Abstract
Resistant starch (RS), fed as high amylose maize starch (HAMS) or butyrylated HAMS (HAMSB), opposes dietary protein-induced colonocyte DNA damage in rats. In this study, rats were fed Western-type diets moderate in fat (19%) and protein (20%) containing digestible starches [low amylose maize starch (LAMS) or low amylose whole wheat (LAW)] or RS [HAMS, HAMSB, or a whole high amylose wheat (HAW) generated by RNA interference] for 11 wk (n = 10/group). A control diet included 7% fat, 13% protein, and LAMS. Colonocyte DNA single-strand breaks (SSB) were significantly higher (by 70%) in rats fed the Western diet containing LAMS relative to controls. Dietary HAW, HAMS, and HAMSB opposed this effect while raising digesta levels of SCFA and lowering ammonia and phenol levels. SSB correlated inversely with total large bowel SCFA, including colonic butyrate concentration (R2 = 0.40; P = 0.009), and positively with colonic ammonia concentration (R2 = 0.40; P = 0.014). Analysis of gut microbiota populations using a phylogenetic microarray revealed profiles that fell into 3 distinct groups: control and LAMS; HAMS and HAMSB; and LAW and HAW. The expression of colonic genes associated with the maintenance of genomic integrity (notably Mdm2, Top1, Msh3, Ung, Rere, Cebpa, Gmnn, and Parg) was altered and varied with RS source. HAW is as effective as HAMS and HAMSB in opposing diet-induced colonic DNA damage in rats, but their effects on the large bowel microbiota and colonocyte gene expression differ, possibly due to the presence of other fiber components in HAW.
Introduction
Colorectal cancer (CRC)11 is a major cause of premature morbidity and mortality in westernized industrial countries and is appearing rapidly in developing economies with greater affluence (1). This time trend supports the importance of environmental influences in its etiology whereas genetic factors are thought to contribute ≤30% of new cases, strengthening the case for control through prevention (2). A study by the European Prospective Investigation into Cancer and Nutrition (EPIC) showed that dietary fiber dose-dependently lowered CRC risk (3), whereas an earlier international comparison of population studies found that greater dietary protein intakes increase risk (4). Further analysis of the EPIC data showed that consumption of red and processed meat increased CRC risk (5). Unrepaired DNA damage is a prerequisite for carcinogenesis and we showed that feeding high-protein diets substantially increased the number of colonic DNA strand breaks in rats (6–8). Significantly greater damage was seen with some protein sources, including red meat and casein, than with others, such as chicken, which is consistent with prospective population studies. Damage was dose-dependently opposed by dietary RS (resistant starch) fed as high amylose maize starch (HAMS) (6–8) or butyrylated HAMS (HAMSB) (9). Resistant starch (RS), the starch and the products of its digestion, escapes from the small intestine and enters the large bowel and so contributes to total dietary fiber intake. In the large bowel, RS is fermented by the microbiota, releasing SCFA. The major SCFA (acetate, propionate, and butyrate) are thought to generally contribute to optimal colonic function, but butyrate is thought to be particularly important (10–12). It is a preferred metabolic substrate for colonocytes and acts to maintain a normal phenotype in these cells through several complementary mechanisms. In our studies on RS, dietary protein, and DNA damage, the strongest protective relationships were with large bowel butyrate (7), consistent with its proposed role in promoting colonic integrity. This suggestion is supported by nutritional studies with HAMSB. Starches acylated to a high degree of substitution (such as HAMSB) pass into the large bowel where the esterified SCFA is released by bacterial action. HAMSB consumption produces a sustained rise in large bowel SCFA, specifically as the esterified acid, and is as effective as HAMS in opposing diet-induced DNA damage (9). Other laboratory studies include experiments in rodents treated with a carcinogen (azoxymethane) where RS significantly lowers precancerous lesions and tumor burden (13–16). Collectively, these experimental data provide mechanistic support for the dose-dependent reduction in CRC risk with greater fiber intake, which was found in the EPIC study (3) and also in an early case-control study that showed attenuation by fiber of excess risk with greater protein consumption (17). In addition to direct effects of butyrate, there are changes in circulating biomarkers that suggest adaptive changes in gene expression (18). RS may also selectively stimulate the growth of beneficial bacterial that are resident in the colon, thereby contributing to a lower risk of diseases, including CRC (19).
RS occurs in foods for a variety of reasons, including the degree of gelatinization of the starch, i.e., the less gelatinized a starch, the lower its ileal digestibility and the higher its RS content. One of the important determinants of gelatinization is the relative content of amylose and amylopectin. The former is a smaller, linear polymer that gelatinizes relatively slowly on heating with water compared with amylopectin. It is also quicker to retrograde on cooling. Both factors contribute to the presence of RS in processed foods made with HAMS (20). Recently, we described a short-term feeding trial in which rats were fed a high amylose wheat flour (HAW) generated by RNA interference and showed that HAW raised large bowel SCFA (21), consistent with greater RS content. HAW is a genetically modified grain and we deemed it important to determine whether it was as effective as other RS sources in opposing diet-induced colonic DNA damage in rats as a prelude to feeding trials in humans. Changes in large bowel bacterial populations and selected colonocyte genes associated with cellular integrity were also measured.
Materials and Methods
Animals and diets.
Sixty male Sprague-Dawley rats of ~200 g weight were obtained from the Animal Resource Centre, Murdoch University, Perth, Australia. They were housed in wire-bottomed cages in a room with controlled temperature (23°C) and lighting (a 12-h-light/-dark cycle) and allowed free access to food and water. The rats were randomly assigned to 1 of 6 groups (n = 10/group) and fed the respective experimental diets for 11 wk. The dietary compositions (Supplemental Table 1) were based on the AIN-93 diet (22). The composition of dietary wheat components (Supplemental Table 2) was determined using standard analytical methods as previously described (21). In the maize starch diets, casein (~80% protein) was the main protein source, with the remainder coming from wheat bran (~20%) (Supplemental Table 2). The control diet contained 7% fat, 13% protein, a highly digestible starch [low amylose maize starch (LAMS); National Starch Food Innovation], and 22% wheat bran as the fiber source. The control treatment is designated C-LAMS. The other diets, all moderate in fat (19%) and protein (20%) (deemed a Western diet), differed primarily with respect to the sources and forms of polysaccharides. The polysaccharide sources were LAMS, HAMS (Hi-maize, National Starch Food Innovation), HAMSB (prepared by National Starch Food Innovation with a degree of substitution of 0.23), HAW, or a commercial low amylose wheat flour (LAW) [CSIRO Plant Industry (21)]. These treatments with a Western diet background are designated W-LAMS, W-HAMS, W-HAMSB, W-LAW, and W-HAW, respectively. LAMS, HAMSB, and HAMS were obtained as purified ingredients (i.e., powder), whereas LAW and HAW were added as a ground meal. Diets were balanced for the high levels of fiber and protein by changing wheat bran, HAMS, or casein levels in the formulations where appropriate (Supplemental Table 2). The fat used in the diets was a blend of palm and canola oils prepared by Goodman Fielder Limited (Australia) that contained ~39% SFA, 46% MUFA, and 15% PUFA. Group food intakes and individual body weights were monitored daily throughout the study. At the completion of the dietary intervention period, rats were anesthetized with 4% halothane/oxygen to allow collection of gut tissues, digesta, and hepatic portal vein blood at the time animals were killed. The rats were not starved before anesthesia and may be regarded as being in a postabsorptive state as sampling started at 0830 h on each day. Experimental procedures were approved by the Animal Ethics Committee of CSIRO Food and Nutritional Sciences and complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Measurement of DNA strand breaks.
