clear that a core functional feature of neural ensembles is their oscillatory activity at various frequencies, and the manner in which this represents the coordination, integration, and transmission of important computations both within and across neural systems (28). This understanding of a fundamental neural network property indicates that the study of “functional disconnectivity” in schizophrenia should also focus on delineating the details of how neuronal oscillations diverge and relate to clinical manifestations in this illness (1,3,29).
The present study had two goals. First, we aimed to evaluate the changes in spontaneous cortical connectivity at rest within the alpha frequency range (8-12Hz) in clinically stable participants with schizophrenia using functional connectivity magnetoencephalographic imaging (fcMEGI). fcMEGI refers to functional connectivity analysis (fc) of source-space reconstructions of MEG sensor data (MEGI). Alpha band oscillations represent a stable idling rhythm in the alert brain (30) and are coherent at large distances (>10cm; 30,31) making them an ideal candidate for functional interactions between distant cortical fields. Changes in alpha oscillatory dynamics have also been discussed as a feature of the cortex in schizophrenia (32,33). We predicted that specific cortical fields in the frontal and temporal lobes would exhibit reduced levels of resting-state functional connectivity in the schizophrenia group, consistent with previous reports in the literature. Second, in order to investigate whether abnormal alpha oscillations in specific cortical sectors are functionally related to clinical presentation, we examined associations between these measures of electromagnetic resting-state functional connectivity and symptom severity. Our goal here was to examine whether impaired alpha-band interactions between brain regions are related to psychopathology in schizophrenia, suggesting novel neural systems-based treatment approaches. We focus specifically on alpha as it is both the dominant oscillation in spontaneous electro- and magnetoencephalogram recordings in ~95% of individuals (34), and overlaps with resting-state networks identified in functional MRI (35,36). Our objective was to isolate the cortical fields that are normally coupled in the alpha range but that are disconnected in patients with schizophrenia, and to examine how decoupling may relate to clinical symptoms.
Methods and Materials
Participants
Thirty clinically stable, persistently ill, volunteer schizophrenia (SZ) participants were recruited from community mental health centers (mean age=38.4 SD=11.1, 7 women). Fifteen healthy comparison (HC) participants matched to the schizophrenia group on age, gender, and education, were recruited from the community via advertisement (mean age=43 SD=12.2, 4 women). Inclusion criteria were: Axis I diagnosis of schizophrenia (Structured Clinical Interview for DSM-IV, SCID, 37) or, for healthy subjects, no Axis I or Axis II psychiatric disorder (SCID-NP, 37); for all subjects, no current/previous substance dependence; good general physical health; age 18-60 years; English as first language; outpatient status (at least 3 months); no significant medication changes (dosage change >10%) during the study. Secondary (comorbid) diagnoses were present in 4 SZ participants (3 with major depressive disorder, 1 dysthymia).
All participants underwent MEG as a neurophysiological assessment at baseline prior to entering a randomized controlled trial of neuroplasticity-based cognitive training in schizophrenia (ClinicalTrials.gov NCT00312962). The MEG scan session which included a battery of auditory tasks (38) followed by a resting-state MEG scan. All procedures were approved by the UCSF Committee on Human Research, and all experiments were conducted in accordance with the Declaration of Helsinki.
Diagnostic and Symptom Assessments
All SZ participants met standard diagnostic criteria for schizophrenia (SCID, 37) and received the following clinical symptom ratings using an extended version of the Positive and Negative Syndrome Scale (PANSS-E, 39): Positive, Negative, Disorganized, Depressed, Anxious, and Other symptoms. The PANSS-E consists of the 30-item PANSS (40) supplemented with 10 items from the Comprehensive Assessment of Symptoms and History (CASH; 41). Ratings were made along a 7-point scale (1= absent, 3= mild, 7= extreme; 43) and represent the consensus of two independent raters performed within two weeks of MEG scanning. In the SZ group, the mean rating on the positive subscale was 2.9 (SD=1.14), negative subscale was 2.8 (SD=0.79), depressed subscale was 3.2 (SD=1.11), disorganized subscale was 2.4 (SD=0.681), anxiety subscale was 1.8 (SD=0.647), and other symptoms was 2.5 (SD=0.78).
MRI Acquisition
Structural (T1-weighted) anatomical images were acquired for source space reconstruction, data visualization and second-level group analyses. Scanning was performed using a 3.0T GE Trio scanner. For each subject, a 3DSPRAGE high-resolution MRI was acquired (160 1mm slices; FOV = 260 mm, matrix = 256 × 256, TE = 6 ms, TR = 35 ms, flip angle = 30°).
Magnetoencephalogram Recording
Four minutes of continuous recording (awake, supine position, eyes closed) were collected from all subjects using a 275-channel whole-head MEG system (MISL, Coquitlam, BC) consisting of 275 axial gradiometers (sampling rate= 600Hz). Three fiducial coils (nasion, left/right preauricular points) were placed to localize the position of the head relative to the sensor array. These points were later co-registered to a T1-weighted MRI in order to generate the head shape. Any participant with excessive within-run head movement based on fiducial coil position (>1cm) or who reported sleeping or feeling sleepy during this scan (<10% of all participants) was re-run.
