Abstract
Cancer pain is an ever-present public health concern. With innovations in treatment, cancer patients are surviving longer, but uncontrollable pain creates a poor quality of life for these patients. Oral cancer is unique in that it causes intense pain at the primary site and significantly impairs speech, swallowing, and masticatory functions. We propose that oral cancer pain has underlying biologic mechanisms that are generated within the cancer microenvironment. A comprehensive understanding of key mediators that control cross-talk between the cancer and peripheral nervous system, and possible interventions, underlies effective cancer pain management. The purpose of this review is to explore the current studies on oral cancer pain and their implications in clinical management for cancer pain in general. Furthermore, we will explore the endogenous opioid systems and novel cancer pain therapeutics that target these systems, which could solve the issue of opiate tolerance and improve quality of life in oral cancer patients.
Keywords: oral cancer, head and neck cancer, cancer pain, cancer pain therapy, opiates, endogenous opioids
Quality-of-Life Studies in oral Cancer Patients Establish Predictors of Poor Outcome
Most cancer patients will experience uncontrollable pain that creates a poor quality of life and limits normal function (Connelly and Schmidt, 2004). For oral cancer patients, pain is rated as the worst symptom, and impairs a patient’s speech, swallowing, eating, drinking, and interpersonal relations (Bjordal et al., 2001). Oral cancer patients experience pain early in the disease. In fact, orofacial pain is the initial symptom that leads to the diagnosis of oral squamous cell carcinoma (SCC) in patients (Marshall and Mahanna, 1997). When compared with oral pre-cancerous lesions for which pain is absent, oral cancerous lesions produce intense orofacial pain exacerbated by function (Lam and Schmidt, 2011). As adjuvant chemotherapy and radiation techniques improve, oral cancer patients are living longer. The overall five-year survival rate for oral cancer patients is 60%. With the improved survival rate, there is an increase in the burden of pain that oral cancer patients must bear. Therefore, oral cancer management requires palliative care to address cancer-associated symptoms. Effective palliative care lies in accurate characterization of symptoms, which requires careful analysis of the available quality-of-life studies. Currently, several quality-of-life studies are available that survey cancer-associated symptoms in oral cancer patients. The unifying conclusion in all published studies is that, outside of survival, pain is the most important concern for oral cancer patients.
The character, severity, and unique features of oral cancer pain likely reflect the anatomy of the oral cavity, the continuous need for orofacial function, the biologic characteristics of oral squamous cell carcinoma (SCC), and the interaction between the carcinoma and the peripheral nervous system. The dense trigeminal innervation of the oral cavity effectively localizes pain at the primary site, whereas cancers of other primary sites, such as the gastrointestinal tract or pelvis, tend to be more visceral (Rigor, 2000). In a meta-analysis of 52 studies that calculated prevalence of cancer pain, head and neck cancer had the highest prevalence of pain, surpassing gynecological, gastrointestinal, lung, breast, and congenital cancer (van den Beuken-van Everdingen et al., 2007). The functional requirements and mechanical stimulation of oral structures during speech, mastication, and swallowing result in severe pain. In oral cancer patients evaluated with the UCSF Oral Cancer Pain Questionnaire, patients consistently report significantly higher function-related, rather than spontaneous, pain intensity and sharpness (Connelly and Schmidt, 2004). They also report sensitivity to touch and restriction of function due to pain. The intensity of pain worsens with oral cancer progression, and patients experience increased functional restriction upon nodal metastasis. Complete surgical resection of the cancer provides near-total relief, which points to the carcinoma and associated cells in the microenvironment as the source of pain (Kolokythas et al., 2007). Oral SCC produces numerous nociceptive mediators that sensitize primary afferent nociceptors in the cancer microenvironment, as we discuss below (Schmidt et al., 2010). The combination of orofacial anatomy and function along with molecular features of oral SCC produces the pain experienced by the oral cancer patient. Although surgical resection alleviates cancer pain, more effective analgesics are still needed; pre-surgical patients often experience extended periods of severe pain, some cancers cannot be excised, some patients are too sick to have surgery, and many patients develop an untreatable recurrence of the cancer or a second primary cancer. A small subset of cancer patients also develop central sensitization resulting in intractable neuropathic pain; however, the incidence in head and neck cancer is unclear (Epstein et al., 2007).