A 6-cm segment of colon was removed from rats at a point 3 cm from the distal end for isolation of colonocytes to enable measurement of DNA single-stranded breaks (SSB) using a single-cell gel electrophoresis (comet) assay (23). Comet tail moment (a product of tail length and the fraction of DNA in the tail) was calculated for 50 cells from each of 3 slides per rat using Comet Score v1.5 software (TriTek). Cells with morphology indicative of apoptosis or necrosis were excluded from analyses.
Colonic mucus layer thickness measurement.
Mucus layer thickness was measured as previously described (24). For each animal, 10 measurements were taken at different points along 3 tissue segments, giving 30 thickness measurements in total. Mean thickness was calculated using an image analysis program (24).
SCFA, phenols, and ammonia.
Frozen fecal and cecal digesta were thawed and then distilled and homogenized with heptanoic acid added as internal standard (to give 5 mmol/kg of feces). The contents were analyzed for SCFA in duplicate by GLC as previously described (25). Portal vein plasma SCFA concentrations were determined by diethyl ether extraction (26). Total SCFA levels were calculated from the sum of acetic, propionic, butyric, isobutyric, caproic, isovaleric, and valeric acid. Phenol levels were determined by a previously described method (27), and ammonia was measured using the indophenol blue procedure (28).
Microbiota analysis.
A custom phylogenetic microarray developed and validated for gut bacteria was used to analyze the microbiota (29). The cecal contents from the 6 dietary treatment groups (n = 10/group) were collected and pooled randomly to 2 groups for each treatment (n = 5/group). Each of the 2 cecal pools from each treatment group were processed as follows and run on the microarray. RNA was extracted from the samples as previously described (30). Prokaryote 16S ribosomal RNA genes were amplified using the primer sets 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and T7/1492R (5′- TCTAATACGACT CACTATAGGG GGYTACCTTGTTACGACTT-3′; the underlined region is modified to include a T7 promoter sequence) (31). The PCR amplicons were purified with the MinElute PCR purification kit (Qiagen) and then added as a template for in vitro transcription-based synthesis of single- stranded RNA (cRNA) using the MEGAScript T7 In Vitro transcription kit (Ambion). After purification with a MEGAclear kit (Ambion), 1 μg of the sample cRNA and 140 ng of standard cRNA were labeled at the same time using Label IT μArray Cy5 reagent (Mirus) for 1 h at 37°C while protected from light and then 0.1 volumes of the 10× stop reagent were added (Mirus) to terminate the labeling reaction. The labeled cRNA (25 μL) was fragmented using 5× fragmentation buffer (Mirus) at 94°C for 15 min so that the effects of fragmentation on signal intensity could be evaluated on the microarray. Without further purification steps that compromised signal intensity, 6 μL (~120 ng) of the labeled cRNA samples in 24 μL hybridization solution were hybridized overnight with the microarray (CombiMatrix) at 42°C as previously described (29).
Gene expression analysis.
Distal colonic tissue samples were removed from RNAlater stabilization reagent (Sigma), placed in 1 mL of TRIzol Reagent (Invitrogen), and homogenized using beads (mix of 2.5-mm glass and 0.1- to 1.0-mm diameter silicon-zirconian beads) in a MiniBeadbeater-8 (BioSpec Products). Total RNA was extracted (using TRIzol Reagent manufacturer’s instructions) and further purified using RNAeasy mini spin columns (QIAGEN) with a DNase on-column digestion per the manufacturer’s instructions. RNA integrity was checked using a Bioanalyzer 2100 (Agilent Technologies) and quantified using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific).
Dietary effects on colonocyte DNA damage and repair pathways were determined by examining candidate rat genes from the 9 replication and repair pathways listed in the Kyoto Encyclopedia of Genes and Genomes (32) and other related genes (e.g., for DNA topological change and chromatin remodelling) listed in Gene Ontology (33). Whole genome expression arrays were used (Affymetrix Rat Gene 1.0 ST Array, Affymetrix). Labeling and hybridization protocols were performed using 100 ng of total RNA using the standard Affymetrix procedure without the ribosomal RNA reduction step. The hybridized probe arrays were stained with a streptavidin and phycoerythrin conjugate, scanned (GeneArray scanner), and analyzed by Affymetrix Microarray Suite software. The relative levels of the differentially expressed genes of interest (as identified by microarray data analysis) were determined by qRT-PCR on RNA that had been reverse transcribed (method described in Supplemental Text; see Supplemental Table 3 for primer details).
Statistical analyses.
Data on body, organ, and digesta weights, digesta pH, and biochemistry are presented as the mean ± SEM for each treatment group. The effect of treatments was determined by 1-way ANOVA and differences between treatments were analyzed post hoc by Tukey’s honestly significant difference test. Relationships between measures were determined by Pearson correlation analysis. Analyses were performed using a SigmaStat statistical software program (SigmaStat 2.0 for Windows, SPSS). For the gene expression microarray array data, 1-way ANOVA and post hoc pairwise comparisons using Tukey’s honestly significant difference test were performed on the normalized gene expression data using R (R version 2.11.0, R Foundation for Statistical Computing). The gene expression qRT-PCR data were normalized (to the endogenous controls) and analyzed for differential expression by the same method.
Principal components analysis (PCA) was carried out on normalized array signal intensities from the microbiota microarray using the Genespring 7.3 software program (Agilent Technologies). P < 0.05 was considered significant for all datasets.
Results
There were no significant differences in final body weight between the treatment groups consuming the Western diet, and body weights of all but the rats consuming W-HAMS and W-HAMSB were significantly greater than for the rats fed C-LAMS (Table 1). Group food intake (measured on a weekly basis) did not differ between any of the treatments (data not shown). Cecal tissue and digesta weights were significantly higher and digesta pH was significantly lower in the W-HAMS and W-HAMSB groups relative to the C-LAMS, W-LAMS, and W-LAW groups. However, whereas cecal tissue weight was significantly higher, digesta mass and pH were unaffected in rats fed W-HAW (Table 1). Although the weight of colonic tissue was unaffected by dietary treatment, the weight of colonic digesta was significantly higher in the W-HAMSB group than in all other groups. Colonic digesta pH was significantly lower in the W-HAMS and W-HAW groups, but not the W-HAMSB group, relative to the C-LAMS, W-LAMS, and W-LAW groups (Table 1). Dietary treatment did not affect the weights of liver, spleen, and heart, but kidney weight was significantly higher in the W-HAMSB and W-LAW groups relative to the C-LAMS group (Table 1). Testicular (epididymal) fat pad weight was significantly greater in the W-LAW group than in the C-LAMS group (Table 1).
