Abstract
Hyperuricemia is linked to gout and features of metabolic syndrome. There is concern that dietary fructose may increase uric acid concentrations. To assess the effects of fructose on serum uric acid concentrations in people with and without diabetes, we conducted a systematic review and meta-analysis of controlled feeding trials. We searched MEDLINE, EMBASE, and the Cochrane Library for relevant trials (through August 19, 2011). Analyses included all controlled feeding trials ≥7 d investigating the effect of fructose feeding on uric acid under isocaloric conditions, where fructose was isocalorically exchanged with other carbohydrate, or hypercaloric conditions, and where a control diet was supplemented with excess energy from fructose. Data were aggregated by the generic inverse variance method using random effects models and expressed as mean difference (MD) with 95% CI. Heterogeneity was assessed by the Q statistic and quantified by I2. A total of 21 trials in 425 participants met the eligibility criteria. Isocaloric exchange of fructose for other carbohydrate did not affect serum uric acid in diabetic and nondiabetic participants [MD = 0.56 μmol/L (95% CI: −6.62, 7.74)], with no evidence of inter-study heterogeneity. Hypercaloric supplementation of control diets with fructose (+35% excess energy) at extreme doses (213–219 g/d) significantly increased serum uric acid compared with the control diets alone in nondiabetic participants [MD = 31.0 mmol/L (95% CI: 15.4, 46.5)] with no evidence of heterogeneity. Confounding from excess energy cannot be ruled out in the hypercaloric trials. These analyses do not support a uric acid-increasing effect of isocaloric fructose intake in nondiabetic and diabetic participants. Hypercaloric fructose intake may, however, increase uric acid concentrations. The effect of the interaction of energy and fructose remains unclear. Larger, well-designed trials of fructose feeding at “real world” doses are needed.
Introduction
Metabolic syndrome predisposes individuals to diabetes and cardiovascular disease and affects over one-quarter of Americans and Canadians, making it an important public health concern (1, 2). Metabolic syndrome is now defined as the presence of any 3 of the following 5 risk factors: elevated waist circumference [central obesity], elevated TG (≥1.7 mmol/L), low HDL cholesterol (<1.0 mmol/L for men, <1.3 mmol/L for women) or drug treatment for low HDL cholesterol, elevated blood pressure (≥130/85 mm Hg) or antihypertensive drug treatment, or elevated fasting glucose (≥5.6 mmol/L) or drug treatment for elevated blood glucose (3). Meanwhile, uric acid is commonly associated with gout, an inflammatory condition affecting 8.3 million Americans (4). Further, hyperuricemia has been shown to be associated with components of metabolic syndrome, including hypertension and diabetes (5–7). Hyperuricemia is associated with obesity, excessive alcohol intake, and kidney failure (5). Uric acid concentrations have increased recently and the prevalence of hyperuricemia in U.S. adults is estimated at 21.4% (4). Proposed mechanisms of uric acid-mediated metabolic syndrome include the inhibition of endothelial NO leading to hypertension; inflammation and oxidative stress in adipocytes leading to insulin resistance; and increased endothelial and smooth muscle oxidative stress (6, 7).
Dietary factors are thought to be important modulators of serum uric acid. Worldwide fructose intake, particularly in the form of high fructose corn syrup, has paralleled the rise in metabolic syndrome and hyperuricemia (8). High fructose consumption is associated with features of metabolic syndrome through an effect on uric acid (9, 10). Fructose metabolism can promote uric acid formation (7). Once absorbed into the cell, unregulated phosphorylation of fructose by fructokinase leads to local ATP depletion and increased AMP production, which in turn increases uric acid (7). Early acute human studies demonstrated elevated serum uric acid after fructose feeding (11). Evidence from longer term fructose feeding trials (12–27) and observational studies (10, 28–32), however, have shown mixed results.
There are currently no recommendations addressing the effect of dietary fructose intake on uric acid and the risk of gout or metabolic syndrome. Various health agencies, including the American Diabetes Association, the European Association for the Study of Diabetes, and the AHA, have discouraged high intakes of fructose based on adverse affects on serum lipids (33–35). To evaluate the need for additional clinical evidence regarding the effects of fructose consumption on uric acid in humans, we conducted a systematic review and meta-analysis of controlled feeding trials to assess the effect of fructose on uric acid.