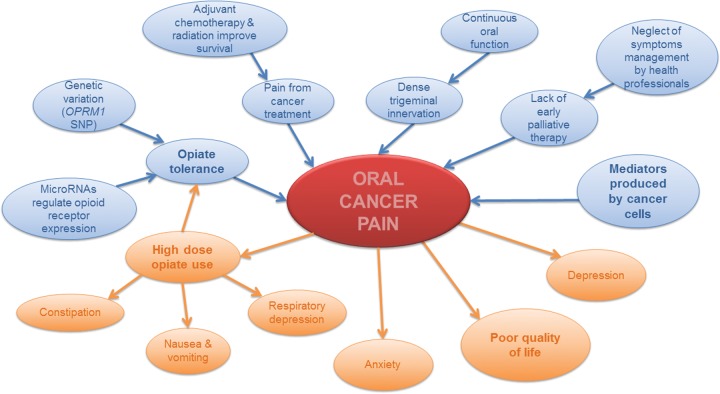
Current palliative care regimens for patients with advanced, incurable cancer include analgesics and neuroleptics; escalating doses of these medications are needed and often become ineffective. Opiates remain the gold standard for treatment of cancer pain, but are often not effective, especially with disease progression and once tolerance develops. The Opioid Escalation Index, which measures the mean increase of the starting opioid dosage, is higher in head and neck cancer than in other cancers, such as lung, breast, pancreas, and gastrointestinal (Mercadante et al., 1997). Studies in other cancers suggest that adding NSAIDs to the treatment regimen improves pain control. A prospective study of 156 cancer patients showed that cancer patients who received ketorolac in addition to opioids had better analgesia and slower opioid escalation than control cancer patients (Mercadante et al., 2002). However, the effects of NSAIDs on oral cancer pain are unknown. The high doses of opiates required for oral cancer pain cause nausea, vomiting, constipation, and respiratory depression, which further reduce the quality of life and can increase mortality. Furthermore, opioid-induced hyperalgesia can develop, causing the paradoxical response of increased pain rather than pain relief (Ramasubbu and Gupta, 2011). Ineffective pain control correlates to worsening depression in patients (Fischer et al., 2010). Multicenter studies have revealed that up to 80% of cancer patients, when evaluated with the Pain Management Index (PMI), experience ineffective pain control with the prescribed doses of analgesics (Di Maio et al., 2004). Cancer pain, particularly oral cancer pain, is associated with marked misery once the cancer cannot be controlled. Effective oral cancer pain management remains elusive due to an incomplete understanding of the neural mechanisms responsible for cancer pain and opiate tolerance, and the lack of new drugs (Fig. 1).
Figure 1.
Causes and consequences of oral cancer pain. The diagram depicts causes of oral cancer pain in blue and consequences in orange. Based on the current laboratory and clinical studies explored in this review, there are 5 main causes that initiate or exacerbate oral cancer pain: (1) mediators in the cancer microenvironment, (2) lack of early palliative therapy, (3) dense trigeminal innervation and continuous oral function, (4) pain from cancer treatment, and (5) opiate tolerance. Oral cancer results in a constellation of symptoms that reduce quality of life, with pain being the most prominent symptom. Pain in turn leads to other symptoms, including anxiety, depression, and side-effects of high-dose opiate use.
While quality-of-life studies reveal significant levels of pain, dysfunction, and anxiety in oral cancer patients, current clinical trials and treatment strategies do not effectively address these symptoms. Clinical health care professionals focus on preparation for surgery and issues of the immediate post-operative period, whereas symptom management is neglected (Chen et al., 2010). Furthermore, palliative care is initiated only for end-stage cancer patients. The mean time from initiation of palliative care to death is 21.9 days in head and neck cancer patients, suggesting that incurable patients may be referred to palliative care institutions too late. The majority of patients (85%) admitted to palliative care had inadequate pain control prior to admission (Lin et al., 2011).
A recent randomized clinical trial compared the effects of early palliative care in addition to standard oncologic care vs. standard oncologic care alone in 151 patients with metastatic non-small-cell lung cancer. The study compared anxiety and depression by the Hospital Anxiety and Depression Scale (HADS) and quality of life according to the Functional Assessment of Cancer Therapy-Lung (FACT-L) scale, and concluded that patients in the early palliative care group had significantly fewer depressive symptoms (16% vs. 38%). Median survival was significantly longer among patients receiving early palliative care, despite the fact that fewer patients in the early palliative care group received aggressive end-of-life care (Temel et al., 2010). The study demonstrates that cancer symptom control improves quality of life, increases survival, and conserves dwindling health care resources.
Mediators of Oral Cancer Pain
Oral cancer pain is sustained by the secretion of nociceptive mediators into the cancer microenvironment. Our review of mediators will include those with direct evidence of nociceptive activity in in vivo oral cancer models. We will discuss endothelin-1 (ET-1), proteases, and nerve growth factor. Other mediators that have been implicated in cancer pain, but for which cause and effect have not been demonstrated and will not be discussed, include protons, transient receptor potential vanilloid (TRPV), substance P, calcitonin gene-related peptide (CGRP), ATP, and bradykinin (Schmidt et al., 2010). Inflammation, a hallmark of cancer, contributes to cancer pain; however, the contribution of the cancer is distinct from the contribution of inflammation to oral cancer pain (Harano et al., 2010). We will therefore focus on the mediators produced and secreted by the cancer.
