Abstract
Background
Pulsed dye laser is the gold standard for treatment of port wine stain birthmarks but multiple treatments are required and complete resolution is often not achieved. Post-treatment vessel recurrence is thought to be a factor that limits efficacy of pulsed dye laser treatment of port wine stains. Imiquimod 5% cream is an immunomodulator with anti-angiogenic effects.
Objective
To determine if application of imiquimod 5% cream after pulsed dye laser improves treatment outcome.
Methods
Healthy patients with port wine stains (n = 24) were treated with pulsed dye laser and then randomized to apply post-treatment placebo or imiquimod 5% cream for 8 weeks. Chromameter measurements (CIE L*a*b* colorspace) for 57 port wine stain sites (multiple sites per subject) were taken at baseline and compared with measurements taken 8 weeks post-treatment. The change in a* and ΔE were measured to quantify treatment outcome.
Results
Two subjects developed minor skin irritation. Other adverse effects weren't noted. Average Δa* was 0.43 for pulsed dye laser + placebo sites (n = 25) and 1.27 for pulsed dye laser + imiquimod sites (n = 32) (p value = 0.0294) indicating a greater reduction in erythema with imiquimod. Average ΔE was 2.59 for pulsed dye laser + placebo and 4.08 for pulsed dye laser + imiquimod (p value = 0.0363), again indicating a greater color improvement with imiquimod.
Limitations
Effects were evaluated after a single treatment and duration of effect is unknown.
Conclusion
Combined selective photothermolysis and anti-angiogenic therapy may enhance port wine stain treatment efficacy.
Keywords: Port wine stain, pulsed dye laser, selective photothermolysis, angiogenesis, imiquimod, vascular malformation
INTRODUCTION
A port wine stain (PWS) is a vascular malformation found in approximately 0.3% of children.1,2 Light-based therapy utilizing the theory of selective photothermolysis3 can lighten these birthmarks, although in the majority of patients, only 10–20% of patients obtain 100% resolution.4,5 Numerous treatments (15–20) are often required, and incomplete resolution and lesion recurrence are common. Over the last few decades, optimization of light-based protocols designed to treat cutaneous vasculature has focused primarily on improving vascular removal by optimizing therapeutic devices (to allow delivery of higher energies and improve safety with epidermal protection) or exploring alternative methods of removal (such as photodynamic therapy). However, improving upon the degree of acute vascular destruction may not be adequate to achieve the desired goal of complete, long-term lesion removal.
We postulate that a critical factor limiting PWS treatment efficacy is post-treatment vessel recurrence as a result of angiogenesis.5 Angiogenesis is a normal process in growth and wound healing, but it is also a contributing factor in a wide range of disease processes.6 Initial interest in angiogenesis following selective laser injury was generated based on observations, noting that acute vascular destruction does not necessarily result in PWS lightening.5 Subsequent studies using laser speckle imaging (LSI) on a rodent dorsal window chamber model, demonstrated an initial shutdown in blood flow followed by reperfusion and vascular remodeling.7 Serial LSI monitoring of PWS patients has also demonstrated the dynamic nature of the post-treatment blood flow response in the clinical setting. Based on the collective data, we hypothesize that the effects of treatment with the pulsed dye laser (PDL) can be enhanced by application of an anti-angiogenic agent.
Imiquimod (Graceway Pharmaceuticals, Bristol, TN) is a topically administered immune response modulator approved by the United States Food and Drug Administration for treatment of external genital warts, superficial basal cell carcinoma, and actinic keratosis.8 It has also been used successfully to treat vascular proliferative lesions such as infantile hemangiomas, pyogenic granulomas, Kaposi's sarcoma and hemangiosarcomas.9–13 A proposed mechanism of action of imiquimod is inhibition of angiogenesis. Imiquimod affects angiogenesis by: 1) induction of anti-angiogenic cytokine including interferon-alpha (IFN-alpha), interleukins (IL) 10 and 12 and tissue inhibitors of metalloproteinases (TIMP), and 2) inhibition of pro-angiogenic factors such as matrix metalloproteinases (MMPs).10, 14
Our objective is to determine if PDL followed by post-treatment application of imiquimod will enhance treatment efficacy.