Methods
This systematic review and meta-analysis followed a similar methodological approach as Sievenpiper et al. (36). We followed the Cochrane Handbook for Systematic Reviews of Interventions for the planning and conduct of this meta-analysis (37). The reporting followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (38, 39).
Study selection.
We conducted a search of MEDLINE (1948 through August 19, 2011), EMBASE (1974 through August 19, 2011), and the Cochrane Central Register of Controlled Trials database (1950 through August 19, 2011) using the search terms: fructose AND (uric acid OR urate). Inclusion criteria is listed in Supplemental Table 1.
Data extraction.
Three reviewers (D.W., V.H., L.C.) independently extracted the following study characteristics: design (parallel or crossover), randomization, blinding, sample size, participant characteristics (age, sex, BMI, and diabetes status), fructose form (solid, liquid, or mixed), dose, control reference carbohydrates (sucrose, starch, glucose), follow-up, and the macronutrient profile of the background diet. The quality of each study was assessed by each reviewer using the Heyland methodological quality score (MQS)13 (40), which assigns a score from 0 to 1 or 0 to 2 in 9 categories of quality related to study design, sampling procedures, and interventions for a total of 13 points. Disagreements were reconciled by consensus through discussion with another investigator (J.L.S.). The fasting serum uric acid concentration (mean ± SD) was extracted as the main endpoint. All trials reported end differences. We calculated missing SD values from available statistics using standard formulae (37). Where these data were not reported preventing calculation, we imputed SD values using a pooled correlation coefficient derived from a meta-analysis of correlation coefficients from those trials reporting sufficient data. We derived correlation coefficients for individual trials according to a standard formula (37, 41). We then input these values into the meta-analysis as transformed Z-scores ± SE, from which we derived the pooled correlation coefficient. If SD coefficients still could not be imputed, then we derived the missing SD from the pooled SD imputed for the other trials (42).
Statistical analyses.
Data were analyzed using Review Manager (RevMan) version 5.0.25 (The Nordic Cochrane Centre, The Cochrane Collaboration). Separate pooled analyses for isocaloric and hypercaloric fructose feeding trials were conducted using the Generic Inverse Variance method using random effects models. Analyses were stratified by diabetes and nondiabetes. The outcome studied was end differences in serum uric acid. Potential sources of methodological heterogeneity were investigated by sensitivity analyses and a priori subgroup analyses, investigating the effect of comparator (starch, sucrose, glucose]) fructose format (fluid, mixed, solid), dose [Canadian Diabetes Association thresholds: ≤60 or >60 g/d (43)], follow-up (≤4 wk, >4 wk), study quality [Heyland MQS <8, ≥8 (40)], randomization, and study design (parallel or crossover). Meta-regressions were performed to assess the significance of subgroup effects (STATA 11.2). Publication bias was investigated by visual inspection of funnel plots and formally tested using Begg (44) and Egger (45) tests.
Results
Search results.
The systematic search and selection of the literature is outlined (Supplemental Fig. 1). A total of 375 reports were identified as eligible from the initial search, of which 340 reports were excluded based on the title or abstract. The remaining 35 reports were retrieved and reviewed, whereas a further 19 were excluded. A total of 16 met the eligibility criteria and were included in this meta-analysis. These 16 reports contained 18 isocaloric trials (12–21, 23–27) and 3 hypercaloric trials (22, 23) and one of the reports contained both an isocaloric and hypercaloric trial (23).
Trial characteristics.