Endothelin-1: A Dual Role in Oral Cancer Pain
The role of ET-1 in oral cancer pain is complex. The nociceptive effects of ET-1 depend on the location of the two endothelin receptor subtypes and the action of those receptor subtypes upon ligand binding. ET-1 is a potent vasoactive peptide that produces nociceptive behavior upon injection in animals and humans (Hans et al., 2007). ET-1 also drives cancer pain (Davar, 2001). ET-1 binds to two G-protein-coupled receptors, the endothelin-A receptor (ETAR) and the endothelin-B receptor (ETBR). ETARs are distributed on peripheral sensory neurons; ETBRs are expressed on non-myelinating Schwann cells of the sciatic nerve and dorsal root ganglion satellite cells (Peters et al., 2004) as well as on keratinocytes, which are known to secrete opioids upon binding and activation.
Patients with oral SCC have high levels of ET-1 in the cancer microenvironment (Pickering et al., 2007; Schmidt et al., 2007) and report severe functional pain upon mechanical stimulation. To parallel the mechanical allodynia that is observed in human oral SCC patients, Pickering and colleagues established a mouse model of cancer pain by inoculating a human oral tongue SCC into the mouse hind paw (Pickering et al., 2008). The role of ET-1 in oral cancer pain has been confirmed and characterized in this mouse model, and the ET-1 concentration has been shown to be a more important factor than tumor volume in establishing cancer pain.
Proteases and Protease-activated Receptors
Proteolytic activity is critical to carcinogenesis, and the cancer microenvironment is replete with both proteases and proteolytic peptide products. Cancer-associated trypsin has been identified in cancers such as ovarian carcinoma, pancreatic cancer, hepatocellular and cholangiocarcinomas, lung neoplasms, colorectal cancers, fibrosarcoma, leukemia, gastric cancer, and oral cancer (Nyberg et al., 2006). Proteases activate cell-surface receptors on primary afferent nociceptors within the cancer microenvironment, either directly or via their peptide products.
Protease-activated receptors (PARs) belong to a family of G-protein-coupled receptors (PAR1 to PAR4) that are activated by proteolytic cleavage. Such cleavage can result from a number of different enzymes, including serine proteases, trypsin, and tryptase. Cleavage exposes a tethered ligand that binds the receptor and initiates signal transduction (Russo et al., 2009). PAR2 activates multiple second-messenger pathways, which sensitize TRPV1 and TRPV4 receptors on nociceptive afferents and result in TRPV1-dependent thermal hyperalgesia and TRPV4-dependent mechanical allodynia, respectively (Amadesi et al., 2006).
PAR2 has recently been implicated in cancer pain by pharmacologic, behavioral, biochemical, and genetic approaches (Lam and Schmidt, 2010). Proteases capable of activating PAR2 on sensory neurons are recovered in the supernatants of human oral SCC cells. Injection of supernatants alone without cancer cells causes marked and prolonged mechanical allodynia in mice. This nociceptive effect is abolished by serine protease inhibition, diminished by mast cell depletion, and absent in PAR2 knockout mice. Serine proteases, such as trypsin from cancer cells and tryptase from mast cells, both of which can activate PAR2, contribute to cancer pain. Fibroblasts in the surrounding stroma of oral carcinoma also produce trypsin. Chronic exposure to serine proteases secreted by human cancer up-regulates PAR2 levels in peripheral neurons (Lam and Schmidt, 2010). The continual release of serine proteases from cancer and non-malignant cells in the microenvironment could produce ongoing excitation of primary nociceptive afferents, leading to mechanical allodynia in oral cancer patients.
Nerve Growth Factor
In the microenvironment of many cancers, sensory neurons are chronically exposed to nerve growth factor (NGF), which is normally secreted to promote the local growth and survival of afferent sensory neurons. Acute peripheral administration of NGF leads to thermal hyperalgesia, whereas chronic administration produces mechanical allodynia. Similarly, a transgenic mouse engineered to overexpress NGF exhibits mechanical hypersensitivity (Davis et al., 1993). Signals from NGF are mediated via a high-affinity receptor tyrosine kinase (TrkA) and a low-affinity p75 receptor on the neuronal membrane (Friedman, 1999). NGF and its high-affinity TrkA receptor can also facilitate proliferation and invasion of multiple cancers, including breast, prostate, pancreatic, and oral cancers (Zhu et al., 1999). Expression of NGF by cancer and regulation of both high- and low-affinity receptors have also been extensively investigated (Kolokythas et al., 2010). NGF secretion by cancer cells into the microenvironment likely leads to a number of changes that contribute to pain. One possible mechanism of NGF-induced cancer pain is its association with perineural involvement, a pathologic term for the invasion and proliferation of a cancer within a nerve, associated with pain and recurrence following surgical resection. NGF is associated with perineural invasion in adenoid cystic carcinoma, a salivary gland malignancy known for its neurotropism, as well as in pancreatic and oral cancer (Ma et al., 2008). Both NGF mRNA and protein levels are also significantly higher in cancer tissues from oral cancer patients and in oral SCC culture (Ye et al., 2011). Anti-NGF with a monoclonal antibody reduces cancer pain and restores function in a bone sarcoma rat model (Sevcik et al., 2005). Furthermore, NGF blockade in two separate mouse oral cancer models decreases tumor proliferation, nociception, and weight loss through modulation of pro-inflammatory cytokines and leptin production. NGF blockade also decreased expression of TRPV1, TRPA1, and PAR-2 nociceptive receptors (Ye et al., 2011). These results unveil anti-NGF as a novel therapeutic for two of the most obstinate oral cancer symptoms, especially in its later stages, namely, pain and cachexia.