METHODS
Study design
To assess the efficacy of combined selective photothermolysis (PDL treatment) and imiquimod, we initiated a single center, eight week, blinded, placebo-controlled clinical feasibility study involving subjects with PWS. Subjects were randomly assigned into two possible treatments arms: PDL + imiquimod 5% cream or PDL + placebo (vehicle) cream. The study was approved by the Investigational Review Board at University of California, Irvine and was registered in the clinicaltrials.gov trial register (Clinicaltrials.gov identifier: NCT00585247). Verbal and written informed consent was obtained for all adult subjects and assent was obtained for subjects under age 18.
Patient enrollment
Healthy adults and children with PWS were enrolled. Prior treatment with PDL was not an exclusion, since PWS generally require multiple treatments. After blinded review of results in 13 subjects suggested efficacy, the protocol was amended to allow subjects to enroll into the trial on two separate occasions. A total of 5 subjects were re-enrolled in this protocol. There was a minimum of a 4 week wash out period between end of study and re-enrollment. At the end of the first enrollment, the subject was unblinded by the independent investigator (sub-investigator). During the next enrollment, the subject was placed into the other treatment arm by the independent investigator. The principal investigator and the subject remained blinded until the completion of the trial. Four of the five subjects had the same PWS area treated during each enrollment while the fifth subject had another portion of the PWS treated. With each re-enrollment, new baseline measurements were obtained and different measurement spots were used.
Study laser treatment and medication
Each subject received a single treatment with the Perfecta 595 nm laser (Candela Corp; Wayland, Massachusetts). Settings used included either a 7 mm or 10 mm spot size, 1.5 ms pulse duration, radiant exposure of 6–12 J/cm2, and cryogen spray cooling (30 ms of cooling with a 30 or 20 ms delay).
Beginning on the first day after treatment, subjects applied one sachet (250 mg) of either 5% imiquimod or placebo (vehicle) cream to < 25 cm2 area of the treated PWS, three times a week, for eight weeks.
Data Collection
The study time line is outlined in Table I. Subjects were assessed for adverse events at 2 week intervals either by office visits or by phone call. Standardized digital photographs (PowerShot S2 IS, Cannon USA; Lake Success, New York) were taken before and up to eight weeks after treatment.
Table I.
Study Timeline
| Baseline (Day 0) |
| - Informed consent obtained and determined subject eligibility |
| - Randomized (1:1) into treatment group (PDL + imiquimod 5% cream) or the placebo group (PDL + vehicle cream) |
| - Photographs |
| - Baseline measurements with chromameter |
| - Treatment with PDL |
| - Laser speckle imaging done prior to and after laser treatment |
| - Study medication dispensed |
| Day 1 |
| - Subject starts treatment with study cream on a 3 times per week schedule |
| Day 14 |
| - Assess for adverse events |
| - Study drug accountability |
| Day 28 |
| - Assess for adverse events |
| - Study drug accountability |
| Day 42 |
| - Assess for adverse events |
| - Study drug accountability |
| End of Study (Day 56) |
| - Assess for adverse events |
| - Photographs |
| - Measurements with chromameter |
| - Study drug accountability and collection |
To quantify changes in skin color, we used a Minolta tristimulus chromameter (CR-400, Konica Minolta; Osaka, Japan) pre- and up to eight weeks post-treatment. The chromameter provides measurements in the CIE (Commission internationale de l'Eclairage) L*a*b* colorspace. This colorspace was developed in part to provide quantitative values, which correspond to human perception of color. L* describes the reflected light intensity and varies from 0 (e.g., black) to 100 (e.g., white); a* describes color saturation and varies from +60 for green to −60 for red; and b* also describes color saturation and varies from +60 for blue to −60 for yellow.15 Multiple sites were measured within the each PWS. Lesion tracings with transparency paper were used with the intent to measure identical spots at each visit. Each subject was not an average of multiple sites; rather, each site was an independent data set.
