Abstract
Nuclear factor-erythroid 2-related factor 2 (Nrf2) plays a pivotal role in maintaining cellular redox homeostasis and eliminating reactive toxic species. Nrf2 is epigenetically suppressed due to CpG hypermethylation in prostate tumors from the transgenic adenocarcinoma of the mouse prostate (TRAMP) model. We previously showed that dietary feeding of a γ-tocopherol–rich mixture of tocopherols (γ-TmT) suppressed prostate tumorigenesis in TRAMP mice associated with higher Nrf2 protein expression. We hypothesized that γ-TmT may maintain Nrf2 through epigenetic inhibition of promoter CpG methylation. In this study, 8-wk-old male TRAMP mice were fed 0.1% γ-TmT or a control diet for 16 wk. The methylation in the Nrf2 promoter was inhibited in the prostate of the γ-TmT group compared with the control group. Protein expressions of DNA methyltransferase (DNMT), including DNMT1, DNMT3A, and DNMT3B, were lower in the prostate of the γ-TmT group than in the controls. TRAMP-C1 cells were treated with 30 μmol/L of γ-TmT or blank medium for 5 d. The methylation in the Nrf2 promoter was inhibited in the γ-TmT–treated cells compared with the untreated cells at d 5, and mRNA and protein expressions of Nrf2 and NAD(P)H:quinone oxidoreductase 1 were higher. Interestingly, only DNMT3B was inhibited in the γ-TmT–treated cells compared with the untreated cells. In the aggregate, our findings demonstrate that γ-TmT could inhibit CpG methylation in the Nrf2 promoter in the prostate of TRAMP mice and in TRAMP-C1 cells, which might lead to higher Nrf2 expression and potentially contribute to the prevention of prostate tumorigenesis in this TRAMP model.
Introduction
Nrf2 (nuclear factor-erythroid 2-related factor 2) is a transcription factor that plays pivotal role in maintaining cellular redox homeostasis and elimination of carcinogens and reactive intermediates (1, 2). Accumulating evidence has demonstrated that Nrf2-decient mice are more susceptible to carcinogenic, inflammatory, and oxidative insults (3, 4). Furthermore, it has been found that Nrf2 and its downstream target GST (glutathione-S-transferase) are suppressed in human and TRAMP (the transgenic adenocarcinoma of the mouse prostate) prostate cancer associated with excessive reactive oxygen species (5). Higher reactive oxygen species levels could cause genetic and epigenetic instability and transduce a variety of signals for tumor cell survival, proliferation, and invasion (5, 6). Although the direct relationship between the loss of Nrf2 and prostate carcinogenesis is yet to be established, maintaining Nrf2 expression appears to be critical in retaining cellular adaptability to environmental and endogenous stresses and to delay or prevent the development of prostate cancer.
The suppression of Nrf2 in prostate tumors of TRAMP mice and TRAMP-C1 cells was found to be caused by CpG hypermethylation in the promoter, especially at the first 5 CpG (7). These CpG are hypermethylated in tumorigenic TRAMP-C1 cells but not in nontumorigenic TRAMP-C3 cells (8). Treatment with DNMT (DNA methyltransferase) inhibitor 5-aza-2′-deoxycytidine and HDAC (histone deacetylase) inhibitor trichostatin A could restore Nrf2 expression in TRAMP-C1 cells (7). However, it may not be feasible to use 5-aza-2′-deoxycytidine as a cancer chemopreventive agent chronically due to its toxicity, and therefore great effort has been made in looking for effective epigenetic interventions through the use of relatively nontoxic natural compounds (9).
