Abstract
Secondary caries is a frequent reason for restoration failure, resulting from acidogenic bacteria and their biofilms. The objectives of this study were to: (1) develop a novel nanocomposite containing nanoparticles of amorphous calcium phosphate (NACP) and quaternary ammonium dimethacrylate (QADM); and (2) investigate its mechanical and antibacterial durability. A spray-drying technique yielded NACP with particle size of 116 nm. The nanocomposite contained NACP and reinforcement glass fillers, with QADM in the resin. Two commercial composites were tested as controls. Composites were inoculated with Streptococcus mutans. After 180-day water-aging, NACP+QADM nanocomposite had flexural strength and elastic modulus matching those of commercial controls (p > 0.1). NACP+QADM nanocomposite reduced the biofilm colony-forming units (CFU) by 3-fold, compared with commercial composites (p < 0.05). Metabolic activity and lactic acid production of biofilms on NACP+QADM were much less than those on commercial composites (p < 0.05). The antibacterial properties of NACP+QADM were maintained after water-aging for 30, 90, and 180 d (p > 0.05). In conclusion, the novel NACP-QADM nanocomposite greatly decreased biofilm metabolic activity, CFU, and lactic acid, while matching the load-bearing capability of commercial composites without antibacterial properties. The NACP-QADM nanocomposite with strong and durable antibacterial properties, together with its previously reported Ca-PO4 release capability, may render it useful for caries-inhibiting restorations.
Keywords: antibacterial nanocomposite, amorphous calcium phosphate nanoparticles, Streptococcus mutans, quaternary ammonium salt, stress-bearing, dental caries
Introduction
Dental composites are increasingly popular because of their esthetics and direct-filling capabilities (Ferracane, 2011). Extensive studies have improved the fillers, resins, and handling and polymerization properties (Bayne et al., 1998; Lim et al., 2002; Spencer and Wang 2002; Watts et al., 2003; Xu X et al., 2006; Drummond, 2008). Nonetheless, composites accumulate more biofilms/plaques than other restoratives (Zalkind et al., 1998; Beyth et al., 2007). Plaques contribute to secondary caries, which is a main reason for restoration failures (Deligeorgi et al., 2001). Replacing failed restorations consumes 50 to 70% of dentists’ time. Replacement dentistry costs $5 billion/year in the USA (Jokstad et al., 2001). To combat caries, antibacterial composites containing quaternary ammonium salts (QAS) were developed (Imazato, 2003, 2009). Resins containing 12-methacryloyloxydodecylpyridinium bromide (MDPB) markedly reduced bacterial viability (Imazato et al., 1994). Other antibacterial resins used agents including methacryloxylethyl cetyl dimethyl ammonium chloride and cetylpyridinium chloride (Beyth et al., 2006; Li et al., 2009; Namba et al., 2009; Xie et al., 2011).
Calcium phosphate (CaP) composites represent another method of caries inhibition. These composites can release supersaturating levels of calcium (Ca) and phosphate (PO4) ions to remineralize tooth lesions (Dickens et al., 2003; Langhorst et al., 2009). Recently, novel CaP and CaF2 nanoparticles were incorporated into composites (Xu H et al., 2006, 2010). Nanoparticles of amorphous calcium phosphate (NACP) with a size of 116 nm were synthesized via a spray-drying technique (Xu et al., 2011). NACP nanocomposite released Ca and PO4 ions similar to those of traditional CaP composites, while possessing much better mechanical properties (Xu et al., 2010). The NACP nanocomposite was “smart” and greatly increased the Ca-PO4 release at acidic pH, when these ions are most needed to combat caries (Xu et al., 2011). When placed in a pH 4 lactic acid solution, the NACP nanocomposite quickly neutralized the acid and increased the pH to above 6, while the pH of commercial restoratives remained at 4 (Moreau et al., 2011). However, little has been reported on combining the best of both worlds: Ca-PO4 release and remineralization, and antibacterial properties of QAS.
In this study, a quaternary ammonium dimethacrylate (QADM) was incorporated into NACP nanocomposite, and the NACP-QADM nanocomposite was water-aged for 180 d and then inoculated with Streptococcus mutans (S. mutans). The objective was to develop a NACP nanocomposite with long-lasting antibacterial properties. It was hypothesized that: (1) the antibacterial properties of NACP-QADM nanocomposite would not decrease with water-aging time; (2) NACP-QADM nanocomposite would greatly reduce biofilm viability and acid production, compared with commercial composites; and (3) NACP-QADM nanocomposite would possess mechanical properties similar to those of controls.
