Abstract
The NHANES has monitored folate status of the U.S. population from prefortification (1988–1994) to postfortification (1999–2010) by measuring serum and RBC folate concentrations. The Bio-Rad radioassay (BR) was used from 1988 to 2006, and the microbiologic assay (MBA) was used from 2007 to 2010. The MBA produces higher concentrations than the BR and is considered to be more accurate. Thus, to bridge assay differences and to examine folate trends over time, we adjusted the BR results to be comparable to the MBA results. Postfortification, assay-adjusted serum and RBC folate concentrations were 2.5 times and 1.5 times prefortification concentrations, respectively, and showed a significant linear trend (P < 0.001) to slightly lower concentrations during 1999–2010. The postfortification prevalence of low serum (<10 nmol/L) or RBC (<340 nmol/L) folate concentrations was ≤1%, regardless of demographic subgroup, compared with 24% for serum folate and 3.5% for RBC folate prefortification, with substantial variation among demographic subgroups. The central 95% reference intervals for serum and RBC folate varied by demographic subgroup during both pre- and postfortification periods. Age and dietary supplement use had the greatest effects on prevalence estimates of low folate concentrations during the prefortification period. In summary, the MBA-equivalent blood folate concentrations in the U.S. population showed first a sharp increase from pre- to postfortification, then showed a slight decrease (17% for serum and 12% for RBC folate) during the 12-y postfortification period. The MBA-equivalent pre- and postfortification reference concentrations will inform countries that plan folic acid fortification or that need to evaluate its impact.
Introduction
The folate status of the U.S. population has been assessed for many years as part of the NHANES by measuring serum and RBC folate concentrations, first (1988–2006) with the Bio-Rad radioassay (BR)9, and then (2007–2010) with the microbiologic growth assay (MBA) using Lactobacillus rhamnosus (formerly known as Lactobacillus casei) (1). Because the MBA produces much higher concentrations than does the BR (2–4), a 2010 roundtable of experts on NHANES folate and vitamin B-12 measurement issues agreed “that an adjustment equation based on a crossover study was necessary for time-trend evaluations” (5). The MBA is considered more accurate because it recovers folate vitamers equally, which is not always the case with clinical protein-binding assays (3, 4, 6). The MBA is sometimes used in research studies and population surveys other than NHANES because it requires only small sample volumes, can be conducted in a high-throughput format, and is a comparatively inexpensive assay (7). It is also the assay against which the accuracy of other assays is evaluated (5, 8). For all of these reasons, it is of interest to scientists and public health officials worldwide to have information on pre- and postfortification folate status of the U.S. population based on MBA-equivalent serum and RBC folate data.
Previous reports have assessed the impact of folic acid fortification on serum and RBC folate concentrations in the general U.S. population and in women of childbearing age during the first few years postfortification by using data generated with the BR (9–12). No reports are yet available for data generated after 2006 or by expressing the data as MBA-equivalent concentrations.
In this article we present the newest serum and RBC folate data generated with the MBA for NHANES 2007–2010 and the new MBA-equivalent blood folate data for 8 previous postfortification years (1999–2006) and 6 prefortification years (1988–1994) by sociodemographic variables. We present long-term trends in folate status of the U.S. population and provide prevalence estimates of low blood folate concentrations for pre- and postfortification time periods. Last, we provide multistratified central 95% reference intervals by age, gender, and race/ethnicity for prefortification, early postfortification (1999–2004), and late postfortification (2005–2010) time periods. A separate article describes the process by which the BR data were adjusted to MBA-equivalent data and the impact of the adjustment (13).
Participants and Methods
Survey design and participants.
The NHANES, which is conducted by the National Center for Health Statistics (NCHS) at the CDC, collects cross-sectional data on the health and nutritional status of the civilian noninstitutionalized U.S. population (14). Prior to 1999, the survey was conducted periodically; since 1999, NHANES has become a continuous survey with data released in 2-y cycles. NHANES obtains a stratified, multistage, probability sample designed to represent the U.S. population on the basis of age, gender, and race/ethnicity. The race/ethnicity categories are based on self-reported data. NCHS personnel first interview survey participants in their homes. During this household interview, interviewers collect information on demographic characteristics, dietary supplement use, and some health-related issues. Participants undergo a physical examination and blood collection in a Mobile Examination Center ~1–2 wk after the household interview. They also complete a 24-h dietary recall. All respondents provided informed consent, and the NHANES protocol was reviewed and approved by the NCHS Research Ethics Review Board. Interview and examination response rates for each survey period are publically available (15).
Laboratory methods.
During NHANES 2007–2010, whole-blood hemolysate and serum samples from participants aged ≥1 y were analyzed for folate by the CDC laboratory by use of the MBA method (16, 17). A short description of the assay and of steps conducted to verify the comparability of results over time is provided elsewhere (13, 18). Long-term quality-control CV for serum folate were 5.9–10% for 2007–2008 and 4.7–8.5% for 2009–2010 (19, 20). For RBC folate, CV were 8.0–14% for 2007–2008 and 7.5–8.2% for 2009–2010 (19, 20). The Bio-Rad Quantaphase I radioassay was used during 1988–1991 and the Quantaphase II was used during 1991–2006 (Bio-Rad Laboratories). Appropriate assay adjustments were applied to the 1988–1991 folate data before their public release to account for method differences between the Quantaphase I and II (21). The performance of the BR has been discussed in previous reports (2, 10). Long-term CV were 4.0–7.0% for serum folate and 4.0–6.0% for RBC folate (10).
Statistical analyses.
Statistical analyses were performed by using SAS (version 9; SAS Institute, Inc.) and SUDAAN (version 9; RTI International) software. In each NHANES survey period we used sample weights to account for differential nonresponse or noncoverage and to adjust for oversampling of some groups. We used the following age groups: 4–11 y (children), 12–19 y (adolescents), 20–59 y (adults; in some analyses 20–39 y and 40–59 y), and ≥60 y (older persons; in some analyses 60–79 y and ≥80 y). Women of childbearing age (15–44 y) were considered as a separate category for most analyses. We used the 3 main race/ethnicity categories Mexican American (MA), non-Hispanic black (NHB), and non-Hispanic white (NHW) that can be compared over the time period covered in this analysis, but we included other racial/ethnic groups in overall estimates.
