Abstract
Phylloquinone (PK) is converted into menaquinone-4 (MK-4) via side chain removal-addition. Stable isotope use is an effective approach to identify the tissue location of this conversion, which is currently unknown. Following a 14-d PK-deficient diet, male Fischer 344 rats (8 mo; n = 15) were fed 1.6 mg deuterium-labeled PK (L-PK) per kg diet for 0 (control), 1 d (PK-1d), and 7 d (PK-7d). Both L-PK and deuterium-labeled MK-4 (L-MK-4) were detected in tissues in PK-1d and PK-7d, although the results varied. Whereas some tissues had an overall increase in MK-4 in response to L-PK, total brain, testes, and fat MK-4 concentrations did not. In contrast, L-MK-4 concentrations increased in all 3 tissues. The deuterium label was found only on the L-MK-4 naphthoquinone ring, confirming the need for side chain removal for the formation of MK-4. Labeled menadione (MD) was detected in urine and serum in PK-1d and PK-7d, confirming its role as an intermediate. A Caco-2 cell monolayer model was used to study the role of the enterocytes in the conversion process. Neither MK-4 nor MD was detected in Caco-2 cells treated with PK. However, when Caco-2 cells were treated with MD, MK-4 was formed. Similarly, MK-4 was formed in response to MD-treated 293T kidney cells, but not HuH7 liver cells. These data demonstrate that MK-4 is the predominant form of vitamin K in multiple tissues, but there appears to be a tissue-specific regulation for the conversion of PK to MK-4.
Introduction
All forms of vitamin K share the 2-methyl-1,4- naphthoquinone ring but differ in the position-3 side chain. The naphthoquinone ring is the active site for vitamin K’s established role as a cofactor for the vitamin K-dependent carboxylase.
Mammals have the ability to convert dietary phylloquinone (PK)7, and menadione (MD; 2-methyl-1,4-napthoquinone), into menaquinone-4 (MK-4) and store the latter in specific tissues (1). It is unlikely that a metabolic pathway leading to MK-4 would have evolved unless MK-4 had unique biological roles. These roles are unlikely to involve the vitamin K-dependent carboxylase, because PK and MK-4 have similar activity as a substrate for this enzymatic activity (2). This suggests that MK-4 plays a role beyond the classical enzyme cofactor role of vitamin K. Indeed, MK-4 has been shown to be the active vitamin K form that inhibits oxidative cell death in primary cultures of oligodendrocyte precursors and immature neurons (3), induces apoptosis induction in leukemia and other malignant cell lines (4, 5), and serves as a ligand for the steroid xenobiotic receptor in bone cells (6).
Recently, UbiA prenyltransferase containing 1 (UBIAD1) was identified as the enzyme catalyzing prenylation of MD with a geranylgeranyl side chain forming MK-4 (7). However, the exact mechanism by which PK is converted to MK-4 and the location of where this conversion occurs are not known. Furthermore, direct evidence identifying MD as the intermediate in the conversion process has been lacking in tissues other than the brain (8).
We used stable isotope technology to address these gaps in knowledge. Specifically, we fed deuterium-labeled PK (L-PK) to Fischer 344 rats to test the hypothesis that the phytyl side chain in the L-PK is cleaved off to form deuterium-labeled unconjugated MD (L-MD). A preformed, unlabeled, geranylgeranyl side chain that is added to the labeled MD to form MK-4 would demonstrate that MK-4 was produced from dietary PK by means of side chain removal-addition. Measurement of L-MD would also support the observation that MD is an intermediate in the PK to MK-4 conversion. A second series of studies was designed to test the hypothesis that enterocytes are the central compartment where the PK’s phytyl side chain is removed, producing MD. To ascertain the role of different cell types in this conversion, we examined the ability of colon cancer cell lines and cultured human liver and kidney cell lines to convert PK to MK-4. The identification of the location and mechanisms by which PK is converted to MK-4 provide insight into the potential unique roles of MK-4.
Materials and Methods
Animals and diets.