Opiate Tolerance
In addition to the above-mentioned nociceptive mediators that are secreted by cancers and activate peripheral afferent nerves, neurologic pathways producing and maintaining opiate tolerance contribute to unrelenting cancer pain. Head and neck cancers are associated with an Opioid Escalation Index higher than that for all other cancers (Mercadante et al., 1997). Oral cancer patients quickly develop opiate tolerance. While opiate tolerance has been extensively studied, successful intervention remains elusive.
Genetic variation of the mu-opioid receptor gene (OPRM1) has been proposed as a possible mechanism for individual variability in response to opiates. A single-nucleotide polymorphism (SNP) 118A>G leads to an exchange of the amino acid asparagine (N) to aspartic acid (D) at position 40 of the extracellular receptor region that alters opioid response in different regions of the brain (Oertel et al., 2009). The most profound effects were in brain regions involved in sensory processing of pain intensity. Specifically, the N40D mu-opioid receptor variant had a reduction of agonist-induced receptor signaling efficacy in the secondary somatosensory area (SII) of post mortem human brain tissue. The mu-opioid receptor-specific agonist DAMGO was only 62% as efficient in homo- and heterozygous carriers of the 118G allele compared with homozygous carriers of the wild-type 118A allele. A mouse model was created with the corresponding human mu-opioid receptor SNP and demonstrated reduced mRNA expression, receptor protein levels, and morphine-mediated anti-nociception compared with wild-type mice (Mague et al., 2009). The effect of the mu-opioid receptor 118A>G SNP was analyzed clinically in post-operative pain control in patients undergoing oral surgery. Sixty patients who underwent sagittal split mandibular osteotomies were enrolled into the study. The patients represented 5 SNPs representative of 4 linkage disequilibrium blocks of the mu-opioid receptor gene. Pain perception before and after the administration of fentanyl, and pre-operative state and trait anxiety measured by STAI, were recorded. The results showed that fentanyl was less effective in individuals with the 118A>G SNP. Furthermore, there was no correlation between state and trait anxiety scores and post-operative fentanyl use. In a separate study, the 118A>G SNP reduced fentanyl-induced analgesia during anesthesia and recovery (Wu et al., 2009).
In addition to mu-opioid receptor gene SNP variations to opioid response, microRNAs (miRNAs) have been identified as a regulatory mechanism for the mu-opioid receptor mRNAs. miRNAs are small non-coding RNA molecules that exert their effect by base-pairing with complementary sequences in the 3′-UTR region of target mRNAs. Binding of miRNAs to these regions results in decreased polypeptide formation from the mRNAs. Hundreds of miRNAs have been identified in humans. In particular, miRNAs function in the nervous system to effect changes in neuronal development, plasticity, metabolism, and apoptosis (Kosik, 2006). One recent study examined the role of let-7 miRNAs in in vitro and in vivo models of opioid tolerance. The level of let-7 miRNA was inversely correlated to the levels of mu-opioid receptor mRNA. Inhibiting let-7 expression resulted in an increase in mu-opioid receptor levels, and, conversely, let-7 expression was regulated by chronic treatment with morphine in both cell culture and the animal model. Knocking down let-7 attenuated the anti-nociceptive tolerance to morphine (He et al., 2010). These results implicate miRNAs as a possible mechanism in opiate tolerance, since previous studies have shown that down-regulation of the mu-opioid receptor occurs with chronic morphine treatment and contributes to tolerance (Bernstein and Welch, 1998).
Endogenous Opioid Systems
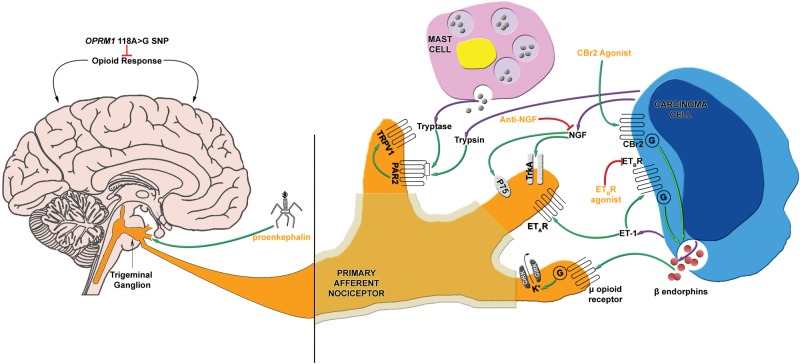
Endogenous analgesia through the production of endogenous opioid peptides has been widely explored as a possible solution to opiate dependence and withdrawal. The promise of endogenous opioid peptides is that they exhibit analgesic abilities like opiate drugs, but do not generate the same adverse effects such as opiate withdrawal (Fig. 2).