We monitored the change in a* (Δa*) and ΔE to quantify PWS treatment outcome.16–29 Δa* indicates a change in the erythema of the vascular lesions. The secondary calculation (ΔE),20 detects all three dimensions of colorspace (L*a*b*) and represents the difference in color between normal and PWS skin. ΔE is calculated as:
ΔL*, Δa*, Δb* are the differences in L*, a*, and b*, respectively, between PWS and normal skin and subscripts “before” and “after” indicate color values acquired prior to and eight weeks after treatment. Starting with subject 6, we collected chromameter data from normal skin sites to enable calculation of ΔE and use a more standardized measure of treatment efficacy. LSI was performed before and after treatment as a method to assess acute post-treatment vascular shutdown.21
The final subject had additional analysis carried out. This subject had 3 testing areas: (1) untreated PWS, (2) PWS treated with PDL alone, and (3) PWS treated with PDL + imiquimod 5% cream. Additional testing areas were included to allow comparison of treatment conditions. At week 4, the subject had three 2 mm biopsies performed. Tissue was frozen and sections processed for immunohistochemistry staining of VEGF, bFGF, MMP-9, and ANG-2.
Resulting images were blinded and graded by a board certified dermatopathologist, who evaluated the specimens' degrees of staining for each of the 4 antibodies in the epidermis, dermis and endothelial cells. The degree of staining was divided by proportion of cells stained into one of four categories: 0%, <10%, 10–50%, or >50%. The intensity was graded on a scale of 0 to 3.
Statistical analyses
The outcomes of the two treatment groups were analyzed as follows, Δ a* for each treatment site was compared using the Mann-Whitney-Wilcoxon non-parametric test. A p less than or equal to .05 indicated statistical significance. The Δ E for each treatment site was also compared using the Mann-Whitney-Wilcoxon non-parametric test. A p less than or equal to .05 indicated statistical significance.
RESULTS
A total of 24 subjects were enrolled (Table II). Enrollment consisted of 22 adults (mean age = 37) and 2 children (youngest was 13 years of age). Most subjects had previous treatment with the PDL (at least 2 months prior) but none of the subjects had treatment with combination PDL and imiquimod prior to enrollment. Baseline a* values were established through measurement of bloodless in vitro human skin and in vivo normal and pretreated PWS skin (Table III). For all subjects, pretreatment a* values were compared with those measured at 8 weeks post treatment. The a* values were measured from 57 independently monitored sites: 25 PDL + placebo and 32 PDL + imiquimod. The first two subjects were not included in the analysis because of chromameter malfunction. When comparing the outcomes using the Mann-Whitney-Wilcoxon test the average Δa* was calculated to be 0.43 (SD=1.63) for PDL + placebo sites and 1.27 (SD=1.76) for PDL + imiquimod sites (Table IV). This statistically significant result (p value = 0.0294) suggests that the addition of imiquimod post PDL improves the reduction of erythema. The ΔE was calculated from 49 independently monitored sites: 25 PDL + placebo and 24 PDL + imiquimod. The average ΔE was calculated to be 2.59 (SD=1.54) for PDL + placebo and 4.08 (SD=3.39) for PDL + imiquimod sites (Table V), again suggesting that imiquimod application improved treatment efficacy (p value = 0.0363).
Table II.
Subject demographics
| Subject Number | Sex | Race | Age | Treatment site | 2nd enrollment |
|---|---|---|---|---|---|
| 1 | Female | Caucasian | 71 | Right hand | |
| 2 | Male | Caucasian | 44 | Left arm | |
| 3 | Female | Caucasian | 31 | Face | |
| 4 | Female | Hispanic | 21 | Right leg | |
| 5 | Female | Asian | 35 | Left hand | |
| 6 | Female | Caucasian | 55 | Left thigh | |
| 7 | Female | Caucasian | 22 | Back | |
| 8 | Female | Caucasian | 13 | Face | |
| 9 | Female | Hispanic | 47 | Right thigh | |
| 10 | Female | Caucasian | 38 | Left leg | |
| 11 | Male | Caucasian | 51 | Left arm/chest | |
| 12 | Male | Caucasian | 14 | Face/ left ear | |
| 13 | Male | Caucasian | 51 | Left arm/chest | Yes |
| 14 | Male | Caucasian | 14 | Face/ left ear | Yes |
| 15 | Female | Caucasian | 14 | Face | Yes |
| 16 | Male | Asian | 23 | Face | |
| 17 | Female | Caucasian | 23 | Left leg | |
| 18 | Male | Asian | 19 | Face | |
| 19 | Male | Asian | 23 | Face | Yes |
| 20 | Male | Hispanic | 45 | Face | |
| 21 | Male | Caucasian | 35 | Face | |
| 22 | Male | Asian | 20 | Face | Yes |
| 23 | Male | Caucasian | 27 | Face | |
| 24 | Male | Caucasian | 62 | Face |
TABLE III.
