Abstract
The NHANES measured serum and RBC folate concentrations by using a radioassay during prefortification (1988–1994) and postfortification (1999–2006) periods followed by the use of a microbiologic assay (MBA) from 2007–2010. The MBA produces higher concentrations than does the radioassay and is considered to be more accurate. To allow for accurate long-term trending (1988–2010), we evaluated different regression models (linear, piecewise linear, and fractional polynomial) to assay-adjust the radioassay results to be comparable to the MBA results. The data used to derive the regression models originated from 2 crossover studies in which the 2 assays were applied to a set of 325 serum and 171 whole-blood samples. Fractional polynomial regression of logarithmically transformed data provided the best fit for serum folate. Linear regression of logarithmically transformed whole-blood data provided an equally good fit compared with the other models and was the simplest to apply for RBC folate. Prefortification serum and RBC folate geometric mean concentrations increased after adjustment from 13.0 to 16.7 nmol/L and from 403 to 747 nmol/L, respectively. Postfortification serum folate concentrations increased from ~30 to ~43 nmol/L, and RBC folate concentrations increased from ~600 to ~1100 nmol/L after adjustment, with some variation across survey cycles. The presented regression equations allow the estimation of more accurate prevalence estimates and long-term trends in blood folate concentrations in the U.S. population by using results that are equivalent to the MBA. This information will be useful to public health officials in the United States who are dealing with folic acid fortification issues.
Introduction
The NHANES measured serum and RBC folate concentrations for >30 y to assess the folate status of the U.S. population. NHANES usually maintains the same assay over many survey periods and tightly controls the performance of the assay so that changes in concentrations over time are attributable to real differences in folate status and are not confounded by changes in assay. However, at times, changes in assays are unavoidable, whether due to the discontinuation of an assay or due to technological advances (1, 2). From 1988 through 2006, folate status has been monitored in NHANES by use of a Bio-Rad radioassay (BR)7, but in 2007 the manufacturer discontinued its production. For NHANES 2007–2010, the CDC selected a traditional microbiologic growth assay (MBA) using Lactobacillus rhamnosus (formerly known as Lactobacillus casei) to measure serum and RBC folate levels (1, 2).
It is known that the MBA produces substantially higher serum and RBC folate concentrations than does the BR (2–5). These assay differences pose challenges in data interpretation across NHANES survey periods (1, 2, 6). Data users need to be able to assess long-term trends in folate status before fortification and during 12 y after the introduction of fortification. If assay differences are not accounted for, users cannot determine whether time trends show actual changes in folate status or are due, in whole or in part, to assay differences. A 2010 roundtable of experts discussed these and other NHANES folate and vitamin B-12 measurement issues, and “the roundtable agreed that an adjustment equation based on a crossover study was necessary for time-trend evaluations” (6). The adjustment of assay differences to assess the prevalence of inadequate folate status and trends over time has also been deemed necessary by previous expert panels reviewing folate biomarker data from NHANES II and III (3, 7). An uninterrupted, continuous, and accurate assessment of the folate status of the U.S. population from pre–folic acid fortification to postfortification (1998 and later) is not only important to U.S. public health officials but also to international public health officials who often compare their country-specific survey results to the U.S. data. Some countries use the MBA to conduct folate population monitoring, and they could compare their postfortification data with the 2007–2010 U.S. data generated with the MBA. However, they also need to be able to compare their prefortification data with the U.S. prefortification data, all of which was generated by using the BR.
In this article we describe the derivation of assay adjustments for the U.S. pre- and postfortification blood folate concentrations generated by the BR in the NHANES 1988–1994 and 1999–2006 to make them comparable to the MBA used in the NHANES 2007–2010. This will allow the comparison of blood folate data across many NHANES survey years. We show the impact of the assay adjustment on population means and on the frequency distribution curves of serum and RBC folate. A separate article described the long-term trends in folate status of the U.S. population using MBA-equivalent data (8).
Participants and Methods
Survey design and participants.
The NHANES collects cross-sectional data on the health and nutritional status of the civilian noninstitutionalized U.S. population by using a stratified, multistage probability sample designed to represent the U.S. population on the basis of age, gender, and race/ethnicity (9). The survey is conducted by the National Center for Health Statistics (NCHS) at the CDC and changed in 1999 from a previously periodic survey to a continuous survey with data released in 2-y cycles. The NHANES race/ethnicity categories are based on self-reported data. All respondents provided their informed consent, and the NHANES protocol was reviewed and approved by the NCHS Research Ethics Review Board.
Laboratory methods.