Male Fischer 344 rats (8 mo old, n = 15) obtained from National Institute of Aging were acclimated for 2 wk with a vitamin K-deficient diet (TD.09686, Harlan Teklad) in suspended wire caging to minimize coprophagy (9). Rats were weight-matched and placed in individual metabolic cages to enable monitoring of food consumption (including spillage) while minimizing coprophagy. Rats were then randomly assigned to 1 of 3 experimental groups of 5 animals each: C (control), which were killed on d 0, and PK-1d (fed 1 d of 1.6 mg deuterium-labeled phylloquinone/kg diet) and PK-7d (fed 7 d of 1.6 mg deuterium-labeled phylloquinone/kg diet), which consumed the vitamin K-deficient diet ad libitum with deuterium-labeled collard greens added as a source of 1.6 mg L-PK (deuterium-labeled PK)/kg diet for 1 and 7 d, respectively. Rats were killed by terminal exsanguinations and serum and urine were collected. Tissues of interest (jejunum, liver, kidney, pancreas, salivary glands, testes, visceral fat, and brain) were harvested and frozen immediately in liquid nitrogen. Urine, serum, and tissues were stored at −80°C until analysis.
Deuterium-labeled collard greens were hydroponically grown in an environmental growth chamber at USDA/Agricultural Research Service Children’s Nutrition Research Center in Houston Texas, as previously described (10). This protocol was approved by the USDA Human Nutrition Research Center on Aging at Tufts University Institutional Animal Care and User Committee.
Cell culture.
Caco-2 cells (passages 20–40) were obtained from the American Type Culture Collection and grown as previously described (11). For experiments, cells (5 × 104) were plated on 12-well Transwells (12-mm diameter, 0.4-μm pore size; Corning Costar) in the presence of DMEM high glucose with L-glutamine plus 20% heat-inactivated FBS, 1% nonessential amino acids, and 1% penicillin-streptomycin solution. The medium was changed every 48 h for 21 d.
Before the transport experiments on Transwells, the integrity of the cell monolayer was tested by determining the diffusion of phenol red from the apical side to the basolateral side as previously described (12). Ten (10) μmol/L PK, MK-4, and MD were solubilized by mixing each with 29 mg/L Tween-40, evaporated under nitrogen, and reconstituted in serum-free medium (SFM) by vortexing (13). Differentiated Caco-2 cells were supplemented on the apical side with 0.5 mL SFM with Tween-40–solubilzed PK, MK-4, or MD for 24 h and 1.5 mL HBSS was added to basolateral side. At 24 h, cells were washed with cold PBS 3 times and then digested with 0.5 mL Trypsin for 10 min at 37°C. The cells were sonicated for 1 min on ice. The homogenized cells were stored at −80°C for further analysis. The HuH7 or 293T cells (American Type Culture Collection) were seeded into 6-well plates at a density of 5 × 105 cells/well in 2 mL DMEM medium with 10% FBS. PK, MK-4, and MD were solubilized by mixing each with 29 mg/L Tween-40 in SFM, as described above, and were added at a final concentration of 10 μmol/L and incubated for 24 h. After incubation, cells were harvested and washed with PBS 3 times and then were lysed by sonication. The sonicated cells (0.5 mL) were stored at −80°C until analysis.
The protein concentration of the cell lysate was determined with a Coomassie Plus Assay kit (Thermo).
Vitamin K analysis.
Rat tissues (100–200 mg wet weight) were homogenized in PBS using a Powergen homogenizer (Fisher Scientific). Concentrations of unlabeled PK, L-PK, unlabeled MK-4, and deuterium-labeled MK-4 (L-MK-4) were measured in tissue homogenates, serum, and cell lysate by atmospheric pressure chemical ionization LC/MS (APCI-LC/MS), as described elsewhere (14). Data were collected using Agilent Chemstation software (Version B.03.01).