Materials & Methods
A spray-drying technique was used to synthesize NACP [Ca3(PO4)2] (Xu et al., 2011). Briefly, calcium carbonate and dicalcium phosphate anhydrous were dissolved in acetic acid to obtain Ca and PO4 concentrations of 8 mmol/L and 5.333 mmol/L, respectively, yielding a Ca/P molar ratio = 1.5. This solution was sprayed into a heated chamber, and an electrostatic precipitator collected the dried particles. This yielded NACP with a mean particle size = 116 nm (Xu et al., 2011).
BisGMA (bisphenol glycidyl dimethacrylate) and TEGDMA (triethylene glycol dimethacrylate) (Esstech, Essington, PA, USA) at mass ratio = 1:1 was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate. The QADM, bis(2-methacryloyloxy-ethyl) dimethyl-ammonium bromide, was synthesized as described recently (Antonucci et al., 2011). Ten mmol of 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA, Sigma-Aldrich, St. Louis, MO, USA) and 10 mmol of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer Labs, Trevose, PA, USA) were combined in ethanol. After 24 hrs of stirring at 60°C, the solvent was removed, yielding QADM as a clear/viscous liquid. QADM was mixed with BisGMA-TEGDMA at a QADM mass fraction = 50%. Preliminary study showed that this mass fraction yielded strong antibacterial properties without compromising mechanical properties. The resin was filled with NACP and glass particles (barium-boroaluminosilicate, mean size = 1.4 µm, Caulk/Dentsply, Milford, DE, USA) silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine. Filler mass fraction was 30% for NACP and 35% for glass, yielding a cohesive paste. Since the resin mass fraction = 35%, the QADM mass fraction in the composite = 17.5%.
A commercial composite served as control (Renamel, Cosmedent, Chicago, IL, USA), and is referred to as “CompositeR”. It had 60% nanofillers of 20 to 40 nm in a methacrylate-ester resin. Another composite, containing 66.7% of silica/ytterbium-trifluoride fillers with fluoride-release (Heliomolar, Ivoclar, Amherst, NY, USA), was referred to as “CompositeF”.
For mechanical testing, the paste was placed into rectangular molds of 2 x 2 x 25 mm. For biofilm testing, disk molds were used (diameter = 9 mm, thickness = 2 mm). Specimens were photo-polymerized (Triad2000, Dentsply, York, PA, USA) for 1 min on each side. Specimens were immersed in distilled water at 37ºC for 1, 30, 90, and 180 d.
Water-aged specimens were fractured in a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC, USA) in three-point flexure (span = 10 mm, crosshead speed = 1 mm/min). Flexural strength (S) was calculated as: S = 3PmaxL/(2bh2), where Pmax is load, L is span, b is specimen width, and h is thickness. Elastic modulus (E) was calculated as: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope.
The degree of conversion (DC) of NACP nanocomposite with or without QADM was measured by near-infrared (NIR) spectroscopy (Nicolet-6700, Thermo, Waltham, MA, USA). NIR spectra were acquired before photo-cure, 5 min post-cure, and 3 d post-cure. DC was calculated from the percentage of change in the integrated peak area of the 6165 cm−1 methacrylate absorption band (Stansbury and Dickens, 2001).
The use of S. mutans (ATCC700610, American Type Culture Collection, Manassas, VA, USA) was approved by the University of Maryland. S. mutans was selected because it is a cariogenic bacterium and is the primary causative agent of caries. The growth medium consisted of brain-heart infusion (BHI) (BD, Franklin Lakes, NJ, USA) supplemented with 0.2% sucrose. A 15-µL quantity of stock bacteria was added to 15 mL of growth medium and incubated (37ºC, 5% CO2) for 16 hrs. Inoculation medium was formed by dilution of this culture 10-fold in growth medium (Cheng et al., 2012a).
For live/dead assay, each disk was placed in a well of 24-well plates and inoculated with 1.5 mL of inoculation medium. The disks were incubated for 3 d to form mature biofilms (Cheng et al., 2012a). The disks were live-/dead-stained (Molecular Probes, Eugene, OR, USA) and examined by epifluorescence microscopy (TE2000-S, Nikon, Melville, NY, USA). The area of green staining (live bacteria) was computed with NIS Elements imaging software (Nikon). The area fraction of live bacteria = green staining area/total area of the image. Six specimens were used for each composite (n = 6) at each time period. Three randomly chosen fields of view were photographed from each specimen, yielding a total of 18 images at each time period.