Non-anticoagulated and EDTA whole-blood samples were collected in the NHANES Mobile Examination Center during 2007–2010 from participants aged ≥1 y (10, 11). An aliquot of EDTA whole blood was diluted (1:11) with 1% ascorbic acid solution to generate a hemolysate for the RBC folate analysis. The serum and whole-blood hemolysate samples were frozen and shipped on dry ice to the National Center for Environmental Health laboratory at the CDC for folate analyses by MBA. A 96-well plate assay using chloramphenicol-resistant cryo-preserved L. rhamnosus was used with slight modifications from a previously published procedure (12, 13): 5-methyltetrahydrofolic acid was used as calibrator, a robotic work station was used to dilute and dispense samples and reagents into the 96-well plate, 4 replicates were prepared per sample at 2 dilutions by using 0.5% sodium ascorbate (1:100 and 1:200 for serum and 1:140 and 1:280 for whole-blood hemolysate), and a polynomial regression (third degree) was used for curve fitting (14–16).
To verify the comparability of results over time, the laboratory used 3 levels of well-characterized in-house-prepared quality-control pools (serum and whole-blood hemolysate) in every assay, analyzed reference materials from the National Institute of Standards and Technology [SRM 1955–serum (17)] and the U.K. National Institute for Biological Standards and Control [RM 03-178–serum (18) and RM 95-528–lyophilized whole-blood hemolysate (19)] several times a year, and participated regularly in the U.K. NEQAS Haematinics scheme proficiency testing program (20). Long-term quality-control CV for serum folate were 5.9–10% at 6.67–56.8 nmol/L for NHANES 2007–2008 and 4.7–8.5% at 8.43–58.6 nmol/L for NHANES 2009–2010 (15, 16). For RBC folate, CV were 8.0–14% at 402–1570 nmol/L for NHANES 2007–2008 and 7.5–8.2% at 421–898 nmol/L for NHANES 2009–2010 (15, 16). The Bio-Rad Quantaphase I radioassay was used during 1988–1991 and the Quantaphase II was used during 1991–2006 (Bio-Rad Laboratories). The NCHS has applied appropriate assay adjustments to the 1988–1991 folate data before their public release to account for method differences between the Quantaphase I and II (3). The performance of the BR has been discussed in previous reports (2, 21). Long-term CV were 4.0–7.0% for serum folate at 5.20–29.9 nmol/L and 4.0–6.0% for RBC folate at 143–1120 nmol/L (21).
Statistical analyses.
Statistical analyses were performed by using SAS (version 9; SAS Institute, Inc.) and SUDAAN (version 9; RTI International) software. Fractional polynomial regressions were performed by using STATA (version 11.2; StataCorp). In each NHANES survey period we used sample weights to account for differential nonresponse or noncoverage and to adjust for planned oversampling of some groups. Analyses were limited to participants 4 y and older because NHANES III did not provide information below that age. The only participants excluded from our analyses were those for whom data were missing: the sample size for assay-adjusted serum folate in NHANES III changed from 23,361 to 23,259 because 2 participants had serum folate values <1 and the adjustment formula requires logarithmic transformation (which produces a negative number) and then calculating the square root; the sample sizes for unadjusted and assay-adjusted RBC folate differed in NHANES III and in survey cycles from 1999 to 2006 because the RBC folate adjustment formula used serum folate, RBC folate, and hematocrit, and sometimes one of these tests was missing for participants.
We examined the relationship between the BR and MBA method by using data from 2 crossover studies conducted with convenience samples from different blood banks, one for serum (4) and one for whole blood (5). After logarithmically transforming the data to address the skewness of the distribution, we evaluated several regression approaches including linear, piecewise linear, and fractional polynomial (22) to determine the best fit. We then applied the regression equations to the BR data in NHANES to generate MBA-equivalent data. We chose a “forward” approach (in which MBA is the predicted variable and BR is the independent variable) rather than a “backward” approach (in which BR is the predicted variable and MBA is the independent variable) for both serum and RBC folate data because the MBA method is considered a more accurate method (6, 23) and the BR method is no longer available.
We calculated descriptive statistics (geometric mean, selected percentiles, and their SE) of unadjusted (BR data as measured) and mathematically adjusted (BR data adjusted to be MBA equivalent) serum and RBC folate concentrations for the prefortification (1988–1994) and postfortification period (1999–2006, by 2-y survey periods). For purposes of comparing the assay-adjusted postfortification data from 1999 to 2006 (generated with the BR and adjusted to the MBA) with the MBA data from 2007 to 2010, we also provided data for these newest 2 NHANES survey periods. We plotted frequency distribution curves for each analyte for unadjusted and assay-adjusted data for the prefortification (1988–1994) and postfortification (combining 2 survey periods at a time, i.e., 1999–2002, 2003–2006, and 2007–2010) periods.
Results
Regression equations.
