Abstract
Objective
To estimate the effects of gestational age and other maternal factors on immunologic responses to influenza vaccination.
Methods
Antepartum and postpartum women receiving influenza vaccination as part of routine clinical care were enrolled through four consecutive vaccination seasons (starting October 2006 through January 2010) Immunologic responses to trivalent inactivated influenza vaccine (TIV) and monovalent H1N1 were assessed, as well as factors influencing vaccine responsiveness. Serum samples were obtained at baseline and 4-8 weeks postvaccination.
Results
Two hundred thirty-nine participants were included in the current analysis. Seroconversion rates to TIV vaccine strains were lowest in the first trimester (54.8%) and immediate postpartum (54.8%), and were highest in the late third trimester (69.6%) and late postpartum (69.4%); these differences were not statistically significant (p=0.23). In a multivariable model, higher baseline antibody levels (p<.001)and prior year flu vaccination (p=0.03) were both significantly associated with reduced odds of seroconversion. Overall, results were consistent when comparing TIV and monovalent pandemic H1N1 responses. Although there was overall no significant association between gestational age at vaccination (p=0.23) or prepregnancy BMI (p=0.16), we observed somewhat lower rates of seroconversion for women vaccinated in the first trimester and for obese women.
Conclusions
Adequate immunologic responses to inactivated influenza vaccines were demonstrated during pregnancy and the postpartum period. No diminution of immunogenicity was observed in the third trimester a time of increased clinical vulnerability to influenza.
Introduction
Recent global reports of pregnant women, especially in the third trimester, being disproportionately affected by 2009 A/H1N1 [1-6] are consistent with reports from past influenza pandemics and support the decade-long public health recommendation to routinely immunize pregnant women with trivalent inactivated influenza vaccine (TIV) in order to protect both women and their infants.[7] Despite these recommendations, vaccination rates, although recently improved [8,9], remain suboptimal and there have been surprisingly few reports of vaccine immunogenicity among pregnant women.[10-15] We report immunologic results from our influenza vaccine cohort study which enrolled pregnant and post-partum women who had received influenza vaccine as part of their routine standard of care.
Material and Methods
Study design
This study was part of the Mount Sinai Viral Immunity in Pregnancy (VIP) project which was funded by a NIH-NIAID contract (Immune Responses to Virus Infections During Pregnancy; Contact No. HHSN266200500028C). The project had two different cohort studies whose overarching aim was to characterize the immunologic adaptations which occur as pregnancy progresses. The vaccination cohort study enrolled antepartum and postpartum women in order to assess factors influencing the immunologic responses to TIV and to evaluate the spread of influenza-like illness among household members. The study design involved a baseline visit with blood draw, a post-vaccination visit with blood draw, and monthly contact visits until the end of flu season (April each year).
The study and all modifications were approved by the Mount Sinai School of Medicine (MSSM) IRB (MSSM # 05-0054) and study recruitment started in the 2006/2007 influenza vaccine season and continued through consecutive influenza vaccine seasons. Consistent with ACIP recommendations, during year 4, we modified our protocol in order to include women receiving either or both of the two different recommended influenza vaccinations – the monovalent inactivated vaccine against the circulating pandemic 2009 H1N1 as well as the standard seasonal 2009/2010 TIV.[8, 16] Patients receiving care at either one of two on-campus practice sites (the faculty practice or the resident-teaching practice) who were receiving TIV for clinical indications were eligible for study participation. There were no exclusions based on maternal co-morbid medical conditions. Subjects were enrolled throughout pregnancy and at two times post-delivery (either within 72 hours of delivery while an inpatient and again at approximately 6 weeks post-partum). Serum samples were obtained pre-vaccination or the day of vaccination and again at 4-8 weeks post-vaccination. The specimen biorepository was linked to comprehensive maternal data (age, weight, co-morbid medical conditions, concomitant medications/vaccinations, obstetrical history, allergies, asthma/atopy, depression/stress assessments, influenza vaccination history, alcohol/drug use, and smoking/second-hand smoke exposures), pregnancy outcome data, and information about the spread of influenza-like illness among pregnant women and their household members.
Assessment of Immunogenicity
Immunologic responses to influenza A were assessed by standard hemagglutination inhibition (HI) methods. HI titer was determined by the ability of serially diluted RDE treated serum to inhibit hemagglutination of chicken (H1 strains) or turkey (H3 strains) red blood cells in round bottom 96 well plates. Viruses used were either pseudotyped (6:2 recombinants) to match vaccine strains (Wisconsin/67/2005, Brisbane 10/2007, Brisbane 59/2007) or wild-type vaccine strains (New Caledonia/20/99, Solomon Islands/03/2006). Appropriate responses were assessed for both H1N1 and H3N2 strains of each year; additionally, response to California 04/2009 was assessed for subjects who received the vaccine for the circulating pandemic H1N1 in 2009-2010.