Data Analysis
Source-space MEG-I reconstructions and functional connectivity metrics were computed using the Nutmeg software suite (http://nutmeg.berkeley.edu; 42). MEG-I can improve both the spatial resolution and signal detection abilities of MEG and overcome limitations inherent to this methodology (e.g. low SNR, radial versus tangential dipole moments; 43) enabling precise reconstructions of oscillatory activity in specific brain regions from MEG data (42,44,45). From the four minute dataset, a 60s, artifact-free segment of the data were selected for analysis (46). Artifact detection was performed qualitatively through a visual inspection of the sensor data after being anonymized and broken into four 60s trials, and only trials without excessive scatter (signal amplitude > 10 pT) due to eyeblink, saccades, head movement or EMG noise were selected for MEGI analysis. MEG sensor data were filtered using a phase-preserving bandpass filter (fourth-order Butterworth; 1-20Hz bandpass) and reconstructed in source space using a minimum-variance adaptive spatial filtering technique (42,47). This approach provides an amplitude estimate at each element (voxel) derived through a linear combination of a spatial weighting matrix with the sensor data matrix. Tomographic reconstructions of the data were created by generating a multisphere head model based on a head shape obtained from each individual subject’s structural MRI. A volume of interest (whole brain VOI) for lead field computation (grid size=2cm; approx. 300 voxels/participant) was automatically generated through a back-transformation of all the points within a spatially normalized MRI that corresponded to locations within the brain and excluding non-cerebral points. Therefore, the timecourse of activity at each voxel used for functional connectivity was computed for every location within the VOI, where each voxel within the VOI itself is an estimate of activity derived from inputs from all sensor recordings
For each subject, alpha frequency bins were selected around a peak power density centered on ~10Hz during the 60s epoch, selected from a broad 1-20Hz band with a frequency resolution of 1.17Hz (512 bins, as in 46). While peaks in the power spectra corresponding to oscillations in other frequency ranges (e.g. theta, low beta) were occasionally identified from subject to subject, only alpha peaks (power density peak between 8-12Hz) were identified from this sampling window in all participants. Functional connectivity estimates were calculated using imaginary coherence (IC), a technique known to reduce overestimation biases in EEG/MEG data generated from common references, cross-talk and volume conduction (46,48). IC is able to sample interactions between source timeseries independent of the class of spatial filter used (46,49). Bivariate imaginary coherence values between two voxels (Ixy) within frequency window f were computed using the following:
Global connectivity (GC) at each voxel was estimated by averaging across a single voxel’s Fisher’s Z-transformed IC values between that voxel and all other voxels in the grid the remaining elements in the reconstruction (46,48).
In a separate cohort of 20 healthy control participants, test-retest reliability of GC maps was evaluated by calculating a fixed-effects average intra-class correlation coefficient (ICC; 50). Good test-retest reliability was verified in the GC maps for both within session (ICC=0.61) and between the baseline scan and a 2-8 week follow-up session (ICC=0.64).
Group Statistics
T1-weighted MRIs were spatially normalized (5mm; SPM2 www.fil.ion.co.uk/spm2) and the transformation matrix from the normalization was then applied to each individual subject’s GC map (GCM). Around 8500 voxels meeting cross-subject alignment were entered into the group analysis. Group contrasts (SZ vs. HC) were conducted using a non-parametric unpaired t-test (SnPM; 51). Average and variance maps were smoothed using a Gaussian kernel (20mm FWHM). Symptom rating scores from the PANSS were correlated with GC values at each voxel (Pearson’s r). All tests were corrected for multiple comparisons using a False Discovery Rate (FDR) modified for dependency (52). We report peak activity at 5% FDR correction whenever possible, although to identify some main effects we used a less stringent 10% FDR threshold (53-55).
Results
Prior to functional connectivity analysis, a broad alpha band range was selected within a 6-14Hz window around the peak frequency (8-12Hz) for each participant. No peak in the power spectrum was consistently identified for bands greater than 12Hz. No significant differences in alpha power were identified between the HC and SZ groups (p=0.36). Although the frequency peak within alpha is known to be lower in schizophrenia (32,33) no significant differences in alpha window size were identified between the two groups for either the highpass (HC=6.60Hz (SD=0.41), SZ=6.68Hz (SD=0.65); p=0.67) or lowpass (HC=13.01 (SD=0.59), SZ=13.16 (SD=0.92); p=0.55) alpha cut-offs. Neuroleptic treatments are not known to affect alpha peaks specifically (56). A voxelwise correlation between medication dosage (chlorpromazine equivalents) and resting-state functional connectivity measures (global IC) yielded no significant results (average Spearman’s rho=0.058).
Global Connectivity Maps
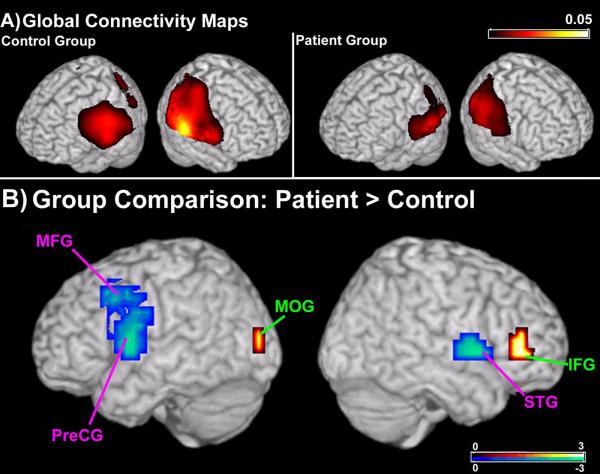
The measures derived from imaginary coherence give us an estimate of global functional connectivity at each voxel, or the mean connectivity at each voxel between that region and the rest of the brain (46). In the alpha range, robust global connectivity across functionally critical brain regions was present in both groups (Figure 1a). The areas that show the maximal global connectivity include regions of parietal, temporal and occipital cortices, including occipital and parietal regions along the midline such as the cuneus and pre-cuneus (Figure 1a).