TABLE 1.
Effects of including different sources of starch in a Western diet moderate in fat and protein on final body, gut tissue, digesta, and organ weights, and digesta pH of rats1
| Variables | C-LAMS | W-LAMS | W-HAMS | W-HAMSB | W-LAW | W-HAW |
| Body weight, g | 479 ± 11a | 538 ± 14b | 523 ± 8ab | 525 ± 17ab | 558 ± 10b | 547 ± 13b |
| Cecum weights, g | ||||||
| Tissue | 1.22 ± 0.08a | 1.01 ± 0.08a | 1.80 ± 0.13b | 1.90 ± 0.16b | 1.13 ± 0.09a | 1.70 ± 0.13b |
| Digesta | 1.57 ± 0.15a | 1.36 ± 0.11a | 3.35 ± 0.48b | 3.97 ± 0.35b | 1.75 ± 0.10a | 2.36 ± 0.27a |
| Cecum pH | 6.79 ± 0.10a | 6.84 ± 0.09a | 6.34 ± 0.11b | 6.22 ± 0.07b | 6.68 ± 0.07a | 6.62 ± 0.11a |
| Colon weights, g | ||||||
| Tissue | 1.86 ± 0.13 | 1.73 ± 0.11 | 1.98 ± 0.20 | 1.87 ± 0.19 | 2.18 ± 0.11 | 2.07 ± 0.21 |
| Digesta | 1.79 ± 0.23a | 1.82 ± 0.13a | 2.25 ± 0.22a | 3.50 ± 0.41b | 1.81 ± 0.18a | 1.85 ± 0.24a |
| Colon pH | 6.74 ± 0.06a | 6.71 ± 0.18a | 6.12 ± 0.05b | 6.38 ± 0.08ab | 6.71 ± 0.05a | 6.28 ± 0.12b |
| Mucus thickness, μm | 600 ± 50 | 680 ± 50 | 550 ± 40 | 580 ± 50 | 590 ± 40 | 600 ± 60 |
| Organ weights, g | ||||||
| Liver | 16.0 ± 0.5 | 17.8 ± 0.8 | 17.5 ± 0.6 | 18.5 ± 1.3 | 18.7 ± 0.6 | 17.6 ± 0.8 |
| Spleen | 0.76 ± 0.04 | 0.84 ± 0.11 | 0.73 ± 0.03 | 0.80 ± 0.04 | 0.86 ± 0.10 | 0.75 ± 0.15 |
| Heart | 1.30 ± 0.03 | 1.47 ± 0.06 | 1.35 ± 0.03 | 1.34 ± 0.04 | 1.40 ± 0.03 | 1.37 ± 0.02 |
| Kidney | 1.34 ± 0.03a | 1.47 ± 0.05ab | 1.46 ± 0.04ab | 1.56 ± 0.05b | 1.55 ± 0.04b | 1.51 ± 0.05ab |
| Epididymal fat | 5.00 ± 0.51a | 7.43 ± 0.72ab | 6.42 ± 0.43ab | 7.27 ± 0.83ab | 8.03 ± 0.40b | 7.42 ± 0.62ab |
Values are mean ± SEM, = 10. Means in a row with superscripts without a common letter differ, P < 0.05. C-LAMS, control diet containing low amylose maize starch; W-HAMS, Western diet containing high amylose maize starch; W-HAMSB, Western diet containing butyrylated high amylose maize starch; W-HAW, Western diet containing high amylose wheat flour; W-LAMS, Western diet containing low amylose maize starch; W-LAW, Western diet containing low amylose wheat flour.
The cecal and colonic SCFA pools and hepatic portal venous SCFA concentrations are presented in Table 2. The levels in the cecum, colon, and portal vein did not differ significantly among the C-LAMS, W-LAMS, or W-LAW groups. The total and individual SCFA pools within the cecum were significantly greater than W-LAMS when W-HAMS, W-HAMSB, or W-HAW was consumed. The W-HAMS, W-HAMSB, and W-HAW diets also resulted in greater cecal acetate, propionate, butyrate, and total SCFA pools than in the C-LAMS group, although these were not always significant. The concentrations of SCFA in the cecum were not significantly changed apart from propionate, which was significantly higher for the W-HAW group than all other groups. The relative proportions of individual SCFA in the cecum also changed in response to diet. The percentage of total SCFA as acetate was significantly lower (and the percentage of butyrate significantly higher) for the W-HAMSB group relative to the W-LAW and W-LAMS groups and the percentage of propionate was significantly higher for the W-HAW group relative to other groups. In the colon, significant differences in SCFA pools in response to treatment were found for propionate, which was significantly higher in the W-HAW group than in the C-LAMS and W-LAMS groups. Colonic concentrations of acetate, propionate, and total SCFA differed significantly. The acetate concentration was lower in the W-HAMSB group than in the C-LAMS and W-HAW groups. The propionate concentration was greater in the W-HAW group than in all other groups and total SCFA was greater in the W-HAW group than in the W-HAMS and W-HAMSB groups. The concentrations of hepatic portal venous acetate and butyrate were significantly higher in rats fed W-HAMS and W-HAMSB, respectively, than in those fed W-LAMS, and propionate concentrations were significantly higher in rats fed W-HAMS and W-HAW.
TABLE 2.