We applied regression equations to serum and RBC folate results analyzed by the BR during 1988–2006 to derive MBA-equivalent data (13, 16, 17). This allowed the assessment of long-term trends in folate status that were unconfounded by assay differences over this long time period. The BR measured folate concentrations that were 31% lower than those from the MBA for whole-blood samples with the MTHFR (5,10-methylenetetrahydrofolate reductase) T/T genotype, but 48% lower for samples with the C/C and C/T genotypes (4). This is because the BR recovered the various folate forms differently compared with the MBA assay (4). MTHFR genotype information is not available for NHANES participants. We could therefore not use genotype-specific regression equations and had to use an “all genotype” equation (13).
The only participants excluded from our analyses were those for whom data were missing: we excluded 2 participants in 1988–1994 because their unadjusted serum folate concentrations were <1 nmol/L and the adjustment formula requires logarithmic transformation (which produces a negative number) and then calculating the square root. Also, we excluded a small fraction (1–2%) of participants in 1988–1994 (556 of 23,402), 1999–2000 (101 of 7491), 2001–2002 (116 of 8336), 2003–2004 (82 of 7618), and 2005–2006 (173 of 7751) because the RBC folate adjustment formula requires serum folate, RBC folate, and hematocrit, and for these participants one of these tests was missing. All analyses presented in this article were performed by using MBA-equivalent data.
We determined pre- and postfortification geometric mean blood folate concentrations by sociodemographic variables [age, gender, race/ethnicity, and family poverty-to-income ratio (PIR)] and dietary supplement use, which are variables known to influence blood folate concentrations. We used geometric means because distributions of blood folate concentrations were skewed. For the postfortification time period (1999–2010), we also determined geometric means by survey period. The prefortification time period (1988–1994) was considered one survey period. We tested which of the variables had a significant effect on blood folate concentrations by using simple linear regression: we used PROC REGRESS in SUDAAN (RTI International) with a subgroup statement, and we used EFFECT statements to test each hypothesis. We limited the postfortification blood folate data to 1999–2008 when we evaluated the effect of supplement use because dietary supplement use information is not yet available for 2009–2010. By using multiple linear regression, we evaluated whether adjustment for the above variables had an effect on the relationship between blood folate concentrations and each variable of interest. Model 1 included the sociodemographic variables for prefortification data and the sociodemographic variables and survey period for postfortification data (1999–2010). Model 2 included the sociodemographic variables and supplement use for prefortification data and the sociodemographic variables, supplement use, and survey period for postfortification data (1999–2008). All statistical comparisons were evaluated at a significance level of α = 0.05.
To visualize pre- and postfortification trends, we plotted the median and IQR of serum and RBC folate concentrations for 1988–2010 by survey period. We described trends of the upper end of the serum and RBC folate distributions over time by plotting the 95th percentile concentrations for 1988–2008 by survey period and by supplement use.
We used the WHO-recommended cutoffs of <10 nmol/L for serum and <340 nmol/L for RBC folate (22) to assess low folate status, and we determined the proportion of low serum and RBC folate concentrations for pre- and postfortification data by sociodemographic variables and dietary supplement use. We also determined proportions by survey period for the postfortification time period.
Because blood folate concentrations varied by age, gender, and race/ethnicity, we calculated multistratified central 95% reference intervals (2.5–97.5th percentiles) for the prefortification (1988–1994), early postfortification (1999–2004), and late postfortification (2005–2010) periods. Six years of NHANES data provided a sufficient sample size to allow 3 levels of stratification and still exceed a cell size of 448 in the majority of subgroups. This cell size is needed for robust estimates of the 2.5th and 97.5th percentiles at an assumed mean design effect of 1.4. We plotted frequency distribution curves of MBA-equivalent serum and RBC folate concentrations for the pre-, early post-, and late postfortification periods.
Results
Variables influencing blood folate concentrations.
During the prefortification period, age, gender, race/ethnicity, PIR, and supplement use affected serum and RBC folate concentrations (P ≤ 0.001) (Table 1). All effects were maintained after we adjusted the prefortification serum and RBC folate concentrations for sociodemographic variables (P-model 1 ≤ 0.001). However, when we also adjusted for supplement use, the difference between males and females in prefortification RBC folate concentrations disappeared (P-model 2 = 0.98), whereas all other effects remained significant.
TABLE 1.
Prefortification (1988–1994) and postfortification (1999–2010) blood folate concentrations by sociodemographic variable, dietary supplement use, and survey period in participants aged ≥4 y (NHANES 1988–2010)1
|
1988–1994 |
1999–2010 |
1988–1994 |
1999–2010 |
|||||
| n | Serum folate | n | Serum folate | n | RBC folate | n | RBC folate | |