Vitamin E refers to a group of lipid-soluble compounds consisting of 8 structurally related tocopherols (α-, β-, γ-, and δ-) and tocotrienols (α-, β-, γ-, and δ-). They are well-known natural antioxidants and are abundant in a variety of foods, including vegetable oils, nuts, and whole grains (10). Epidemiological studies revealed that a higher serum γ-tocopherol level is associated with a reduced risk of prostate cancer (11), but large-scale clinical trials with α-tocopherol supplementation demonstrated inconsistent efficacy against prostate cancer (12, 13). γ-TmT (γ-tocopherol-rich mixture of tocopherols) is a by-product of the refining of soybean oil and typically contains 57% γ-tocopherol, 24% δ-tocopherol, 13% α-tocopherol, and 1.5% β-tocopherol. γ-TmT has been shown to inhibit carcinogenesis in different types of cancer, including prostate, colon, lung, and mammary (14–17).
We reported that dietary feeding of 0.1% γ-TmT could inhibit prostate tumorigenesis in TRAMP mice along with higher Nrf2 expression, but the potential mechanisms remain unknown (17). Hence, the present study was undertaken to investigate whether γ-TmT would maintain Nrf2 expression by inhibiting CpG methylation in TRAMP mice and TRAMP-C1 cells.
Methods and Materials
Mice.
Female heterozygous C57BL/TGN TRAMP mice, line PB Tag 8247NG, and male C57BL/6 mice were purchased from Jackson Laboratory. TRAMP females were crossed with C57BL/6 males and the first or second generation of transgenic males was chosen for the study. The genotype of the offspring was determined by a PCR-based method (18). Mice were housed in cages with wood chip bedding in a temperature-controlled room (20–22°C) with a 12-h-light/-dark cycle, with a relative humidity of 45–55% in Rutgers Animal Care Facility. The study was carried out using an IACUC-approved protocol at Rutgers University.
Mouse study design.
To test whether higher Nrf2 expression in TRAMP prostates after γ-TmT treatment was associated with decreased promoter methylation, we repeated the treatment of 0.1% γ-TmT diet in 8-wk-old TRAMP mice for 16 wk, as previously performed (17). Eight-week-old male TRAMP mice were randomly assigned to treatment (n = 7) and control (n = 6) groups. Mice in the treatment group were fed 0.1% mixed tocopherols in an AIN-93M diet (19). γ-TmT was purchased from Cognis and contained 130.0 mg of α-tocopherol, 15.0 mg of β-tocopherol, 243.0 mg of δ-tocopherol, and 568.0 mg of γ-tocopherol/g. At wk 24, mice were killed by CO2 asphyxiation and the genitourinary apparatus including the prostate, the seminal vesicles, and the bladder were collected, snap-frozen in liquid nitrogen, and stored in −80oC for further analysis.
Archived prostate tissues of TRAMP mice at the age of 12 wk (n = 4), 18 wk (n = 3), and 24 wk (n = 5) and nontransgenic C57BL/6 mice at the age of 12 wk (n = 2) and 24 wk (n = 2) were used for DNA extraction to determine the methylation status of the Nrf2 promoter at different ages. These tissues were from our unpublished study and were kept in −80°C for <1 y before DNA collection. Mice were fed Purina Mouse Chow 5015.
Cell culture and treatment.
TRAMP-C1 cells were cultured in DMEM containing 10% FBS and antibiotics. Cells were grown at 37oC in a humidified 5% CO2 atmosphere. γ-TmT was dissolved in DMSO to make a stock solution containing 100 mmol/L total tocopherols consisting of 13.0 mmol/L α-tocopherol, 1.5 mmol/L β-tocopherol, 25.0 mmol/L δ-tocopherol, and 60.5 mmol/L γ-tocopherol. TRAMP-C1 cells were treated with 30 µμmol/L of γ-TmT in DMEM containing 1% FBS for 5 d and harvested.
Bisulfite sequencing.
Genomic DNA was isolated from TRAMP prostate tissues and TRAMP-C1 cells using a QIAamp mini kit (Qiagen). DNA (800 ng) was bisulfite converted using an EZ DNA Methylation-Gold kit (Zymo Research). TA cloning was performed as previously described (7). For each sample, 5–10 clones were chosen for sequencing. Plasmid DNA was sequenced using T7 primer (Genewiz) at the Rutgers Sequencing Core facility. The methylation percentage was calculated as the number of methylated CpG over the total number of CpG examined.