Disks with biofilms were immersed in 2.5% glutaraldehyde for 4 hrs at 4ºC. They were then subjected to graded-ethanol dehydrations and rinsed with 100% hexamethyldisilazane. Specimens were gold-coated and examined via scanning electron microscopy (SEM, Quanta-200, FEI, Hillsboro, OR, USA).
For MTT assay, disks were placed in 24-well plates, inoculated with 1.5 mL inoculation medium, and cultured for 3 d. Each disk was transferred to new 24-well plates for the MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Antonucci et al., 2011; Cheng et al., 2012a). MTT is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. A total of 1 mL of MTT was added to each well and incubated for 1 hr. Disks were transferred to new 24-well plates, and 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. The DMSO solution from each well was used, and the absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA).
For lactic acid measurement, disks with 3-day biofilms were placed in new 24-well plates, and 1.5 mL of buffered-peptone water (BPW) supplemented with 0.2% sucrose was added. The disks were incubated for 3 hrs to allow the biofilms to produce acid. Then the BPW solutions were stored for lactate analysis. The microplate reader was used to measure the absorbance at 340 nm, and standard curves were prepared with a lactic acid standard (Supelco, Bellefonte, PA, USA) (Cheng et al., 2012a).
For colony-forming unit (CFU) counts, the biofilms were dispersed and diluted, and each viable bacterium resulted in a single colony on an agar plate. Biofilms on disks were harvested by sonication (3510R-MTH, Branson, Danbury, CT, USA). The bacterial suspensions were serially diluted, spread onto BHI agar plates, and incubated for 3 d. The colony number was counted and used, along with the dilution factor, to calculate the CFU (Cheng et al., 2012a).
We performed one- and two-way analyses of variance (ANOVA) to detect the significant effects of the variables. Tukey’s multiple comparison was used at a p = 0.05.
Results
Flexural strength and elastic modulus (n = 6) showed a moderate decrease during the first month of aging, with little decrease during 1-6 mos (Fig. 1). After 180-day immersion, the strength and modulus of NACP-QADM nanocomposite were similar to those of commercial control composites (p > 0.1).
Figure 1.
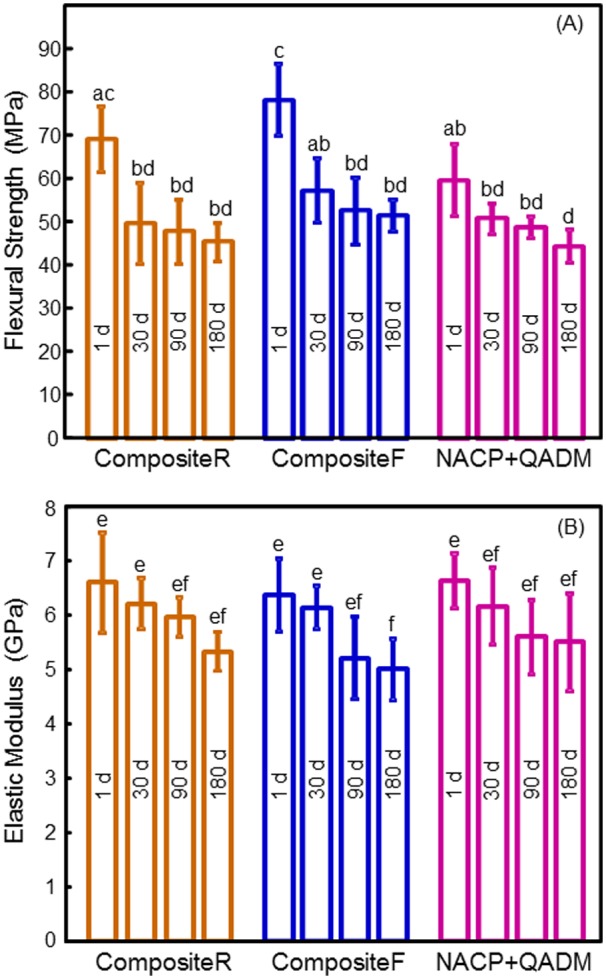
Mechanical properties of composites in water immersion. (A) Flexural strength. (B) Elastic modulus. Each value is the mean of 6 measurements, with the error bar showing one standard deviation (mean ± SD; n = 6). In each plot, values with dissimilar letters are significantly different (p < 0.05). There was a moderate decrease during the first 30 d, with little decrease from 30 to 180 d. At 180 d, the NACP-QADM nanocomposite had values similar to those of the commercial composites (p > 0.1).