Total MD (which included both conjugated and unconjugated forms of MD) in urine and cell lysate were measured by HPLC, as described elsewhere (15). The assay was adapted for measurement of the % enrichment of unconjugated MD in rat urine using APCI-LC/MS and detection of unconjugated MD in rat serum using APCI-LC/tandem MS (APCI-LC/MS/MS). MD was extracted 3 times using 2 mL iso-octane each time, which was a modification of the method previously described (15). For protein precipitation, cell lysate, urine, or serum samples were treated with 10% trichloroacetic acid (wt:v, final concentration) (16). Protein precipitation treatment releases unconjugated, fat-soluble MD from its binding proteins and makes it readily accessible for liquid-liquid extraction. The LC/MS consisted of an Agilent HP series 1100 G1946D MSD with an APCI source connected to an Agilent series 1200 HPLC instrument. The mobile phase consisted of 100% methanol. The flow rate was set at 0.6 mL/min. One cycle was 24 min. The MS ion source was negative APCI. Data were collected using Agilent Chemstation software (Version B.03.01). The APCI-LC/MS/MS consisted of an AB SCIEX QTRAP 5500 MS/MS with an APCI source connected to an Agilent series 1200 HPLC instrument. A ProntoSil C30 column was used (5 μm, 250 mm × 4.6 mm) (MAC-MOD Analytical). The MS/MS ion source was negative APCI with the temperature set at 400°C. Multiple Reaction Monitoring was used to detect isotopomers of parent compounds and fragments of MD [m/z (parent:fragment) 172:146] and L-MD [m/z (parent:fragment) 173:147, 174:148, 175:149]. The mobile phase consisted of 100% methanol. The flow rate was set at 0.6 mL/min. One cycle was 24 min. Data were collected using Analyst 1.5.1. The detection methods for the various forms of vitamin K measured in this study are summarized in Table 1.
TABLE 1.
Confirmation of deuterium labeling in each form of vitamin K
| Major isotopomers (m/z) |
Detection methods | ||||
| Vitamin K | Sources | Molecular weight | Unlabeled | Labeled | |
| PK1 | Diet, tissues, and serum | 451 | 451 | 459–4632 | APCI-LC/MS |
| MK-4 | Tissues | 445 | 445 | 446–449 | APCI-LC/MS |
| MD | Urine | 172 | 172 | 173–174 | APCI-LC/MS |
| Serum | 172 | 172 | 173–175 | APCI-LC/MS/MS | |
Tissue and serum PK isotopomer distribution matched that of PK in diet. APCI-LC/MS, atmospheric pressure chemical ionization LC/MS; MD, menadione; MK-4, menaquinone-4; PK, phylloquinone.
459–463 corresponds to 63% of total deuterium label in PK.
Urinary protein and creatinine determination.
The presence of protein in urine was determined by visual reading of colorimetric reactions on reagent strip (Bayer Multistix-10 SG). Urinary creatinine was measured using the modified Jaffe method on an Olympus AU400 analyzer (Olympus).
Statistical methods.
Data are reported as means ± SD. Mean differences in body weight were analyzed using 1-way ANOVA. Otherwise, nonparametric statistics were applied, because the outcome distributions were skewed and did not normalize following log-transformation. The main effect of the number of days consuming labeled collard greens on the tissue concentrations of vitamin K and urinary MD was analyzed using a Kruskal-Wallis test. If the overall K statistic was significant (P < 0.05), between-group comparisons were analyzed using a Wilcoxon’s rank-sum test. To account for the 3 independent comparisons within each outcome, pairwise analyses were considered significant at P < 0.017. All analyses were carried out using SAS v 9.1.
Results
Mean body weights (final: 430.9 ± 33.5 g) did not differ among the groups (overall P = 0.83). We measured PK, MK-4, and MD in a variety of biological matrices in response to intake of L-PK in collard greens (Table 1). The most abundant isotopomers of L-PK in serum (m/z 459–463) corresponded to 63% of the total deuterium label in PK in the collard greens. This indicates that deuterium atoms were located at 8–12 of the hydrogen positions in the individual PK molecules, which is consistent with labeling of the naphthoquinone ring and the side chain (10). In contrast, the most abundant isotopomers for unlabeled and labeled MK-4 corresponded to m/z 445 and 446–449, respectively. The isotopomer profile of L-MK-4 corresponded to that of the labeled MD, in which the deuterium atoms were located at 1–3 of the hydrogen positions in the individual MD molecules. These results indicate that the side chain of the PK molecule is cleaved to form MD and a new unlabeled side chain is added to form MK-4, as proposed by others (8).