Immunologic Endpoints
Adequacy of serologic responses (seroconversion and seroprotection) were assessed using the criteria adopted by regulatory agencies to support influenza vaccine licensure.[17] Seroconversion rates were defined as the proportion of subjects with a ≥ 4-fold increase in reciprocal HI antibody titer at post-vaccination visit versus pre-vaccination, or a reciprocal HI titer of ≥ 40 from a starting value <10. Seroconversion rates has been accepted as a surrogate for clinical vaccine efficacy since studies have demonstrated a strong correlation between a fourfold rise in serum HI antibody titer and disease protection. By convention, seroprotection rates were defined as the proportion of participants with HI titers ≥ 1:40. Geometric means of reciprocal HI titers were calculated for baseline and post-vaccination samples, and the geometric mean fold-rises (geometric mean of the within-subject fold increases from pre-and post—vaccination) were also calculated. In addition to HI titers, immunostaining was performed to determine subtype of IgG antibody response for the pandemic H1N1 in the 2009/2010 cohort. Briefly, Madin-Darby canine kidney epithelial cells were infected with California 04/2009 pseudotyped virus at MOI = 5, and then cultured for 18-24 hrs on 96 well flat bottom plates. Cells, which now express viral proteins HA/NA were fixed before incubation with serial dilutions of patient serum. A peroxidase-conjugated secondary antibody that is specific against total IgG, IgG1, IgG3 or IgG4 was used to identify IgG antibody sub-types.
Statistical Methods
We calculated descriptive statistics, including geometric mean titers, seroconversion rates by timing of vaccination during gestation, and seroprotection rates according to vaccine strains and patient characteristics, by vaccine year and overall. For the geometric means, a titer of less than 10 was interpreted as 5.
We constructed a multivariable adjusted model for seroconversion, starting with a large pool of variables of interest and then reducing the number of variables using a backward elimination, ending with those that were statistically significant given the other terms in the model, or of primary clinical interest. Predictors of seroconversion tested included both vaccine-related characteristics as well as maternal characteristics. The vaccine- related variables included: (i) nine combinations of year of administration and strain (see Figure 1), (ii) baseline HI titer values stratified into 0-20, 40, and 80 and above, and (iii) whether the woman had a vaccine in the previous year. The maternal characteristics included in the final model were (i) age (stratified into less than 23, 23-29, and over 30), (ii) obesity (BMI > or = 30 kg/m2), and (iii) education (<high school, high school degree, > high school). Statistical models accounted for the fact that women had multiple vaccines administered at the same time (e.g. multiple strains in the inactivated seasonal vaccine and, in year 4, an additional monovalent H1N1 vaccine) by using generalized linear models as implemented in proc genmod of SAS version 9.2. We started out by fitting an unstructured 3 by 3 correlation matrix in which it was assumed that there was a common correlation between any two vaccine strains administered at the same time, but another correlation between the pandemic vaccine and each of the two seasonal Brisbane vaccines in year 4. The resulting pattern indicated, as expected, a positive correlation between the strains administered at the same time, but based on small sample sizes, we noted a slightly negative correlation between the pandemic and seasonal vaccine. To assure that the results were not unduly influenced by this implausible negative correlation between the pandemic and seasonal vaccines, for the final model, we set these correlations to zero.
Results
Characteristics of study volunteers
Two hundred and eighty one subjects were enrolled and 245 completed both baseline and post-vaccination study visits and also had serial specimens available. Those that did not have a post-vaccination specimen did not differ from the study population in any of the dimensions summarized in Table 1 (data not shown). In addition, 6 HIV-infected pregnant subjects were eliminated from the current analysis. The description of the 239 subjects is summarized in Table 1. Each year, the goal was to complete enrollment prior to the onset of the peak clinical flu season. In fact, most subjects were vaccinated by the end of November (Year 1: 89.2%; Year 2: 84.3%; Year 3: 93.0%; Year 4 seasonal: 92.3%; Year 4 H1N1: 66.7%). In total, only 8 subjects reported a possible influenza-like illness (defined as a febrile illness with either cough or sore throat) at the time of the post-vaccination blood draw and only 1 of these cases was confirmed by a health-care provider.
Table 1.