At 5 min post-cure, the degree of conversion (n = 3) was 87.3 ± 2.1% for NACP-QADM nanocomposite, and 77.9 ± 7.2% for NACP nanocomposite without QADM (p < 0.05). After 3 d, the degree of conversion was 94.5 ± 3.8% for NACP-QADM nanocomposite, and 83.0 ± 4.2% for NACP nanocomposite without QADM (p < 0.05).
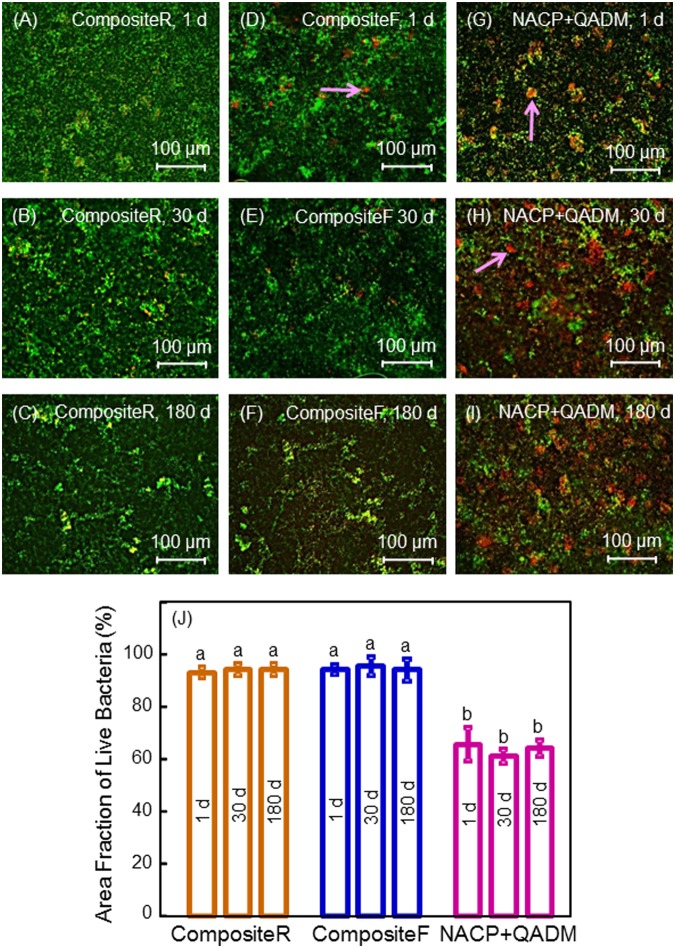
Representative live-/dead-stained images (Fig. 2) showed that CompositeR was completely covered by dense and primarily live biofilms. Live bacteria were stained green, and dead bacteria were stained red. In some areas, the live and compromised bacteria were closely associated; hence the red color was mingled with green to yield yellow/orange colors. Examples of these staining colors are indicated by the arrows. Compared with CompositeR and CompositeF, NACP+QADM had much more red/yellow/orange staining. The area fraction of live bacteria is plotted in Fig. 2J. NACP-QADM greatly reduced live bacteria coverage compared with controls (p < 0.05). The antibacterial activity of NACP-QADM was maintained during 1-180 d (p > 0.1).
Figure 2.
Live/dead staining of 3-day biofilms on composites. Live bacteria were stained green, and dead bacteria were stained red. Live and dead bacteria in close proximity showed yellow/orange colors. The images shown in (A-I) are representative of each group. CompositeR was covered by a dense biofilm with green staining. CompositeF had some compromised bacteria. NACP+QADM had much more dead bacteria staining than the controls. The area fraction of live bacteria staining is plotted in (J) (mean ± SD; n = 6). There was little difference in biofilm viability vs. aging time, indicating that the antibacterial activity of NACP-QADM nanocomposite was not lost in water immersion.