Trial characteristics are shown in Table 1. Nine of the isocaloric trials were in 119 prediabetic and diabetic participants with 12 comparison arms and 9 of the isocaloric trials were in 271 nondiabetic participants with 8 comparison arms. The 3 hypercaloric trials were in 35 nondiabetic participants with 3 comparison arms. The mean age of participants was 42.6 y old (23–62 y old) in the isocaloric trials and 24.4 y old (24–24.6 y old) in the hypercaloric trials. Mean baseline uric acid was 317 μmol/L (232–398 μmol/L) in isocaloric trials and 312 μmol/L (300–322 μmol/L) in hypercaloric trials. Eight (44%) isocaloric trials and all 3 hypercaloric trials were randomized. Twelve (67%) isocaloric and all 3 hypercaloric trials used crossover designs. Starch (11 trial arms), sucrose (6 trial arms), or glucose (4 trial arms) were used as the comparator-carbohydrate in the isocaloric trials, whereas weight-maintaining control diets were used as the comparator in the hypercaloric trials. Fructose was administered in liquid, solid, or mixed formats at a mean dose of 94 g/d [25–213 g/d or 5–33% energy (E)] in the isocaloric trials and 215 g/d (213–219 g/d or +35% excess E) in the hypercaloric trials. Thirteen (72%) of the isocaloric trials and all 3 of the hypercaloric trials exceeded the dose threshold of 60 g/d proposed by the Canadian Diabetes Association (13, 15–17, 19, 20, 22, 23, 26). Nine (50%) isocaloric and all 3 hypercaloric trials used metabolically controlled designs. Background diets in the isocaloric and hypercaloric trials provided a wide range of macronutrient profiles. Isocaloric trial diets consisted of 43–92% E carbohydrate, 0–42% E fat, and 8–20% E protein. All hypercaloric trial diets consisted of 55% E carbohydrate, 30% E fat, and 15% E protein. The mean follow-up was 14.4 wk (1–95 wk) in the isocaloric trials, whereas all 3 hypercaloric trials lasted 1 wk. The Heyland MQS in the isocaloric trials ranged from 4 to 8 with 9 trials (50%) considered to be of higher quality (MQS ≥8). All hypercaloric trials were of higher quality with an MQS score of 8.
TABLE 1.
Characteristics of included trials
| Study | Participants1 | Age2 | Baseline plasma uric acid3 | Design4 | Setting | Feeding control5 | Randomization | Fructose dose6 | Fructose form7 | Comparator8 | Diet energy CHO:fat:protein | Follow-up | MQS9 |
| n | y | μmol/L | g/d (%E) | wk | |||||||||
| Isocaloric trials | |||||||||||||
| No diabetes | |||||||||||||
| Crapo (15) | 11 N; 4M, 7F | 39.5 ± 11.4 | 339 ± 98.6 | C | IP, USA | Supp | No | ~81 (13.2) | MX | Sucrose | 55:30:15 | 2 | 7 |
| Forster (17) | 6 N; 4M, 2F | 23 (20–26) | 309 ± 77 | C | IP, GER | Met | No | 162 (33) | MX | Glucose | 92:00:08 | 2.9 | 7 |
| Hallfrisch (19) | 12 N; 12M, 0F | 39.8 ± 8.3 | 324 ± 82.3 | P | IP/OP, USA | Met | No | ~101 (15) | SO | Starch | 43:42:15 | 5 | 7 |
| 12 HI; 12M, 0F | 39.5 ± 7.3 | 371 ± 82 | P | IP/OP, USA | Met | No | ~101 (15) | SO | Starch | 43:42:15 | 5 | 7 | |
| Huttunen (20) | 68 N; 35 fructose, 33 sucrose | 27.5 ± 7.0 | 320 ± 57.4 | P | OP, FIN | Supp | No | 69.1 | MX | Sucrose | — | 95 | 5 |
| Koh (21) | 9 N; 3M, 6F | 50 ± 15 | 333 ± 53.5 | C | IP/OP, USA | PM | No | ~78.5 (15) | MX | Glucose | 52:32:16 | 4 | 8 |