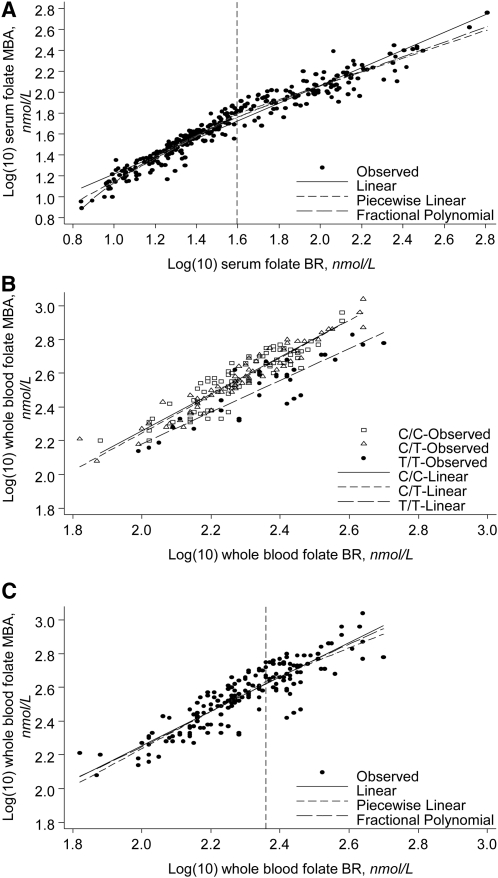
We used data from a serum folate crossover study performed between the BR and the MBA (4) to relate the 2 assays. We examined several regression methods beyond the piecewise linear model (with a predefined knot) performed by Fazili et al. (4), including linear (least-squares), piecewise linear (using a grid search to find the knot), and fractional polynomial. In this crossover study (n = 325), the BR results were 29% lower than the MBA results. Each regression model gave a similar R2 of ~0.95, but the regression line to fit the data varied slightly among models, particularly at the tails of the distribution (Fig. 1A). The linear regression model tended to overpredict at the tails of the serum folate distribution. The piecewise linear regression model [with the predefined knot (not shown) or grid search defined knot] provided better estimates at the tails, which is important for the estimation of prevalence at low or high cutoffs. Upon visual inspection, the fractional polynomial regression method gave a slightly better fit than did the other regression approaches, especially at lower serum folate values. It resulted in the following equation that regressed BR serum folate results (nmol/L) to match MBA results (serum folateadjusted, nmol/L) (9):
where x = log10serum folate (note: **designates exponential).
FIGURE 1.
Regression models for blood folate data generated by the BR and the MBA. (A) Finding the best fit for serum folate data in a convenience set of serum samples (n = 325) (4). The dashed vertical line represents the knot for the piecewise linear regression determined through a grid search (39 nmol/L). The correlation coefficients (R2) were as follows: linear, 0.94; piecewise linear, 0.95; and fractional polynomial, 0.95. The regression equations with x = log10BR and y = MBA were as follows: linear, y = 10**(0.847 · x + 0.372); piecewise linear, y = 10**(1.0847 · x + 0.0636) if x ≤1.595 and y = 10**(0.6591 · x + 0.7424) if x >1.595; and fractional polynomial, y = 10**(0.0188 · x3 – 2.7109 · x−1/2 + 3.8276). (B) Linear regression model showing the influence of MTHFR genotype on whole-blood folate in a convenience set of whole-blood samples (n = 171) (5). The correlation coefficients (R2) were as follows: C/C, 0.83; C/T, 0.92; and T/T, 0.80. The regression equations with x = log10BR and y = MBA were as follows: C/C, y = 10**(1.0937 · x + 0.0707); C/T, y = 10**(1.1137 · x + 0.0180); and T/T, y = 10**(0.9441 · x + 0.2924). (C) Finding the best fit for whole-blood folate data in a convenience set of whole-blood samples (n = 171) (4). The dashed vertical line represents the knot for the piecewise linear regression determined through a grid search (229 nmol/L). The correlation coefficients (R2) were as follows: linear, 0.80; piecewise linear, 0.80; and fractional polynomial, 0.80. The regression equations with x = log10BR and y = MBA were as follows: linear, y = 10**(1.0179 · x + 0.2175); piecewise linear, y = 10**(1.1108 · x + 0.0147) if x ≤2.36 and y = 10**(0.8216 · x + 0.6972) if x >2.36; and fractional polynomial, y = 10**(−15.4588 · x−2 * logex – 1.1317 · x−2 + 5.2092). Note: **designates exponential. BR, Bio-Rad radioassay; MBA, microbiologic assay.