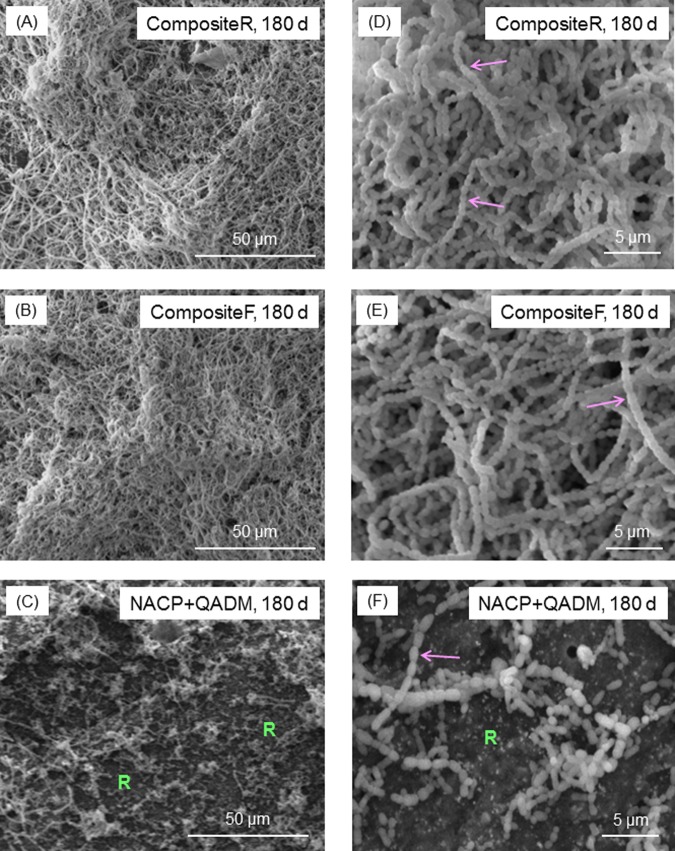
The biofilm structure was examined via SEM (Fig. 3). CompositeR and CompositeF had dense biofilms (Figs. 3A, 3B). NACP-QADM had less biofilm, where “R” indicates resin composite not covered by biofilms (Fig. 3C). Higher magnification (Figs. 3D, 3E) revealed that the S. mutans grew in chains (arrows). The chains twisted in 3 dimensions and were long or continuous in the biofilm architecture. The chains were much shorter on NACP-QADM in (Fig. 3F), with each chain containing 3 to 10 cells.
Figure 3.
SEM micrographs of typical biofilms. (A-C) Lower magnification. (D-F) Higher magnification. Each type of composite, aged for 1 to 180 d, had a similar biofilm appearance. The images shown here are for composites aged for 180 d, to demonstrate the long-term antibacterial activity of the NACP-QADM nanocomposite. CompositeR and CompositeF had dense biofilms. NACP-QADM had much less biofilm coverage. In (C) and (F), “R” indicates the resin composite surface not covered by biofilms. Arrows indicate the chain structure of S. mutans biofilms. The chains are much shorter on NACP-QADM in (F), along with individual cells that did not form a chain. Each bacterial cell had the shape of a short rod with a length of about 1 µm (arrow in F).
The MTT metabolic activity, CFU, and lactic acid production of biofilms did not vary significantly over aging times (Fig. 4). NACP-QADM yielded much lower MTT than commercial composites (p < 0.05) (Fig. 4A). NACP-QADM greatly reduced the CFU (Fig. 4B) and lactic acid (Fig. 4C), compared with commercial composites (p < 0.05). Therefore, NACP-QADM nanocomposite substantially decreased biofilm growth and acid production, and its antibacterial properties were maintained during immersion.
Figure 4.