Average a* values in control tissues
| Average a* | |
|---|---|
| Description | Indicative of erythema color |
| Bloodless Tissue (Cadaver skin) | 0.92 |
| Normal Skin | 9.28 |
| PWS Skin | 15.50 |
TABLE IV.
The change in a* values between baseline and 8 week measurements. Δ* indicates a change in the erythema of the vascular lesions. P = 0.0294.
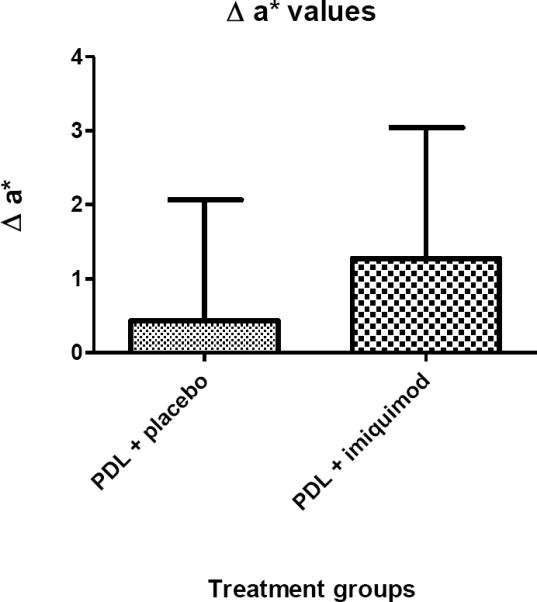 |
TABLE V.
The change in E between baseline and 8 week measurements. P = 0.0363 ΔE detects all three dimensions of colorspace (L*a*b*) and represents the difference in color between normal and PWS skin. ΔE is calculated as:
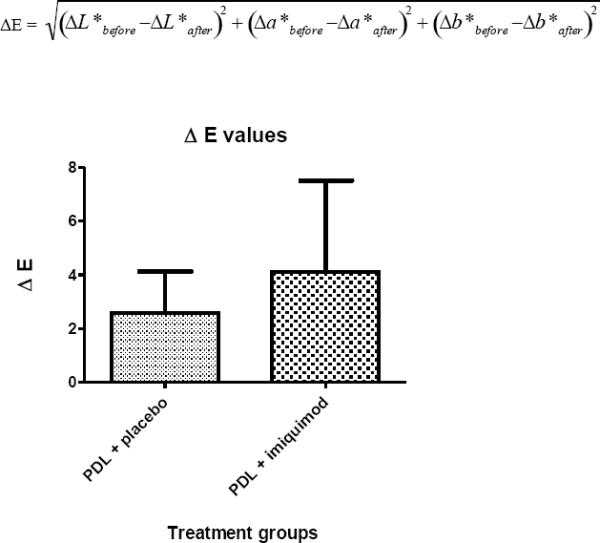 |
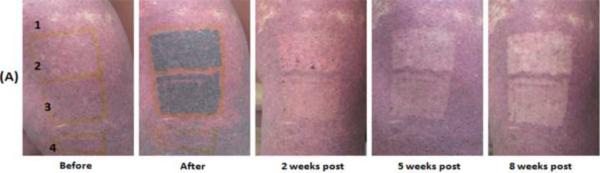
Figure 1 provides images taken from a subject in the PDL + imiquimod group. Due to the large area of PWS skin, we evaluated not only the intended test condition (PDL + imiquimod) but also additional test and control conditions. Site 1 received imiquimod alone; site 2 received PDL + imiquimod; site 3 received PDL alone; and site 4 is an untreated PWS site. The Δa* for each site was: site 1 (imiquimod alone) =1.41; site 2 (PDL + imiquimod) =2.68; site 3 (PDL alone) =2.37; and site 4 (untreated PWS) = −1.63. Positive numbers indicate a reduction of erythema; a greater positive number indicated enhanced treatment response. The corresponding ΔE value was 1.09, 11.47, 9.97, and 4.83, for sites 1–4 respectively. Enhanced lightening in the PDL + imiquimod site is particularly impressive as this demonstrates augmentation of an already dramatic PDL response (a result which is not often achieved with a single PDL treatment).