Data from a separate whole-blood folate (WBF) crossover study (5) were used to examine the same types of regression methods mentioned above. The BR results in this crossover study (n = 171) were 45% lower than the MBA results; however, there was a different relationship between the 2 assays depending on the MTHFR (5,10-methylenetetrahydrofolate reductase) C677T polymorphism. Whole-blood samples from persons with the T/T genotype showed a smaller difference between the 2 assays (31%) than did whole-blood samples from persons with the C/C and C/T genotypes (48%) (Fig. 1B). The reason for this was that the BR underrecovered 5-methyltetrahydrofolic acid and overrecovered some other folate forms, whereas the MBA showed close to complete recovery for the major folate forms (5). Regardless of the genotype, however, the difference between the 2 assays increased with increasing WBF concentration. Because MTHFR genotype information is not available for NHANES 1999–2010, we cannot use genotype-specific regression equations as recommended by Fazili et al. (5).
Using the crossover data for all genotypes, the linear, piecewise linear, and fractional polynomial regression models all gave a similar R2 of ~0.80 and a similar fit for the logarithmically transformed WBF data (Fig. 1C). We selected the linear regression model because it performed as well as the other models, even at the tails of the distribution, and was easier to apply. It resulted in the following equation that regressed BR WBF results (nmol/L) to match MBA results (WBFadjusted, nmol/L) (9):
where x = log10WBF. The regression line representing this equation lay below the regression lines for the C/C and C/T genotype and above the regression line for T/T genotype. At low folate concentrations, predicted values using this “all genotype” equation were similar to values using the C/C and C/T regression equations. At high folate concentrations, predicted values were lower compared with the C/C and C/T equations. Regardless of folate concentration, predicted values were always higher compared with the T/T equation.
Because NHANES data are reported as RBC folate, additional steps, as described below and in the analytic note released with the NHANES 2007–2008 (9) and 2009–2010 data (10), are necessary to calculate the MBA-equivalent concentrations:
1) The BR RBC folate results (nmol/L) have to be converted to BR WBF results (nmol/L) before adjustment to match MBA results: WBF = (RBC folate · HCT/100) + serum folate · [1 − (HCT/100)], using the hematocrit (HCT; %) and BR serum folate (nmol/L).
2) The BR serum folate results (nmol/L) have to be regressed by using the fractional polynomial regression equation to match MBA results (serum folateadjusted, nmol/L): serum folateadjusted = 10**(0.0188 · x3 − 2.7109 · x−1/2 + 3.8276), where x = log10serum folate.
3) Last, the adjusted WBF results (WBFadjusted, nmol/L) have to be back-converted to adjusted RBC folate results (RBC folateadjusted, nmol/L) by using adjusted serum folate (serum folateadjusted, nmol/L) and HCT results (%): RBC folateadjusted = {WBFadjusted – [serum folateadjusted · (1.0 − (HCT/100)]}/(HCT/100).
Blood folate concentrations before and after assay adjustments.
Serum and RBC folate concentrations were much higher in 2007–2010 using the MBA method compared with previous postfortification years (1999–2006) that used the BR method (Table 1). However, after adjustment of the 1999–2006 BR data, all six 2-y survey periods produced similar geometric means and selected percentiles for serum and RBC folate. For serum folate, the effect of the assay adjustment was larger on the left tail (lower concentrations) and center of the distribution compared with the right tail (higher concentrations) of the distribution for each of the 4 postfortification survey periods. For RBC folate, the effect of the assay adjustment was similar throughout the entire distribution for the 4 survey periods.