| nmol/L | nmol/L | nmol/L | nmol/L | |||||
| Total | 23,359 | 16.7 ± 0.5 | 46,873 | 41.0 ± 0.3 | 22,846 | 747 ± 10 | 46,759 | 1120 ± 7 |
| Age group | ||||||||
| 4–11 y | 4627 | 29.6 ± 0.7 | 7379 | 56.1 ± 0.4 | 4498 | 851 ± 16 | 7380 | 1130 ± 7 |
| 12–19 y | 2956 | 15.5 ± 0.6 | 10,614 | 39.5 ± 0.4 | 2888 | 642 ± 12 | 10,574 | 971 ± 8 |
| 20–39 y | 6467 | 13.0 ± 0.4 | 10,044 | 35.1 ± 0.3 | 6335 | 674 ± 9 | 10,017 | 1020 ± 7 |
| 40–59 y | 4258 | 15.5 ± 0.5 | 8966 | 38.9 ± 0.4 | 4180 | 763 ± 13 | 8945 | 1150 ± 10 |
| 60–79 y | 3882 | 22.0 ± 0.6 | 7739 | 49.0 ± 0.5 | 3796 | 898 ± 15 | 7718 | 1350 ± 11 |
| ≥80 y | 1169 | 26.2 ± 1.0 | 2131 | 57.8 ± 1.0 | 1149 | 965 ± 22 | 2125 | 1490 ± 19 |
| P2 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 12,3 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 22,4 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Gender | ||||||||
| Male | 11,130 | 15.8 ± 0.5 | 23,021 | 38.9 ± 0.3 | 10,877 | 734 ± 9 | 22,966 | 1090 ± 8 |
| Female | 12,229 | 17.7 ± 0.5 | 23,852 | 43.2 ± 0.3 | 11,969 | 759 ± 12 | 23,793 | 1150 ± 8 |
| Age 15–44 y | 5254 | 14.0 ± 0.5 | 9994 | 37.6 ± 0.3 | 5153 | 686 ± 12 | 9968 | 1060 ± 9 |
| P5 | <0.001 | <0.001 | 0.001 | <0.001 | ||||
| P-model 13,5 | <0.001 | <0.001 | 0.001 | <0.001 | ||||
| P-model 24,5 | 0.008 | <0.001 | 0.982 | <0.001 | ||||
| Race/ethnicity | ||||||||
| MA | 7017 | 15.0 ± 0.5 | 11,822 | 37.4 ± 0.4 | 6820 | 696 ± 15 | 11,801 | 1020 ± 10 |
| NHW | 8533 | 17.5 ± 0.6 | 19,241 | 43.1 ± 0.4 | 8344 | 786 ± 12 | 19,171 | 1190 ± 10 |
| NHB | 6813 | 13.1 ± 0.3 | 10,722 | 34.3 ± 0.3 | 6695 | 573 ± 6 | 10,703 | 900 ± 5 |
| P6 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 13,6 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 24,6 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| PIR | ||||||||
| <1.0 | 6154 | 15.6 ± 0.6 | 10,772 | 37.7 ± 0.4 | 6009 | 687 ± 14 | 10,753 | 1020 ± 10 |
| 1.0–1.9 | 5909 | 15.8 ± 0.5 | 11,690 | 39.6 ± 0.3 | 5785 | 703 ± 12 | 11,656 | 1080 ± 9 |
| 2.0–3.9 | 6462 | 16.8 ± 0.5 | 11,318 | 41.4 ± 0.4 | 6299 | 758 ± 11 | 11,290 | 1130 ± 9 |
| ≥4.0 | 2756 | 19.0 ± 0.8 | 9520 | 43.3 ± 0.5 | 2707 | 830 ± 13 | 9488 | 1190 ± 10 |
| P7 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 13,7 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 24,7 | 0.004 | <0.001 | 0.001 | <0.001 | ||||
| Supplement use8 | ||||||||
| Yes | 7949 | 24.3 ± 0.7 | 15,294 | 49.9 ± 0.4 | 7776 | 923 ± 14 | 15,211 | 1320 ± 10 |
| No | 15,376 | 13.1 ± 0.3 | 23,356 | 35.4 ± 0.3 | 15,038 | 649 ± 7 | 23,287 | 994 ± 7 |
| P9 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| P-model 13,9 | NA | NA | NA | NA | ||||
| P-model 24,9 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Survey period | ||||||||
| 1999–2000 | NA | 7411 | 45.8 ± 1.0 | NA | 7393 | 1180 ± 24 | ||
| 2001–2002 | NA | 8242 | 42.7 ± 0.6 | NA | 8220 | 1160 ± 17 | ||
| 2003–2004 | NA | 7692 | 40.3 ± 0.7 | NA | 7618 | 1080 ± 16 | ||
| 2005–2006 | NA | 7639 | 41.1 ± 0.7 | NA | 7578 | 1140 ± 11 | ||
| 2007–2008 | NA | 7705 | 39.2 ± 0.9 | NA | 7728 | 1120 ± 23 | ||
| 2009–2010 | NA | 8184 | 37.9 ± 0.5 | NA | 8222 | 1040 ± 14 | ||
| P10 | NA | <0.001 | NA | <0.001 | ||||
| P-model 13,10 | NA | <0.001 | NA | <0.001 | ||||
| P-model 24,10 | NA | <0.001 | NA | <0.001 | ||||
Values are geometric means ± SE. Serum and RBC folate concentrations were measured from 1988–2006 by the Bio-Rad radioassay and assay-adjusted to be comparable to the microbiologic assay that was used from 2007–2010 (13, 16, 17). MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white; NA, not applicable; PIR, poverty-to-income ratio.
Test comparing group means across age categories.
-value for multiple linear regression model 1 including age, gender, race/ethnicity, and PIR for prefortification data and age, gender, race/ethnicity, PIR, and survey period for postfortification data (1999–2010).
value for multiple linear regression model 2 including age, gender, race/ethnicity, PIR, and supplement use for prefortification data and age, gender, race/ethnicity, PIR, supplement use, and survey period for postfortification data (1999–2008).
Test comparing males and females.
Test comparing group means across race/ethnicity categories.
Test of linear trend across PIR categories.
Geometric means by dietary supplement use were limited to data from 1999–2008 for the postfortification period.
Test comparing group means across supplement use categories.
Test of linear trend across survey periods.
During the postfortification period, age, gender, race/ethnicity, PIR, and supplement use had a significant (P < 0.001) effect on serum and RBC folate concentrations (Table 1). All effects were maintained after we adjusted the postfortification serum and RBC folate concentrations for sociodemographic variables and survey period (P-model 1 < 0.001) or for sociodemographic variables, survey period, and supplement use (P-model 2 < 0.001; data limited to 1999–2008).
Postfortification geometric means showed a significant (P-trend < 0.001) linear trend to lower concentrations from 1999 to 2010, with serum and RBC folate declining up to 17% and 12%, respectively, from 1999–2000 to 2009–2010 (Table 1). The linear trend remained significant for serum and RBC folate after adjustment for sociodemographic variables (P-trend model 1 < 0.001) or when additionally adjusted for supplement use (P-trend model 2 < 0.001; data limited to 1999–2008), indicating that potential changes in these variables over time did not explain the decrease in blood folate concentrations.
Long-term trends in blood folate concentrations.