Figure 1.
Port Wine Stain treated in the PDL + imiquimod group. Site 1 received imiquimod alone; site 2 received PDL + imiquimod; site 3 received PDL alone; and site 4 is an untreated PWS site. The Δa* for each site was: site 1 (imiquimod alone) =1.41; site 2 (PDL + imiquimod) =2.68; site 3 (PDL alone) =2.37; and site 4 (untreated PWS) = −1.63.
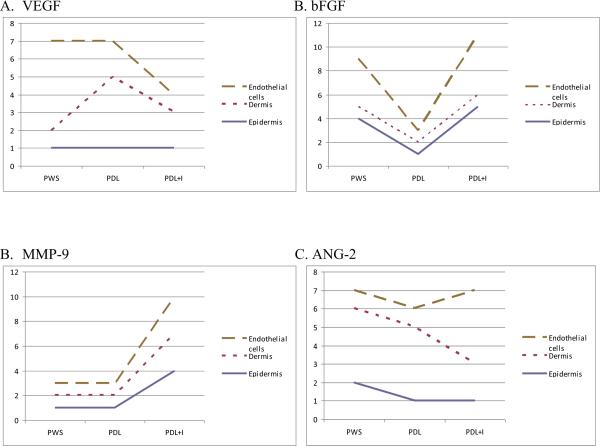
Table VI summarizes the data, from a single subject, evaluating the immunohistochemical assessment of angiogenesis mediators of an untreated PWS compared to PWS treated with PDL alone and also PDL+ imiquimod. As expected, the degree of staining of the mediators (VEGF, bFGF, MMP-9, ANG-2) found in the epidermis was lower compared to dermal staining; this was especially true for VEGF. VEGF staining was decreased in both endothelial cells and the dermis with PDL+ imiquimod compared to PDL alone. bFGF was was found in all 3 structures (epidermis, dermis, endothelial cells) and it was found in lower levels with the PDL alone compared to PDL + imiquimod. With MMP-9 there were low levels of staining in an untreated PWS and no change was seen with treatment of the PDL. The MMP-9 increased in the PDL + imiquimod sample. ANG-2 levels decreased with PDL, although there was a variable response seen with PDL + imiquimod.
TABLE VI.
Immunohistochemistry of angiogenic mediators
 |
Adverse events
The treatment was well tolerated by all subjects. Two subjects receiving PDL + imiquimod required a (one and four week) rest period beginning at the second week post-PDL therapy, due to mild erythema and crusting. Both of these subjects (ages 14 and 21) demonstrated only mild sun damage on clinical exam. After the rest period, imiquimod dosing was resumed without incident. No other adverse effects were reported.
DISCUSSION
Our preliminary findings offer important considerations for therapeutic applications of light and may indicate an important paradigm shift for this field. Based on the average Δa* values, the addition of imiquimod post PDL improved the reduction of erythema. Average ΔE values suggest improved efficacy with PDL + imiquimod compared to PDL alone. Imiquimod appears to minimize post-laser treatment angiogenesis. The addition of imiquimod was safe, few minor adverse events were reported during the study.
Only two subjects reported irritation. Application of the imiquimod more than three times a week may have resulted in more subjects with irritation. We chose the three times a week application because this regimen specifically has been reported to have anti-angiogenic effect.22
Our study size was small and the treatment areas were not uniform between patients, but each measurement site was independently monitored. The laser settings were variable but the calculation of Δa and ΔE allowed for each site to be measured independently. Finally the duration of effect of our results is not known as the study was only 8 weeks long. Further experiments are required, but the initial results are intriguing and suggest that treatment optimization should focus on both initial vascular destruction and modulation of the biological repair processes.