Participant Characteristics
| Year | 1 | 2 | 3 | 4 | Years 1-4 | ||
|---|---|---|---|---|---|---|---|
| n=35 | n=50 | n=68 | TIV or H1N1 (n=86) (Seasonal: n=78) (H1N1: n=51) | TIV Only (n=35) | TIV and H1N1 (n=43) | n=239 | |
| Maternal age (years) | 26.5 (6.4) | 24.6 (5.0) | 24.6 (5.5) | 25.7 (6.2) | 25 (5.3) | 26.7 (6.9) | 25.4 (5.9) |
| Race | |||||||
| White | 3 (8.6) | 1 (2.0) | 8 (11.8) | 7 (8.1) | 2 (5.7) | 5 (11.6) | 19 (8.0) |
| Black | 11 (31.4) | 20 (40.0) | 28 (41.2) | 26 (30.2) | 13 (37.1) | 10 (23.3) | 85 (35.6) |
| Latina | 17 (48.6) | 25 (50.0) | 26 (38.2) | 49 (57.0) | 18 (51.4) | 26 (60.5) | 117 (49) |
| American Indian | 2 (5.7) | 1 (2.0) | 3 (4.4) | - | - | - | 6 (2.5) |
| Asian | 1 (2.9) | 1 (2.0) | 1 (1.5) | 2 (2.3) | 1 (2.9) | 1 (2.3) | 5 (2.1) |
| Other | 1 (2.9) | 2 (4.0) | 2 (2.9) | 2 (2.3) | 1 (2.9) | 1 (2.3) | 7 (2.9) |
| Education | |||||||
| Less than high school | 13 (37.1) | 22 (44.0) | 14 (20.6) | 26 (30.2) | 9 (25.7) | 15 (34.9) | 75 (31.4) |
| Finished high school | 5 (14.3) | 7 (14.0) | 17 (25.0) | 17 (19.8) | 10 (28.6) | 7 (16.3) | 46 (19.3) |
| More than high school | 17 (48.6) | 21 (42.0) | 37 (54.4) | 43 (50.0) | 16 (45.7) | 21 (48.8) | 118 (49.4) |
| Marital status | |||||||
| Married or living with partner | 23 (65.7) | 21 (42.0) | 40 (58.8) | 45 (52.3) | 16 (45.7) | 24 (55.8) | 129 (54.0) |
| Divorced, widowed, separated, single, and never married | 12 (34.3) | 29 (58.0) | 28 (41.2) | 41 (47.7) | 19 (54.3) | 19 (44.2) | 110 (46.0) |
| Parity | |||||||
| 0 | 14 (40.0) | 20 (40.0) | 28 (41.2) | 43 (50.0) | 17 (48.6) | 22 (51.2) | 105 (43.9) |
| 1 | 13 (37.1) | 13 (26.0) | 23 (33.8) | 21 (24.4) | 9 (25.7) | 9 (20.9) | 70 (29.3) |
| 2 or more | 8 (22.9) | 17 (34.0) | 17 (25.0) | 22 (25.6) | 9 (25.7) | 12 (27.9) | 64 (26.78) |
| Spontaneous abortions | |||||||
| 0 | 23 (65.7) | 36 (72.0) | 53 (77.9) | 62 (72.1) | 25 (71.4) | 31 (72.1) | 174 (72.8) |
| 1 | 8 (22.9) | 10 (20.0) | 11 (16.2) | 15 (17.4) | 7 (20) | 7 (16.3) | 44 (18.4) |
| 2 or more | 4 (11.4) | 4 (8.0) | 4 (5.9) | 9 (10.5) | 3 (8.6) | 5 (11.6) | 21 (8.8) |
| Induced abortions | |||||||
| 0 | 24 (68.6) | 27 (54.0) | 36 (52.9) | 43 (50.0) | 15 (42.9) | 24 (55.8) | 130 (54.4) |
| 1 | 6 (17.1) | 14 (28.0) | 17 (25.0) | 21 (24.4) | 9 (25.7) | 9 (20.9) | 58 (24.3) |
| 2 or more | 5 (14.3) | 9 (18.0) | 15 (22.1) | 22 (25.6) | 11 (31.4) | 10 (23.3) | 51 (21.4) |
| BMI (kg/m2)* | |||||||
| Less than 18 | 1 (2.9) | 4 (8.7) | 3 (4.5) | 2 (2.4) | 1 (2.9) | 1 (2.3) | 10 (4.3) |
| 18-24.9 | 12 (34.3) | 15 (32.6) | 33 (49.3) | 38 (44.7) | 16 (47.1) | 19 (44.2) | 98 (42.1) |
| 25-29.9 | 8 (22.9) | 13 (28.3) | 16 (23.9) | 22 (25.9) | 9 (26.5) | 13 (30.2) | 59 (25.3) |
| Greater than 30 | 14 (40.0) | 14 (30.4) | 15 (22.4) | 23 (27.1) | 8 (23.5) | 10 (23.3) | 66 (28.3) |
| Pregnancy-induced hypertension | 1 (2.9) | 3 (6.0) | 3 (4.4) | 5 (5.8) | 1 (2.9) | 3 (7) | 12 (5.0) |
| Gestational diabetes | 3 (8.6) | 2 (4.0) | 1 (1.5) | 4 (4.7) | 1 (2.9) | 3 (7) | 10 (4.2) |
| Preeclampsia | 1 (2.9) | 5 (10.0) | 4 (5.9) | 9 (10.5) | 2 (5.7) | 5 (11.6) | 19 (8.0) |
| Preterm delivery | 3 (8.6) | 5 (10.0) | 4 (5.9) | 2 (2.3) | - | 1 (2.3) | 14 (5.9) |
| Gestational age at delivery | 38.4 (2.7) | 37.5 (3.5) | 38.4 (2.5) | 38.2 (2.9) | 38.3 (2.2) | 38.2 (3.3) | 38.2 (2.9) |
| Asthma (ever) | 9 (25.7) | 10 (20.0) | 10 (14.7) | 23 (26.7) | 9 (25.7) | 10 (23.3) | 52 (21.8) |
| Depression | 6 (17.1) | 10 (20.0) | 15 (22.4) | 11 (12.8 | 4 (11.4) | 6 (14) | 42 (17.7) |
TIV, trivalent inactivated influenza vaccine; BMI, body mass index.
Data are n (%).
Based on self-reported prepregnancy weight. Five patients did not report a prepregnancy weight, so the earliest recorded first-trimester weight in the prenatal record was utilized to assign a BMI category.