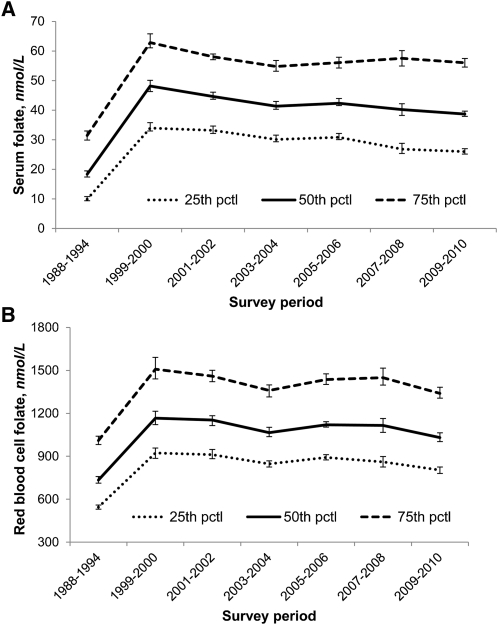
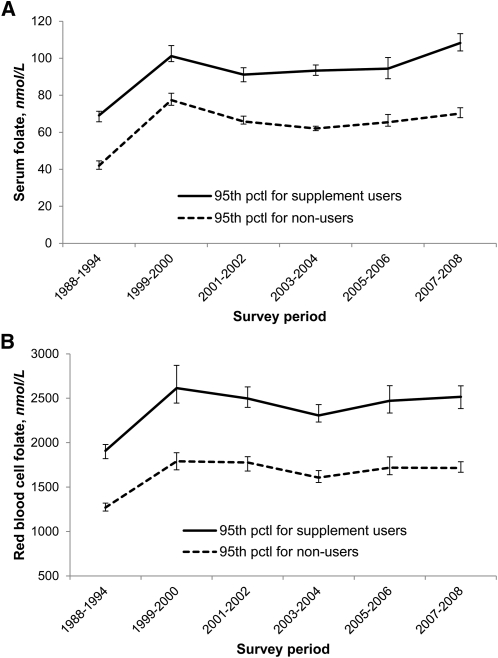
Geometric mean serum and RBC folate concentrations postfortification were 2.5 times and 1.5 times prefortification concentrations, respectively (Table 1). Median serum and RBC folate concentrations sharply increased from pre- to postfortification, then showed small fluctuations during 12 y of postfortification (Fig. 1). The upper end of the serum and RBC folate distributions (95th percentiles) showed––as expected––an increase from pre- to postfortification in both users and nonusers of dietary supplements, and then small fluctuations from 1999 to 2008 (Fig. 2). During each survey period (pre- and postfortification), serum folate concentrations were ~30 nmol/L higher in supplement users than in nonusers, and RBC folate concentrations were ~750 nmol/L higher.
FIGURE 1.
Selected percentiles for pre- and postfortification serum (A) and RBC (B) folate concentrations by survey period (NHANES 1988–2010). Concentrations measured from 1988–1994 and from 1999–2006 by the Bio-Rad radioassay were adjusted to make them comparable to the 2007–2010 concentrations measured by microbiologic assay (13, 16, 17). Error bars represent 95% CIs. Sample sizes (n) for serum folate were as follows: 23,359 (1988–1994), 7411 (1999–2000), 8242 (2001–2002), 7692 (2003–2004), 7639 (2005–2006), 7705 (2007–2008), and 8184 (2009–2010). Sample sizes (n) for RBC folate were as follows: 22,846 (1988–1994), 7393 (1999–2000), 8220 (2001–2002), 7618 (2003–2004), 7578 (2005–2006), 7728 (2007–2008), and 8222 (2009–2010). pctl, percentile.
FIGURE 2.
Pre- and postfortification 95th percentile serum (A) and RBC (B) folate concentrations for users (solid line) and nonusers (dashed line) of dietary supplements by survey period (NHANES 1988–2010). Concentrations measured from 1988 to 1994 and from 1999 to 2006 by the Bio-Rad radioassay were adjusted to make them comparable to the 2007–2010 concentrations measured by microbiologic assay (13, 16, 17). Error bars represent 95% CIs. Sample sizes (n) were the same as shown in Figure 1. pctl, percentile.
Pre- and postfortification prevalence estimates of low blood folate concentrations.
The prefortification prevalences of low serum (<10 nmol/L) and RBC (<340 nmol/L) folate concentrations were 24% and 3.5%, respectively (Table 2). Prevalence varied by demographic subgroup, with the biggest difference observed in different age groups: compared with all other age groups, children (age 4–11 y) had the lowest prevalence (2.8% for serum and 0.7% for RBC folate), whereas young adults (age 20–39 y) had the highest prevalence (33% for serum and 4.5% for RBC folate). The difference in prevalence between supplement users (13.5% for serum and 1.5% for RBC folate) compared with nonusers (37.2% for serum and 5.7% for RBC folate) was also high. Postfortification, the prevalence of low serum and RBC folate concentrations was very low (generally ≤1%), regardless of age, gender, race/ethnicity, PIR, dietary supplement use, or survey period (Table 2).
TABLE 2.
Prefortification (1988–1994) and postfortification (1999–2010) proportions of low blood folate concentrations by sociodemographic variable, dietary supplement use, and survey period in participants aged ≥4 y (NHANES 1988–2010)1
|
Serum folate <10 nmol/L |
RBC folate <340 nmol/L |
|||
| 1988–1994 | 1999–2010 | 1988–1994 | 1999–2010 | |
| % | ||||
| Total | 23.8 ± 1.0 | 0.7 ± 0.1 | 3.5 ± 0.3 | 0.1 ± 0.0 |
| Age group | ||||
| 4–11 y | 2.8 ± 0.4 | —2 | 0.7 ± 0.1 | —2 |
| 12–19 y | 24.7 ± 1.9 | 0.3 ± 0.1 | 4.5 ± 0.7 | —2 |
| 20–39 y | 33.0 ± 1.4 | 1.0 ± 0.1 | 4.5 ± 0.5 | 0.2 ± 0.0 |
| 40–59 y | 26.7 ± 1.2 | 1.0 ± 0.1 | 4.2 ± 0.5 | 0.2 ± 0.1 |
| ≥60 y | 14.4 ± 1.0 | 0.4 ± 0.1 | 2.1 ± 0.3 | 0.1 ± 0.04 |
| Gender | ||||
| Male | 25.8 ± 1.2 | 0.8 ± 0.1 | 3.2 ± 0.3 | 0.1 ± 0.0 |
| Female | 21.9 ± 0.9 | 0.7 ± 0.1 | 3.9 ± 0.3 | 0.1 ± 0.0 |
| Age 15–44 y | 30.1 ± 1.3 | 0.9 ± 0.1 | 5.5 ± 0.5 | —2 |
| Race/ethnicity | ||||
| MA | 26.4 ± 1.1 | 0.7 ± 0.1 | 3.2 ± 0.4 | 0.2 ± 0.13 |
| NHB | 22.6 ± 1.1 | 0.7 ± 0.1 | 2.8 ± 0.3 | 0.1 ± 0.03 |
| NHW | 32.2 ± 1.0 | 1.2 ± 0.2 | 10.3 ± 0.6 | 0.4 ± 0.1 |
| PIR | ||||
| <1.0 | 26.9 ± 1.6 | 1.0 ± 0.2 | 5.2 ± 0.7 | 0.3 ± 0.1 |
| 1.0–1.9 | 25.3 ± 1.2 | 1.0 ± 0.1 | 4.7 ± 0.6 | 0.2 ± 0.13 |
| 2.0–3.9 | 23.6 ± 0.9 | 0.7 ± 0.1 | 3.1 ± 0.3 | 0.1 ± 0.03 |
| ≥4.0 | 19.9 ± 1.6 | 0.5 ± 0.1 | 2.1 ± 0.4 | —2 |
| Supplement use4 | ||||
| Yes | 13.5 ± 1.0 | 0.4 ± 0.1 | 1.5 ± 0.2 | 0.0 ± 0.03 |
| No | 37.2 ± 1.2 | 1.0 ± 0.1 | 5.7 ± 0.5 | 0.2 ± 0.0 |
| Survey period | ||||
| 1999–2000 | NA | 0.8 ± 0.1 | NA | —2 |
| 2001–2002 | NA | 0.6 ± 0.1 | NA | —2 |
| 2003–2004 | NA | 0.8 ± 0.1 | NA | 0.1 ± 0.03 |
| 2005–2006 | NA | 0.8 ± 0.1 | NA | 0.1 ± 0.03 |
| 2007–2008 | NA | 0.5 ± 0.1 | NA | 0.2 ± 0.13 |
| 2009–2010 | NA | 0.8 ± 0.2 | NA | 0.3 ± 0.1 |
Values are percentages ± SE. Serum and RBC folate concentrations were measured by the Bio-Rad radioassay from 1988 to 2006 and assay-adjusted to be comparable to the microbiologic assay, which was used from 2007 to 2010 (13, 16, 17). values are the same as in Table 1. MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white; NA, not applicable; PIR, poverty-to-income ratio.