The anti-angiogenic effect of imiquimod occurs through the activation of toll-like receptor 7 (TLR 7). TLR 7 induces anti-angiogenic cytokines (IFN-alpha, IL-10, IL-12, IL-18), reduces angiogenic stimulators (MMP-9 and bFGF), and locally upregulates endogenous inhibitors of MMP (TIMP). In addition, imiquimod induces endothelial cell apoptosis. This cascade of events has the potential to halt the post-laser treatment vessel recurrence seen in PWS birthmarks. Imiquimod has been used successfully as a single-agent treatment for hemangiomas, thus one might consider whether imiquimod alone would be effective in treating PWS.14 PWS are stable vascular lesions consisting of dermal, dilated, capillary-like vessels with no abnormal endothelial proliferation.23 In contrast, hemangiomas or other benign vascular tumors, are characterized by rapid vascular proliferation that may be followed by involution.24–27 Due to the slowly proliferating nature of PWS vasculature, it is likely the use of an anti-angiogenic agent alone would have limited effect on PWS vessels. Addition of an anti-angiogenic agent is more useful as an adjunct to PDL induced selective photothermolysis, which is effective for acute destruction of PWS vasculature, but limited by vessel repair during the wound healing phase.
Other anti-angiogenic agents may have utility in treatment of cutaneous vascular lesions. Imiquimod was chosen for this study because of its commercial availability, ease of topical administration, and good safety record. Our results are statistically significant but we do think even more impressive results may be obtained as studies reveal which angiogenesis mediators are stimulated by laser therapy and thus, should be targeted for reduction.
Recently there has been research directed toward the anti-angiogenic effect of rapamycin (Pfizer, New York, NY), an immunosuppressive medication with inhibitory action against the mammalian target of rapamycin (mTOR).23,28–30 Using an in vivo window chamber model (rodent and hamster), investigators have demonstrated significant decrease in revascularization with laser (PDL or Nd:YAG) and topical rapamycin compared to laser alone.23,29 When topical rapamycin was applied to normal human skin in situ, similarly there was suppression of reformation and reperfusion of vessels in the area treated.30 Other macrolides inhibiting the mTOR pathway have been evaluated including tacrolimus (Astellas Pharma, Deerfield, Illinois) and temsirolimus (Pfizer). There is emerging evidence that temsirolimus may have increased solubility and thus have superior efficacy compared to rapamycin.31 Further evaluation of this family of angiogenesis inhibitors is needed.
In this protocol, the immunohistochemical analysis of angiogenesis mediators was limited in that only a single subject was evaluated. The initial trends confirm that known angiogenesis mediators are present in the dermis and endothelial cells of untreated PWS and are modified by PDL and imiquimod. Further evaluation of angiogenesis promoters and inhibitors in unmanipulated and post-treatment tissue is necessary. Additional research may indicate potential therapeutic targets for a wide range of dermatologic diseases including benign vascular tumors (hemangiomas, angiofibromas), malignant vascular tumors (Kaposi's sarcoma, angiosarcoma) and inflammatory conditions with a prominent vascular component (rosacea, psoriasis). Successful therapies targeting angiogenesis have been developed and are now a standard-of-care treatment in oncology and ophthalmology. Use of anti-angiogenic agents for treatment of skin conditions has been limited, but this is now changing and it is likely that angiogenesis targeting therapies will play an increasing role in dermatology.
Other methods of vascular destruction (including other lasers or photodynamic therapy) could also be combined with post-treatment anti-angiogenic therapy in an effort to enhance results. Safety and efficacy of other combined approaches should also be studied in the future.
In summary, we provide preliminary data that treatment efficacy of selective photothermolysis of PWS may be enhanced by post-treatment application of imiquimod, an immunomodulating agent with anti-angiogenic activity. This combined therapy may have significant impact in the fields of biomedical optics and dermatology.
Acknowledgements
This work was supported in part by grants obtained from the National Institutes of Health (AR51443 to KMK), the A. Ward Ford foundation (BC) and Graceway Pharmaceuticals. Imiquimod and placebo cream were provided by Graceway Pharmaceuticals. Institutional support was provided by the National Institutes of Health Laser Microbeam and Medical Program (LAMMP, a P41 Technology Research Resource) and the Arnold and Mabel Beckman Foundation.