Western-blot analyses.
DNMT are key enzymes catalyzing the addition of the methyl group to cytosine and play a critical role in establishing DNA methylation patterns (20). To investigate whether inhibition of methylation in the Nrf2 promoter was related to downregulation of any of DNMT, we determined the protein expression of DNMT in the prostate tissues of TRAMP mice and TRAMP-C1 cells. Two prostate specimens in the same group were combined for protein extraction. The detailed procedure of Western blotting was previously described (7). Protein bands were visualized by Supersignal West Femto (Pierce) and documented by Gel Documentation 2000 system (Bio-Rad). Protein expressions were semiquantitated by densitometry using ImageJ program. Antibodies against DNMT1, DNMT3A, and DNMT3B were purchased from Imgenex. Antibodies against Nrf2 (sc-722), NQO1 [NAD(P)H:quinone oxidoreductase 1; sc-16464], and β-actin (sc-1616) were purchased from Santa Cruz Biotechnology.
RNA extraction and RT-PCR.
RNA was extracted using a Qiagen RNeasy mini kit and converted to cDNA (TaqMan). Conditions for qPCR were described (18). Relative expression was analyzed by a ΔΔCt method using RQ Manager 1.2 and GAPDH expression was used as internal control. The forward and reverse primers for Nrf2 amplification were 5′-TCACACGAGATGAGCTTAGGGCAA-3′ and 5′-TACAGTTCTGGGCGGCGACTTTAT-3′. Primers for Nqo1 were 5′-AAGAGCTTTAGGGTCGTCTTGGCA-3′ and 5′-AGCCTCCTTCATGGCGTAGTTGAA-3′. Primers for GAPDH were 5′-TCAACAGCAACTCCCACTCTTCCA-3′ and 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′.
Statistical analyses.
Data are mean ± SEM. Palpable tumor incidence was evaluated using the Fisher exact test. The methylation percentages of the Nrf2 promoter in the archived prostate samples were compared using 1-way ANOVA followed by Tukey’s Studentized range test. For all other determinations, Student’s t-test or Welch’s t-test was used. SAS, version 9.2, was used for all statistical analyses. All P values correspond to 2-sided hypothesis tests and P < 0.05 was regarded as significant.
Results
CpG methylation in the Nrf2 promoter increases during prostate tumorigenesis in TRAMP mice.
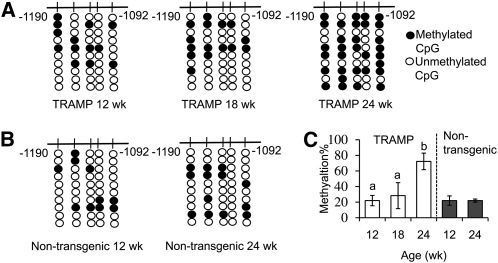
In the present study, we examined the methylation pattern of the first 5 CpG in the prostate of TRAMP and nontransgenic mice at different ages. In the prostate of TRAMP mice, the methylation of these CpG significantly increased from 12 to 24 wk (Fig. 1A,C), whereas in the prostate of nontransgenic mice, the methylation remained unchanged (Fig. 1B,C).
FIGURE 1.
Methylation patterns of the first 5 CpG in the Nrf2 promoter in archived prostate samples from TRAMP (A) and nontransgenic (B) mice and the methylation percentage (C) at various ages. In C, data are mean ± SEM, n = 3–5 (TRAMP) or 2 (nontransgenic). In TRAMP mice, means without a common letter differ, P < 0.05. TRAMP, transgenic adenocarcinoma of the mouse prostate.
Dietary 0.1% γ-TmT inhibits CpG hypermethylation in the Nrf2 promoter in the prostate of TRAMP mice.