Estimate suppressed because the relative SE was ≥40%.
Relative SE was >30% but <40%.
Because information on dietary supplement use is not yet available for 2009–2010, postfortification prevalence estimates were limited to data from 1999 to 2008.
Reference intervals and distributions of blood folate concentrations for pre-, early post-, and late postfortification time periods.
We combined 6 y each of NHANES serum (Table 3) and RBC folate (Table 4) data to allow the generation of multistratified central 95% reference intervals by age, gender, and race/ethnicity. For children (age 4–11 y) and adolescents (age 12–19 y), we observed little difference in serum folate reference intervals between males and females (pre- and postfortification), race/ethnic groups (pre- and postfortification), and early versus late postfortification periods. For adults (age 20–59 y) and older persons (age ≥60 y), we observed little change in serum folate reference intervals from early to late postfortification. However, women generally had higher serum folate concentrations than did men (pre- and postfortification) and NHW persons had higher concentrations than did NHB or MA persons (pre- and postfortification).
TABLE 3.
Central 95% reference intervals for serum folate concentrations by demographic variable in participants age ≥4 y (NHANES 1988–2010)1
|
Prefortification (1988–1994) |
Early postfortification (1999–2004) |
Late postfortification (2005–2010) |
||||
| Demographic variable | n | Serum folate | n | Serum folate | n | Serum folate |
| nmol/L | nmol/L | nmol/L | ||||
| MA | ||||||
| Male | ||||||
| 4–11 y | 837 | 8.4–76.5 | 601 | 29.9–101 | 599 | 28.4–104 |
| 12–19 y | 497 | 2.4–44.8 | 1134 | 17.4–77.5 | 632 | 14.0–81.3 |
| 20–59 y | 1639 | 1.7–43.8 | 953 | 12.4–68.7 | 1041 | 10.5–64.6 |
| ≥60 y | 520 | 2.9–61.6 | 488 | 15.4–97.0 | 3322 | 12.5–101 |
| Female | ||||||
| 4–11 y | 857 | 8.4–64.2 | 590 | 30.9–94.4 | 586 | 29.4–95.9 |
| 12–19 y | 512 | 2.2–50.9 | 1134 | 20.5–73.4 | 612 | 14.3–74.1 |
| 20–59 y | 1680 | 1.6–53.0 | 1105 | 14.2–77.6 | 1123 | 12.7–81.4 |
| ≥60 y | 475 | 4.0–75.3 | 495 | 15.3–101 | 3972 | 13.1–120 |
| NHB | ||||||
| Male | ||||||
| 4–11 y | 768 | 7.7–60.1 | 578 | 30.7–101 | 4402 | 23.7–104 |
| 12–19 y | 481 | 1.8–42.1 | 1037 | 13.5–72.2 | 622 | 15.5–78.0 |
| 20–59 y | 1451 | 1.3–43.0 | 827 | 9.7–63.9 | 968 | 10.2–64.7 |
| ≥60 y | 476 | 2.1–57.6 | 3402 | 12.2–104 | 476 | 10.3–110 |
| Female | ||||||
| 4–11 y | 740 | 7.1–60.8 | 582 | 27.2–101 | 453 | 24.5–103 |
| 12–19 y | 552 | 1.4–50.2 | 965 | 15.5–70.6 | 571 | 12.9–65.3 |
| 20–59 y | 1856 | 1.2–50.6 | 935 | 12.2–82.9 | 1088 | 11.0–78.9 |
| ≥60 y | 489 | 2.5–73.2 | 3752 | 12.2–113 | 465 | 11.2–137 |
| NHW | ||||||
| Male | ||||||
| 4–11 y | 608 | 9.8–81.8 | 475 | 29.0–109 | 586 | 25.2–107 |
| 12–19 y | 3412 | 2.4–55.0 | 809 | 17.6–81.4 | 646 | 15.8–85.7 |
| 20–59 y | 1648 | 1.5–55.4 | 1907 | 13.7–84.4 | 2231 | 11.9–85.3 |
| ≥60 y | 1411 | 2.9–75.0 | 1354 | 19.7–114 | 1542 | 15.1–131 |
| Female | ||||||
| 4–11 y | 598 | 9.9–84.6 | 460 | 30.9–103 | 531 | 31.8–109 |
| 12–19 y | 4242 | 2.2–55.4 | 808 | 16.9–86.6 | 576 | 16.2–84.9 |
| 20–59 y | 1972 | 1.7–69.6 | 2153 | 14.1–97.6 | 2367 | 12.3–107 |
| ≥60 y | 1531 | 4.1–89.8 | 1347 | 20.6–119 | 1449 | 16.6–158 |
Values are reference intervals (2.5–97.5th percentile). Serum folate concentrations were measured by the Bio-Rad radioassay from 1988 to 1994 and from 1999 to 2006 and assay-adjusted to be comparable to the microbiologic assay, which was used from 2007 to 2010 (13, 16, 17). MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white.
Cell size is smaller than required ( = 448) to estimate the 2.5th and 97.5th percentiles with sufficient precision at an assumed mean design effect of 1.4.
TABLE 4.