TABLE 1.
Descriptive statistics for weighted unadjusted and assay-adjusted serum and RBC folate concentrations in participants aged ≥4 y as measured by the BR and the MBA (NHANES 1988–2010)1
| NHANES survey cycle and assay | Percentile4 |
|||||
| Data | n2 | Mean3 | 5th | 50th | 95th | |
| nmol/L | ||||||
| Serum folate | ||||||
| 1988–1994 BR | Unadjusted | 23,361 | 13.0 ± 0.3 | 4.6 ± 0.1 | 12.5 ± 0.3 | 39.0 ± 1.2 |
| Adjusted | 23,359 | 16.7 ± 0.5 | 3.3 ± 0.3 | 18.4 ± 0.5 | 56.8 ± 1.4 | |
| 1999–2000 BR | Unadjusted | 7411 | 31.7 ± 0.7 | 12.9 ± 0.5 | 32.1 ± 0.7 | 74.6 ± 1.7 |
| Adjusted | 7411 | 45.8 ± 1.0 | 19.2 ± 0.8 | 48.2 ± 1.0 | 93.4 ± 1.5 | |
| 2001–2002 BR | Unadjusted | 8242 | 29.1 ± 0.4 | 12.9 ± 0.4 | 29.4 ± 0.4 | 61.3 ± 1.1 |
| Adjusted | 8242 | 42.7 ± 0.6 | 19.2 ± 0.7 | 44.6 ± 0.6 | 80.8 ± 1.0 | |
| 2003–2004 BR | Unadjusted | 7692 | 27.4 ± 0.5 | 12.2 ± 0.3 | 27.0 ± 0.5 | 63.9 ± 1.5 |
| Adjusted | 7692 | 40.3 ± 0.7 | 17.8 ± 0.5 | 41.4 ± 0.7 | 83.3 ± 1.4 | |
| 2005–2006 BR | Unadjusted | 7639 | 27.9 ± 0.5 | 12.4 ± 0.4 | 27.7 ± 0.4 | 64.0 ± 2.0 |
| Adjusted | 7639 | 41.1 ± 0.7 | 18.1 ± 0.7 | 42.3 ± 0.6 | 83.5 ± 2.0 | |
| 2007–2008 MBA | Unadjusted | 7705 | 39.2 ± 0.9 | 14.7 ± 0.4 | 40.2 ± 1.0 | 92.6 ± 1.2 |
| 2009–2010 MBA | Unadjusted | 8184 | 37.9 ± 0.5 | 14.4 ± 0.3 | 38.7 ± 0.4 | 90.0 ± 1.3 |
| RBC folate | ||||||
| 1988–1994 BR | Unadjusted | 23,402 | 403 ± 5 | 196 ± 4 | 395 ± 6 | 867 ± 15 |
| Adjusted | 22,846 | 747 ± 10 | 360 ± 6 | 734 ± 12 | 1630 ± 29 | |
| 1999–2000 BR | Unadjusted | 7491 | 636 ± 12 | 346 ± 9 | 626 ± 13 | 1190 ± 29 |
| Adjusted | 7393 | 1180 ± 24 | 639 ± 17 | 1170 ± 24 | 2250 ± 52 | |
| 2001–2002 BR | Unadjusted | 8336 | 627 ± 9 | 350 ± 7 | 620 ± 8 | 1180 ± 21 |
| Adjusted | 8220 | 1160 ± 17 | 645 ± 12 | 1150 ± 17 | 2200 ± 41 | |
| 2003–2004 BR | Unadjusted | 7700 | 585 ± 8 | 331 ± 7 | 575 ± 9 | 1090 ± 21 |
| Adjusted | 7618 | 1080 ± 16 | 609 ± 12 | 1070 ± 17 | 2070 ± 43 | |
| 2005–2006 BR | Unadjusted | 7751 | 616 ± 6 | 343 ± 4 | 603 ± 5 | 1170 ± 21 |
| Adjusted | 7578 | 1140 ± 11 | 630 ± 6 | 1120 ± 9 | 2200 ± 44 | |
| 2007–2008 MBA | Unadjusted | 7728 | 1120 ± 23 | 569 ± 11 | 1120 ± 24 | 2200 ± 59 |
| 2009–2010 MBA | Unadjusted | 8223 | 1040 ± 14 | 545 ± 8 | 1030 ± 15 | 2020 ± 51 |
BR, Bio-Rad radioassay; MBA, microbiologic assay.
The sample size for adjusted serum folate in NHANES III changed from 23,361 to 23,359 because 2 participants had serum folate values <1 and the adjustment formula requires logarithmic transformation (which produces a negative number) and then calculating the square root. The sample sizes for unadjusted and adjusted RBC folate differed in NHANES III and in survey cycles from 1999 to 2006 because the RBC folate adjustment formula used serum folate, RBC folate, and hematocrit and sometimes one of these measurements was missing for participants.
Values are geometric means ± SE.
Values are percentile estimates ± SE.
As expected, assay adjustment of the prefortification data from 1988 to 1994 resulted in higher geometric means and selected percentiles, but serum and RBC folate concentrations were still significantly lower than in the postfortification data. As seen with the postfortification data, the effect of the assay adjustment was different across the entire distribution for serum folate but similar for RBC folate.
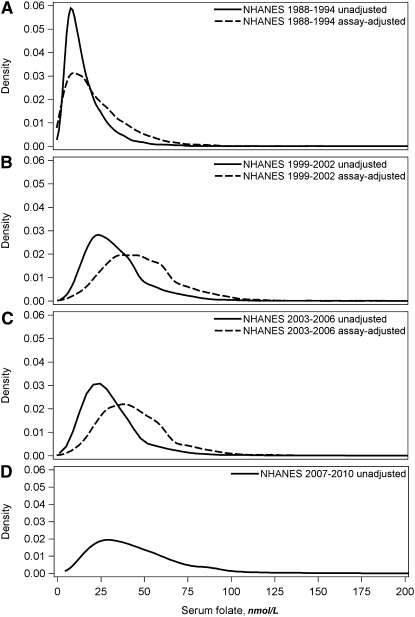
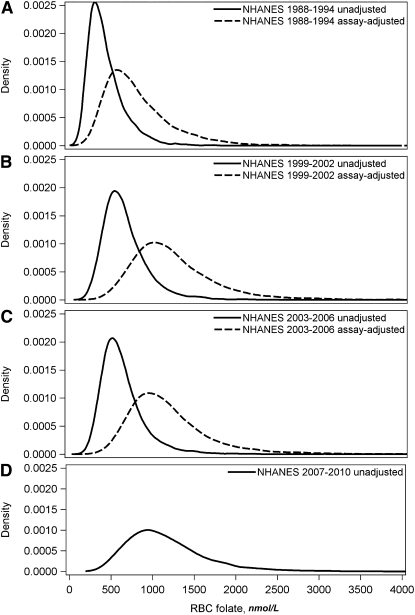
Frequency distribution curves for serum (Fig. 2) and RBC folate (Fig. 3) also showed that assay adjustment of the pre- and postfortification BR data changed the entire distribution to show higher concentrations. The assay-adjusted distribution curves for 1999–2006 were similar to the unadjusted 2007–2010 distribution curve produced by the MBA.