As expected, there were no labeled forms of vitamin K in tissues obtained from the C group (no collard intake) (Table 2). There was also more unlabeled MK-4 than unlabeled PK. In PK-1d and PK-7d, the total PK concentrations were higher in jejunum, liver, and serum compared with the C group (P ≤ 0.009 for all between-group comparisons). For the PK-1d and PK-7d groups, the testes and brain had detectable amounts of labeled and unlabeled MK-4, but no PK, even though the diet contained PK only. There were higher concentrations of total MK-4 and L-MK-4 in the kidney and salivary glands of the PK-7d group compared with the PK-1d and C groups (all between-group P ≤ 0.016). In other tissues, such as the brain, testes, and visceral fat, there were no overall differences in total MK-4 concentrations among the 3 groups (all overall P ≥ 0.18); however, there were higher L-MK-4 concentrations in brain and testes of the PK-7d group compared with the C and PK-1d groups.
TABLE 2.
Vitamin K concentrations in serum and tissues of untreated male Fischer 344 rats and those fed 1.6 mg L-PK /kg diet for 1 or 7 d1
| Labeled vitamin K |
Total2 |
||||||
| Tissue | Form of vitamin K | C | PK-1d | PK-7d | C | PK-1d | PK-7d |
| nmol/kg wet tissue or L serum | nmol/kg wet tissue or L serum | ||||||
| Jejunum | PK | NDa,3 | 172 ± 49.4b | 136 ± 69.8b | 5.1 ± 2.6a | 179 ± 50.3b | 139 ± 71.4b |
| MK-4 | NDa | 37.7 ± 4.3b | 57.3 ± 9.2c | 64.8 ± 47.7 | 94.0 ± 14.1 | 107 ± 21.7 | |
| Liver | PK | NDa | 195 ± 51.1b | 209 ± 112b | 3.6 ± 0.8a | 199 ± 52.0b | 213 ± 114b |
| MK-4 | ND | ND | ND | ND | ND | ND | |
| Serum | PK | NDa | 28.7 ± 18.1b | 7.7 ± 2.7b | NDa | 28.7 ± 18.1b | 7.7 ± 2.7b |
| MK-4 | ND | ND | ND | ND | ND | ND | |
| Kidney | PK | NDa | 8.3 ± 3.0b | 15.2 ± 3.2b | 2.6 ± 5.9a | 8.6 ± 3.4ab | 16.2 ± 3.6b |
| MK-4 | NDa | 27.3 ± 5.0b | 43.2 ± 9.2c | 9.2 ± 20.7a | 27.3 ± 5.0ab | 43.2 ± 9.3b | |
| Pancreas | PK | NDa | 1.8 ± 4.0a | 38.5 ± 8.0b | 22.8 ± 9.6a | 15.5 ± 4.4a | 54.8 ± 6.4b |
| MK-4 | NDa | 101 ± 10.8b | 364 ± 43.0c | 979 ± 165a | 1230 ± 38.5b | 1060 ± 201ab | |
| Salivary glands | PK | ND | 0.1 ± 0.3 | 1.7 ± 3.7 | ND | 0.5 ± 0.9 | 1.3 ± 3.7 |
| MK-4 | NDa | 311 ± 46.8b | 757 ± 112c | 135 ± 38.1a | 467 ± 47.6b | 904 ± 140c | |
| Testes | PK | ND | ND | ND | ND | ND | ND |
| MK4 | NDa | NDa | 55.9 ± 12.6b | 428 ± 93.9 | 339 ± 72.2 | 318 ± 43.5 | |
| Visceral fat | PK | ND | ND | 8.5 ± 14.4 | 25.1 ± 9.2 | 14.7 ± 2.2 | 24.0 ± 21.3 |
| MK4 | NDa | 73.3 ± 10.3b | 68.6 ± 23.9b | 121 ± 53.1 | 115 ± 13.1 | 109 ± 49.8 | |
| Brain | PK | ND | ND | ND | ND | ND | ND |
| MK-4 | NDa | 15.4 ± 6.0b | 38.6 ± 8.3c | 67.5 ± 20.4 | 82.6 ± 18.2 | 63.9 ± 14.9 | |
Values are means ± SD, = 5. For a variable, means in a row with superscripts without a common letter differ, P < 0.017 (Wilcoxon's rank-sum test). C, control; L-PK, deuterium-labeled phylloquinone; MK-4, menaquinone-4; ND, not detected (<0.05 nmol/kg wet tissue or L serum); PK, phylloquinone; PK-1d, fed 1 d of 1.6 mg deuterium-labeled phylloquinone/kg diet; PK-7d, fed 7 d of 1.6 mg deuterium-labeled phylloquinone/kg diet.