Effects of including different sources of starch in a Western diet moderate in protein and fat on individual and total SCFA levels in cecal and colonic digesta, and hepatic portal vein plasma of rats1
| Variables | C-LAMS | W-LAMS | W-HAMS | W-HAMSB | W-LAW | W-HAW |
| Cecum SCFA | ||||||
| Pool, μmol | ||||||
| Acetate | 94 ± 14a | 67 ± 11a | 188 ± 32b | 158 ± 18b | 107 ± 15ab | 151 ± 22b |
| Propionate | 16 ± 2a | 11 ± 2a | 33 ± 4b | 32 ± 5b | 16 ± 2a | 42 ± 7b |
| Butyrate | 62 ± 7a | 39 ± 6a | 136 ± 26b | 167 ± 28b | 69 ± 7a | 104 ± 27b |
| Total | 178 ± 22a | 121 ± 18a | 377 ± 56b | 369 ± 51b | 200 ± 24ab | 308 ± 47b |
| Concentration, mmol/kg tissue | ||||||
| Acetate | 75 ± 6 | 65 ± 7 | 72 ± 8 | 59 ± 3 | 78 ± 7 | 80 ± 10 |
| Propionate | 13 ± 1a | 10 ± 1a | 13 ± 1a | 12 ± 1a | 12 ± 1a | 22 ± 3b |
| Butyrate | 51 ± 4 | 37 ± 4 | 50 ± 5 | 61 ± 6 | 51 ± 4 | 52 ± 9 |
| Total | 144 ± 9 | 117 ± 11 | 143 ± 10 | 135 ± 9 | 146 ± 11 | 161 ± 15 |
| Percentage of total, % | ||||||
| Acetate | 52 ± 2a | 56 ± 2a | 50 ± 2a | 44 ± 1b | 53 ± 1a | 49 ± 3ab |
| Propionate | 9 ± 1a | 9 ± 1a | 9 ± 1a | 9.0 ± 1a | 8 ± 0a | 14 ± 2b |
| Butyrate | 35 ± 2a | 31 ± 2a | 35 ± 3a | 44 ± 1b | 35 ± 1a | 32 ± 4a |
| Colon SCFA | ||||||
| Pool, μmol | ||||||
| Acetate | 57 ± 7 | 56 ± 4 | 65 ± 7 | 59 ± 5 | 48 ± 7 | 78 ± 14 |
| Propionate | 9 ± 1a | 8 ± 1a | 12 ± 2ab | 14 ± 2ab | 9 ± 1a | 21 ± 4b |
| Butyrate | 24 ± 4 | 26 ± 3 | 28 ± 7 | 45 ± 6 | 26 ± 7 | 33 ± 7 |
| Total | 93 ± 10 | 94 ± 8 | 110 ± 14 | 123 ± 11 | 87 ± 15 | 137 ± 18 |
| Concentration, mmol/kg tissue | ||||||
| Acetate | 49 ± 4a | 47 ± 3ab | 42 ± 3ab | 30 ± 2b | 43 ± 5ab | 57 ± 7a |
| Propionate | 8 ± 1a | 7 ± 1a | 7 ± 1a | 7 ± 1a | 8 ± 1a | 15 ± 2b |
| Butyrate | 21 ± 4 | 22 ± 2 | 17 ± 3 | 23 ± 3 | 24 ± 5 | 27 ± 5 |
| Total | 81 ± 7ab | 78 ± 5ab | 70 ± 5a | 63 ± 5a | 78 ± 11ab | 103 ± 11b |
| Percentage of total, % | ||||||
| Acetate | 60 ± 3ab | 60 ± 2ab | 62 ± 3a | 49 ± 2b | 57 ± 3ab | 55 ± 4ab |
| Propionate | 10 ± 1a | 9 ± 1a | 10 ± 1a | 11 ± 1a | 10 ± 1a | 14 ± 1b |
| Butyrate | 25 ± 3 | 27 ± 2 | 23 ± 3 | 36 ± 2 | 28 ± 3 | 26 ± 4 |
| Portal vein SCFA | ||||||
| Concentration, μmol/L | ||||||
| Acetate | 399 ± 14ab | 338 ± 23a | 528 ± 66b | 465 ± 36ab | 405 ± 43ab | 449 ± 42ab |
| Propionate | 52 ± 6ab | 39 ± 6a | 71 ± 10ab | 95 ± 14b | 59 ± 11ab | 93 ± 13b |
| Butyrate | 223 ± 30ab | 166 ± 15a | 264 ± 39ab | 354 ± 38b | 204 ± 29ab | 251 ± 61ab |
| Total | 674 ± 43a | 542 ± 40a | 863 ± 91b | 914 ± 81b | 668 ± 78ab | 793 ± 89ab |
| Percentage of total, % | ||||||
| Acetate | 60 ± 3 | 63 ± 2 | 62 ± 3 | 52 ± 2 | 62 ± 2 | 58 ± 4 |
| Propionate | 8 ± 1a | 7 ± 1a | 8 ± 1ab | 10 ± 1ab | 8 ± 1ab | 12 ± 1b |
| Butyrate | 32 ± 3 | 30 ± 1 | 30 ± 3 | 38 ± 1 | 30 ± 1 | 30 ± 5 |
Values are mean ± SEM, = 9–10. Labeled means without a common letter differ, P < 0.05. C-LAMS, control diet containing low amylose maize starch; W-HAMS, Western diet containing high amylose maize starch; W-HAMSB, Western diet containing butyrylated high amylose maize starch; W-HAW, Western diet containing high amylose wheat flour; W-LAMS, Western diet containing low amylose maize starch; W-LAW, Western diet containing low amylose wheat flour.
Levels of ammonia and phenols within the cecal and colonic digesta differed significantly between dietary treatments (Table 3). W-HAMS, W-HAMSB, and W-HAW had similar effects on both of the protein fermentation products, lowering concentrations or pools significantly relative to one or more of the C-LAMS, W-LAMS, and W-LAW treatments. No significant effects were found for pools of ammonia in the cecum or pools of phenols in the colon.
TABLE 3.
Effects of including different sources of starch in a Western diet moderate in protein and fat on ammonia and phenol levels in cecal and colonic digesta of rats1
| Variables | C-LAMS | W-LAMS | W-HAMS | W-HAMSB | W-LAW | W-HAW |
| Cecum ammonia | ||||||
| Pool, mmol | 24.0 ± 3.7 | 15.2 ± 2.4 | 13.6 ± 3.2 | 16.4 ± 1.6 | 19.3 ± 2.9 | 17.5 ± 2.0 |
| Concentration, mmol/kg tissue | 15.6 ± 2.7a | 13.0 ± 1.7a | 4.8 ± 0.7b | 6.7 ± 0.7b | 13.7 ± 1.5a | 8.9 ± 0.8b |
| Cecum phenols | ||||||
| Pool, mmol | 10.6 ± 1.9a | 8.0 ± 2.2ab | 4.9 ± 0.8b | 5.1 ± 0.5b | 7.8 ± 1.6ab | 4.2 ± 0.7b |
| Concentration, mmol/kg tissue | 8.5 ± 1.3a | 7.7 ± 1.9a | 2.0 ± 0.2b | 2.0 ± 0.2b | 5.7 ± 1.1a | 2.4 ± 0.4b |
| Colon ammonia | ||||||
| Pool, mmol | 10.0 ± 0.9a | 12.1 ± 2.2a | 3.9 ± 1.2b | 6.2 ± 1.0b | 9.8 ± 0.9a | 4.9 ± 1.0b |
| Concentration, mmol/kg tissue | 7.8 ± 0.6a | 9.5 ± 0.9a | 2.3 ± 0.6b | 3.3 ± 0.6b | 8.9 ± 0.5a | 3.4 ± 0.7b |
| Colon phenols | ||||||
| Pool, mmol | 5.8 ± 1.0 | 5.1 ± 0.9 | 3.1 ± 1.2 | 2.4 ± 0.7 | 4.3 ± 0.7 | 3.5 ± 1.6 |
| Concentration, mmol/kg tissue | 5.3 ± 1.2a | 4.4 ± 0.9a | 2.4 ± 1.2ab | 1.4 ± 0.3b | 4.0 ± 0.8a | 2.5 ± 1.1ab |
Values are mean ± SEM, = 4–10. Means in a row with superscripts without a common letter differ, P < 0.05. C-LAMS, control diet containing low amylose maize starch; W-HAMS, Western diet containing high amylose maize starch; W-HAMSB, Western diet containing butyrylated high amylose maize starch; W-HAW, Western diet containing high amylose wheat flour; W-LAMS, Western diet containing low amylose maize starch; W-LAW, Western diet containing low amylose wheat flour.