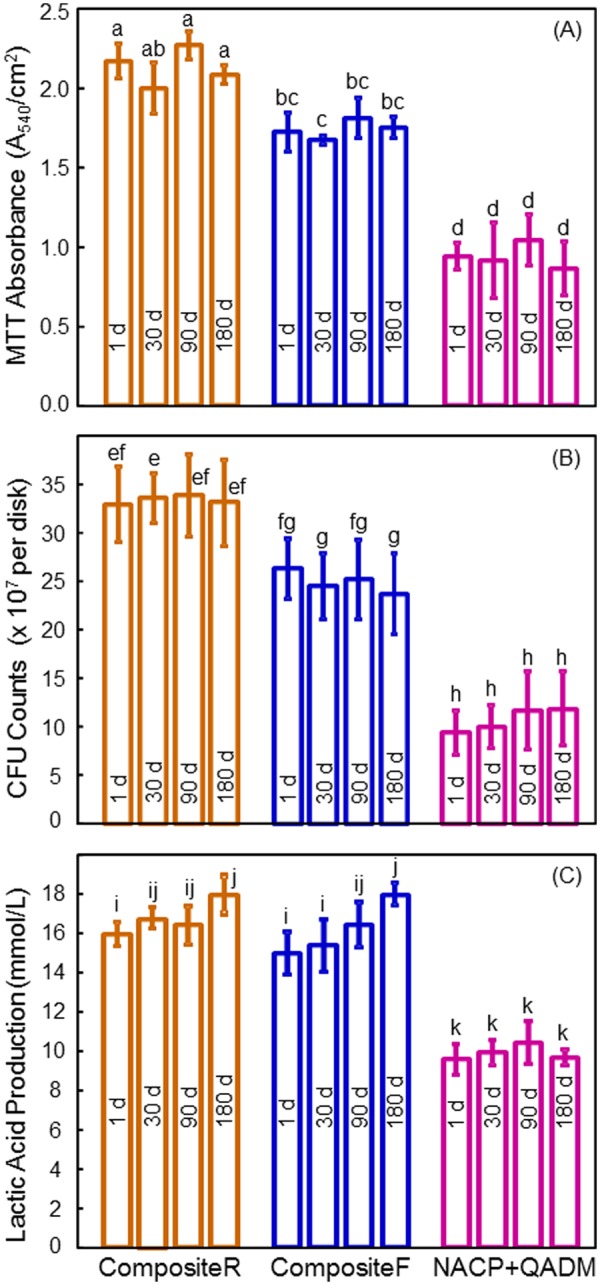
Biofilm viability, growth, and acid production. (A) MTT metabolic activity, (B) CFU counts, and (C) lactic acid production of 3-day biofilms on the composites water-aged for 1 to 180 d. Each value is mean ± SD (n = 6). In each plot, values with dissimilar letters are significantly different (p < 0.05). For the MTT assay, a higher absorbance indicates a higher formazan concentration, which in turn indicates a higher metabolic activity in the biofilm. The NACP-QADM nanocomposite had biofilm metabolic activity and lactic acid production that were about 1/2 of those on the commercial composites, and CFU counts about 1/3 of those on commercial composites (p < 0.05). Aging for 1 to 180 d did not reduce the antibacterial potency of the NACP-QADM nanocomposite (p > 0.1).
Discussion
In this study, we developed a novel NACP-QADM nanocomposite and demonstrated that its strong anti-biofilm activity was maintained after 180 d of water-aging. Cariogenic bacteria such as S. mutans can metabolize carbohydrates to acid, which leads to demineralization. Therefore, dental caries is a dietary carbohydrate-modified bacterial infectious disease caused by acid from biofilms (Deng and ten Cate, 2004; Featherstone, 2004). Hence, it is highly desirable for the next-generation composites to be bioactive, and to possess remineralizing as well as antibacterial capabilities. However, little has been reported on antibacterial CaP composites. Recent studies showed that while CaP composite reduced the coverage of planktonic S. mutans (Moreau et al., 2011), CaP composite had no antibacterial activity against biofilms (Cheng et al., 2012a; Cheng et al., 2012b). Therefore, antibacterial agents should be incorporated to develop antibacterial CaP composites. The present study showed that incorporating QADM was a promising method to achieve long-term antibacterial capability for CaP composite. After 180-day immersion, the NACP-QADM nanocomposite substantially reduced S. mutans biofilm viability, CFU, and acid production, while possessing mechanical properties similar to those of commercial control composites.
Previous studies investigated QAS monomethacrylates, such as MDPB (Imazato, 2003) and QAS chloride (Li et al., 2009). The QADM of the present study has three merits. First, as a dimethacrylate, QADM has reactive groups on both ends of the molecule, which could be incorporated into the resin with less negative impact on the mechanical properties. Indeed, NACP-QADM nanocomposite maintained good mechanical properties after 180-day immersion. The degree of conversion was slightly higher for NACP-QADM nanocomposite than for that without QADM. It appeared that QADM slightly reduced the viscosity of the resin, thereby improving the mobility of the reactive species and hence the conversion. Second, the synthetic method of QADM is fairly straightforward, because the reaction products were generated at quantitative amounts and required no further purification (Antonucci et al., 2011; Cheng et al., 2012a; Cheng et al., 2012b). Third, QADM is a low-viscosity monomer that is miscible with common dimethacrylates and is expected to have minimal monomer leachability due to the reactive groups on both ends of the molecule, compared with QAS monomethacylates. A preliminary agar disk diffusion test showed no inhibition zone for the cured BisGMA-TEGDMA-QADM disks, indicating that QADM was copolymerized with BisGMA-TEGDMA without significant leach-out. In addition, while color measurement is beyond the scope of this study, visual examination revealed no difference between resins with and those without QADM. Furthermore, the NACP-QADM nanocomposite contained NACP for the purpose of remineralization, while none of the previously reported antibacterial composites contained CaP fillers.