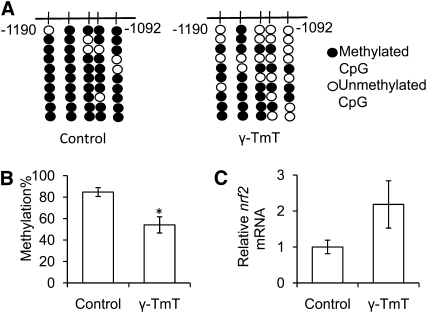
The palpable tumor incidence (Table 1) was significantly lower in the γ-TmT group than in the control group, which is consistent with the previous study (17). The methylation of the first 5 CpG in the Nrf2 promoter was significantly lower in the γ-TmT group than in the control group (Fig. 2A,B). Nrf2 mRNA expression tended to be higher in the γ-TmT group than in the control group (P = 0.069) (Fig. 2C).
TABLE 1.
Palpable tumor incidence in TRAMP mice fed control or 0.1% γ-TmT diet for 16 wk1
| Current study |
Previous study2 |
|||
| Group | n | Palpable tumor incidence | n | Palpable tumor incidence |
| Control | 6 | 4/6 | 17 | 13/17 |
| γ-TmT | 7 | 0/7* | 11 | 2/11* |
*Differs from the control group, < 0.05. TRAMP, transgenic adenocarcinoma of the mouse prostate; γ-TmT, γ-tocopherol-rich mixture of tocopherols.
Adapted from (17).
FIGURE 2.
Methylation patterns of the first 5 CpG in the Nrf2 promoter (A), the overall methylation percentage (B), and the mRNA expression of Nrf2 (C) in the prostate of TRAMP mice fed a control or 0.1% γ-TmT diet. Data are mean ± SEM, n = 4 (control) or 7 (γ-TmT). *Different from control, P < 0.05. TRAMP, transgenic adenocarcinoma of the mouse prostate; γ-TmT, γ-tocopherol-rich mixture of tocopherols.
γ-TmT reverses CpG hypermethylation in the Nrf2 promoter in TRAMP-C1 cells.
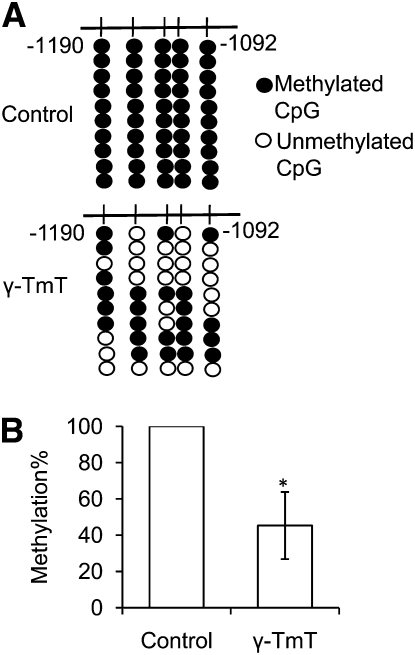
From the above in vivo study, which demonstrated that γ-TmT inhibited CpG methylation in the Nrf2 promoter, we next investigated whether γ-TmT could reverse CpG hypermethylation in TRAMP-C1 cells. The methylation of the first 5 CpG in the Nrf2 promoter was inhibited in the γ-TmT–treated cells compared with the untreated cells at d 5 (Fig. 3). Cell viability was not affected by the treatment (data not shown).
FIGURE 3.
The methylation pattern (A) and the overall methylation percentage (B) of the first 5 CpG in the Nrf2 promoter in TRAMP-C1 cells following treatment with 30 μmol/L of γ-TmT for 5 d. Data are mean ± SEM, n = 3. *Different from control, P < 0.05. TRAMP, transgenic adenocarcinoma of the mouse prostate; γ-TmT, γ-tocopherol-rich mixture of tocopherols.
γ-TmT induces mRNA and protein expressions of Nrf2 and Nqo1 in TRAMP-C1 cells.
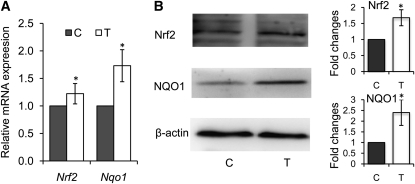
We examined the expression of Nrf2 to see whether reduced promoter methylation could reactivate gene expression. The mRNA and protein expressions of Nrf2 and NQO1 were induced in TRAMP-C1 cells treated with 30 μmol/L of γ-TmT compared with the control cells on d 5 (Fig. 4).