Figure 1.
Global connectivity maps (GCMs) and data from a group comparison (non-parametric unpaired t-test) between patient and control GCMs. (A) In both the SZ and HC group, strong alpha imaginary coherence is seen in the GCMs in the posterior regions of the brain. (B) In patients with schizophrenia, greater connectivity (in red) is present in a region of the right inferior frontal gyrus (IFG) and left medial occipital gyrus (MOG). In addition, multiple cortical fields in the patient group had lower levels of functional connectivity (in blue). Connectivity of regions in the left frontal lobe, including cortex along the middle frontal gyrus (MFG) and pre-central gyrus (PreCG) were reduced in patients with schizophrenia. In the right hemisphere, connectivity of the superior temporal gyrus (STG) was significantly reduced in the patient group. Areas with greater connectivity in the control group are abbreviated in pink, areas with greater connectivity in the patient group are abbreviated in green. Statistical maps are thresholded and superimposed over a rendering of the MNI template brain through MRICro (http://www.sph.sc.edu/comd/rorden/mricro.html).
A direct comparison between the global connectivity maps in the SZ and HC groups (Figure 1b) reveals regions that show relative changes in connectivity patterns in patients with schizophrenia.
Increases in functional connectivity in the SZ group were restricted to a region of the occipital lobe, as well as right pre-frontal cortex (Figure 1b, in red). Greater global connectivity scores in the SZ patients (p<0.05, 10% FDR correction) were observed near the medial occipital gyrus (MOG; Figure 1b) in the left hemisphere in Brodmann’s Area (BA) 19 (Table 1). SZ subjects also showed an overall increase in connectivity in the right inferior frontal gyrus (IFG; Figure 1b), near BA45 (Table 1; p<0.05; 10% FDR).
Table 1.
| Region | Abbrev | Hemi | BA | x | y | z | p | T |
|---|---|---|---|---|---|---|---|---|
| Group Comparison | ||||||||
| Pre-Central Gyrus | Pre-CG | L | 6 | −55 | 5 | 10 | 0.008 | 2.57 |
| Middle Frontal Gyrus | MFG | L | 6/9 | −40 | 10 | 40 | 0.009 | 2.16 |
| Superior Temporal | ||||||||
| Gyrus | STG | R | 22 | 60 | 5 | 0 | 0.004 | 2.69 |
| Inferior Frontal Gyrus | IFG | R | 45 | 55 | 40 | 0 | 0.007 | 2.99 |
| Middle Occipital Gyrus | MOG | L | 19 | −40 | −90 | 10 | 0.007 | 2.55 |
| Positive Symptoms | r | |||||||
| Inferior Parietal Lobe | IPL | L | 40 | −60 | −35 | 25 | 0.002 | −0.55 |
| Negative Symptoms | ||||||||
| Middle Frontal Gyrus | MFG | L | 9/10 | −40 | 50 | 25 | 0.004 | −0.51 |
| Depressed Symptoms | ||||||||
| Anterior Cingulate | ||||||||
| Cortex | ACC | L/R | 32 | 5 | 30 | 30 | 0.002 | −0.54 |
| Other Symptoms | ||||||||
| Middle Frontal Gyrus | MFG | R | 8 | 40 | 25 | 50 | 0.002 | −0.54 |
Decreases in functional connectivity in the SZ group were found in multiple areas of the frontal and temporal lobes (Figure 1b, in blue). In the left hemisphere, decreased global connectivity values in SZ subjects were seen in BA6 and BA9 in left middle frontal gyrus (MFG; p<0.05, 10% FDR), and a region of left precentral gyrus extending ventrally to the upper bank of the sylvian fissure (Pre-CG; p<0.05, 10% FDR). In the right hemisphere, decreased connectivity (p<0.05, 10% FDR) was found over the superior temporal gyrus (STG; Table 1).
Correlation: Global Connectivity Measures and Clinical Symptom Severity
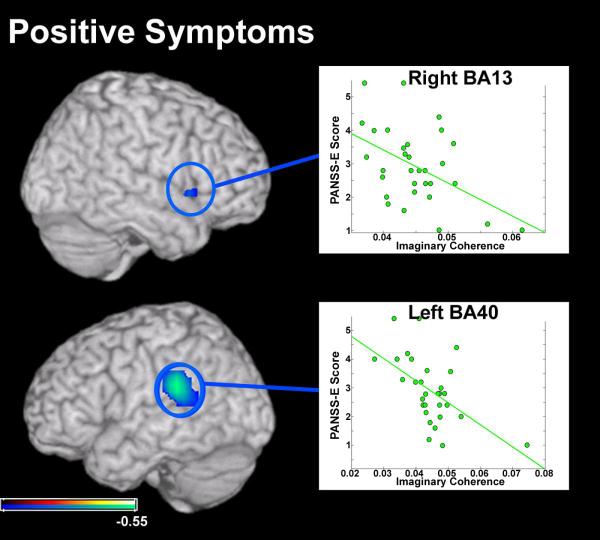
For ratings of positive symptoms, the SZ participants recruited for this study fell within the range of absent to moderate-severe (1-5.5) on the PANSS-E. A significant negative correlation was found between positive symptom ratings and global connectivity scores in the left inferior parietal lobe (r=−0.5511, p<0.01 5% FDR; Figure 2a). This region, in BA40 (Table 1), overlaps areas known to be involved in speech comprehension and production (57). A similar association between low global connectivity and high positive symptom ratings was identified in a region within the right anterior insula (BA13; overlapping areas of reduced connectivity in the group analysis) although this correlation was weak (r=−0.4792, p<0.05 uncorrected; Figure 2b).