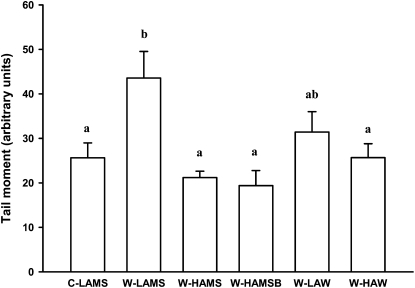
The number of SSB in colonic DNA (Fig. 1) was significantly greater (by 70%) in rats fed W-LAMS than in those fed C-LAMS. However, SSB numbers were significantly lower than for the W-LAMS treatment when W-HAMS, W-HAMSB, or W-HAW, but not W-LAW, was consumed.
FIGURE 1.
Effects of including different sources of starch in Western diets moderate in protein and fat on DNA SSB (comet assay tail moments) in colonocytes of rats. Values are mean ± SEM, n = 9–10. Values without a common letter differ, P < 0.05. C-LAMS, control diet containing low amylose maize starch; SSB, single-strand break; W-HAMS, Western diet containing high amylose maize starch; W-HAMSB, Western diet containing butyrylated high amylose maize starch; W-HAW, Western diet containing high amylose wheat flour; W-LAMS, Western diet containing low amylose maize starch; W-LAW, Western diet containing low amylose wheat flour.
The thickness of the colonic mucus layer did not significantly differ between treatment groups (data not shown).
Significant inverse relationships were found between SSB and the concentrations of SCFA within the hepatic portal vein (acetate: R2 = 0.32, P = 0.04; propionate: R2 = 0.33, P = 0.04; butyrate: R2 = 0.28, P = 0.07; total SCFA: R2 = 0.36, P = 0.02) and cecal concentrations of butyrate (R2 = 0.40, P = 0.009) and total SCFA (R2 = 0.40, P = 0.009) but not acetate (R2 = 0.25) or propionate (R2 = 0.22). There were no significant relationships between SSB and cecal SCFA pools or between SSB and colonic concentrations or pools of SCFA. When pools of SCFA in the cecum and colon were combined, an inverse relationship was found between total SCFA and SSB (R2 = 0.46, P = 0.002). A positive relationship was observed between SSB and the concentration of ammonia within the colon (R2 = 0.40, P = 0.014) but not the cecum (R2 = 0.17). There was no significant relationship with pools of ammonia within the colon or cecum or with concentrations or pools of phenols within the cecum or colon.
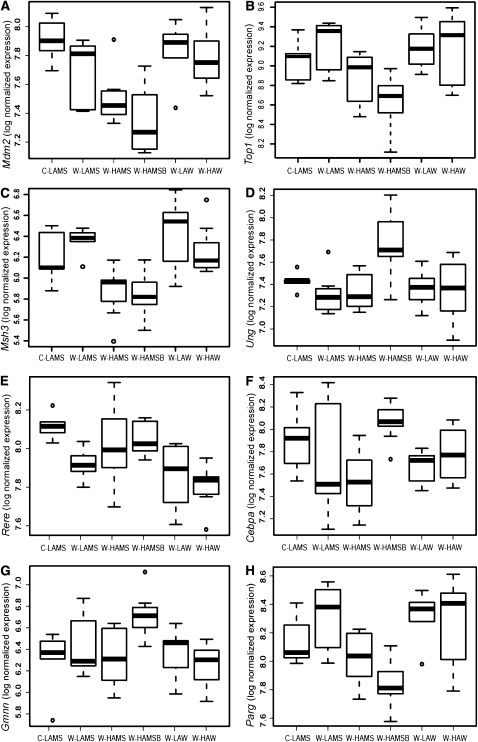
Different diets were associated with changes in expression of some candidate DNA damage and repair genes. Box plots depicting the impact of diet on the expression of 8 genes associated with DNA damage, repair, and cell growth displaying significant alteration by one or more of the diets, as detected by microarray, are shown in Figure 2. The expression of Mdm2 was significantly downregulated by W-HAMS and W-HAMSB compared to C-LAMS. Mdm2 was also downregulated in rats by W-HAMSB compared to W-LAW and W-HAW. When the effects of W-HAMSB were compared to the effects of W-LAMS, W-LAW, and W-HAW, there was significant downregulation of Top1, Parg, and Msh3. Furthermore, Msh3 was also downregulated by W-HAMS compared with W-LAMS, W-LAW, and W-HAW. In contrast, W-HAMSB resulted in the significant upregulation of Gmnn, Cebpa, and Ung. Expression of Gmnn was significantly higher in rats consuming W-HAMSB than those receiving the C-LAMS, W-HAMS, and W-HAW diets. Cebpa expression was significantly higher in the W-HAMSB group than in the W-HAMS group and Ung was significantly upregulated in the W-HAMSB group compared with the W-LAMS and W-HAW groups. When the W-HAW treatment was compared to the C-LAMS and W-HAMSB diets, the greatest DNA damage and repair-related transcriptional response was the significant downregulation of Rere. The above microarray differential expression data were confirmed by qRT-PCR (Supplemental Fig. 1), except for Msh3, where differences were reduced and only the elevated expression in the W-LAW group was clearly evident.
FIGURE 2.
Boxplots showing effects of including different sources of starch in Western diets moderate in protein and fat on colonic gene expression of Mdm2 (A), Top1 (B), Msh3 (C), Ung (D), Rere (E), Cebpa (F), Gmnn (G), and Parg (H) in rats. Standard gene symbols are used (32, 33). The limits of the boxes represent the lower and upper quartile range, and the solid line within the box represents the median value. Whiskers extend to the lowest and highest values within 1.5 times the quartile range and values outside this range are shown as circles. C-LAMS, control diet containing low amylose maize starch (n = 5); W-HAMS, Western diet containing high amylose maize starch (n = 8); W-HAMSB, Western diet containing butyrylated high amylose maize starch (n = 9); W-HAW, Western diet containing high amylose wheat flour (n = 8); W-LAMS, Western diet containing low amylose maize starch (n = 7); W-LAW, Western diet containing low amylose wheat flour (n = 6).