Figure 2.
Mediators of oral cancer pain and current novel analgesic therapy. Mediators that have a known role in oral cancer pain include: ET-1, which activates ETAR and ETBR; proteases, which activate PAR2; and NGF, which activates p75 and TrkA receptors. In addition to nociceptive mediators, the OPRM1 118A>G SNP affects opioid response, which in turn affects cancer pain. Currently established endogenous analgesic mechanisms include the endogenous opioid and cannabinoid systems. Release of beta-endorphin, an endogenous opioid, could be stimulated by activation of the endothelin axis (ETBR) or cannabinoid system (CBr2). There are several potential analgesic therapies targeting oral cancer pain. These include virus-mediated gene transfer to express proenkephalin, anti-NGF with a neutralizing antibody, and ETBR agonism.
Opioid Receptors and Peptides
In 1973, opioid receptors were identified by three separate groups (Pert and Snyder, 1973; Simon et al., 1973; Terenius, 1973). Since then, the endogenous opioid system has become well-characterized. Molecular characterization and cloning of the mu-, delta-, and kappa-opioid receptors occurred in the 1990s (Chen et al., 1993). Opioid receptors are G-protein-coupled receptors belonging to the subfamily of rhodopsin. They are comprised of 7 alpha-helical transmembrane domains and an extracellular N-terminus with multiple glycosylation sites (Law et al., 2000). Mu-, delta-, and kappa-opioid receptors are highly homologous to each other but are diverse at the N- and C-termini and extracellular loops (Pil and Tytgat, 2003). Activation of opioid receptors causes inhibition of cAMP production and voltage-gated calcium-channels, and stimulation of inwardly rectifying potassium channels and the MAP kinase pathway. These combined effects result in inhibition of neuronal activity.
Endogenous opioid peptides are derived from either the pro-opiomelanocortin (POMC), pro-enkephalin (PENK), or pro-dynorphin (PDYN) family. These precursors form the final active peptides, beta-endorphin, met-enkephalin, leu-enkephalin, dynorphin, and neo-endorphin (Przewlocki and Przewlocka, 2001). Each endogenous opioid peptide exhibits different affinities for the 3 opioid receptors. Opioid receptors and endogenous opioid peptides are widely distributed in the central nervous system (CNS) and peripheral tissues (Niikura et al., 2010). They are also present in peripheral neurons and can contribute to anti-nociception.
Many studies have shown that endogenous opioids in the central nervous system are involved in the anti-nociceptive process. Beta-endorphin administration to the lateral brain ventricle and intrathecally produces a strong anti-nociceptive response that is blocked by naloxone (Przewlocki and Przewlocka, 2001). Interestingly, however, endogenous opioids are also expressed in non-neuronal, peripheral tissues, like immunocytes and keratinocytes, and could result in an anti-nociceptive effect (Khodorova et al., 2003). Keratinocytes have been identified as a source of beta-endorphin production. Activation of the endothelin B receptor (ETBR) and cannabinoid 2 receptor (CBr2) in keratinocytes induces secretion of beta-endorphin and elicits anti-nociception in vivo (Khodorova et al., 2003; Ibrahim et al., 2005). More importantly for cancer pain, oral SCC cells, which are malignant keratinocytes, also secrete endogenous opioids.
Endothelin Receptors and Opioid Release
Several studies have proposed harnessing endogenous opioids as pharmacologic solutions to opiate tolerance. Specifically, one study demonstrated that treatment with an ETBR agonist in a mouse oral SCC model results in mechanical anti-nociception through secretion of beta-endorphin (Quang and Schmidt, 2010b). Oral SCC consists of malignant keratinocytes that bear ETBRs and secrete opioids which can modulate the activity of the surrounding primary afferent nociceptors in skin. In addition, ET-1 activation of ETBRs on keratinocytes leads to analgesia that is reversed with naloxone, implicating the keratinocytes as a source of opioid released upon ETBR activation (Khodorova et al., 2002, 2003).
Surprisingly, in parallel with the role of ETBR activation, increased production of β-endorphin and leu-enkephalin occurs in oral SCC cell culture treated with an endothelin A receptor (ETAR) antagonist (Quang and Schmidt, 2010a). In the cancer mouse model, significant mechanical nociception begins at 4 days after inoculation of SCC cells and lasts up to 30 days when the mice are sacrificed. Local administration of naloxone methiodide significantly blocks the anti-nociceptive effect of the ETBR agonist or ETAR antagonist.