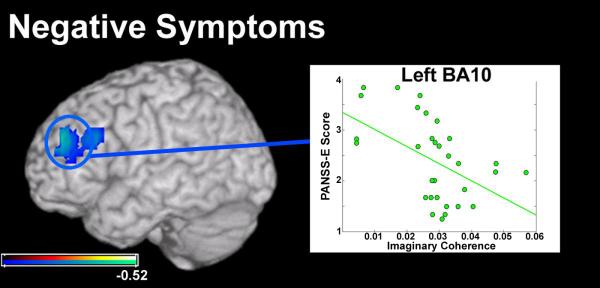
For ratings of negative symptoms, SZ participants fell within the range of absent to moderate (1-4) (Figure 3). A significant relationship was found between negative symptom ratings and decreased connectivity in the left pre-frontal cortex in BA9/ BA10 of the middle frontal gyrus (Table 1, Figure 3; r=−0.5164, p<0.01; 5% FDR). This region of BA9 overlaps with regions of left dorsolateral pre-frontal cortex (DLPFC) with reduced functional connectivity seen in the group comparison at relaxed statistical threshold (p<0.05, uncorrected).
Figure 3.
Results from a pairwise correlation between global connectivity (GC) measures in the patient group with negative symptom ratings from the PANSS-E. One region of left PFC, in Brodmann’s Area 10 (BA10) was significantly correlated with negative symptoms in these patients. Left BA10 in this analysis overlapped a region in aPFC with lower functional connectivity in the patient group. The direction of this trend suggests that low global connectivity in BA10 is related to high negative symptom ratings on the PANSS-E in this group. Conventions as in previous figures.
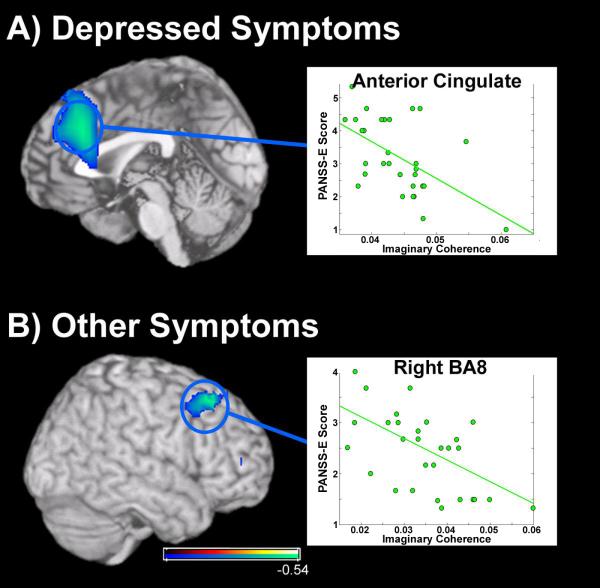
For ratings of depression, participants with schizophrenia fell within the range of absent to moderate-severe (1-5.5). SZ subjects with high ratings of depression had lower global connectivity over medial pre-frontal cortex (Figure 4a; r=−0.5432, p<0.01; 5% FDR) centered on anterior cingulate cortex (ACC), an area known to play a strong role in cognitive control, self-evaluation and mood (58,59). Finally, we found a significant association in the “other” category of the PANSS-E (inattention, poor abstraction, etc.) and decreased functional connectivity in the right middle frontal gyrus (Figure 4b), over BA8 (r=−0.5432, p<0.01; 5% FDR). Symptom ratings fell within the range of absent to moderate (1-4) in this “other” category. No significant relationship was observed between global connectivity scores and disorganized symptoms or excited symptoms (10% FDR correction; Table 2).
Figure 4.
Results from a pairwise correlation between global connectivity (GC) measures in the patient group with extended scores from the PANSS-E. A) Relationship between alpha-band functional connectivity and level of depression (as assessed by the PANSS-E) in patients with schizophrenia. Low IC values in a large region of cortex along the medial wall (in the anterior cingulate) were associated with high ratings for depression in patients with schizophrenia. B) Relationship between GC measures and assessment ratings of “other” impairments as assessed by the PANSS-E, including cognitive deficits. In the right hemisphere, a negative relationship was identified between global connectivity of the right MFG, in Brodmann’s Area 8 (BA8) and these symptoms in patients with schizophrenia. Conventions as previous figures.