Central 95% reference intervals for RBC folate concentrations by demographic variable in participants aged ≥4 y (NHANES 1988–2010)1
|
Prefortification (1988–1994) |
Early post-fortification (1999–2004) |
Late post-fortification (2005–2010) |
||||
| Demographic variable | n | RBC folate | n | RBC folate | n | RBC folate |
| nmol/L | nmol/L | nmol/L | ||||
| MA | ||||||
| Male | ||||||
| 4–11 y | 810 | 473–1660 | 598 | 667–2180 | 603 | 673–1820 |
| 12–19 y | 482 | 305–1430 | 1129 | 555–1840 | 628 | 500–1700 |
| 20–59 y | 1603 | 314–1410 | 948 | 514–1930 | 1041 | 427–1810 |
| ≥60 y | 504 | 311–1770 | 485 | 527–2730 | 3332 | 534–2320 |
| Female | ||||||
| 4–11 y | 823 | 407–1370 | 587 | 678–2000 | 593 | 667–1800 |
| 12–19 y | 500 | 318–1480 | 1130 | 575–2100 | 613 | 529–1730 |
| 20–59 y | 1644 | 318–1670 | 1100 | 560–2310 | 1125 | 501–2250 |
| ≥60 y | 454 | 319–2070 | 492 | 578–3180 | 3962 | 520–2680 |
| NHB | ||||||
| Male | ||||||
| 4–11 y | 756 | 382–1300 | 577 | 642–1550 | 4432 | 572–1610 |
| 12–19 y | 467 | 234–1140 | 1035 | 471–1380 | 621 | 449–1540 |
| 20–59 y | 1421 | 246–1160 | 824 | 431–1600 | 968 | 429–1770 |
| ≥60 y | 470 | 238–1640 | 3402 | 450–2240 | 480 | 449–2660 |
| Female | ||||||
| 4–11 y | 723 | 313–1220 | 576 | 609–1620 | 453 | 594–1490 |
| 12–19 y | 544 | 218–1120 | 963 | 438–1440 | 566 | 443–1420 |
| 20–59 y | 1827 | 223–1480 | 930 | 448–1950 | 1089 | 431–1990 |
| ≥60 y | 487 | 248–1870 | 3742 | 480–2500 | 464 | 442–2400 |
| NHW | ||||||
| Male | ||||||
| 4–11 y | 593 | 475–1850 | 471 | 800–2230 | 588 | 688–1930 |
| 12–19 y | 3272 | 336–1620 | 805 | 577–1920 | 644 | 488–1890 |
| 20–59 y | 1616 | 326–1710 | 1902 | 600–2320 | 2224 | 540–2190 |
| ≥60 y | 1381 | 370–2210 | 1343 | 650–3250 | 1535 | 614–3110 |
| Female | ||||||
| 4–11 y | 577 | 462–1620 | 457 | 747–2170 | 534 | 685–1790 |
| 12–19 y | 4182 | 318–1360 | 802 | 595–2070 | 573 | 540–1910 |
| 20–59 y | 1930 | 314–1906 | 2142 | 558–2660 | 2361 | 514–2530 |
| ≥60 y | 1502 | 370–2618 | 1336 | 661–3270 | 1454 | 627–3405 |
Values are reference intervals (2.5–97.5th percentile). RBC folate concentrations were measured by the Bio-Rad radioassay from 1988 to 1994 and from 1999 to 2006 and assay-adjusted to be comparable to the microbiologic assay, which was used from 2007 to 2010 (13, 16, 17). MA, Mexican American; NHB, non-Hispanic black; NHW, non-Hispanic white.
Cell size is smaller than required ( = 448) to estimate the 2.5th and 97.5th percentiles with sufficient precision at an assumed mean design effect of 1.4.
We noticed different effects for RBC folate reference intervals. Regardless of age, we observed race/ethnicity differences (pre- and postfortification), with NHW persons having higher RBC folate concentrations than NHB and MA persons. Also, regardless of age for most subgroups, we observed a slight decrease in RBC folate reference intervals from early to late postfortification.
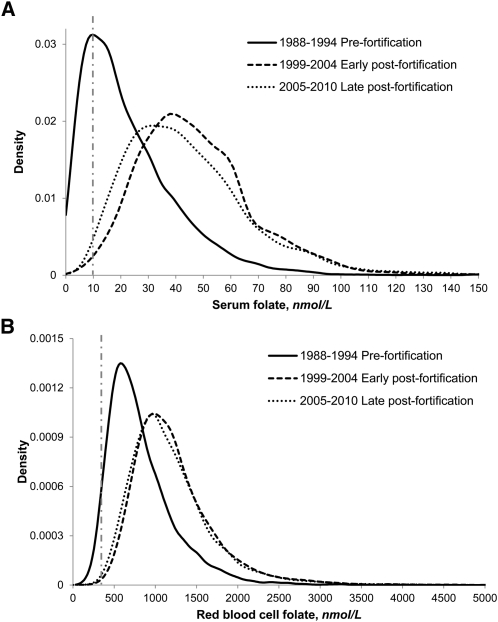
The frequency distribution curves of serum and RBC folate concentrations showed a distinct shift of the entire distribution to higher concentrations from the pre- to the early postfortification period, then a minor shift to slightly lower concentrations from the early to the late postfortification period for serum folate, but hardly any change in RBC folate (Fig. 3). To allow a detailed review of multistratified (by age, gender, and race/ethnicity) distribution curves for serum (Supplemental Tables 1–3) and RBC folate (Supplemental Tables 4–6) for pre-, early post-, and late postfortification time periods, we provided selected percentiles from the 2.5th to the 97.5th percentile.
FIGURE 3.
Frequency distribution curves for serum (A) and RBC (B) folate concentrations for prefortification (1988–1994), early postfortification (1999–2004), and late postfortification (2005–2010) time periods (NHANES 1988–2010). Concentrations measured from 1988–2006 by the Bio-Rad radioassay were adjusted by regressing them to be comparable to the 2007–2010 concentrations measured by microbiologic assay (13, 16, 17). The vertical dashed lines represent the 10-nmol/L low serum (A) and 340-nmol/L low RBC (B) folate cutoffs. Sample sizes (n) for serum folate were as follows: 23,359 (1988–1994), 23,345 (1999–2004), and 23,528 (2005–2010). Sample sizes (n) for RBC folate were as follows: 22,846 (1988–1994), 23,231 (1999–2004), and 23,528 (2005–2010).