Fecal microbial population profiles were determined using a custom phylogenetic microarray and PCA showed that the profiles fell into 3 defined groups: C-LAMS and W-LAMS, W-HAMS and W-HAMSB, and W-LAW and W-HAW (Supplemental Fig. 2). A heat map showing some of the major differences in abundance of selected species and genera in response to diet is presented in Supplemental Figure 3. Lactic acid producing bacteria of clostridial cluster XVI were in significantly higher abundance in the W-HAW group compared to the WHAMS and W-HAMSB groups, and Eubacterium ventriosum and Roseburia cecicola, which belong to clostridial cluster XIVa, were significantly higher in both W-HAMS and W-HAMSB groups. Bacteria of clostridial cluster IV, Spirochaeta sp., and the uncultured gram-negative Cytophaga-Flavobacteria-Bacteroides group were also significantly higher in abundance in rats consuming the RS diets compared with the C-LAMS and W-LAMS diets (data not shown).
Discussion
The current data confirm our earlier findings of more colonocyte SSB in rats fed diets high in protein and also that HAMS and HAMSB opposed this damage (6–9, 24). These data are consistent with findings from population studies showing increased CRC risk with greater fat and protein consumption but a lowering of risk by dietary fiber. The present study adds new knowledge in showing that W-HAW, but not W-LAW, also significantly lowered SSB compared to W-LAMS, although the W-HAW and W-LAW groups were not significantly different from each other. Previous animal studies on diet and CRC have generally used genotoxic agents to induce damage (e.g., azoxymethane). In keeping with the current data, they have shown that aberrant crypt foci or adenoma formation was increased by high dietary protein and that this was opposed by RS (13–16). The mechanisms behind increased SSB and other genetic damage probably involve increased exposure to cytotoxic agents generated by bacteria. One possibility for proteins containing heme iron (such as red meat) is the formation of DNA adducts through bacterial generation of damaging reactive species (34). However, casein (fed in this study) induces damage but does not contain heme, suggesting involvement of other genotoxic protein products such as ammonia and phenols (6, 24). Higher dietary protein can raise large bowel digesta ammonia in rats (35) and increase CRC risk through enhancing colonocyte proliferation (36). Ammonia is absorbed from the colon in its unprotonated form (i.e., at more alkaline pH). Our earlier studies showed that cecal ammonia level was inversely related to pH, consistent with diminished absorption. In the present study, SSB correlated positively with colonic digesta ammonia concentration. The apparent anomaly between current and previous findings can be reconciled by the fact that the levels throughout the large bowel may be influenced by the type of dietary fiber (35). There was no significant relationship between SSB and large bowel phenol levels, although levels of ammonia and phenols were significantly lowered by the RS treatments.
Recently, we showed dose-dependent changes in circulating concentrations of several CRC-related biomarkers in rats fed HAMS and red or white (chicken) meat (18). The present data, demonstrating colonic transcriptional changes in genes associated with DNA damage and repair, indicate that these previous findings may reflect altered gene expression. The expression of genes involved in DNA damage recovery were downregulated in rats fed W-HAMSB. These were Mdm2, which encodes a key negative regulator of the p53 tumor suppressor protein that is overexpressed in neoplasia (37); Parg, which is stimulated by DNA strand breaks (38), markers of topological stress, e.g., Top1 (39); and Gmnn, which can kill cancer cells without harming normal ones (40). In contrast, expression of the base-excision repair pathway gene Ung and the cell cycle damage checkpoint gene Cepba were higher in rats fed HAMSB. The changes suggest that protective mechanisms were operating through different regulatory pathways for W-HAW and W-HAMS and /or W-HAMSB with differential expression of Mdm2, Top1, Parg, Gmnn, and Ung. The HAW diet had the most significant impact on the chromatin remodeling gene, Rere. These results suggest there are fewer interruptions to DNA integrity by the moderate fat/protein base with the inclusion of HAMS, HAMSB, or HAW to the diet. For W-HAMSB, the underlying protective mechanism probably involves the fine maintenance of genomic homeostasis while preventing replication of damaged cells, whereas for W-HAW, chromatin restructuring seems to be a key feature, suggesting that protection could occur at a different stage of the cell cycle process.
This study was carried out over 3 mo and confirms that dietary effects on large bowel SCFA and DNA damage were sustained in the longer term. As expected, SCFA levels were highest in rats fed RS and lowest in those fed LAMS, consistent with its high digestibility. Large bowel SCFA were intermediate in rats fed H-LAW, supporting the suggestion that RS is higher in whole grain foods compared with refined ones (41). This may contribute to the protective effect of whole grains in lowering the risk of diet-related disease through the generation of SCFA (42). However, for maximal effect, a high amylose content may be necessary (43). Digesta total SCFA and butyrate were highest in rats fed W-HAMSB, consistent with acylated starches supplying SCFA both as the esterified acid and through fermentation of undigested starch. There were substantial differences between W-HAW and the other RS types. Changes in SCFA and pH were distributed more evenly through the large bowel in rats fed W-HAW. This may be of some value, because most chronic large bowel disease (including CRC) is localized in the distal colon and rectum, where SCFA supply is lowest (44). It has been suggested that a combination of RS plus nonstarch polysaccharide (NSP) is optimal in ensuring the supply of SCFA to these viscera (45). Our data support this, because HAW contained both fiber polysaccharides integrally, whereas the other diets (apart from W-LAW) required the addition of fiber. This may also explain a difference in SCFA between W-HAW and other RS treatments – the significantly higher propionate levels. The fermentation of NSP mixtures gives different SCFA patterns relative to when they are fed separately (46) and HAW had an altered NSP content as well as more amylose.
In contrast to earlier studies, where higher dietary protein induced a dose-dependent thinning of the mucus layer (7), there was no effect of diet on mucus barrier thickness. This may reflect the higher level of indigestible matter (as NSP) in this study, because indigestible styrofoam particles increase small intestinal mucin secretion in rats (47). The cereal NSP in the present study might have similar effects.
Feeding of RS alters the composition of the large bowel microbiota (48–51) and HAMS can function as a prebiotic in animals (48). In humans, a diet high in RS and NSP raises fecal levels of a range of bacteria, including Ruminococcus bromii, thought to be important for starch degradation and SCFA production (49). In this study, phylogenetic array analysis followed by PCA showed that the gut microbiota profiles fell into 3 distinct groupings. One consisted of the C-LAMS and W-LAMS treatments, suggesting relatively little effect of increasing the fat and protein content of the diet on the large bowel microbiota. The profiles of the W-HAMS– and W-HAMSB–treated rats also clustered together, indicating little effect of the extra butyrate delivered by HAMSB relative to HAMS. The microbiota profiles of rats receiving the wheat treatments comprised the third grouping. These groupings suggest the feeding of RS alters the relative proportions of the gut microbiota but also that the further addition of mixed forms of NSP from a whole meal flour leads to other changes in the microbiota profiles. Our analyses also revealed a common feature of the microbial profiles associated with the RS diets: the high abundance of clostridial cluster IV bacteria (except Faecalibacterium prausnitzii). The butyrate-producing ability of many bacteria in this cluster is expected to have contributed to the increased butyrate and SCFA produced in response to RS diets.