| Madero (27) | 109 N; 7M, 102F | 38.8 ± 8.8 | 329 ± 8.3 | P | OP, MEX | Supp | Yes | ~60 (13) | MX | Starch | 55:30:15 | 6 | 6 |
| Ngo Sock (23) | 11 N; 11M, 0F | 24.6 ± 2.0 | 330 ± 29.8 | C | OP, SUI | Met | Yes | ~213 (26) | LQ | Glucose | 55:30:15 | 1 | 8 |
| Reiser (26) | 11 N; 11M, 0F; 10 HI; 10M, 0F | 43.5 (23–64) | 312 ± 82.3 | C | OP, USA | Met | No | 167 (20) | MX | Starch | 51:36:13 | 5 | 4 |
| Diabetes/prediabetes | |||||||||||||
| Anderson (12) | 14 DM2; 14M, 0F | 60 ± 4 | 360 ± 82.3 | C | OP, USA | PM | No | ~55 (12) | MX | Starch | 55:25:20 | 23 | 8 |
| Bantle (13) | 12 DM1; 6M, 6F; 12 DM2; 5M, 7F | 62 (36–80) | 244 ± 82.3 | C | IP/OP, USA | Met | Yes | ~136.5 (21) | MX | Starch | 55:30:15 | 1.1 | 8 |
| 12 DM1; 6M, 6F; 12 DM2; 5M, 7F | 62 (40–72) | 250 ± 82.3 | C | IP/OP, USA | Met | Yes | ~136.5 (21) | MX | Sucrose | 55:30:15 | 1.1 | 8 | |
| Blayo (14) | 11 DM1; 3 DM2 | 46.9 ± 13.1 | 232 ± 82.3 | P | OP, FRA | Supp | Yes | ~25 (5) | MX | Starch | 55:30:15 | 52 | 7 |
| 8 DM1; 4 DM2 | 46.9 ± 13.1 | 244 ± 82.3 | P | OP, FRA | Supp | Yes | ~25 (5) | MX | Sucrose | 55:30:15 | 52 | 7 | |
| Crapo (16) | 7 DM2; 3M, 4F | 50.9 ± 8.4 | 357 ± 158 | C | IP, USA | Met | No | ~97.5 (13.2) | MX | Sucrose | 55:30:15 | 2 | 7 |
| Grigo resco (18) | 8 DM2; 5M, 3F | 40 ± 6.9 | 354 ± 102 | C | OP, FRA | Supp | Yes | 30 (8) | LQ | Starch | 50:30:20 | 8 | 8 |
| Koh (21) | 9 prediabetes; 3M, 6F | 50 ± 15 | 398 ± 35.7 | C | OP, FRA | PM | No | ~78.5 (15) | MX | Glucose | 52:32:16 | 4 | 8 |
| Osei (24) | 13 DM2; 5M, 8F | 54 ± 10.8 | 274 ± 111 | C | OP, FRA | Met | Yes | 60 (7.5) | MX | Starch | 55:35:15 | 26 | 8 |
| Osei (25) | 18 DM2; 3M, 15F | 57 ± 3.0 | 340 ± 144 | P | O, USA | Supp | Yes | 60 (10) | MX | Starch | 55:35:15 | 12 | 8 |
| Hypercaloric trials | |||||||||||||
| No diabetes | |||||||||||||
| Le (22) | 8 N; 8M, 0F | 24.7 ± 5.2 | 300 ± 22.6 | C | OP, SUI | Met | Yes | ~213 (+35) | LQ | Diet alone | 55:30:15 | 1 | 8 |
| 16 OffDM; 16M, 0F | 24 ± 2.7 | 322 ± 20.0 | C | OP, SUI | Met | Yes | ~219 (+35) | LQ | Diet alone | 55:30:15 | 1 | 8 | |
| Ngo Sock (23) | 11 N; 11M, 0F | 24.6 ± 2.0 | 313 ± 29.8 | C | OP, SUI | Met | Yes | ~213 (+35) | LQ | Diet alone | 55:30:15 | 1 | 8 |
C, crossover; CHO, carbohydrate; DM1, type 1 diabetes mellitus; DM2, type 2 diabetes mellitus; E, energy; F, female; Fin, Finland; Fra, France; Ger, Germany; HI, hyperinsulinemic; IP, inpatient; LQ, liquid; M, male; Met, metabolic; Mex, Mexico; MQS, methodological quality score, MX, mixed; N, normal; OffDM, offspring of type 2 diabetes mellitus; OP, outpatient; P, parallel; PM, partial metabolic; SUI, Switzerland; Supp, supplement; SO, solid; USA, United States.
Values are mean ± SD or mean (range).
Baseline or control treatment (comparator) concentrations are mean ± SD.
Designs were either C or P.
Met feeding control represents the provision of all meals, snacks, and study supplements (test sugars and foods) consumed during the study under controlled conditions. PM feeding control represents the provision of some meals and snacks and all study supplements (test sugars and foods) consumed during the study under controlled conditions. Supp feeding control represents the provision of study supplements.