Total is the sum of the labeled and unlabeled measures.
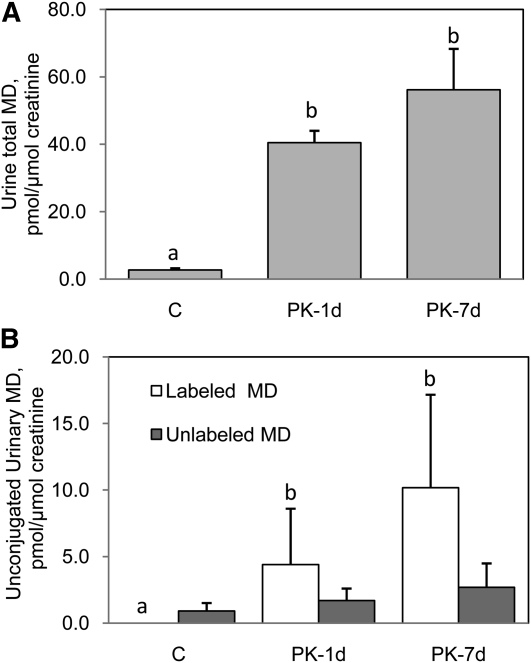
Urine total MD ( and L-MD ( concentrations were higher in the PK-1d and PK-7d groups than in the C group (P ≤ 0.009). Unlabeled unconjugated MD excretion did not differ among the 3 groups (P = 0.25) (Fig. 1B). The presence of L-MD in serum was confirmed using APCI-LC/MS/MS. It was detected in serum of rats in the PK-1d (n = 4/5) and PK-7d (n = 3/5) groups but not in the C group (n = 0/5). However, we were unable to calculate the L-MD concentration, because we did not have an appropriate internal standard for use in serum. In contrast, there was no MK-4 (labeled or unlabeled) detected in serum (Table 2).
FIGURE 1.
Total (A) and unconjugated (B) MD concentrations in urine of untreated male Fischer 344 rats and those fed 1.6mg L-PK /kg diet for 1 or 7d. Urine total MD concentrations include both conjugated and unconjugated forms. Values are means ± SD, n = 5. Labeled means for a variable without a common letter differ, P < 0.017 (Wilcoxon rank sum test). C, control; L-PK, deuterium-labeled phylloquinone; MD, menadione; PK-1d, fed 1 d of 1.6 mg L-PK/kg diet; PK-7d, fed 7 d of 1.6 mg L-PK/kg diet.
The Caco-2 cells were harvested and tested for the formation of MK-4 and MD. When treated with 10 μmol PK/L, only PK was measured in the Caco-2 cells (2200 ± 570 pmol/mg cell protein). Neither MK-4 nor MD was detected. However, MK-4 was detected when Caco-2 cells were treated with 10 μmol/L of either MD (15.3 ± 0.4 pmol/mg cell protein) or MK-4 (1700 ± 60 pmol/mg cell protein). MK-4 epoxide (MK-4O) was detected in response to MK-4. However, we were unable to calculate the concentration due to technical challenges. HuH7 and 293T cells were also unable to convert PK to MK-4 (Table 3). However, MK-4O formed in HuH7 cells, but not 293T cells, when treated with PK.