Table 2.
| Region | Abbrev | Hemi | BA | x | y | z | r | p |
|---|---|---|---|---|---|---|---|---|
| Disorganized Symptoms | ||||||||
| Superior Temporal Gyrus | STG | L | 22 | −40 | −55 | 10 | 0.455 | 0.012 |
| Inferior Frontal Gyrus | IFG | R | 44 | 60 | 15 | 15 | −0.387 | 0.035 |
| Excited Symptoms | ||||||||
| Cuneus | CU | L | 7 | −20 | −60 | 55 | 0.385 | 0.036 |
| Middle Temporal Gyrus | MTG | L | 39 | −50 | −75 | 5 | 0.442 | 0.015 |
Discussion
We present here, for the first time, direct evidence for functional disconnection between specific cortical regions in schizophrenia as demonstrated through disrupted spontaneous alpha oscillations--electromagnetic fluctuations that represent long-range communication between groups of neurons. Patterns of reduced alpha-band functional connectivity were correlated with symptoms of psychosis, depressed mood, and impaired cognition in these individuals. Our findings suggest that disconnectivity in the alpha range between key cortical regions reflects a core neurophysiologic correlate of clinical symptoms in schizophrenia, and thus may be a useful treatment target through pharmacological or behavioral interventions (3).
Functional Connectivity: Differences between patients and healthy comparison subjects
In the group comparison, connectivity of a large region of left DLPFC and pre-central gyrus was globally reduced in the SZ group (Figure 1b). This observation is congruent with previous reports of functional connectivity deficits of this region in schizophrenia using fMRI (18,60) as well as reductions in coherence in left hemisphere electrodes in EEG (26,61). Impaired function within DLPFC is thought to affect cognitive control, which in turn appears to be associated with many of the behavioral manifestations of schizophrenia (8).
As in left DLPFC, reductions in fMRI functional connectivity (10) and EEG alpha coherence (61) of the right temporal lobe have also been reported in individuals with schizophrenia both at rest and during behavior. Abnormal connectivity of the right MTG is believed to be related to impairments in auditory processing and attention (10), while abnormal activity within the right temporal lobe is often associated with auditory hallucinations (62-64).
Higher mean global IC values were identified in the patient group over the right inferior frontal gyrus (IFG) and the left medial occipital gyrus (MOG; Figure 1b). Increases in BOLD signal during a continuous performance task over the right IFG have been reported in patients with schizophrenia (65) as have been increased correlations between occipital EEG electrodes even in the absence of visual stimulation (66). Although a handful of neuroimaging studies have attributed increased neural activity in schizophrenia to an inefficiency in cortical processing (67) or heightened internal conflict and distractibility (18,21), these hypotheses have yet to be directly tested.
Functional Connectivity and Symptom Severity in Schizophrenia Subjects
Positive symptoms were negatively correlated with global IC values of the left inferior parietal lobe (IPL; Figure 2), a region which intersects the superior parietal-temporal lobe border (area sPT) near the sylvian fissure. Area sPT is a major component of a language network (57) and fMRI functional connectivity of this region during behavior correlates with the severity of auditory hallucinations (68). Abnormal auditory perceptual experiences may occur as a result of diminished functional connectivity of this area.
Figure 2.
Results from a pairwise correlation between global connectivity (GC) measures in the patient group with positive symptom ratings from the Positive and Negative Symptom Scale, Extended (PANSS-E). Voxels that are negatively correlated with symptom strength are circled in blue and color scaled blue to azure. Connectivity of a region in the right hemisphere in Brodmann’s Area 13 (BA13) was negatively correlated with positive symptoms assessed in these patients. We also identified a negative relationship between GC measures and positive symptoms in a region of the left inferior parietal lobe, in Brodmann’s Area 40 (BA40). Statistical maps (Pearson’s r) are thresholded (p<0.05, uncorrected) and superimposed over a rendering of the MNI template brain through MRICro.
A strong relationship was seen in our subjects between reduced left BA9/10 connectivity and negative symptoms (Figure 3). This region, in left DLPFC, plays a strong role in the control of executive faculties, intention and motivation (69-71). Functional imaging studies have correlated low neural activity in this region to negative symptoms in patients with schizophrenia (72). It is thus plausible that compromised functional integrity of this region could contribute to behavioral, emotional, and social withdrawal in this patient population.
In anterior cingulate cortex (ACC), reduced functional connectivity was significantly correlated with symptoms of depression and anxiety (Figure 4a). Patients with major depressive disorder have significantly reduced connectivity between ACC and limbic structures (amygdala, dorsomedial thalamus) in both active-state (73,74) and resting-state (75) fMRI studies. Our findings indicate that a common neurophysiological mechanism contributes to depressive symptoms in both schizophrenia and major depression.
The “other” symptoms category rates a range of symptoms, including cognitive features (in attention, disorientation; 40). In the SZ group, functional connectivity of right DLPFC (including BA8) was negatively correlated with these ratings (Figure 4b). Right-lateralized DLPFC function is thought to play a role in memory retrieval (76,77) and cognitive control (78,79). Estimates of right DLPFC functional connectivity derived from fMRI studies show reduced interactions between this region in patients with schizophrenia across a wide range of cognitive tasks (80-81). Our data suggest that a relationship between right DLPFC functional connectivity and cognition may also be present in the brain’s resting-state, indicating a potential treatment target (82).
Functional Significance of Atypical Alpha Connectivity in Schizophrenia
Alpha oscillations are a stable rhythm thought to be generated through reciprocal excitatory and inhibitory neuronal interactions (30,83). Coherent activity between cortical sources cannot be explained by thalamic inputs alone (84-86), suggesting that these oscillations are a reliable marker of long-range cortico-cortical interactions in the brain (87,88). Our data is consistent with previous EEG studies outlining the topology and magnitude of compromised oscillatory activity and non-linear alpha interdependence in schizophrenia (89-91). While deviations in oscillatory power across many frequency bands (including alpha) have been identified using MEG (92-94), this is the first investigation to use MEG source data (MEGI) to examine spontaneous alpha functional connectivity directly in schizophrenia. Disruptions in alpha coherence represent a lack of synchrony between brain regions, which itself may be due to either a reduction in local computational processing and/or reduced neural synchrony across cortical fields.