Gestational Age Effects – Geometric Mean Titers and Seroconversion Rates
Geometric mean titers pre and post vaccination are presented in Table 2, according to vaccination year, strain, and timing of vaccination; this table provides descriptive information without formal statistical testing. Averaged over all strains, geometric mean titers were higher post versus pre-vaccination regardless of the gestation time period when a woman was vaccinated. Vaccination in the late postpartum and late third trimester resulted in the largest post versus pre vaccination titer ratios. During our study period, three viral strains appeared in two successive flu seasons: Wisconsin H3N2 2006/2007 & 2007/2008, Brisbane H3N2 2008/2009 & 2009/2010, and Brisbane H1N1 2008/2009 & 2009/2010. There were no notable differences in geometric mean titers post-vaccination according to timing or year of vaccination for these repeating strains. The effect of timing of vaccination on seroconversion is presented in Table 3, which summarizes for each pregnancy time period the number of women, the average seroconversion rates, and the odds ratio based on a generalized linear model adjusting for the other terms in the model; data is summarized separately for all TIV strains combined and for monovalent pandemic H1N1. Averaged over all strains, the crude rate of seroconversion was lowest in the first trimester (54.8%) and immediate postpartum (54.8%), and highest in the late third trimester (69.6%) and late postpartum (69.4%). The low rate of seroconversion in trimester 1 was even more dramatic for pandemic H1N1 (28.6%). However, this was based on a very small number of subjects. There were no statistically significant differences in odds of seroconversion according to time of vaccination (p=0.23).
Table 2.
Geometric Mean Titers by Strain* and Year: Prevaccination and Postvaccination
| T1† Geometric Mean Titer | T2† Geometric Mean Titer | T3.1† Geometric Mean Titer | T3.2† Geometric Mean Titer | T4† Geometric Mean Titer | T5† Geometric Mean Titer | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Year | n | Pre | Post | n | Pre | Post | n | Pre | Post | n | Pre | Post | n | Pre | Post | n | Pre | Post |
| A/Wisconsin/67//2005 | 2006/2007 H3N2 | 2 | 14.1 | 80.0 | 16 | 27.1 | 70.3 | 11 | 22.7 | 58.4 | 5 | 20.0 | 121.3 | 1 | 80.0 | 160.0 | |||
| A/New Caledonia/20/99 | 2006/2007 H1N1 | 2 | 10.0 | 56.6 | 16 | 18.3 | 140.5 | 11 | 15.5 | 132.4 | 5 | 20.0 | 139.3 | 1 | 80.0 | 160.0 | |||
| A/Wisconsin/67/2005 | 2007/2008 H3N2 | 11 | 40.0 | 109.6 | 16 | 36.7 | 160.0 | 4 | 33.6 | 134.5 | 7 | 48.8 | 195.0 | 11 | 27.4 | 160.0 | 1 | 20.0 | 160.0 |
| A/Solomon Islands/3/2006 | 2007/2008 H1N1 | 11 | 20.0 | 141.1 | 16 | 16.1 | 207.5 | 4 | 16.8 | 134.5 | 7 | 48.8 | 262.5 | 11 | 15.5 | 233.5 | 1 | 40.0 | 160.0 |
| A/Brisbane/10/2007 | 2008/2009 H3N2 | 9 | 18.5 | 80.0 | 19 | 14.9 | 103.3 | 9 | 10.0 | 80.0 | 4 | 20.0 | 113.1 | 18 | 10.0 | 37.0 | 9 | 13.6 | 50.4 |
| A/Brisbane/59/2007 | 2008/2009 H1N1 | 9 | 6.8 | 58.8 | 18 | 11.7 | 50.4 | 9 | 9.3 | 46.7 | 4 | 5.0 | 226.3 | 18 | 5.4 | 25.2 | 9 | 9.3 | 63.5 |
| A/Brisbane/10/2007 | 2009/2010 H3N2 | 11 | 16.6 | 37.6 | 33 | 17.3 | 89.2 | 13 | 34.1 | 122.6 | 8 | 25.9 | 73.4 | 6 | 22.5 | 48.0 | 4 | 10.0 | 190.3 |
| A/Brisbane/59/2007 | 2009/2010 H1N1 | 11 | 12.1 | 58.4 | 33 | 12.3 | 80.0 | 15 | 21.0 | 76.4 | 8 | 25.9 | 95.1 | 4 | 20.0 | 95.1 | 5 | 15.2 | 242.5 |
| A/California/7/09 | 2009 Pandemic H1N1 | 7 | 5.0 | 13.5 | 16 | 8.8 | 40.0 | 10 | 7.1 | 40.0 | 8 | 5.0 | 56.6 | 3 | 5.0 | 72.7 | 7 | 8.2 | 59.4 |
| Total All Strains and Years | 73 | 14.76 | 62.5 | 183 | 16.12 | 90.37 | 86 | 16.62 | 79.36 | 56 | 20 | 120.36 | 73 | 12.21 | 63.8 | 36 | 11.44 | 84.76 | |
The trivalent inactivated vaccine (TIV) has two influenza A antigens and 1 influenza B antigen. These data are presented by number of influenza A strains, not number of individual participants. For most participants, specimens were available to test both influenza A vaccine strains each year.