Funding Sources: This work was supported in part by grants obtained from the American Society for Laser Medicine and Surgery (KMK) and Graceway Pharmaceuticals. Imiquimod and placebo cream were provided by Graceway Pharmaceuticals. Institutional support was provided by the National Institutes of Health Laser Microbeam and Medical Program (LAMMP, a P41 Technology Research Resource), NIH EB009571 (BC) and the Arnold and Mabel Beckman Foundation.
Kristen M. Kelly: Grants from Graceway, Candela, DUSA. Equipment from Candela, Solta Medical, and DUSA
Abbreviations and Acronyms
- PDL
Pulsed dye laser
- PWS
Port wine stain
- FDA
Food and Drug Administration
- LSI
Laser speckle imaging
- TLR7
Toll-like receptor 7
- IFN
Interferon
- IL
Interleukin
- TIMP
Tissue-Inhibitors of Metalloproteinases
- MMP
Matrix-Metalloproteinases
- VEGF
vascular endothelial growth factor
- bFGF
Basic fibroblast growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Anne Marie Tremaine, Jennifer Armstrong, Arisa Ortiz, Bernard Choi, Yu-Chih Huang and Ronald Harris: None
REFERENCES
- 1.Van Aalst JA, Bhuller A, Sadove AM. Pediatric vascular lesions. J Craniofac Surg. 2003;14:566–83. doi: 10.1097/00001665-200307000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–22. [PubMed] [Google Scholar]
- 3.Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol. 2007;57:677–82. doi: 10.1016/j.jaad.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Huikeshoven M, Koster PH, de Borgie CA, Beek JF, van Gemert MJ, van der Horst CM. Redarkening of port-wine stains 10 years after pulsed-dye-laser treatment. N Engl J Med. 2007;356:2745–6. doi: 10.1056/NEJMoa064329. [DOI] [PubMed] [Google Scholar]
- 5.Kelly KM, Choi B, McFarlane S, Motosue A, Jung B, Khan MH, et al. Description and analysis of treatments for port-wine stain birthmarks. Arch Facial Plast Surg. 2005;7:287–94. doi: 10.1001/archfaci.7.5.287. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 7.Choi B, Jia W, Channual J, Kelly KM, Lotfi J. The importance of long term monitoring to evaluate the microvasculature response to light-based therapies. J Invest Dermatol. 2008;128:485–8. doi: 10.1038/sj.jid.5700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagstaff AJ, Perry CM. Topical imiquimod: a review of its use in the management of anogenital warts, actinic keratoses, basal cell carcinoma and other skin lesions. Drugs. 2007;67:2187–210. doi: 10.2165/00003495-200767150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Célestin Schartz NE, Chevret S, Paz C, Kerob D, Verola O, Morel P, et al. Imiquimod 5% cream for treatment of HIV-negative Kaposi's sarcoma skin lesions: A phase I to II, open-label trial in 17 patients. J Am Acad Dermatol. 2008;58:585–91. doi: 10.1016/j.jaad.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Li V, Li W, Talcott K, Zhai A. Imiquimod as an antiangiogenic agent. J Drugs Dermatol. 2005;4:708–17. [PubMed] [Google Scholar]
- 11.Marra D, Haynes HA, Li V. Antiangiogenic treatment of pyogenic granuloma with imiquimod. J Am Acad Dermatol. 2004;50(Supp P57) [Google Scholar]
- 12.Welsh O, Olazaran Z, Gomez M, Salas J, Berman B. Treatment of infantile hemangiomas with short-term application of imiquimod 5% cream. J Am Acad Dermatol. 2004;51:639–42. doi: 10.1016/j.jaad.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Hazen P, Carney J, Engstrom C, Turgeon K, Reep M, Tanphaichitr A. Proliferating hemangioma of infancy: successful treatment with topical 5% imiquimod cream. Pediatr Dermatol. 2005;22:254–6. doi: 10.1111/j.1525-1470.2005.22318.x. [DOI] [PubMed] [Google Scholar]
- 14.Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int J Dermatol. 2005;44:14–9. doi: 10.1111/j.1365-4632.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 15.Alaluf W, Atkins D, Barrett K, Blount M, Carter N, Heath A. The impact of epidermal melanin on objective measurements of human skin color. Pigment Cell Res. 2002;15:119–26. doi: 10.1034/j.1600-0749.2002.1o072.x. [DOI] [PubMed] [Google Scholar]