FIGURE 2.
Frequency distribution curves for unadjusted and assay-adjusted serum folate data for NHANES 1988–1994 (A; n = 23,361 unadjusted and 23,359 adjusted), for 1999–2002 (B; n = 15,653 unadjusted and adjusted), and for 2003–2006 (C; n = 15,331 unadjusted and adjusted) and for unadjusted data for NHANES 2007–2010 (D; n = 15,889). Adjustments were performed by regressing Bio-Rad radioassay data from 1988 to 1994 and from 1999 to 2006 to microbiologic assay–equivalent data.
FIGURE 3.
Frequency distribution curves for unadjusted and assay-adjusted RBC folate data for NHANES 1988–1994 (A; n = 23,402 unadjusted and 22,846 adjusted), for 1999–2002 (B; n = 15,827 unadjusted and 15,613 adjusted), and for 2003–2006 (C; n = 15,451 unadjusted and 15,196 adjusted) and for unadjusted data for NHANES 2007–2010 (D; n = 15,951). Adjustments were made by regressing Bio-Rad radioassay data from 1988 to 1994 and from 1999 to 2006 to microbiologic assay–equivalent data.
Discussion
This article describes how to adjust U.S. pre- and postfortification blood folate concentrations obtained with a defunct radioassay to make them comparable to the MBA and the impact of the adjustment on population means and on the frequency distribution curves of serum and RBC folate. The introduction of the U.S. folic acid fortification program in the late 1990s was an important public health intervention that required a careful impact assessment at the time and still requires continuous monitoring of population blood folate concentrations to ensure that neither folate inadequacy reemerges nor folate oversupply occurs in the general population and in at-risk groups. Monitoring biochemical indicators such as blood folate concentrations over time is superior to monitoring only dietary folate intake because it is not affected by several uncertainties in intake data that are difficult to measure (e.g., underestimation of intake, changing market composition of folate-fortified foods).
The ability to adjust the folate biomarker concentrations to MBA-equivalent data is also important from the perspective of interpreting prevalence results because the MBA is the method by which cutoffs for assessing inadequate folate status have been derived (23). It is also a generally accepted assay against which the accuracy of other assays is evaluated (6, 23). Finally, the MBA used in NHANES 2007–2010 produces equivalent (±10%) results for serum folate compared with the CDC liquid chromatography–MS/MS method (2), which, in turn, has been shown to produce results equivalent to National Institute of Standards and Technology–developed liquid chromatography–MS/MS methods (17). The key to a strong biomarker monitoring program is the availability of accurate and precise laboratory methods that generate results that are consistent across laboratories, methods, and over time, as well as interpretable from the known biological relationship of commonly used cutoffs to inadequate status. These have long been recognized as necessary prerequisites to assessing population prevalence estimates and time trends in folate status (1, 3, 6, 7).
Unfortunately, for decades the clinical folate community has had major problems with assays that readily lend themselves to clinical laboratory settings but that do not generate comparable results: in some cases, they suffer from poor precision or produce inaccurate results and undergo frequent reformulations by the manufacturer (24, 25). Consequently, public health officials planning for NHANES had to face difficult decisions with regard to which folate assay to best choose for this important and unique population survey that generates reference data and prevalence estimates for the United States but is also a cornerstone for countries worldwide that may not afford to carry out their own national folate status assessment or may want to compare their data to U.S. data.