T1, Less than 13 weeks of gestation (first trimester); T2, 13–less than 28 weeks of gestation (second trimester); T3.1, 28–less than 34 weeks of gestation (early third trimester); T3.2, 34 weeks of gestation or more (late third trimester); T4, immediate postpartum (within 72 hours); and T5, approximately 6 weeks postpartum.
Table 3.
Seroconversion Rates by Trimester and Postpartum Period
| Time Period | Averaged Overall All Strains, All Years | Pandemic H1N1 Only | |||||
|---|---|---|---|---|---|---|---|
| n | % Seroconverted* | Odds Ratio† 95% CI | n | % Seroconverted* | Odds Ratio†† 95% CI | ||
| First trimester (less than 13 weeks) | 73 | 54.8 | 0.48 (0.16, 1.41) | 7 | 28.6 | 0.27 (0.03, 2.12) | |
| Second trimester (13-less than 28 weeks) | 183 | 62.8 | 0.76 (0.29, 2.05) | 16 | 62.5 | 1.11 (0.22, 5.62) | |
| Early third trimester (28-less than 34 weeks) | 86 | 58.1 | 0.74 (0.26, 2.14) | 10 | 60.0 | 1.00 (0.17, 5.98) | |
| Late third trimester (34 weeks or more) | 56 | 69.6 | 1.41 (0.43, 4.58) | 8 | 87.5 | 4.67 (0.40, 53.95) | |
| Immediate postpartum (less than 72 hours) | 73 | 54.8 | 0.55 (0.18, 1.71) | 3 | 66.7 | Ref | |
| Later postpartum (approximately 6 weeks) | 36 | 69.4 | ref | 7 | 57.1 | ||
CI, confidence interval.
Crude seroconversion rate averaged over all strains and separately for pandemic H1N1
Adjusted for strain, baseline antibody titer, vaccination in the last year, age at vaccination, education, and obesity.
Unadjusted
Multivariable Model – Maternal Predictor of Seroconversion
Relations between immunological and patient characteristics and odds of seroconversion are presented in Table 4. Baseline antibody titer was strongly related to odds of seroconversion (p<.001), with the odds of seroconversion falling dramatically with increasing baseline antibody level. Women who received an influenza vaccination in the previous year had significantly (p = 0.03) lower odds of seroconversion than those who did not. Seroconversion rates also varied significantly (p<.001) among the nine vaccine strains administered during our study period; 2006/7 A Wisconsin had the lowest seroconversion rates (37%), the 2007/8 Solomon Islands the highest seroconversion rates (78%), and all the others were in the narrow range 56%-65%. There were no other significant predictors of seroconversion among the factors examined. As noted previously, differences between trimesters were not significant (p=.23). In the multivariable adjusted model, maternal obesity did not reach statistical significance (p=.16); however, obese women (OR = 0.68, 95% CI 0.41, 1.14) had slightly though non-significantly lower odds of seroconversion.
Table 4.
Multivariable Model*
| n (Vaccine Strains) | n (Women) | % Seroconversion | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| Age group (p=.31) | ||||
| Younger than23 | 192 | 90 | 63.5 | ref |
| 23-29 | 179 | 86 | 63.7 | 1.06 (0.62-1.78) |
| 30 or older | 136 | 63 | 53.7 | 0.68 (0.38, 1.21) |
| Education (p=.12) | ||||
| Less than high school | 162 | 75 | 57.4 | 0.89 (.53-1.52) |
| High school | 97 | 46 | 73.2 | 1.66 (.93-3.0) |
| Post-high school | 248 | 118 | 58.5 | ref |
| Obese (p=.16) | ||||
| Nonobese | 371 | 173 | 64.4 | ref |
| Obese | 136 | 66 | 51.5 | 0.68 (0.41-1.14) |
| Baseline hemagglutinin inhibition titer (p<.001) | ||||
| 0-20 | 354 | 204 | 69.8 | ref |
| 40 | 85 | 71 | 57.7 | 0.42 (.23-.74) |
| 80 or higher | 68 | 50 | 19.1 | 0.07 (0.03, 0.16) |
| Self-reported receipt of influenza vaccine in the prior year (p=.03) | ||||
| Yes | 153 | 71 | 48.4 | 0.51 (.30-.85) |
| No | 321 | 154 | 66.7 | ref |
| Uncertain | 33 | 14 | 63.6 | 1.32 (.45-3.84) |
| Vaccine strain by year (p<.001) | ||||
| 2006/2007 A/New Caledonia/20/99 | 35 | 62.9 | ||
| 2006/2007 A/Wisconsin/67/2005 | 35 | 37.1 | ||
| 2007/2008 A/Wisconsin/67/2005 | 50 | 64 | ||
| 2007/2008 A/Solomon Islands/03/2006 | 50 | 78 | ||
| 2008/2009 A/Brisbane 10/2007 | 68 | 64.7 | ||
| 2009/2010 A/Brisbane 10/2007 | 75 | 56 | ||
| 2008/2009 A/ Brisbane 59/2007 | 67 | 58.2 | ||
| 2009/2010 A/ Brisbane 59/2007 | 76 | 61.8 | ||
| 2009 Pandemic A/California 04/2009 | 51 | 60.8 |
P-values are for each term, adjusting for all other terms in the model.