Modulation of ET-1 receptors in the management of cancer pain might have additional benefits. ETAR antagonism has been shown to prevent morphine tolerance (Bhalla et al., 2003). Theoretically, the combination of ETAR antagonism, which produces anti-nociception and simultaneously prevents morphine tolerance, and ETBR agonism, which leads to local opioid release, might hold promise for the treatment of oral cancer pain.
Cannabinoid Receptors and Endogenous Analgesia
Another endogenous analgesic mechanism that could be exploited for oral cancer pain treatment is the cannabinoid system. The two cannabinoid receptors, CBr1 and CBr2, both contribute to analgesia. CBr1 is expressed at central and peripheral nerve terminals and in keratinocytes after being synthesized in dorsal root ganglia (Munro et al., 1993). CBr2 is found on immune cells and keratinocytes (Ibrahim et al., 2006). CBr2 activation stimulates beta-endorphin release, leading to endogenous analgesia.
The link between cannabinoids and cancer pain was made by Kehl et al., who demonstrated that cannabinoids produce anti-nociception through CBr1 in a murine model of bone sarcoma pain (Kehl et al., 2003). The only soft-tissue carcinoma model exploring the analgesic effects of cannabinoids is by Guerrero et al., who created an oral cancer pain model by inoculating oral carcinoma cells into the hind paws of mice (Guerrero et al., 2008). These authors demonstrated that activation of CBr2 leads to the release of opioids from the carcinoma cells (Saghafi et al., 2011). Similar to the findings with the endothelin receptors, these findings target cannabinoid receptor activation as a possible endogenous approach to oral cancer pain treatment.
Opioid Gene Delivery
Fink and colleagues have made use of herpes simplex virus (HSV) vector-mediated endogenous opioid peptide gene delivery to dorsal root ganglia (DRG) in a rat model (Braz et al., 2001; Goss et al., 2001; Hao et al., 2009; Meunier et al., 2005). HSV vector-mediated gene delivery is ideal because it is a double-stranded DNA virus, and once it is inoculated into the epithelium, it is then carried by retrograde axonal transport to the DRGs, where the viral genome establishes a life-long latent state. Since chronic morphine treatment causes a decrease in met-enkephalin, and this effect likely contributes to morphine dependence, this group examined the effect of overexpressing pro-enkephalin in DRGs of a rat model using vector-mediated gene delivery. They inoculated a non-replicating HSV vector containing the pro-enkephalin gene, which produces met-enkephalin and leu-enkephalin, into the footpads of rats. Enkephalin acts through both the mu- and delta-opioid receptors. Overexpression of pro-enkephalin produced a significant decrease in nocifensive behavior to a formalin footpad test. This anti-nociceptive effect was reversed by naltrexone, an opioid receptor antagonist (Goss et al., 2001). Significant anti-nociceptive effects were also seen in animal models of arthritis induced by Freund’s adjuvant (Braz et al., 2001), facial pain (Meunier et al., 2005), bone cancer pain (Goss et al., 2002), and neuropathic pain induced by selective ligation of the L5 spinal nerve (Hao et al., 2009). The use of HSV-vector-mediated pro-enkephalin gene delivery has since gone on to phase 1 clinical trials. Cancer patients who had a less than 12-month projected survival and pain greater than 4 out of 10 on a visual analogue scale, despite treatment with greater than 200 mg/day of morphine, were enrolled into the study (Wolfe et al., 2009). The results regarding safety of virus injection and pain control are pending from this group.
To date, the published studies illustrate the emerging role of endogenous opioid peptides as an alternative treatment strategy for cancer pain. Because oral SCC consists of malignant keratinocytes, and keratinocytes are capable of secreting opioids, endogenous analgesia could serve as a viable treatment approach for oral cancer patients.
Conclusion
Oral cancer pain is unique due to its intense, function-related pain at the primary site. It is poorly responsive to opiates, and opiate tolerance is a significant clinical problem. If surgery is not curative, patients suffer progressive pain through their final months. An expansive understanding of oral cancer pain is not available; however, using rodent cancer models, investigators have been able to characterize the mechanisms within the cancer microenvironment that drive oral cancer pain. Novel pharmacological pain treatments targeted to mechanisms that are confined to the cancer microenvironment would avoid the systemic effects of opiates that are so debilitating and reduce the burden on care providers and the health care system.