A recent article by Yetley and Johnson (1), which was written as part of proceedings from a 2010 roundtable of experts discussing NHANES folate and vitamin B-12 measurement issues, provides detailed background on the history of folate biomarker measurements in NHANES. The traditional folate MBA was chosen during the early NHANES surveys; however, poor precision and low throughput led to a reassessment of the applied technology (7). Radioassays became widely available in the 1980s and offered improved precision and throughput compared with the MBA (24). The Bio-Rad Quantaphase I radioassay, which was initially calibrated against the MBA, was used toward the end of NHANES II (1978–1980) and during the first phase of NHANES III (1988–1991). However, a recalibrated Bio-Rad Quantaphase II assay (using a spectrophotometrically verified folic acid calibrator) was introduced in 1993 and was used in the second phase of NHANES III (1991–1994) and in NHANES 1999–2006 (3). The recalibrated assay, although it provided excellent precision and stability, resulted in a 30% downward shift in measured folate concentrations and necessitated the use of adjusted cutoffs to assess folate inadequacy (3). In 2007 the manufacturer discontinued the BR, and NHANES 2007–2010 used a new version of the MBA, which is superior to older MBA in precision, stability, throughput, and user friendliness (14, 25).
The excellent stability of the Quantaphase II BR over time has allowed public health officials to assess changes in population blood folate concentrations associated with the initiation of the U.S. folic acid fortification program (21). However, the differences in folate concentrations between the BR and MBA assays have led to questions about how to appropriately use folate cutoffs and how to compare data from these 2 assays. The regression equations developed in this study will help bridge the BR and MBA and allow continued long-term trending of blood folate concentrations in the U.S. population beyond the availability of the BR. Also, this study allows other studies that use the MBA to compare their data with those of the NHANES.
As with other statistical adjustments, regression equations have limitations. Depending on the regression model used, we observed a difference in fit mainly at the tails of the distribution. As discussed in the 2010 roundtable on “NHANES Monitoring of Biomarkers of Folate and Vitamin B-12 Status” (2), the tails of the distribution are a high priority for folate status assessment. We therefore chose the regression model that not only provided overall the best fit for the data but also provided the best fit at the tails of the distribution.
A second limitation of statistical adjustments is that a mean adjustment is applied, despite the fact that there is sample-to-sample variability. However, the higher the correlation is between the 2 methods, the better the quality of the statistical adjustment. For serum folate we obtained a high degree of correlation (R2 = ~0.95) in the crossover study between results produced by the BR and the MBA, which indicated that the major difference between the 2 assays was due to a systematic bias and that there was little random bias. The correlation was weaker (R2 = ~0.80) for WBF in the crossover study, which may be due to ≥2 factors. First, the analysis of WBF requires the hemolysis of RBC and the deconjugation of folate polyglutamates, both of which are known to increase sample-to-sample variability (24, 25). Second, the relationship between the BR and MBA is different for samples with the MTHFR T/T genotype compared with either C/C or C/T genotypes, and ideally genotype-specific regression equations should be used (5). However, this was not feasible for NHANES because the MTHFR genotype of the participants was not known. Users of the NHANES RBC folate data need to be aware of the genotype effects and interpret their results with caution.
The MBA also has limitations. Although the assay can be very consistent within a laboratory, it may produce different results between laboratories if it is calibrated differently or if a different type of microorganism is used (14). In a recent NHANES 2007–2008 subset analysis, we compared results from 3 laboratories conducting the MBA. We found that the 2 laboratories using the chloramphenicol-resistant L. rhamnosus microorganism were able to generate comparable results as long as they both used the same folate calibrator. However, calibration with folic acid produced folate results that were 22–32% higher than did calibration with 5-methyltetrahydrofolate (14). The third laboratory using the wild-type L. rhamnosus microorganism produced different results than the other 2 laboratories, and the difference was not due to a calibration bias (14). Other national and international surveys can compare their folate population data to those of the United States by harmonizing their MBA through use of the same microorganism and calibrator used in NHANES.
In summary, we hope to have conveyed the importance of choosing the best available method to conduct long-term population biochemical monitoring, to perform crossover studies when changes in methods are unavoidable, and to carefully examine the comparison data to assess the best statistical adjustment approach so that differences over time can be attributed to real changes in folate status that are not confounded by assay differences. Conducting assay adjustments when necessary is consistent with recommendations of several previous expert committees. The regression equations presented here will allow continued long-term trending of blood folate concentrations in the U.S. population, and they will enable other studies using an MBA harmonized to the assay used by CDC to compare their data with NHANES.
Acknowledgments
The authors acknowledge contribution from the following laboratory members: Daniel Rabinowitz, Neelima Paladugula, Bridgette Haynes, and Donna LaVoie (CDC National Center for Environmental Health). C.M.P., D.A.L., and C.T.S. designed the overall research project; C.M.P., J.P.H., R.A.D.-A., D.A.L., and C.T.S. conducted most of the research; C.M.P., J.P.H., and D.A.L. analyzed most of the data; and C.M.P. wrote the initial draft, which was modified after feedback from all coauthors, and had primary responsibility for content. All authors read and approved the final manuscript.