TABLE 3.
Concentrations of forms of vitamin K in human liver (HuH 7) and kidney (293T) cells cultured with PK, MK-4, or MD for 24 h1
| Cell line | Addition to media | PK | PK epoxide | MK-4 | MK-4O | MD |
| pmol/mg cell protein | ||||||
| HuH7 | ||||||
| None | ND | ND | ND | ND | ND | |
| PK | 3800 ± 846 | 14,900 ± 3100 | ND | 11.9 ± 4.8 | ND | |
| MK-4 | ND | ND | 29.0 ± 2.3 | 8300 ± 155 | ND | |
| MD | ND | ND | ND | 8.9 ± 1.0 | ND | |
| 293T | ||||||
| None | ND | ND | ND | ND | ND | |
| PK | 9900 ± 2210 | 2500 ± 534 | ND | ND | ND | |
| MK-4 | ND | ND | 414 ± 94 | 3200 ± 592 | ND | |
| MD | ND | ND | 5.5 ± 0.3 | 4.3 ± 0.6 | ND | |
Values are means ± SD, = 2 independent experiments. MD, menadione; MK-4, menaquinone-4; MK-4O, menaquinone-4 epoxide; ND, not detected (< 0.3 pmol/mg cell protein for MD, and < 0.5 pmol/mg cell protein for all other vitamin K forms); PK, phylloquinone.
Discussion
Within 7 d of daily L-PK intake, L-MK-4 was detected in various tissues of male Fischer 344 rats. The L-MK-4 carried the deuterium-label on the naphthoquinone ring, but not on the side chain, confirming the need for side chain removal for its formation. The L-MD detected in serum and urine originated from L-PK in collards, providing direct evidence that MD is an intermediate in the conversion of dietary PK to MK-4 in tissues.
The tissue-specific differences in the conversion of PK to MK-4 were unexpected. The vitamin K source in rat unpurified diet is typically in the form of MD. When labeled MD was fed to vitamin K-deficient chicks (17) and Wistar rats (18), labeled MK-4 was the predominant form in tissues. However, in humans, MD is not a dietary source of vitamin K. Instead, vitamin K is primarily consumed in the form of PK. To the best of our knowledge, this is the first use of a deuterium-labeled food source to measure the conversion in multiple tissues, thereby providing a model for the metabolic fate of dietary PK in the human diet. PK is the primary circulating form of vitamin K in humans (19) and in rodents fed PK-containing purified diet. MK-4 does not appear in circulation in response to dietary PK intake, as confirmed in this study. Furthermore, no MK-4 (total or labeled) was detected in liver. Whereas some tissues appear to accumulate MK-4 with the increased amounts of PK in the diet, other tissues appeared to be highly regulated. In our study, the total MK-4, which contained both labeled and unlabeled forms, was higher than the L-MK-4 in the testes, brain, and visceral fat. The total MK-4 concentrations in these tissues did not change in response to the L-PK, whereas the L-MK-4 concentrations did. Although rats were on a vitamin K-restricted diet for 14 d before intake of the deuterium label, it is plausible that the MD in the unpurified diet fed to rats before the start of the experiment was converted to MK-4 and stored in these tissues before or during vitamin K restriction. However, the unlabeled MK-4 was replaced with L-MK-4 while maintaining the same total MK-4 concentrations. This suggests that MK-4 concentrations are regulated in certain tissues, whereas other tissues appear to fluctuate more according to short-term PK restriction. The mechanism by which this putative regulation occurs is unknown.