Regarding the antimicrobial mechanism, it is suggested that QAS resins can cause bacterial lysis by binding to the cell membrane and causing cytoplasmic leakage (Beyth et al., 2006). When the negatively charged bacterial cell comes into contact with the positively charged (N+) sites of the QAS resin, the electric balance of the cell membrane is disturbed, and the bacterium explodes under its own osmotic pressure (Namba et al., 2009). One potential limitation of resins containing QAS monomers is that the deposit of salivary proteins on composite surfaces could decrease the efficacy of “contact-inhibition”, thereby reducing the antibacterial potency (Imazato, 2003). Further studies are needed to investigate the antibacterial properties of NACP-QADM nanocomposite with protein adsorption. Regarding the antibacterial durability, because QAS monomers are immobilized in the composite, its antibacterial capability is long-lasting (Imazato, 2003, 2009; Li et al., 2009; Xie et al., 2011). For example, the antibacterial effect of MDPB composite was maintained after water-aging for 3 mos (Imazato et al., 1994), and a QAS-containing adhesive showed no decrease in anti-biofilm effect after water-aging for 1 mo (Li et al., 2009). In addition, a QAS nanoparticle-containing composite maintained its antibacterial effect after water-aging for 1 mo (Beyth et al., 2006). In the present study, the NACP-QADM nanocomposite was water-aged for a longer time of 6 mos, and its anti-biofilm effect did not decrease, compared with that at 1 d.
In addition to antibacterial properties, it is important for dental composite to have load-bearing properties. As shown previously (Xu et al., 2011), the NACP had a high surface area; hence the nanocomposite could release high levels of Ca-PO4 at a low NACP filler level, thereby making room in the resin for reinforcement glass fillers. Previous CaP composites contained CaP particles of several microns in sizes, without glass reinforcement (Dickens et al., 2003; Langhorst et al., 2009). In the present study, the photo-cured NACP-QADM nanocomposite contained 35% glass fillers; hence it relied on the stable glass, not the releasing NACP, for reinforcement. As a result, NACP-QADM nanocomposite had mechanical properties similar to those of commercial composites (Renamel and Heliomolar) after 180-day immersion. Heliomolar is indicated for Classes I and II posterior restorations and Classes III and IV anterior restorations. The new NACP-QADM nanocomposite with similar mechanical properties may also be suitable for these applications, with additional functions of Ca-PO4 release and antibacterial capabilities. Furthermore, the NACP-QADM nanocomposite had a filler level of 65%, similar to those of commercial control composites and higher than the 40% filler level of a previous ACP composite (Skrtic et al., 2000). Further studies are needed to investigate the polymerization shrinkage of NACP-QADM nanocomposite and other properties, including dentin bond strength.
In summary, novel NACP-QADM nanocomposite was developed with strong antibacterial capabilities that were maintained after water-aging for 180 d. Strength and modulus of NACP-QADM nanocomposite after 180-day immersion matched those of commercial control composites without antibacterial properties. Incorporation of QADM into NACP nanocomposite greatly reduced S. mutans biofilm viability, metabolic activity, CFU, and acid production. The antibacterial results were not significantly different after water-aging for 1, 30, 90, and 180 d. The durable antibacterial properties, plus the Ca-PO4 release and acid neutralization properties previously reported, indicate that the novel NACP-QADM nanocomposite may be useful in restorations to inhibit secondary caries.
Acknowledgments
We thank Drs. L.C. Chow and L. Sun of the American Dental Association Foundation (ADAF), J.M. Antonucci, N.J. Lin, and S. Lin-Gibson of the National Institute of Standards and Technology, and Prof. A.F. Fouad of the University of Maryland School of Dentistry for discussions. We thank Esstech (Essington, PA) and Ivoclar Vivadent (Amherst, NY) for donating the materials and the Core Imaging Facility of the University of Maryland for technical support.