FIGURE 4.
The mRNA (A) and protein (B) expression of Nrf2 and NQO1 in TRAMP-C1 cells following the treatment with 30 μmol/L of γ-TmT for 5 d. Three independent experiments were carried out. Data are mean ± SEM, n = 3. *Different from control, P < 0.05. C, control; TRAMP, the transgenic adenocarcinoma of mouse prostate; γ-TmT, γ-tocopherol-rich mixture of tocopherols; T, γ-TmT treated.
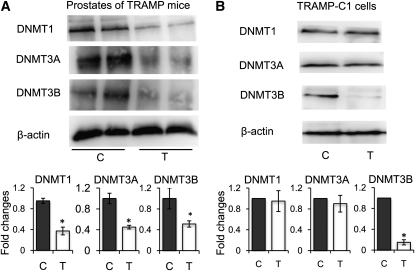
γ-TmT suppresses the expression of DNMT in the prostate of TRAMP mice and TRAMP-C1 cells.
In the prostate of TRAMP mice, the protein levels of DNMT, including DNMT1, DNMT3A, and DNMT3B, were all lower in the γ-TmT group than in the control group (Fig. 5A). Interestingly, only DNMT3B was suppressed when TRAMP-C1 cells were treated with 30 μmol/L of γ-TmT for 5 d (Fig. 5B). These results suggest the potential role of DNMT in CpG methylation and demethylation regulated by γ-TmT.
FIGURE 5.
Protein expressions of DNMT1, DNMT3A, and DNMT3B in the prostate of TRAMP mice fed a control or 0.1% γ-TmT diet (A) and in TRAMP-C1 cells (B) treated with 30 μmol/L of γ-TmT for 5 d. Values are mean ± SEM, n = 4–7 (TRAMP mice) or 3 (TRAMP-C1 cells). *Different from control, P < 0.05. C, control; γ-TmT, γ-tocopherol-rich mixture of tocopherols; T, γ-TmT treated.
Discussion
γ-TmT has been shown to inhibit prostate tumorigenesis in TRAMP mice associated with higher Nrf2 expression (17); however, the molecular mechanism remains unclear. In the present study, we show that γ-TmT treatment prevented CpG hypermethylation in the Nrf2 promoter in vivo and reversed its hypermethylation in vitro, which might contribute to higher Nrf2 expression. Tocopherols are extensively studied with respect to their antioxidative, antiinflammatory, and antiproliferative effects (21), yet to the best of our knowledge, their effects on epigenetic modification have not been reported.
The progression of prostate tumorigenesis in the TRAMP model is associated with abnormal DNA methylation events with both locus-specific hypermethylation and global genomic hypomethylation (22). The present study demonstrated that methylation of the first 5 CpG in the Nrf2 promoter increased during prostate cancer development in TRAMP mice, especially at the late stage (Fig. 1A,C). These CpG are critical in regulating the expression of Nrf2, and the increased methylation may contribute to the lower Nrf2 expression in prostate tumors of TRAMP mice, as previously reported (7). NRF2 is also found to be repressed in human prostate cancer (5); however, future studies are warranted to investigate whether NRF2 inactivation in human prostate cancer is caused by CpG hypermethylation.
Prostate cancer in TRAMP mice appears to involve excessive oxidative stress, accompanied by increased damage to DNA, protein, and lipid (6). Because Nrf2 plays a central role in adapting the cells to environmental and endogenous stresses (1), the loss of Nrf2 expression would potentially make the prostate of TRAMP mice more vulnerable to insults, because Nrf2-targeted enzymes such as the SOD, UGT1A1, NQO1, and GST family are also lost during tumorigenesis (5, 17, 23). γ-TmT inhibited CpG methylation (Figs. 2A,B and ) and elevated Nrf2 and its downstream antioxidant enzyme NQO1 in TRAMP-C1 cells (Fig. 4), which could potentially contribute to the prevention against prostate cancer.