There has been debate around the potential for greater SCFA production through fermentation to induce a colonic epithelial hyperproliferation and, hence, contribute to CRC. This proposition is supported by the greater incidence of polyps in mutant CRC-prone mice when fed fermentable NSP as apple pomace (52). However, >90% of those tumors were in the small intestine and are not relevant to CRC. The current data and those from other studies from this and other laboratories show that the changes due to RS consumption are consistent with improved bowel health. A previous short-term study with HAW in rats showed higher large bowel SCFA levels and the current data suggest that this new RS source HAW offers benefits similar to HAMS and HAMSB.
Acknowledgments
The authors thank Ben Scherer, Emma Watson, Debbie Davies, and Corinna Bennett for assistance during the animal study and Thu Ho for laboratory assistance with the rat gene expression arrays. M.A.C., A.R.B., M.K.M., T.J.L., P.L.M., and D.L.T. designed research; M.A.C., C.A.K., C.S.M., R.A.D., J.M.S., S.K., A.R., and S.T. conducted research; M.A.C., C.A.K., C.S.M., R.A.D., J.M.S., A.R.B., S.K., S.T., J.M.C., and D.L.T. analyzed data; M.A.C., C.A.K., C.S.M., R.A.D., J.M.S., S.K., A.R.B., M.K.M., T.J.L., P.L.M., A.R., S.T., J.M.C., and D.L.T. wrote the paper; and M.A.C. and D.L.T. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by CSIRO. CSIRO receives support from a joint venture with Arista, which indirectly provides assistance to CSIRO researchers.
Supplemental Tables 1–3, Supplemental Figures 1–3, and Supplemental Text are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: C-LAMS, control diet containing low amylose maize starch; CRC, colorectal cancer; cRNA, single-stranded RNA; EPIC, European Prospective Investigation into Cancer and Nutrition; HAMS, high amylose maize starch; HAMSB, butyrylated high amylose maize starch; HAW, high amylose wheat flour; LAMS, low amylose maize starch; LAW, low amylose wheat flour; NSP, nonstarch polysaccharide; PCA, principal components analysis; RS, resistant starch; SSB, single-strand break; W-HAMS, Western diet containing high amylose maize starch; W-HAMSB, Western diet containing butyrylated high amylose maize starch; W-HAW, Western diet containing high amylose wheat flour; W-LAMS, Western diet containing low amylose maize starch; W-LAW, Western diet containing low amylose wheat flour.
Literature Cited
- 1.Mascie-Taylor CG, Karim E. The burden of chronic disease. Science. 2003;302:1921–2 [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare Cancer in Australia: an overview; 2006. [cited 2010 Sep 21]. Available from: http://www.aihw.gov.au/publications/index.cfm/title/1046
- 3.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, et al. Dietary fiber in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–501 [DOI] [PubMed] [Google Scholar]
- 4.Cassidy A, Bingham SA, Cummings JH. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994;69:937–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, Overvad K, Olsen A, Tjønneland A, Clavel F, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. J Natl Cancer Inst. 2005;97:906–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol Ther. 2006;5:267–72 [DOI] [PubMed] [Google Scholar]
- 7.Toden S, Bird AR, Topping DL, Conlon MA. Dose-dependent reduction of dietary protein-induced colonocyte DNA damage by resistant starch in rats correlates more highly with caecal butyrate than with other short chain fatty acids. Cancer Biol Ther. 2007;6:253–8 [DOI] [PubMed] [Google Scholar]
- 8.Toden S, Bird AR, Topping DL, Conlon MA. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat: attenuation by high amylose maize starch. Carcinogenesis. 2007;28:2355–62 [DOI] [PubMed] [Google Scholar]
- 9.Bajka BH, Clarke JM, Cobiac L, Topping DL. Butyrylated starch protects colonocyte DNA against dietary protein-induced damage in rats. Carcinogenesis. 2008;29:2169–74 [DOI] [PubMed] [Google Scholar]
- 10.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64 [DOI] [PubMed] [Google Scholar]
- 11.Brouns F, Kettlitz B, Arrigoni E. Resistant starch and the “butyrate revolution”. Trends Food Sci Technol. 2002;13:251–61 [Google Scholar]
- 12.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43 [DOI] [PubMed] [Google Scholar]
- 13.Le Leu RK, Brown IL, Hu Y, Esterman A, Young GP. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol Ther. 2007;6:1621–6 [DOI] [PubMed] [Google Scholar]
- 14.Clarke JM, Topping DL, Bird AR, Young GP, Cobiac L. Effects of high-amylose maize starch and butyrylated high-amylose maize starch on azoxymethane-induced intestinal cancer in rats. Carcinogenesis. 2008;29:2190–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassand P, Maziere S, Champ M, Meflah K, Bornet F, Narbonne JF. Effects of resistant starch- and vitamin A-supplemented diets on the promotion of precursor lesions of colon cancer in rats. Nutr Cancer. 1997;27:53–9 [DOI] [PubMed] [Google Scholar]
- 16.Bauer-Marinovic M, Florian S, Müller-Schmehl K, Glatt H, Jacobasch G. Dietary resistant starch type 3 prevents tumor induction by 1,2 dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis. 2006;27:1849–59 [DOI] [PubMed] [Google Scholar]
- 17.Potter JD, McMichael AJ. Diet and cancer of the colon and rectum: a case-control study. J Natl Cancer Inst. 1986;76:557–69 [DOI] [PubMed] [Google Scholar]
- 18.Toden S, Belobrajdic DP, Bird AR, Topping DL, Conlon MA. Effects of dietary beef and chicken with and without high amylose maize starch on blood malondialdehyde, interleukins, IGF-1, insulin, leptin, MMP-2 and TIMP-2 concentrations in rats. Nutr Cancer. 2010;62:454–65 [DOI] [PubMed] [Google Scholar]
- 19.Le Leu RK, Brown IL, Hu Y, Bird AR, Jackson M, Esterman A, Young GP. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J Nutr. 2005;135:996–1001 [DOI] [PubMed] [Google Scholar]
- 20.Brown IL. Applications and uses of resistant starch. J AOAC Int. 2004;87:727–32 [PubMed] [Google Scholar]
- 21.Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T, Kosar-Hashemi B, Li Z, Rahman S, Morell M. High amylose wheat generated by RNA-interference improves indices of large bowel health in rats. Proc Natl Acad Sci USA. 2006;103:3546–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 23.Anderson D, Hambly RJ, Yu TW, Thomasoni F, Shuker DE. The effect of potassium diazoacetate on human peripheral lymphocytes, human adenocarcinoma Colon caco-2 cells, and rat primary colon cells in the comet assay. Teratog Carcinog Mutagen. 1999;19:137–46 [DOI] [PubMed] [Google Scholar]
- 24.Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch attenuates colonic DNA damage induced by higher dietary protein in rats. Nutr Cancer. 2005;51:45–51 [DOI] [PubMed] [Google Scholar]
- 25.Patten GS, Abeywardena MY, McMurchie EJ, Jahangiri A. Dietary fish oil increases acetylcholine- and eicosanoid-induced contractility of isolated rat ileum. J Nutr. 2002;132:2506–13 [DOI] [PubMed] [Google Scholar]
- 26.Murase M, Kimura Y, Nagata Y. Determination of portal short-chain fatty acids in rats fed various dietary fibers by capillary gas chromatography. J Chromatogr B Biomed Appl. 1995;664:415–20 [DOI] [PubMed] [Google Scholar]
- 27.Murray KE, Adams RF. Determination of simple phenols in in faeces and urine by high-performance liquid chromatography. J Chromatogr. 1988;431:143–9 [DOI] [PubMed] [Google Scholar]
- 28.Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–2 [PubMed] [Google Scholar]
- 29.Kang S, Denman SE, Morrison M, Yu Z, Dore J, Leclerc M, McSweeney CS. Dysbiosis of faecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis. 2010;16:2034–42 [DOI] [PubMed] [Google Scholar]
- 30.Kang S, Denman SE, Morrison M, Yu Z, McSweeney C. An efficient RNA extraction method for estimating gut microbial diversity by polymerase chain reaction. Curr Microbiol. 2009;58:464–71 [DOI] [PubMed] [Google Scholar]
- 31.Salzman NH. Jong HH, Paterson Y. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148:3651–60 [DOI] [PubMed] [Google Scholar]
- 32. Kyoto Encyclopedia of Genes and Genomes [cited 2009 Jun 10]. Available from: http://www.genome.jp/kegg/pathway.html.