Footnotes
This study was supported by NIH/NIDCR R01 DE17974 and DE14190 (HX), by a seed fund (HX) from the University of Maryland School of Dentistry, and by the School of Stomatology at the Capital Medical University in China (KZ).
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. (2011). Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater 28:219-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. (1998). A characterization of first-generation flowable composites. J Am Dent Assoc 129:567-577 [DOI] [PubMed] [Google Scholar]
- Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. (2006). Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 27:3995-4002 [DOI] [PubMed] [Google Scholar]
- Beyth N, Domb AJ, Weiss EI. (2007). An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent 35:201-206 [DOI] [PubMed] [Google Scholar]
- Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, et al. (2012a). Antibacterial amorphous calcium phosphate nanocomposite with quaternary ammonium salt and silver nanoparticles. Dent Mater [Epub ahead of print 02/01/2012] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Weir MD, Limkangwalmongkol P, Hack GD, Xu HH, Chen QM, et al. (2012b). Tetracalcium phosphate composite containing quaternary ammonium dimethacrylate with antibacterial properties. J Biomed Mater Res B [Epub ahead of print 12/21/2011] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligeorgi V, Mjör IA, Wilson NH. (2001). An overview of reasons for the placement and replacement of restorations. Prim Dent Care 8:5-11 [DOI] [PubMed] [Google Scholar]
- Deng DM, ten Cate JM. (2004). Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res 38:54-61 [DOI] [PubMed] [Google Scholar]
- Dickens SH, Flaim GM, Takagi S. (2003). Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater 19:558-566 [DOI] [PubMed] [Google Scholar]
- Drummond JL. (2008). Degradation, fatigue, and failure of resin dental composite materials. J Dent Res 87:710-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone JD. (2004). The continuum of dental caries – evidence for a dynamic disease process. J Dent Res 83(Spec Iss C):C39-C42 [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (2011). Resin composite – state of the art. Dent Mater 27:29-38 [DOI] [PubMed] [Google Scholar]
- Imazato S. (2003). Review: Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 19:449-457 [DOI] [PubMed] [Google Scholar]
- Imazato S. (2009). Bioactive restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J 28: 11-19 [DOI] [PubMed] [Google Scholar]
- Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. (1994). Incorporation of bacterial inhibitor into resin composite. J Dent Res 73:1437-1443 [DOI] [PubMed] [Google Scholar]
- Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. (2001). Quality of dental restorations. FDI Commission Projects 2-95. Int Dent J 51:117-158 [DOI] [PubMed] [Google Scholar]
- Langhorst SE, O’Donnell JN, Skrtic D. (2009). In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study; Dent Mater 25:884-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. (2009). Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res 88: 372-376 [DOI] [PubMed] [Google Scholar]
- Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. (2002). Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater 18:436-444 [DOI] [PubMed] [Google Scholar]
- Moreau JL, Sun L, Chow LC, Xu HH. (2011). Mechanical and acid neutralizing properties and inhibition of bacterial growth of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res B Appl Biomater 98:80-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, et al. (2009). Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater 25:424-430 [DOI] [PubMed] [Google Scholar]
- Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. (2000). Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res 53:381-391 [DOI] [PubMed] [Google Scholar]
- Spencer P, Wang Y. (2002). Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62:447-456 [DOI] [PubMed] [Google Scholar]
- Stansbury JW, Dickens SH. (2001). Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater 17:71-79 [DOI] [PubMed] [Google Scholar]
- Watts DC, Marouf AS, Al-Hindi AM. (2003). Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater 19:1-11 [DOI] [PubMed] [Google Scholar]
- Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. (2011). Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater 27:487-496 [DOI] [PubMed] [Google Scholar]
- Xu HH, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. (2006). Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res 85:722-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, et al. (2010). Strong nanocomposites with Ca, PO4 and F release for caries inhibition. J Dent Res 89:19-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Moreau JL, Sun L, Chow LC. (2011). Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater 27:762-769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ling L, Wang R, Burgess JO. (2006). Formation and characterization of a novel fluoride-releasing dental composite. Dent Mater 22:1014-1023 [DOI] [PubMed] [Google Scholar]
- Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. (1998). Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. J Esthet Dent 10:187-190 [DOI] [PubMed] [Google Scholar]