Some natural phytochemicals have been shown to reactivate the expression of silenced genes in tumor cells through epigenetic modifications (9, 24). Possible mechanisms could be related to inhibition of DNMT and/or HDAC. For instance, green tea polyphenols, sulforaphane, and curcumin have been reported to inhibit both DNMT and HDAC (24–26). In the present study, we showed that dietary γ-TmT feeding suppressed the expressions of all 3 DNMT in TRAMP mice (Fig. 5A). Lower expression of DNMT could prevent promoter CpG hypermethylation in the prostate of TRAMP mice, including the Nrf2 promoter during the early stages of tumorigenesis. Furthermore, metabolites of tocopherols are hypothetical HDAC inhibitors as predicted by molecular modeling (24). We speculate that histone modifications might also contribute to the lower CpG methylation and higher Nrf2 expression after γ-TmT treatment in vivo. Further study is needed to explore the effect of γ-TmT on HDAC and histone modifications.
The human body preferentially retains α-tocopherol despite the high γ-tocopherol intake from the typical American diet (21). This is achieved in part by the selectivity of the hepatic α-tocopherol transfer protein, which facilitates the entrance of α-tocopherol into the circulatory system, while the non-α-tocopherols undergo fast metabolism mediated by the cytochrome P450 (27). In immunodeficient mice fed 0.1% γ-TmT, α-tocopherol remained the most abundant form in the prostate, though its concentration was not greater than in the control group (28). The concentrations of γ- and δ-tocopherol in the prostate increased by 2- to 3-fold following 0.1% γ-TmT treatment. Another study showed that the urinary excretions of tocopherol metabolites such as γ- and δ-carboxymethyl hydroxychromans dramatically increased in immunodeficient mice following 0.17–0.3% of γ-TmT feeding (29). These tocopherol levels reported in mice suggest that the observed epigenetic effect in the prostate of TRAMP mice in the present study may be attributed to the single or combined effects of γ- and δ-tocopherol and their metabolites.
As a proof-of-concept, we demonstrated that γ-TmT could reverse hypermethylation of the Nrf2 promoter using TRAMP-C1 cells (Fig. 3). However, DNMT3B, but not DNMT1 or DNMT3A, was suppressed in the γ-TmT–treated cells at d 5 (Fig. 5B). There are several possible explanations for the differences between the in vivo and in vitro results. First, the TRAMP study revealed primary prevention, in which γ-TmT blocked the expression of DNMT proteins and the methylation of the Nrf2 promoter during prostate tumorigenesis. The in vitro study was carried out in a prostate cancer cell line, in which the Nrf2 promoter is already hypermethylated (7), and the γ-TmT treatment reversed the hypermethylation. Second, prostate tumors from TRAMP mice are a heterogeneous population (8) compared with the TRAMP-C1 cells, which are relatively homogenous and therefore could result in different outcomes upon γ-TmT treatment. Third, the concentrations of different tocopherols and their metabolites in prostate tissues and TRAMP-C1 cells might be different. It has been reported that only a small portion of tocopherols in cell culture medium can be metabolized by human prostate cancer cells (30). Hence, it is likely that TRAMP-C1 cells are mostly exposed to the parent tocopherols as they are supplemented in the medium. In contrast, mice can metabolize tocopherols extensively in vivo, generating high concentrations of carboxychromanol metabolites (29), some of which have been shown to possess superior biological activity compared with the parent tocopherols (31–33).
In summary, in our present study, we showed that γ-TmT prevents CpG methylation in the Nrf2 promoter in vivo in the prostate of TRAMP mice and reverses hypermethylation of the Nrf2 promoter in vitro in TRAMP-C1 cells, associated with lower DNMT protein expressions. These epigenetic modifications might contribute to higher Nrf2 expression, which potentially plays a role in the prevention of prostate tumorigenesis in TRAMP mice.