Seroprotection Rates
Seroprotection rates (pre and post-vaccination titers > 1:40) are summarized in Figures 1 & 2; these figures provide descriptive information without formal statistical testing. Higher baseline (pre-vaccination) seroprotection rates were observed when the same antigen was utilized in consecutive years. Overall, for H3N2 strains, the seropotection rates post-vaccination varied from 65% to 95%. Overall for H1N1 strains, the seroprotection rates post-vaccination varied from 75% to 98%.
Figure 1.
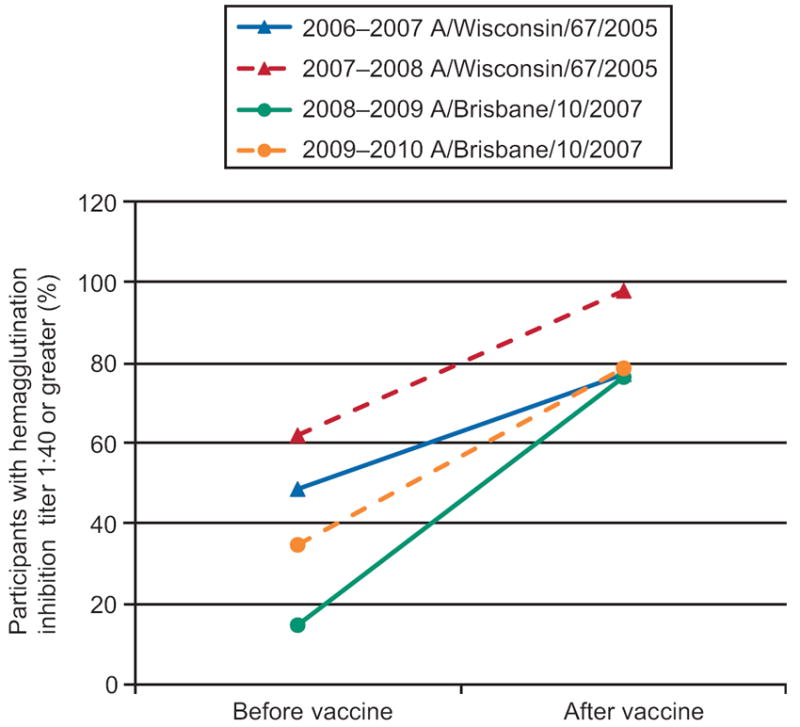
Seroprotection rates for Influenza A(H3N2 strains).
Figure 2.
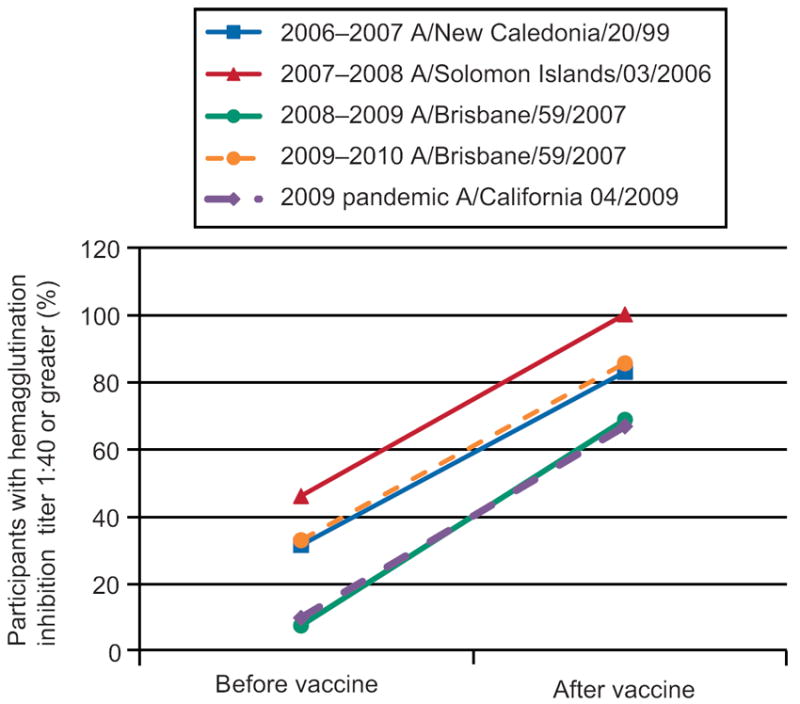
Seroprotection rates for Influenza A (H1N1 strains).
IgG Antibody Class Switching
Only 16% of the subjects receiving the monovalent H1N1 vaccine had background levels of antibody to the pandemic H1N1 strain allowing us to measure IgG antibody class without interference from pre-existing antibodies. Peroxidase-conjugated secondary antibodies specific for total IgG, IgG1, IgG3 or IgG4 were used to evaluate immunoglobulin class switching. Immunostaining is more sensitive than the HI method to detect antibody responses, and some subjects with negative vaccine responses by HI assay showed clearly detectable staining post vaccination (data not shown). The results (data not shown) demonstrated an overwhelming IgG1 response to the vaccine at all vaccination time points assessed (both antepartum and post-partum). No change in IgG subtype preference was observed at any time point.