Footnotes
This work was supported by National Institutes of Health grant R21 DE018561 and R01 DE019796.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, et al. (2006). Protease-activated receptor 2 sensitizes trpv1 by protein kinase cepsilon- and a-dependent mechanisms in rats and mice. J Physiol 575(Pt 2):555-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein MA, Welch SP. (1998). Mu-opioid receptor down-regulation and camp-dependent protein kinase phosphorylation in a mouse model of chronic morphine tolerance. Brain Res Mol Brain Res 55:237-242 [DOI] [PubMed] [Google Scholar]
- Bhalla S, Matwyshyn G, Gulati A. (2003). Endothelin receptor antagonists restore morphine analgesia in morphine tolerant rats. Peptides 24:553-561 [DOI] [PubMed] [Google Scholar]
- Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biorklund A, et al. (2001). A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope 111:1440-1452 [DOI] [PubMed] [Google Scholar]
- Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. (2001). Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci 21:7881-7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Yu WP, Chu TL, Hung HC, Tsai MC, Liao CT. (2010). Prevalence and correlates of supportive care needs in oral cancer patients with and without anxiety during the diagnostic period. Cancer Nurs 33:280-289 [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Yu L. (1993). Molecular cloning of a rat kappa opioid receptor reveals sequence similarities to the mu and delta opioid receptors. Biochem J 295(Pt 3):625-628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly ST, Schmidt BL. (2004). Evaluation of pain in patients with oral squamous cell carcinoma. J Pain 5:505-510 [DOI] [PubMed] [Google Scholar]
- Davar G. (2001). Endothelin-1 and metastatic cancer pain. Pain Med 2: 24-27 [DOI] [PubMed] [Google Scholar]
- Davis BM, Lewin GR, Mendell LM, Jones ME, Albers KM. (1993). Altered expression of nerve growth-factor in the skin of transgenic mice leads to changes in response to mechanical stimuli. Neuroscience 56:789-792 [DOI] [PubMed] [Google Scholar]
- Di Maio M, Gridelli C, Gallo C, Manzione L, Brancaccio L, Barbera S, et al. (2004). Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer 90:2288-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JB, Elad S, Eliav E, Jurevic R, Benoliel R. (2007). Orofacial pain in cancer: Part II—Clinical perspectives and management. J Dent Res 86:506-518 [DOI] [PubMed] [Google Scholar]
- Fischer DJ, Villines D, Kim YO, Epstein JB, Wilkie DJ. (2010). Anxiety, depression, and pain: differences by primary cancer. Support Care Cancer 18:801-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SM. (1999). Optic nerve avulsion secondary to a basketball injury. Ophthalmic Surg Lasers 30:676-677 [PubMed] [Google Scholar]
- Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. (2001). Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther 8:551-556 [DOI] [PubMed] [Google Scholar]
- Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X, et al. (2002). Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol 52:662-665 [DOI] [PubMed] [Google Scholar]
- Guerrero AV, Quang P, Dekker N, Jordan RC, Schmidt BL. (2008). Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci Lett 433:77-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans G, Deseure K, Robert D, De Hert S. (2007). Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett 418:117-121 [DOI] [PubMed] [Google Scholar]
- Hao S, Hu J, Fink DJ. (2009). Transgene-mediated enkephalin expression attenuates signs of naloxone-precipitated morphine withdrawal in rats with neuropathic pain. Behav Brain Res 197:84-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano N, Ono K, Hidaka K, Kai A, Nakanishi O, Inenaga K. (2010). Differences between orofacial inflammation and cancer pain. J Dent Res 89:615-620 [DOI] [PubMed] [Google Scholar]
- He Y, Yang C, Kirkmire CM, Wang ZJ. (2010). Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 30:10251-10258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. (2005). Cb2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA 102:3093-3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. (2006). Cb2 cannabinoid receptor mediation of antinociception. Pain 122:36-42 [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, et al. (2003). A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain 103:175-186 [DOI] [PubMed] [Google Scholar]
- Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. (2002). Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci 22:7788-7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, et al. (2003). Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med 9:1055-1061 [DOI] [PubMed] [Google Scholar]
- Kolokythas A, Connelly ST, Schmidt BL. (2007). Validation of the University of California San Francisco oral cancer pain questionnaire. J Pain 8:950-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokythas A, Cox DP, Dekker N, Schmidt BL. (2010). Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J Oral Maxillofac Surg 68:1290-1295 [DOI] [PubMed] [Google Scholar]
- Kosik KS. (2006). The neuronal microRNA system. Nat Rev Neurosci 7:911-920 [DOI] [PubMed] [Google Scholar]
- Lam DK, Schmidt BL. (2010). Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain 149:263-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Schmidt BL. (2011). Orofacial pain onset predicts transition to head and neck cancer. Pain 152:1206-1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. (2000). Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40:389-430 [DOI] [PubMed] [Google Scholar]