Discussion
To our knowledge, this article presents the first analysis of more than a decade of post–folic acid fortification trends in serum and RBC folate in a representative sample of the U.S. population aged ≥4 y using MBA-equivalent data. The evaluation of data from 12 y postfortification (1999–2010) and comparing it with prefortification (1988–1994) data was only possible because of the availability of regression equations that allowed us to express all data on an MBA-equivalent basis, even though some of the data (1988–2006) were generated with the BR (13). Two past (21, 23) and one recent (5) expert panel who assessed changes in folate assays as well as a recent expert panel on 25-hydroxyvitamin D assays (24) have all come to the conclusion that when laboratory methods change, data need to be adjusted to allow for meaningful interpretation and trend analysis. The new MBA-equivalent data allow U.S. public health officials for the first time to directly assess the prevalence of inadequate blood folate concentrations pre- and postfortification by using updated cutoffs from a 2005 WHO Technical Consultation (22). The data also allow officials in other countries where the MBA is used to compare their country-specific population data to those of the U.S. population. However, other countries that evaluate the impact of fortification must also consider the extent of their fortification (e.g., food vehicles and levels of fortification) and the percentage of their population that fortification is reaching when comparing biomarker data.
We confirmed findings from previous analyses (9–12, 25) that showed differences in blood folate concentrations by sociodemographic subgroup. The new finding in this analysis is that the difference in prefortification RBC folate concentrations between males and females disappeared after adjustment for supplement use. It has been shown that a higher percentage of females use supplements compared with males (26), and this may explain the higher blood folate concentrations we observed in females. However, during postfortification, when RBC folate concentrations were ~50% higher in both males and females compared with the prefortification time period, adjustment for supplement use did not remove the gender difference.
The relative increases in serum (2.5 times) and RBC (1.5 times) folate concentrations from pre- to postfortification using MBA-equivalent data were very similar to earlier reports using the original BR data (10, 12). Those reports, limited to 6 y (1999–2004) and 8 y (1999–2006) of postfortification data, found a significant linear trend showing slightly lower concentrations during the postfortification period and discussed potential reasons for the decrease, such as changes in consumer behavior and reduced folate content of fortified breads. The yearly USDA per capita disappearance data also show a slight decline in daily per capita dietary folate equivalents (DFE) between 2000 (927 DFE) and 2006 (874 DFE), supporting the idea that the declining postfortification blood folate concentrations could be explained by declining intakes (27). Our analysis showed that adjustment for potential changes in sociodemographic variables and dietary supplement use over the course of 12 y postfortification did not explain the decreases in serum and RBC folate concentrations of ≤17% and 12%, respectively. Despite this significant decrease, however, the prevalence of low blood folate concentrations remained ≤1% throughout the entire 12-y time period and did not exceed 1% for any demographic or socioeconomic subgroup. Thus, these declines are unlikely to have biological significance with respect to folate status and are not of public health concern.
We have shown that the central 95% reference intervals varied over time as well as with demographic subgroup. This underscores the difficulty of using reference intervals to derive cutoffs for assessing prevalence of low status. The currently \recommended WHO cutoffs for low serum (<10 nmol/L) and RBC (<340 nmol/L) folate concentrations have both been derived from inflection point analyses of cross-sectional data in which the one-carbon metabolite homocysteine, a functional indicator of inadequate folate status, starts to increase (28). These cutoffs are higher than the traditional cutoffs of <7 nmol/L for serum and <305 nmol/L for RBC folate as defined by the 1998 Institute of Medicine Committee that reviewed the DRI for folate (29). The Institute of Medicine committee used small, conventional metabolic and depletion/repletion studies as the basis for their cutoffs. Even when using the higher, more conservative WHO cutoffs, we found ≤1% of the U.S. population with inadequate folate status during 12 y postfortification.
For the prefortification time period, it is surprising to see how different the prevalence estimates of low serum (24%) and RBC (3.5%) folate concentrations are, considering that the WHO cutoff for serum folate was derived from the same study population as the cutoff for RBC folate by looking at the value at which an optimum total homocysteine concentration is achieved. Possibly, the different within-person to between-person ratios for serum (0.192) compared with RBC (0.043) folate may affect prevalence estimates to some degree because of a wider spread in the tails of the serum folate distribution compared with the RBC folate distribution (1).
The prevalence of low blood folate concentrations (<10 nmol/L for serum folate and <340 nmol/L for RBC folate) during prefortification varied by sociodemographic subgroup and was lower in supplement users (14% for serum and 1.5% for RBC folate) compared with nonusers (37% for serum and 5.7% for RBC folate). Data from the Framingham cohort study, which used a slightly higher cutoff for low RBC folate (<363 nmol/L), showed similar prefortification estimates of 1.6% (supplement users) and 4.9% (nonusers) as well as similar postfortification estimates of 0% (supplement users) and 1.9% (nonusers) (30). A second Framingham cohort analysis (31), which used a lower cutoff for serum folate (<6.8 nmol/L), showed lower prefortification estimates of 3.9% (supplement users) and 22% (nonusers) but similar postfortification estimates of 0% (supplement users) and 1.7% (nonusers). An MBA was used to measure blood folate concentrations in the Framingham cohort study, which may explain the similarity of prevalence estimates compared to the NHANES MBA-equivalent data.
The major limitation with this analysis relates to the use of statistically adjusted data and was discussed as part of an article that presented equations on how to adjust U.S. pre- and postfortification blood folate concentrations generated by the BR to make them comparable to the MBA (13). Another limitation is that when we generated the assay-adjusted MBA-equivalent RBC folate data, we were not able to use genotype-specific regression equations to account for the different relationship between the BR and MBA assays for samples with the MTHFR T/T genotype compared with the C/C or C/T genotype (13). The different genotype frequencies by race/ethnicity make it difficult to provide an accurate description of race/ethnicity differences.
The strengths of this article include the following: its large pre- and postfortification data sets that allow multiple levels of stratification; its design that crosses over multiple postfortification survey periods, allowing a more accurate estimation of folate time trends; its use of cutoffs for low blood folate concentrations that have been updated by WHO; and its results expressed as MBA-equivalents, which makes them directly relevant to the assay method generally accepted as accurate for assessing folate status.
In summary, we have presented the newest serum and RBC folate data generated with the MBA for NHANES 2007–2010 as well as MBA-equivalent blood folate data for 8 previous postfortification years (1999–2006) and 6 prefortification years (1988–1994). After the introduction of folic acid fortification, there was a sharp increase in blood folate concentrations, more than doubling serum folate concentrations and increasing RBC folate concentrations by ~50%. Over the next 12 y postfortification, serum folate concentrations decreased by ~17% and RBC folate concentrations by ~12%. These decreases did not affect the very low postfortification prevalence (≤1%) of inadequate blood folate concentrations. The current data provide an invaluable cornerstone for U.S. and foreign public health officials in guiding and evaluating folic acid fortification policy and in assessing population folate status.