Discussion
We assessed seroconversion rates to TIV through four consecutive flu vaccination seasons (starting October 2006 through January 2010) as well as to the pandemic 2009 monovalent H1N1 vaccine and observed rates generally similar to what has been reported among non-pregnant adults.[17] Pregnant women demonstrated adequate immunologic responses to inactivated influenza vaccines throughout pregnancy and post-partum. When comparing seroconversion rates in the different gestational time periods to post-partum rates, the highest vaccine response rates were observed in the late third trimester (≥ 34 weeks of gestation) and the lowest response rates were observed in the first trimester (<13 weeks of gestation) and in the immediate post-partum period (within 72 hours of delivery); however, these differences were not statistically significant. It is unlikely that any of the observed antibody responses were confounded by exposure to wild-type circulating virus since almost all subjects were vaccinated prior to the onset of peak flu season and only 8 subjects reported a possible influenza like illness at the time of their post-vaccination visit.
Our multivariable model (Table 4) examined both maternal and vaccine characteristics as potential predictors of seroconversion. Only baseline antibody titer and prior year vaccination were strongly related to odds of seroconversion. Seroconversion rates also varied significantly among the nine vaccine strains administered during our study period. Women over 30 and obese women were observed to have non-significant but lower odds of seroconversion. Obesity, an increasing prevalent global health problem had emerged as a probable independent risk factor for Pandemic 2009 H1N1 influenza disease severity.[18] Obesity has been linked to diminished hepatitis vaccine responsiveness [19] possibly mediated by immunologic alterations related to adipocyte dysfunction [20], or alternatively due to improper vaccination technique.[21] We utilized influenza vaccine as our model because of its routine use during pregnancy. However, it is not an ideal model to study primary immunologic responses because of the likelihood of significant past exposure to the vaccine antigens and/or related strains either from prior vaccinations or from prior wild-type infections. Neutralizing antibodies from previous exposures may block access to B cells or deliver suppressive signals. In fact, in our cohort, a high level of circulating baseline antibodies was the strongest predictor of diminished vaccine responsiveness.
Our study has focused on the third trimester of pregnancy as this has been recognized as a time of immunologic vulnerability. Suppression of T cell activation has been suggested to be the basis of increased disease susceptibility and/or increased disease severity to certain infections including Listeria monocytogenes [22], Plasmodium falciparum [23], Varicella zoster [24], seasonal influenza[7] and most recently the novel H1N1 influenza.[1-6] Alterations in B cell function have been less well-studied during pregnancy; however, significant suppression of B cell lymphopoiesis has been reported[25] and steroid hormones have been implicated in changes of B cell function[26] including possible changes in isotype switching.[27]
The availability of subjects who received the monovalent H1N1 vaccine afforded us the unique opportunity to measure vaccine responses in a naïve population without background antibody interference. Although we enrolled only a very small number of first trimester H1N1 vaccinees, our data suggests the possibility of a diminished first trimester immune response which warrants further investigation. Despite the existing clinical recommendations for influenza vaccination throughout gestation [7], women in the first trimester continue to be excluded from participation in clinical trials of pregnancy-related influenza vaccine immunogenicity.[14] Among our H1N1 vaccinees we were also able to assess IgG class switching. Immunoglobulin class switching is strongly influenced by the cytokine milieu[28] which changes during pregnancy in a predictable fashion.[29] Th1 cytokines IFNγ and IL12 drive a switch to the IgG1 subtype while Th2 cytokines such as IL4 direct a switch to IgG2 and IgG4. As pregnancy progressed, if we had observed a shift away from IgG1 to other subtypes, this would have provided indirect support for a shift from Th1 to Th2 dominance which has been posited to occur. In addition, transport across the placenta varies by class – (IgG1>IgG4>IgG3>IgG2) and a switch in IgG class could potentially influence the protection afforded to the newborn.[30] We did not observe a change in IgG subtype; at all gestational time points tested, IgG1 overwhelmingly dominated the response.
In summary, our observational cohort study provides practical guidance to clinicians faced with the need to counsel pregnant and post-partum patients about the benefits of influenza vaccination and also further elucidates our understanding of the immunologic alterations which characterize normal gestation. Vaccine responsiveness to inactivated influenza vaccines antigens was demonstrated throughout gestation with no diminution seen in the third trimester, a time strongly associated with increased influenza-related morbidity and mortality. Although our study was not designed and powered to identify the ideal time to vaccinate women during pregnancy, our data does suggest the possibility of lower seroconversion rates in the first trimester as well as in the immediate post-partum period. In addition, obesity may also be associated with lower seroconversion rates. Future studies specifically designed to assess the gestational age effect on vaccine responsiveness and among obese pregnant women are warranted by our observations and would help to refine influenza and other vaccination recommendations for pregnant and post-partum women.
Acknowledgments
The authors thank Heidi Hess, PA, and Rachel Gerber, PA, for recruitment and retention of study participants, as well as Bhakti Rawal and Sharon Czelusniak for technical support in the laboratory.
Supported by the National Institute of Allergies and Infectious Diseases - Division of Allergy, Immunology and Transplantation of the National Institutes of Health [grant number N01-AI-50028].