Relationship between MEG and fMRI resting-state connectivity
In schizophrenia, where the prevailing model of the disease is built upon an a priori assumption of dysfunctional connectivity (95,96), it is difficult to make a distinction between deficits in functional connectivity and abnormal levels of activity within a cortical field. Due to temporal limitations of fMRI, functional connectivity metrics of cerebral blood flow are restricted to models of functionally significant networks at extremely low frequency ranges (<0.1Hz; 97). Active-state fMRI studies can be contaminated by non-neural, physiological interactions unrelated to brain activity (98,99). Resting-state imaging paradigms provide a unique advantage to resolving this conflict between abnormal activity and connectivity. It is not clear how reductions in fMRI connectivity relate to lowered MEG connectivity in 8-12Hz oscillations. Simultaneous EEG/fMRI studies have shown that reduced alpha power correlates with increased BOLD signal change in posterior brain regions (100,101). Interestingly, although these functional connectivity metrics in schizophrenia are derived from very divergent imaging modalities, they highlight a common theme of lack of coordination or integration of normal patterns of cortical information processing.
Limitations of study
It is important to note that in any psychiatric disorder there is a considerable degree of heterogeneity within the population that is being studied. Our sample included subjects ranging from recent onset to those who had been ill for decades. Clinical heterogeneity in our sample may also play a role in any observed associations—or lack of associations--between coherence and symptom ratings. Further, in these kinds of studies, it is difficult to dissect out neurophysiologic findings which represent the schizophrenia “endophenotype” from those which represent the cumulative effects of illness burden or which represent the current clinical state. Future work will need to examine if the reductions in functional connectivity that we report here are altered over the course of successful treatment.
Changes in fcMEGI we observe in the schizophrenia group could be arguably interpreted as an “inability to rest” for patients in general (102), potentially impacting all studies of activity and connectivity in schizophrenia using task-evoked designs (103). However, we observe no differences in alpha power or distribution of alpha peak which would indicate heightened alpha activity. Furthermore, a strong relationship between regional connectivity and clinical symptoms that we observe suggest that if there is an “inability to rest”, it is manifested in patterns of functional interactions and is of pathological importance.
As a clinically stable cohort, all of the participants were medicated, making it difficult to discern how their psychiatric medications impact MEG alpha coherence at rest. We found no significant correlation between level of medication and functional connectivity. Therefore, it is reasonable to assume that the reductions in alpha-band connectivity we identify here were only marginally affected by medication and that these effects would also be observed in an unmedicated patient sample.
Conclusions
The current study provides novel and compelling evidence for how disrupted long-range neural functional connectivity (as evidenced through deviations in coherence of resting-state alpha) contributes to characteristic clinical manifestations in schizophrenia. Although this relationship is complex, an understanding of the oscillatory mechanisms through which networked brain regions normally cooperate and how these networks relate to disruptions in cognition and affect in psychiatric illnesses could lead to innovations in treatment conceptualization and development.
Acknowledgements
This work was supported by the San Francisco VA Medical Center, the National Institutes of Health (R01 MH068725-01A1 to S.V., R01 grants DC004855, DC006435, DC10145, NS67962 and NSF grant BCS 926196 to S.S.N.), the National Institutes of Health/National Center for Research Resources (UCSF-CTSI UL1 RR024131 to S.S.N.), the Dystonia Medical Research Foundation (L.B.N.H.) and UCSF/REAC grants (S.S.N.). The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registry: clinicaltrials.gov NCT00312962
References
- 1.Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 2.Ford JM, Mathalon DH. Neural synchrony in schizophrenia. Schizophr Bull. 2008;34:904–906. doi: 10.1093/schbul/sbn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 4.Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb. Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- 5.Schlösser R, Gesierich T, Kaufmann B, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- 6.Honey GD, Pomarol-Clotet E, Corlett PR, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrity AG, Pearlson GD, McKiernan K, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 8.Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DI, Mathalon DH, Ford JM, et al. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leocani L, Comi G. EEG coherence in pathological conditions. J Clin Neurophysiol. 1999;16:548–555. doi: 10.1097/00004691-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Ford MR, Goethe JW, Dekker DK. EEG coherence and power in the discrimination of psychiatric disorders and medication effects. Biol. Psychiatry. 1986;21:1175–1188. doi: 10.1016/0006-3223(86)90224-6. [DOI] [PubMed] [Google Scholar]
- 13.Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR. Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biol. Psychiatry. 2003;54:1181–1192. doi: 10.1016/s0006-3223(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 14.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Liu Z, Liang M, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr. Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bluhm RL, Miller J, Lanius RA, et al. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavrilescu M, Rossell S, Stuart GW, et al. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med. 2009:1–10. doi: 10.1017/S0033291709991632. [DOI] [PubMed] [Google Scholar]