Acknowledgments
The authors acknowledge contributions from the following laboratory members: Daniel Rabinowitz, Neelima Paladugula, Bridgette Haynes, and Donna LaVoie (CDC National Center for Environmental Health). C.M.P., J.P.H., D.A.L., and R.L.B. designed the overall research project; C.M.P., J.P.H., D.A.L., and M.Z. conducted most of the research; C.M.P., J.P.H., and D.A.L. analyzed most of the data; and C.M.P. wrote the initial draft, which was modified after feedback from all coauthors, and had primary responsibility for content. All authors read and approved the final manuscript.
Footnotes
Supported by funding from the Office of Dietary Supplements, NIH. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the CDC and Prevention/Agency for Toxic Substances and Disease Registry, the NIH, or the Department of Health and Human Services.
Supplemental Tables 1–6 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: BR, Bio-Rad radioassay; DFE, dietary folate equivalents; MA, Mexican American; MBA, microbiologic assay; NCHS, National Center for Health Statistics; NHB, non-Hispanic black; NHW, non-Hispanic white; PIR, poverty-to-income ratio.
Literature Cited
- 1.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr. 2011;94 Suppl:322S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, et al. Biomarkers of folate status in the National Health and Nutrition Examination Survey (NHANES): a roundtable summary. Am J Clin Nutr. 2011;94 Suppl:303S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and BioRad radioassay. Clin Chem. 2007;53:781–4 [DOI] [PubMed] [Google Scholar]
- 4.Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. Influence of 5,10-methylene-tetrahydrofolate reductase polymorphism on whole blood folate concentrations measured by LC-MS/MS, microbiologic assay and BioRad radioassay. Clin Chem. 2008;54:197–201 [DOI] [PubMed] [Google Scholar]
- 5.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr. 2011;94 Suppl:297S–302S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackmore S, Pfeiffer C, Hamilton MS, Lee A. Recoveries of folate species from serum pools sent to participants of the UK NEQAS Haematinics scheme in February and March 2004. Clin Chim Acta. 2005;355:S457 [Google Scholar]
- 7.Pfeiffer CM, Fazili Z, Zhang M. Folate analytical methodology. : Bailey LB, editor Folate in health and disease. 2nd ed Boca Raton, FL: CRC Press; 2010. p. 517–74 [Google Scholar]
- 8.Shane B. Folate status assessment history: implications for measurement of biomarkers in NHANES. Am J Clin Nutr. 2011;94 Suppl:337S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganji V, Kafai MR. Trends in serum folate, RBC folate, and circulating total homocysteine concentrations in the United States: analysis of data from National Health and Nutrition Examination Surveys, 1988–1994, 1999–2000, and 2001–2002. J Nutr. 2006;136:153–8 [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 11.Boulet SL, Yang Q, Mai C, Mulinare J, Pfeiffer CM. Folate status in women of childbearing age by race/ethnicity–United States 1999–2000, 2001–2002, and 2003–2004. MMWR Morb Mortal Wkly Rep. 2007;55:1377–80 [PubMed] [Google Scholar]
- 12.McDowell MA, Lacher DA, Pfeiffer CM, Mulinare J, Picciano MF, Rader JI, Yetley EA, Kennedy-Stephenson J, Johnson CL. Blood folate levels: the latest NHANES results. Hyattsville, MD: National Center for Health Statistics; 2008. NCHS Data Briefs, no. 6 [PubMed] [Google Scholar]
- 13.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Yetley EA, Zhang M, Yetley EA, Rader JI, Sempos CT, et al. Changes in measurement procedure from a radioassay to a microbiologic assay necessitates adjustment of serum and RBC folate concentrations in the U.S. population from the NHANES 1988–2010. J Nutr. 2011;142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Survey, 2007–2008 [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/overviewbrochure_0708.pdf
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics Response rates and CPS population totals, National Health and Nutrition Examination Survey [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm
- 16.Centers for Disease Control and Prevention, National Center for Health Statistics 2007–2008 serum and red blood cell folate [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/FOLATE_E.htm
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics 2009–2010 serum and red blood cell folate [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/FOLATE_F.htm
- 18.Pfeiffer CM, Zhang M, Lacher DA, Molloy AM, Tamura T, Yetley EA, Picciano M-F, Johnson CL. Comparison of serum and red blood cell folate microbiologic assays for national population surveys. J Nutr. 2011;141:1402–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics Total folate by microbiological assay [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/folate_b12_d_met.pdf
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics Total folate by microbiological assay [cited 2011 Dec 17]. Available from: http://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/FOLATE_F_met.pdf
- 21.Raiten DJ, Fisher KD. Assessment of folate methodology used in the third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). J Nutr. 1995;125 Suppl:1371S–98S [DOI] [PubMed] [Google Scholar]
- 22.de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29:S238–44 [DOI] [PubMed] [Google Scholar]
- 23.Senti FR, Pilch SM. Analysis of folate data from the Second National Health and Nutrition Examination Survey (NHANES II). J Nutr. 1985;115:1398–402 [DOI] [PubMed] [Google Scholar]
- 24.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagel AN, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140 Suppl:2030S–45S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright JD, Bialostosky K, Gunter EW, Carroll MD, Najjar MF, Bowman BA, Johnson CL. Blood folate and vitamin B12: United States, 1988–1994. Vital Health Stat. 11. 1998;243:1–78 [PubMed] [Google Scholar]
- 26.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano M-F. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USDA Economic Research Service U.S. food supply: nutrients and other food components, per capita per day [cited 2011 Dec 17]. Available from: http://www.ers.usda.gov/Data/FoodConsumption/NutrientAvailIndex.htm
- 28.Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G. The use of a combination of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food Nutr Bull. 2008;29:S67–73 [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine, Food and Nutrition Board Dietary reference intakes: thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington: National Academy Press; 1998 [PubMed] [Google Scholar]
- 30.Choumenkovitch SF, Jacques PF, Nadeau MR, Wilson PWF, Rosenberg IH, Selhub J. Folic acid fortification increases red blood cell folate concentrations in the Framingham study. J Nutr. 2001;131:3277–80 [DOI] [PubMed] [Google Scholar]
- 31.Jacques PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54 [DOI] [PubMed] [Google Scholar]