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
References
- 1.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 2.The ANZIC Influenza Investigators Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louie JK, Acosta M, Jamieson DJ, Honein MA for the California Pandemic (H1N1) Working Goup. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 4.Fine AM, Dentinger C, Johnson T, et al. 2009 Pandemic Influenza A (H1N1) in Pregnant Women Requiring Intensive Care - New York City, 2009. MMWR. 2010;59(11):321–6. [PubMed] [Google Scholar]
- 5.Siston AM, Rasmmussen SA, Honein MA, et al. Pandemic 2009 Influenza A(H1N1) Virus Illness Among Pregnant Women in the United States. JAMA. 2010;303(15):1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 Pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2010.12.033. In press, corrected proof. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 8.Ahluwalia IB, Jamieson DJ, D’Angelo DV, et al. Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women--10 states, 2009-10 influenza season. Morbidity and Mortality Weekly Report. 2010;59(47):1541–5. [PubMed] [Google Scholar]
- 9.Ding H, Santibanez TA, Jamieson DJ, et al. Influenza vaccination coverage among pregnant women-National 2009 H1N1 Flu Survey (NHFS) American Journal of Obstetrics and Gynecology. 2011;204(6, Supplement 1):S96–S106. doi: 10.1016/j.ajog.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Sumaya CV, Gibbs RS. Immunization of Pregnant Women with Influenza A/New Jersey/76 Virus Vaccine: Reactogenicity and Immunogenicity in Mother and Infant. J Infect Dis. 1979;140(2):141–6. doi: 10.1093/infdis/140.2.141. [DOI] [PubMed] [Google Scholar]
- 11.Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glazen WP. Maternal Immunization with Influenza or Tetanus Toxoid Vaccine for Passive Antibody Protection in Young Infants. J Infect Dis. 1993;168(3):647–56. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 12.Steinhoff MC, Omer SB, Roy E, et al. Influenza Immunization in Pregnancy - Antibody Responses in Mothers and Infants. N Engl J Med. 2010;362(17):1644–46. doi: 10.1056/NEJMc0912599. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Hisano M, Isojima S, et al. Relationship of Th1/Th2 cell balance with the immune response to influenza vaccine during pregnancy. Journal of Medical Virology. 2009;81(11):1923–8. doi: 10.1002/jmv.21620. [DOI] [PubMed] [Google Scholar]
- 14.Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an Inactivated Monovalent 2009 H1N1 Influenza Vaccine in Pregnant Women. J Infect Dis. 2011;204(6):854–63. doi: 10.1093/infdis/jir440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandemic influenza A (H1N1) 2009 virus vaccine - conclusions and recommendations from the October 2009 meeting of the immunization Strategic Advisory Group of Experts. Weekly Epidemiological Record. 2009;84(49):505–8. [PubMed] [Google Scholar]
- 16.United States Food and Drug Administration, Guidance for Industry. Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. Available at http://www.fda.gov/cber/guidelines.htm.
- 17.Jackson L, Gaglani MJ, Keyserling HL. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infectious Diseases. 2010;10(1):71. doi: 10.1186/1471-2334-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical Aspects of Pandemic 2009 Influenza A (H1N1) Virus Infection. N Engl J Med. 2010;362:1708–19. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 19.Weber DJ. Obesity as a Predictor of Poor Antibody Response to Hepatitis B Plasma Vaccine. JAMA. 1985;254(22):3187–9. [PubMed] [Google Scholar]
- 20.Schäffler A, Schölmerich J. Innate immunity and adipose tissue biology. Trends in Immunology. 2010;31(6):228–35. doi: 10.1016/j.it.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman JN. The importance of injecting vaccines into muscle. BMJ. 2000;321(7271):1237–8. doi: 10.1136/bmj.321.7271.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braden CR. Listeriosis. Pediatric Infectious Disease Journal. 2003;22(8):745–6. doi: 10.1097/01.inf.0000079439.30496.57. [DOI] [PubMed] [Google Scholar]
- 23.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(1):30–5. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 24.Harger JH, Ernest JM, Thurnau GR, et al. Risk Factors and Outcome of Varicella Zoster Virus Pneumonia in Pregnant Women. J Infect Dis. 2002;185(4):422–7. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- 25.Medina KG, Smithson, Kincade P. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178(5):1507–15. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109(12):1625–33. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai T, Zan H, Zhang J, Hawkins JS, Xu Z, Casali P. Estrogen Receptors Bind to and Activate the HOXC4/HoxC4 Promoter to Potentiate HoxC4-mediated Activation-induced Cytosine Deaminase Induction, Immunoglobulin Class Switch DNA Recombination, and Somatic Hypermutation. Journal of Biological Chemistry. 2010;285(48):37797–37810. doi: 10.1074/jbc.M110.169086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stavnezer J. Immunoglobulin class switching. Current Opinion in Immunology. 1996;8(2):199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 29.Kraus TA, Sperling RS, Engel SM, et al. Peripheral Blood Cytokine Profiling During Pregnancy and Post-partum Periods. American Journal of Reproductive Immunology. 2010;64(6):411–26. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 30.Pentšuk N, van der Laan JW. An interspecies comparison of placental antibody transfer: New insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2009;86(4):328–44. doi: 10.1002/bdrb.20201. [DOI] [PubMed] [Google Scholar]