- 23.Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. [Accessed April 21, 2010];Auditory Hallucinations in Schizophrenia Are Associated with Reduced Functional Connectivity of the Temporo-Parietal Area. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.11.017. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20060103. [DOI] [PubMed] [Google Scholar]
- 24.Merrin EL, Floyd TC, Fein G. EEG coherence in unmedicated schizophrenic patients. Biol. Psychiatry. 1989;25:60–66. doi: 10.1016/0006-3223(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 25.Merrin EL, Floyd TC. Negative symptoms and EEG alpha activity in schizophrenic patients. Schizophr. Res. 1992;8:11–20. doi: 10.1016/0920-9964(92)90056-b. [DOI] [PubMed] [Google Scholar]
- 26.Tauscher J, Fischer P, Neumeister A, Rappelsberger P, Kasper S. Low frontal electroencephalographic coherence in neuroleptic-free schizophrenic patients. Biol. Psychiatry. 1998;44:438–447. doi: 10.1016/s0006-3223(97)00428-9. [DOI] [PubMed] [Google Scholar]
- 27.Winterer G, Egan MF, Radler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr. Res. 2001;49:129–143. doi: 10.1016/s0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 28.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 29.Uhlhaas PJ, Haenschel C, Nikolić D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunez PL. A preliminary physiology of macro-neocortical dynamics and brain function. In: Uhl C, editor. Analysis of neurophysiological brain functioning. Springer-Verlag; Berlin: 1999. pp. 41–72. [Google Scholar]
- 32.Omori M, Koshino Y, Murata T, et al. Quantitative EEG in never-treated schizophrenia patients. Biol Psychiatry. 1995;5:303–309. doi: 10.1016/0006-3223(95)00300-6. [DOI] [PubMed] [Google Scholar]
- 33.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- 34.Nunez PL. A study of origins of the time dependencies of scalp EEG: i--theoretical basis. IEEE Trans Biomed Eng. 1981;28:271–80. doi: 10.1109/tbme.1981.324700. [DOI] [PubMed] [Google Scholar]
- 35.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jann K, Dierks T, Boesch C, Kottlow M, Strik W, Koenig T. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Neuroimage. 2009;45:903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- 38.Dale CL, Findlay AM, Adcock RA, et al. Timing is everything: neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int J Psychophysiol. 2010;75:183–193. doi: 10.1016/j.ijpsycho.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole JH, Tobias FC, Vinogradov S. The functional relevance of affect recognition errors in schizophrenia. J Int Neuropsychol. 2000;6:649–658. doi: 10.1017/s135561770066602x. [DOI] [PubMed] [Google Scholar]
- 40.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 41.Andreasen NC, Flaum MC, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 42.Dalal SS, Guggisberg AG, Edwards E, et al. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menninghaus E, Lütkenhöner B. How silent are deep and radial sources in neuromagnetic measurements? In: Bumgartner, et al., editors. Biomagnetism: Fundamental Research and Clinical Applications. Elsevier Science, IOS Press; 1995. pp. 352–356. [Google Scholar]
- 44.Wipf DP, Owen JP, Attias HT, Sekihara K, Nagarajan SS. Robust Bayesian estimation of the location, orientation, and time course of multiple correlated neural sources using MEG. Neuroimage. 2010;49(1):641–655. doi: 10.1016/j.neuroimage.2009.06.083. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinkley LBN, Owen JP, Fisher M, Findlay AM, Vinogradov S, Nagarajan SS. Cognitive impairments in schizophrenia as assessed through activation and connectivity measures of magnetoencephalography (MEG) data. Front Hum Neurosci. 2010;3:73. doi: 10.3389/neuro.09.073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guggisberg AG, Honma SM, Findlay AM, et al. Mapping functional connectivity in patients with brain lesions. Ann. Neurol. 2008;63:193–203. doi: 10.1002/ana.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson S, Vrba J. Functional neuroimaging by synthetic aperture magnetometry (SAM) In: Yoshimoto, et al., editors. Recent advances in biomagnetism. Tohoku University Press; Sendai, Japan: 1999. pp. 302–305. [Google Scholar]
- 48.Nolte G, Bai O, Wheaton L, et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 49.Martino J, Honma SM, Findlay AM, et al. Resting functional connectivity in patients with brain tumors in eloquent areas. Ann Neurol. 2011 doi: 10.1002/ana.22167. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1:30–46. [Google Scholar]
- 51.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamini Y, Yekutieli D. The control of false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 53.Durka PJ, Zygierewicz J, Klekowicz H, Ginter J, Blinowska KJ. On the statistical significance of event-related EEG desynchronization and synchronization in the time-frequency plane. IEEE Trans on Biomed Eng. 2004;51:1167–1175. doi: 10.1109/TBME.2004.827341. [DOI] [PubMed] [Google Scholar]
- 54.Lutcke H, Frahm J. Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cereb Cortex. 2008;18:508–515. doi: 10.1093/cercor/bhm090. [DOI] [PubMed] [Google Scholar]
- 55.Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of AB in mild cognitive impairment: an example of brain reserve. J. Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cañive JM, Lewine JD, Edgar JC, et al. Spontaneous brain magnetic activity in schizophrenia patients treated with aripiprazole. Psychopharmacol Bull. 1998;34:101–105. [PubMed] [Google Scholar]
- 57.Hickok G, Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 58.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 59.Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. (Regul. Ed.) 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 61.Merrin EL, Floyd TC. Negative symptoms and EEG alpha in schizophrenia: a replication. Schizophr Res. 1996;19:151–161. doi: 10.1016/0920-9964(96)88522-7. [DOI] [PubMed] [Google Scholar]