- 16.Jung B, Choi B, Shin Y, Durkin AJ, Nelson JS. Determination of optimal view angles for quantitative facial image analysis. J Biomed Opt. 2005;10:024002. doi: 10.1117/1.1895987. [DOI] [PubMed] [Google Scholar]
- 17.Jung B, Choi B, Durkin AJ, Kelly KM, Nelson JS. Characterization of port wine stain skin erythema and melanin content using cross-polarized diffuse reflectance imaging. LasersSurg Med. 2004;34:174–81. doi: 10.1002/lsm.10242. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Kim M, Jung B, Choi B, Verkruysse W, Jeong MY, et al. Determination of an optimized conversion matrix for device independent skin color image analysis. Lasers Surg Med. 2005;37:138–43. doi: 10.1002/lsm.20219. [DOI] [PubMed] [Google Scholar]
- 19.Chang CJ, Hsiao YC, Mihm MC, Nelson JS. Pilot study examining the combined use of pulsed dye laser and topical imiquimod versus laser alone for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40:605–10. doi: 10.1002/lsm.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Horst CM, Koster PH, de Borgie CA, Bossuyt PM, van Gemert MJ. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye. Engl J Med. 1998;338:1028–33. doi: 10.1056/NEJM199804093381504. [DOI] [PubMed] [Google Scholar]
- 21.Huang YC, Ringold TL, Nelson JS, Choi B. Noninvasive blood flow imaging for real-time feedback during laser therapy of port wine stain birthmarks. Lasers Surg Med. 2008;40:167–73. doi: 10.1002/lsm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li VW, Li WW, Talcott KE, Ahai AW. Imiquimod as an antiangiogenic agent. J Drugs Dermatol. 2005 Nov-Dec;4(6):708–17. [PubMed] [Google Scholar]
- 23.Phung TL, Oble DA, Jia W, Benjamin LE, Mihm MC, Jr., Nelson JS. Can the wound healing response of human skin be modulated after laser treatment and the effects of exposure extended? Implications on the combined use of the pulsed dye laser and a topical angiogenesis inhibitor for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40:51. doi: 10.1002/lsm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paller A, Mancini A. Hurwitz Clinical Pediatric Dermatology. 4th edition Saunders; New York, NY: 2011. Vascular disorders of infancy and childhood; pp. 307–344. [Google Scholar]
- 25.Bruckner AL, Frieden IJ. Hemangiomas of infancy. J Am Acad Dermatol. 2003;48:477–93. doi: 10.1067/mjd.2003.200. [DOI] [PubMed] [Google Scholar]
- 26.Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol. 2008;58:261–85. doi: 10.1016/j.jaad.2007.10.492. [DOI] [PubMed] [Google Scholar]
- 27.Dohil MA, Baugh WP, Eichenfield LF. Vascular and pigmented birthmarks. Pediatr Clin North Am. 2000;47:783–812. doi: 10.1016/s0031-3955(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 29.Jia W, Sun V, Tran N, Choi B, Liu SW, Mihm MC, Jr, et al. Long-term blood vessel removal with combined laser and topical rapamycin antiangiogenic therapy: implications for effective port wine stain treatment. Laser Surg Med. 2010;42:105–12. doi: 10.1002/lsm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loewe R, Oble DA, Valero T, Zukerberg L, Mihm MC, Nelson JS. Stem cell maker upregulation in normal cutaneous vessels following pulsed-dye laser exposure and its abrogation by concurrent rapamycin administration: implications for treatment of port-wine stain birthmarks. J Cutan Pathol. 2010;37(Supp 1):76–82. doi: 10.1111/j.1600-0560.2010.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia W, Sun V, Liu T, Choi B, Nelson S. Comparison of antiangiogenic agents for inhibiting reperfusion of photocoagulated blood vessels in an animal model [abstract]. Proceedings of the 2011 Annual Conference of the American Society for Laser Medicine and Surgery; 2011 Apr 1–3; Grapevine TX: ASLMS; p. 35. 2011. Abstract nr 9. [Google Scholar]