Acknowledgments
Y.H. discussed, designed, conducted the research, analyzed the data, and wrote the manuscript; T.O.K. discussed, designed, conducted the research, and wrote the manuscript; N.S. and C.S.Y. discussed and designed the research; A.N.K. discussed and designed the research, wrote the manuscript and had primary responsibility for the final content; and L.S., C.L-L.S., and T-Y.W. conducted the research. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grant R01-CA152826.
Literature Cited
- 1.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89 [DOI] [PubMed] [Google Scholar]
- 3.Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents: targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–7 [DOI] [PubMed] [Google Scholar]
- 4.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–62 [DOI] [PubMed] [Google Scholar]
- 6.Tam NN, Nyska A, Maronpot RR, Kissling G, Lomnitski L, Suttie A, Bakshi S, Bergman M, Grossman S, Ho SM. Differential attenuation of oxidative/nitrosative injuries in early prostatic neoplastic lesions in TRAMP mice by dietary antioxidants. Prostate. 2006;66:57–69 [DOI] [PubMed] [Google Scholar]
- 7.Yu S, Khor TO, Cheung KL, Li W, Wu TY, Huang Y, Foster BA, Kan YW, Kong AN. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS ONE. 2010;5:e8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30 [PubMed] [Google Scholar]
- 9.Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010;1:101–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–55 [PubMed] [Google Scholar]
- 11.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, Morris JS, Comstock GW. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–23 [DOI] [PubMed] [Google Scholar]
- 12.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, Albanes D, Taylor PR, Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–85 [DOI] [PubMed] [Google Scholar]
- 13.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila). 2009;2:143–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert JD, Lu G, Lee MJ, Hu J, Ju J, Yang CS. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol Nutr Food Res. 2009;53:1030–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, Lee MJ, Yang CS, Newmark HL, Suh N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res. 2009;15:4242–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, Newmark H, Kong AN. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu TY, Saw CL, Khor TO, Pung D, Boyanapalli SS, Kong AN. doi: 10.1002/mc.20841. In vivo pharmacodynamics of indole-3-carbinol in the inhibition of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: Involvement of Nrf2 and cell cycle/apoptosis signaling pathways. Mol Carcinog. Epub 2011 Aug 11. [DOI] [PubMed] [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 20.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21 [DOI] [PubMed] [Google Scholar]
- 21.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morey Kinney SR, Smiraglia DJ, James SR, Moser MT, Foster BA, Karpf AR. Stage-specific alterations of DNA methyltransferase expression, DNA hypermethylation, and DNA hypomethylation during prostate cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Res. 2008;6:1365–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavis CK, Morey Kinney SR, Foster BA, Karpf AR. Expression level and DNA methylation status of glutathione-S-transferase genes in normal murine prostate and TRAMP tumors. Prostate. 2009;69:1312–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007;17:363–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khor TO, Huang Y, Wu TY, Shu L, Lee J, Kong AN. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol. 2011;82:1073–8 [DOI] [PubMed] [Google Scholar]
- 26.Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126:2520–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traber MG. Regulation of xenobiotic metabolism, the only signaling function of alpha-tocopherol? Mol Nutr Food Res. 2010;54:661–8 [DOI] [PubMed] [Google Scholar]
- 28.Xi Zheng X-XC. Khor TO, Huang Y, DiPaola RS, Goodin S, Lee M-J, Yang CS, Kong A-N, Conney AH. Inhibitory effect of a γ-tocopherol-rich mixture of tocopherols on the formation and growth of LNCaP prostate tumors in immunodeficient mice. Cancers. 2011;3:3762–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li GX, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, Yang CS. delta-Tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila). 2011;4:404–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conte C, Floridi A, Aisa C, Piroddi M, Galli F. Gamma-tocotrienol metabolism and antiproliferative effect in prostate cancer cells. Ann N Y Acad Sci. 2004;1031:391–4 [DOI] [PubMed] [Google Scholar]
- 31.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–22 [DOI] [PubMed] [Google Scholar]
- 33.Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15 [DOI] [PubMed] [Google Scholar]