- 62.Woodruff PW, Wright IC, Bullmore ET, et al. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry. 1997;154:1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- 63.Bentaleb LA, Beauregard M, Liddle P, Stip E. Cerebral activity associated with auditory verbal hallucinations: a functional magnetic resonance imaging case study. J Psychiatry Neurosci. 2002;27:110–115. [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman RE, Anderson AW, Varanko M, Gore JC, Hampson M. Time course of regional brain activation associated with onset of auditory/verbal hallucinations. Br J Psychiatry. 2008;193:424–425. doi: 10.1192/bjp.bp.107.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacDonald AW, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. 2005. [DOI] [PubMed] [Google Scholar]
- 66.Jin S, Ham B, Kim SY. Functional clustering in EEG photic and auditory driving in schizophrenia. Int J Psychophysiol. 2005;56:249–259. doi: 10.1016/j.ijpsycho.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 68.Lawrie SM, Buechel C, Whalley HC, et al. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol. Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 69.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 70.Gilbert AM, Fiez JA. Integrating rewards and cognition in the frontal cortex. Cogn Affect Behav Neurosci. 2004;4:540–552. doi: 10.3758/cabn.4.4.540. [DOI] [PubMed] [Google Scholar]
- 71.Ichihara-Takeda S, Funahashi S. Reward-period activity in primate dorsolateral prefrontal and orbitofrontal neurons is affected by reward schedules. J Cogn Neurosci. 2006;18:212–226. doi: 10.1162/089892906775783679. [DOI] [PubMed] [Google Scholar]
- 72.Wolkin A, Sanfilipo M, Wolf AP, et al. Negative symptoms and hypofrontality in chronic schizophrenia. Arch. Gen. Psychiatry. 1992;49:959–965. doi: 10.1001/archpsyc.1992.01820120047007. [DOI] [PubMed] [Google Scholar]
- 73.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 74.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol. Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 75.Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Curr. Opin. Neurol. 2000;13:415–421. doi: 10.1097/00019052-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 78.Konishi S, Nakajima K, Uchida I, et al. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 79.Milham MP, Banich MT, Webb A, et al. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- 80.Salgado-Pineda P, Caclin A, Baeza I, et al. Schizophrenia and frontal cortex: where does it fail? Schizophr. Res. 2007;91:73–81. doi: 10.1016/j.schres.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 81.Woodward ND, Waldie B, Rogers B, et al. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr. Res. 2009;109:182–190. doi: 10.1016/j.schres.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 82.Eriksson J, Larsson A, Nyberg L. Item-specific training reduces prefrontal cortical involvement in perceptual awareness. J. Cog. Neurosci. 2008;20:1777–1787. doi: 10.1162/jocn.2008.20064. [DOI] [PubMed] [Google Scholar]
- 83.Nunez PL. Wavelike properties of the alpha rhythm. IEEE Trans Biomed Eng. 1974;21:473–482. [Google Scholar]
- 84.da Silva FH Lopes, Vos JE, Mooibroek J, van Rotterdam A. Relative contributions of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50:449–456. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- 85.van Rotterdam A, da Silva FH Lopes, van den Ende J, Viergever MA, Hermans AJ. A model of the spatial-temporal characteristics of the alpha rhythm. Bulletin of Mathematical Biology. 1982;44:283–305. doi: 10.1007/BF02463252. [DOI] [PubMed] [Google Scholar]
- 86.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 87.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 88.Nikulin VV, Brismar T. Long-range temporal correlations in electroencephalographic oscillations: Relation to topography, frequency band, age and gender. Neuroscience. 2005;130:549–558. doi: 10.1016/j.neuroscience.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Breakspear M, Terry J, Friston K, Williams L, Brown K, Brennan J, Gordon E. A disturbance of nonlinear interdependence in scalp EEG of subjects with first episode schizophrenia. Neuroimage. 2003;20:466–478. doi: 10.1016/s1053-8119(03)00332-x. [DOI] [PubMed] [Google Scholar]
- 90.Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AWF, Williams LM, Breakspear M. Small world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30:403–416. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Breakspear M, Terry J. Detection and description of nonlinear interdependence in normal multichannel human EEG. Clin Neurophysiol. 2002;113:735–753. doi: 10.1016/s1388-2457(02)00051-2. [DOI] [PubMed] [Google Scholar]
- 92.Cañive JM, Lewine JD, Edgar JC, et al. Magnetoencephalographic assessment of spontaneous brain activity in schizophrenia. Psychopharmacol Bull. 1996;32:741–750. [PubMed] [Google Scholar]
- 93.Sperling W, Martus P, Kober H, Bleich S, Kornhuber J. Spontaneous, slow and fast magnetoencephalographic activity in patients with schizophrenia. Schizophr. Res. 2002;58:189–199. doi: 10.1016/s0920-9964(02)00238-4. [DOI] [PubMed] [Google Scholar]
- 94.Rutter L, Carver FW, Holroyd T, et al. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 96.Friston KJ. The disconnection hypothesis. Schizophr. Res. 1998;30(2):115–125. doi: 10.1016/s0920-9964(97)00140-0. 1998. [DOI] [PubMed] [Google Scholar]
- 97.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp. 2008;29:740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang C, Glover GH. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage. 2009;47:1381–1393. doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laufs H, Kleinschmidt A, Beyerle A, et al. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 102.Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 103.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]