Footnotes
Supported by funding from the Office of Dietary Supplements, NIH. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the CDC /Agency for Toxic Substances and Disease Registry, the NIH, or the Department of Health and Human Services.
Abbreviations used: BR, Bio-Rad radioassay; HCT, hematocrit; MBA, microbiologic assay; NCHS, National Center for Health Statistics; WBF, whole-blood folate.
Literature Cited
- 1.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr. 2011;94 Suppl:322S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, et al. Biomarkers of folate status in the National Health and Nutrition Examination Survey (NHANES): a roundtable summary. Am J Clin Nutr. 2011;94 Suppl:303S–12S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raiten DJ, Fisher KD. Assessment of folate methodology used in the third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). J Nutr. 1995;125:1371S–98S [DOI] [PubMed] [Google Scholar]
- 4.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and BioRad radioassay. Clin Chem. 2007;53:781–4 [DOI] [PubMed] [Google Scholar]
- 5.Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. Influence of 5,10-methylene-tetrahydrofolate reductase polymorphism on whole blood folate concentrations measured by LC-MS/MS, microbiologic assay and BioRad radioassay. Clin Chem. 2008;54:197–201 [DOI] [PubMed] [Google Scholar]
- 6.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr. 2011;94 Suppl:297S–302S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senti FR, Pilch SM. Analysis of folate data from the Second National Health and Nutrition Examination Survey (NHANES II). J Nutr. 1985;115:1398–402 [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Yetley EA, Zhang M, Yetley EA, Rader JI, Sempos CT, et al. Trends in serum and red blood cell folate in the U.S. population from pre- to post-fortification: National Health and Nutrition Examination Survey 1988–2010. J Nutr. 2011 [Google Scholar]
- 9.Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Survey, 2007–2008 [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/overviewbrochure_0708.pdf
- 10.Centers for Disease Control and Prevention, National Center for Health Statistics. 2007–2008 Serum and red blood cell folate [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/FOLATE_E.htm.
- 11.Centers for Disease Control and Prevention, National Center for Health Statistics. 2009–2010 Serum and red blood cell folate [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/FOLATE_F.htm.
- 12.O’Broin S, Kelleher B. Microbiological assay on microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45:344–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53 [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer CM, Zhang M, Lacher DA, Molloy AM, Tamura T, Yetley EA, Picciano M-F, Johnson CL. Comparison of serum and red blood cell folate microbiologic assays for national population surveys. J Nutr. 2011;141:1402–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. Total folate by microbiological assay [cited 2011 Dec 17]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/folate_b12_d_met.pdf.
- 16.Centers for Disease Control and Prevention, National Center for Health Statistics. Total folate by microbiological assay [cited 2011 Dec 17]. Available from: http://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/FOLATE_F_met.pdf.
- 17.Satterfield MB, Sniegoski LT, Sharpless KE, Welch MJ, Hornikova A, Zhang N-F, Pfeiffer CM, Fazili Z, Zhang M, Nelson BC. Development of a new standard reference material: SRM 1955 (homocysteine and folate in human serum). Anal Bioanal Chem. 2006;385:612–22 [DOI] [PubMed] [Google Scholar]
- 18.Thorpe SJ, Heath A, Blackmore S, Lee A, Hamilton M, O'Broin S, Nelson BC, Pfeiffer CM. An international standard for serum vitamin B12 and serum folate: International collaborative study to evaluate a batch of lyophilized serum for B12 and folate content. Clin Chem Lab Med. 2007;45:380–6 [DOI] [PubMed] [Google Scholar]
- 19.Thorpe SJ, Sands D, Heath AB, Hamilton M, Blackmore S, Barrowcliffe T. An international standard for whole blood folate: evaluation of a lyophilised haemolysate in an international collaborative study. Clin Chem Lab Med. 2004;42:533–9 [DOI] [PubMed] [Google Scholar]
- 20.U.K. National External Quality Assessment Scheme for Haematinic Assays. [cited 2012 Feb 5]. Available from: http://www.ukneqas-haematinics.org.uk/content/Pageserver.asp.
- 21.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 22.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modeling. Appl Stat. 1994;43:429–67 [Google Scholar]
- 23.Shane B. Folate status assessment history: implications for measurement of biomarkers in NHANES. Am J Clin Nutr. 2011;94 Suppl:337S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackmore S, Pfeiffer CM, Lee A, Fazili Z, Hamilton MS. Assessment of the accuracy of serum folate assays and a proficiency testing consensus mean by an isotope dilution liquid chromatography tandem-mass spectrometry reference method. Clin Chem. 2011;57:986–94 [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer CM, Fazili Z, Zhang M. Folate analytical methodology. : Bailey LB, editor Folate in health and disease. 2nd ed Boca Raton, FL: CRC Press; 2010 [Google Scholar]