Unlike longer chain menaquinones (20), gut bacteria are not needed for the synthesis of tissue MK-4 (1, 21). Overall evidence from the literature supports the side chain removal-addition model of dietary PK to tissue MK-4 conversion. To confirm this model in the brain, Okano et al. (8) used L-PK carrying the label on the naphthoquinone ring but not the phytyl side chain. The resulting compound was orally given to mice and the naphthoquinone ring-labeled MK-4 was detected in cerebra. In contrast, MK-4 labeled on both the ring and the geranylgeranyl side chain was not detected, which indicated that the phytyl side chain of PK is replaced by a geranylgeranyl side chain to produce MK-4. The major limitation of the study (18) was the inability to measure MD as the intermediate in the conversion. Similarly, the study was limited to measurement of the brain only. In our study, we were able to directly measure the conversion of PK to MK-4 in multiple tissues using stable isotopes. In addition, we were able to confirm the intermediate MD in serum and urine.
There are 2 possible routes regarding tissue localization of the conversion of PK to MK-4. In the first route, phytyl side chain removal as well as the subsequent geranylgeranyl side chain prenylation are combined enzymatic activities specific to certain tissues (1, 8). The alternative route requires the phytyl side chain to be removed in a central body compartment and MD, or its epoxide, to be released into circulation, where it is prenylated in target tissues with a preformed geranylgeranyl side chain, forming MK-4 (18). Collectively, the data suggest that MD formed from PK may take place in a limited number of tissues, as well as a central compartment. The subsequent prenylation with the preformed geranylgeranyl side chain occurs in target tissues. The detection of L-MD in serum suggests that the phytyl side chain removal step may partially take place in a central compartment. Alternatively, MK-4 may be exported from tissues into the blood stream via HDL. Although we measured L-PK and some L-MK-4 in the jejunum in our rat study in response to L-PK intake, neither MK-4 nor MD was detected in our Caco-2 cell culture model treated with PK. This suggests L-MK-4 in the jejunum comes from a nonenterocyte conversion. Furthermore, PK is the predominant form of vitamin K in the jejunum. Similar results were noted when we further explored this with HuH7 and 293T cells. However, MK-4O was detected in HuH7 cells, but not 293T cells, in response to PK. It is possible that PK is first converted to MK-4O from MD, as suggested by others (1).
UBIAD1 was recently identified as a human prenyltransferase enzyme responsible for the geranylgeranyl side chain addition step of the conversion process from PK to MK-4 (7). However, the evidence regarding UBIAD1’s role as the enzyme responsible for the phytyl side chain removal was weak. Indeed, our data suggest that Caco-2 and 293Tcells are capable of converting MD, but not PK, to MK-4, because they presumably lack the enzymatic activity that cleaves the side chain of PK to form MD. More research is required to confirm if UBIAD1 is indeed the enzyme responsible for both steps.
In conclusion, MK-4 is the predominant form of vitamin K in multiple tissues, but there appears to be a tissue-specific regulation for the conversion of PK to MK-4. To convert PK to MK-4, the phytyl side chain in PK is removed and a geranylgeranyl side chain is added to the MD nucleus, forming MK-4. However, our data do not support the involvement of enterocytes in this conversion.
Acknowledgments
The authors thank Dr. Gregory G. Dolnikowski for his invaluable guidance on the use of LC/MS/MS and Dr. Donald Smith for his support of the animal study. A.A. and X.F. analyzed data, wrote the paper, and contributed to study design; J.W.P. analyzed data and contributed to the study design; S.L.B. designed the research and had primary responsibility for final content; S.W.C. and J.S. contributed to the study design, the interpretation of the data, and preparation of the manuscript; and M.K.S., M.A.G., and B.M. analyzed data and contributed to its interpretation. All authors read and approved the final manuscript.
Footnotes
Supported by the USDA Agricultural Research Service under Cooperative Agreements no. 58-1950-7-707 and no. 58-6250-0-008, and by the NIH (DK69341). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Abbreviations used: APCI-LC/MS, atmospheric pressure chemical ionization LC/MS; APCI-LC/MS/MS, atmospheric pressure chemical ionization LC/tandem MS; L-MD, deuterium-labeled unconjugated menadione; L-MK-4, deuterium-labeled menaquinone-4; L-PK, deuterium-labeled phylloquinone; MD, menadione; MK-4, menaquinone-4; MK-4O, menaquinone-4 epoxide; PK, phylloquinone; PK-1d, fed 1 d of 1.6 mg deuterium-labeled phylloquinone/kg diet; PK-7d, fed 7 d of 1.6 mg deuterium-labeled phylloquinone/kg diet; SFM, serum-free medium; UBIAD1, UbiA prenyltransferase containing 1.