- 33. Gene Ontology [cited 2009 Jun 10]. Available from: http://geneontology.org.
- 34.Bingham SA, Pignatelli B, Pollock JR, Ellul A, Malaveille C, Gross G, Runswick S, Cummings JH, O’Neill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17:515–23 [DOI] [PubMed] [Google Scholar]
- 35.Lupton JR, Marchant LJ. Independent effects of fiber and protein on colonic luminal ammonia concentration. J Nutr. 1989;119:235–41 [DOI] [PubMed] [Google Scholar]
- 36.Lin HC, Visek WJ. Large intestinal pH and ammonia in rats: dietary fat and protein interactions. J Nutr. 1991;121:832–43 [DOI] [PubMed] [Google Scholar]
- 37.Perry ME. The regulation of the p53-mediated stress response by MDM2 and MDM4. Cold Spring Harb Perspect Biol. 2010;2:a000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dantzer F, Amé JC, Schreiber V, Nakamura J, Ménissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006;409:493–510 [DOI] [PubMed] [Google Scholar]
- 39.Miao ZH, Player A, Shankavaram U, Wang YH, Zimonjic DB, Lorenzi PL, Liao ZY, Liu H, Shimura T, Zhang HL. Non-classic functions of human topoisomerase I: genome-wide and pharmacologic analyses. Cancer Res. 2007;67:8752–61 [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Depamphilis ML. Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 2009;69:4870–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livesey G, Wilkinson JA, Roe M, Faulks R, Clark S, Brown JC, Kennedy H, Elia M. Influence of the physical form of barley grain on the digestion of its starch in the human small intestine and implications for health. Am J Clin Nutr. 1995;61:75–81 [DOI] [PubMed] [Google Scholar]
- 42.Topping D, Bird A, Toden S, Conlon M, Noakes M, King R, Mann G, Li Z, Morell M. Resistant starch as a contributor to the health benefits of whole grains. : Marquart L, Jacobs D, McIntosh G, Poutanen K, Reicks M, Whole grains and health. Ames (IA): Blackwell Publishers; 2007. p. 219–27 [Google Scholar]
- 43.Bird AR, Vuaran MS, King RA, Noakes M, Keogh J, Morell MK, Topping DL. Wholegrain foods made from a novel high-amylose barley variety (Himalaya 292) improve indices of bowel health in human subjects. Br J Nutr. 2008;99:1032–40 [DOI] [PubMed] [Google Scholar]
- 44.Cats A, De Vries EG, Mulder NH, Kleibeuker JH. Regional differences of physiological functions and cancer susceptibility in the human large intestine. Int J Oncol. 1996;9:1055–69 [DOI] [PubMed] [Google Scholar]
- 45.Muir JG, Yeow EG, Keogh J, Pizzey C, Bird AR, O’Dear K, Macrae FA. Combining wheat bran with resistant starch has more beneficial effects on fecal indices than does wheat bran alone. Am J Clin Nutr. 2004;79:1020–8 [DOI] [PubMed] [Google Scholar]
- 46.Topping DL, Mock S, Trimble RP, Storer GB, Illman RJ. Effects of varying the content and proportions of gum arabic and cellulose on caecal volatile fatty acid concentrations in the rat. Nutr Res. 1988;8:1013–20 [Google Scholar]
- 47.Morita T, Tanabe H, Ito H, Sugiyama K, Kiriyama S. Long-term ingestion of insoluble dietary fiber increases luminal mucin content but has no effect on nutrient absorption in rats. Biosci Biotechnol Biochem. 2008;72:767–72 [DOI] [PubMed] [Google Scholar]
- 48.Brown I, Warhurst M, Arcot J, Playne M, Illman RJ, Topping DL. Fecal starch and volatile fatty acid excretion and Bifidobacterium longum numbers are higher in pigs fed a high amylose starch than in those fed amylopectin. J Nutr. 1997;127:1822–7 [DOI] [PubMed] [Google Scholar]
- 49.Abell GC, Cooke CM, Bennett CN, Conlon MA, McOrist AL. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol. 2008;66:505–15 [DOI] [PubMed] [Google Scholar]
- 50.Silvi S, Rumney CJ, Cresci A, Rowland IR. Resistant starch modifies gut microflora and microbial metabolism in human flora-associated rats inoculated with faeces from Italian and UK donors. J Appl Microbiol. 1999;86:521–30 [DOI] [PubMed] [Google Scholar]
- 51.Bird AR, Vuaran M, Crittenden R, Hayakawa T, Playne MJ, Brown IL, Topping DL. Comparative effects of a high amylose starch and a fructooligosaccharide on fecal bifidobacteria numbers and short-chain fatty acids in pigs fed Bifidobacterium animalis. Dig Dis Sci. 2009;54:947–54 [DOI] [PubMed] [Google Scholar]
- 52.Mandir N, Englyst H, Goodlad RA. Resistant carbohydrates stimulate cell proliferation and crypt fission in wild-type mice and in the ApcMin/+ mouse model of intestinal cancer association with enhanced polyp development. Br J Nutr. 2008;100:711–21 [DOI] [PubMed] [Google Scholar]