- Lin YL, Lin IC, Liou JC. (2011). Symptom patterns of patients with head and neck cancer in a palliative care unit. J Palliat Med 14:556-559 [DOI] [PubMed] [Google Scholar]
- Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. (2008). Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroen Hepatol 23:1852-1859 [DOI] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. (2009). Mouse model of oprm1 (a118g) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci USA 106:10847-10852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JA, Mahanna GK. (1997). Cancer in the differential diagnosis of orofacial pain. Dent Clin North Am 41:355-365 [PubMed] [Google Scholar]
- Mercadante S, Dardanoni G, Salvaggio L, Armata MG, Agnello A. (1997). Monitoring of opioid therapy in advanced cancer pain patients. J Pain Symptom Manage 13:204-212 [DOI] [PubMed] [Google Scholar]
- Mercadante S, Fulfaro F, Casuccio A. (2002). A randomised controlled study on the use of anti-inflammatory drugs in patients with cancer pain on morphine therapy: effects on dose-escalation and a pharmacoeconomic analysis. Eur J Cancer 38:1358-1363 [DOI] [PubMed] [Google Scholar]
- Meunier A, Latremoliere A, Mauborgne A, Bourgoin S, Kayser V, Cesselin F, et al. (2005). Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther 11:608-616 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61-65 [DOI] [PubMed] [Google Scholar]
- Niikura K, Narita M, Butelman ER, Kreek MJ, Suzuki T. (2010). Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci 31:299-305 [DOI] [PubMed] [Google Scholar]
- Nyberg P, Ylipalosaari M, Sorsa T, Salo T. (2006). Trypsins and their role in carcinoma growth. Exp Cell Res 312:1219-1228 [DOI] [PubMed] [Google Scholar]
- Oertel BG, Kettner M, Scholich K, Renne C, Roskam B, Geisslinger G, et al. (2009). A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem 284:6530-6535 [DOI] [PubMed] [Google Scholar]
- Pert CB, Snyder SH. (1973). Opiate receptor: demonstration in nervous tissue. Science 179:1011-1014 [DOI] [PubMed] [Google Scholar]
- Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, et al. (2004). Endothelin and the tumorigenic component of bone cancer pain. Neuroscience 126:1043-1052 [DOI] [PubMed] [Google Scholar]
- Pickering V, Jay Gupta R, Quang P, Jordan RC, Schmidt BL. (2008). Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur J Pain 123: 293-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering V, Jordan RC, Schmidt BL. (2007). Elevated salivary endothelin levels in oral cancer patients—a pilot study. Oral Oncol 43:37-41 [DOI] [PubMed] [Google Scholar]
- Pil J, Tytgat J. (2003). Serine 329 of the mu-opioid receptor interacts differently with agonists. J Pharmacol Exp Ther 304:924-930 [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Przewlocka B. (2001). Opioids in chronic pain. Eur J Pharmacol 429:79-91 [DOI] [PubMed] [Google Scholar]
- Quang PN, Schmidt BL. (2010a). Endothelin-A receptor antagonism attenuates carcinoma-induced pain through opioids in mice. J Pain 11:663-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quang PN, Schmidt BL. (2010b). Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. Pain 149:254-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu C, Gupta A. (2011). Pharmacological treatment of opioid-induced hyperalgesia: a review of the evidence. J Pain Palliat Care Pharmacother [Epub ahead of print, August 11, 2011] (in press). [DOI] [PubMed] [Google Scholar]
- Rigor BM., Sr (2000). Pelvic cancer pain. J Surg Oncol 75:280-300 [DOI] [PubMed] [Google Scholar]
- Russo A, Soh UJ, Trejo J. (2009). Proteases display biased agonism at protease-activated receptors: location matters! Mol Interv 9:87-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghafi N, Lam DK, Schmidt BL. (2011). Cannabinoids attenuate cancer pain and proliferation in a mouse model. Neurosci Lett 488:247-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, et al. (2007). Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain 11:406-414 [DOI] [PubMed] [Google Scholar]
- Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL. (2010). Mechanism of cancer pain. Mol Interv 10:164-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. (2005). Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 115:128-141 [DOI] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I. (1973). Stereospecific binding of the potent narcotic analgesic (3h) etorphine to rat-brain homogenate. Proc Natl Acad Sci USA 70:1947-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. (2010). Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742 [DOI] [PubMed] [Google Scholar]
- Terenius L. (1973). Characteristics of the “receptor” for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol Toxicol (Copenh) 33:377-384 [DOI] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. (2007). Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 18:1437-1449 [DOI] [PubMed] [Google Scholar]
- Wolfe D, Mata M, Fink DJ. (2009). A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther 16:455-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WD, Wang Y, Fang YM, Zhou HY. (2009). Polymorphism of the micro-opioid receptor gene (oprm1 118a>g) affects fentanyl-induced analgesia during anesthesia and recovery. Mol Diagn Ther 13:331-337 [DOI] [PubMed] [Google Scholar]
- Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan J, et al. (2011). Nerve growth factor links oral cancer progression, pain and cachexia. Molecular Cancer Therapeutics [Epub ahead of print, July 12, 2011] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, et al. (1999). Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol 17:2419-2428 [DOI] [PubMed] [Google Scholar]