Literature Cited
- 1.Davidson RT, Foley AL, Engelke JA, Suttie JW. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr. 1998;128:220–3 [DOI] [PubMed] [Google Scholar]
- 2.Suttie J. Vitamin K in Health and Disease Series. Vitamin K in health and disease. Boca Raton, Florida: CRC Press; 2009 [Google Scholar]
- 3.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Miyazawa K, Kasuga I, Yokoyama T, Minemura K, Ustumi K, Aoshima M, Ohyashiki K. Apoptosis induction of vitamin K2 in lung carcinoma cell lines: the possibility of vitamin K2 therapy for lung cancer. Int J Oncol. 2003;23:627–32 [PubMed] [Google Scholar]
- 5.Yaguchi M, Miyazawa K, Katagiri T, Nishimaki J, Kizaki M, Tohyama K, Toyama K. Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia. 1997;11:779–87 [DOI] [PubMed] [Google Scholar]
- 6.Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278:43919–27 [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–21 [DOI] [PubMed] [Google Scholar]
- 8.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–9 [DOI] [PubMed] [Google Scholar]
- 9.Booth SL, Peterson JW, Smith D, Shea MK, Chamberland J, Crivello N. Age and dietary form of vitamin K affect menaquinone-4 concentrations in male Fischer 344 rats. J Nutr. 2008;138:492–6 [DOI] [PubMed] [Google Scholar]
- 10.Dolnikowski GG, Sun Z, Grusak MA, Peterson JW, Booth SL. HPLC and GC/MS determination of deuterated vitamin K (phylloquinone) in human serum after ingestion of deuterium-labeled broccoli. J Nutr Biochem. 2002;13:168–74 [DOI] [PubMed] [Google Scholar]
- 11.Hubatsch I, Ragnarsson EG, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2:2111–9 [DOI] [PubMed] [Google Scholar]
- 12.Fleet JC, Wood RJ. Specific 1,25(OH)2D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am J Physiol. 1999;276:G958–64 [DOI] [PubMed] [Google Scholar]
- 13.Anwar K, Kayden HJ, Hussain MM. Transport of vitamin E by differentiated Caco-2 cells. J Lipid Res. 2006;47:1261–73 [DOI] [PubMed] [Google Scholar]
- 14.Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81:5421–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Rajabi A, Peterson J, Choi SW, Suttie J, Barakat S, Booth SL. Measurement of menadione in urine by HPLC. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2457–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang F, Huang L, Taylor A. Degradation of native and oxidized beta- and gamma-crystallin using bovine lens epithelial cell and rabbit reticulocyte extracts. Curr Eye Res. 1994;13:423–31 [DOI] [PubMed] [Google Scholar]
- 17.Martius C. The metabolic relationships between the different K vitamins and the synthesis of the ubiquinones. Am J Clin Nutr. 1961;9:97–103 [DOI] [PubMed] [Google Scholar]
- 18.Thijssen HH, Drittij-Reijnders MJ, Fischer MA. Phylloquinone and menaquinone-4 distribution in rats: synthesis rather than uptake determines menaquinone-4 organ concentrations. J Nutr. 1996;126:537–43 [DOI] [PubMed] [Google Scholar]
- 19.Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm. 2008;78:1–22 [DOI] [PubMed] [Google Scholar]
- 20.Ramotar K, Conly JM, Chubb H, Louie TJ. Production of menaquinones by intestinal anaerobes. J Infect Dis. 1984;150:213–8 [DOI] [PubMed] [Google Scholar]
- 21.Ronden JE, Drittij-Reijnders MJ, Vermeer C, Thijssen HH. Intestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the rat. Biochim Biophys Acta. 1998;1379:69–75 [DOI] [PubMed] [Google Scholar]