Doses were administered on a g/d, percentage energy, or g/kg body weight basis. Doses preceded by approximate symbol represent average doses calculated based on the average reported energy intake or weight of participants. If these data were not available, then the average dose was based on a 2000-kcal intake preceded by approximate symbol represent average doses.
Fructose was provided in 1 of 3 forms: LQ form, where all or most of the fructose was provided as beverages or crystalline fructose to be added to beverages; SO form, where fructose was provided as solid foods (fruit in the one case); or MX form, where all or most of the fructose was provided as a mix of beverages, solid foods (not fruit), and/or crystalline fructose.
Comparator refers to the reference carbohydrate (starch, sucrose, or glucose) in the isocaloric trials and diet alone (weight maintaining, background diet) in the hypercaloric trials. Fructose was exchanged for the reference carbohydrate providing an energy matched comparison in the isocaloric trials, whereas it was added to the diet alone providing excess energy relative to the diet alone in the hypercaloric trials.
Study quality was assessed by the Heyland MQS. Trials scored ≥8 were considered to be of higher quality.
Isocaloric feeding trials.
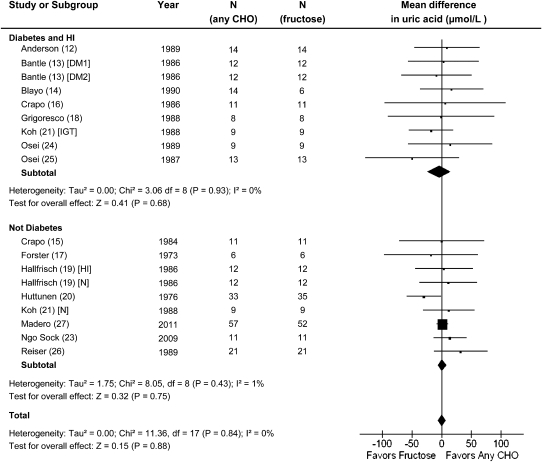
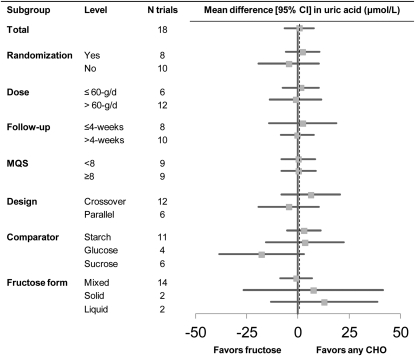
The effect of fructose in isocaloric exchange for other carbohydrate on uric acid was not significant in prediabetic/diabetic participants mean difference (MD) = −4.09 (95% CI: −23.7, 15.6)] and nondiabetic participants [MD = 1.28 (95% CI: −6.65, 9.22)] (Fig. 1). The lack of effect was found consistently across trials without any evidence of inter-study heterogeneity (I2 = 0% in both groups). Systematic removal of trials during sensitivity analyses did not alter the conclusions. There was no significant effect in any of the a prioi subgroups and no significant evidence of heterogeneity (Fig. 2).
FIGURE 1.
Forest plots of feeding trials investigating the effect of isocaloric exchange of fructose for carbohydrate on uric acid in people with and without diabetes. Three pooled effect estimates (diamonds) are shown: one each for trials in individuals with diabetes, no diabetes, and their combination. Paired analyses were applied to all crossover trials. Data are for weighted MD with 95% CI in uric acid (μmol/L). Data are expressed as weighted MD with 95% CI using generic inverse variance random effects models. Inter-study heterogeneity was tested by Cochrane’s Q statistic (chi-square) at a significance level of P < 0.10 and quantified by I2, where I2 ≥ 50% is considered to be evidence of substantial heterogeneity and ≥75%, considerable heterogeneity. CHO, any carbohydrate comparator; DM1, type 1 diabetes mellitus; DM2, type 2 diabetes mellitus; IGT, impaired glucose tolerance; HI, hyperinsulemia; MD, mean difference; N, normal.
FIGURE 2.
Subgroup analyses in the isocaloric feeding trials investigating the effect of isocaloric exchange of fructose for carbohydrate on uric acid in people with and without diabetes. Points for each subgroup level are the pooled effect estimates expressed as weighted MD with 95%CI using generic inverse variance random effect models. The dashed line represents the pooled effect estimate for the total analysis. CHO, any carbohydrate comparator; MD, mean difference.
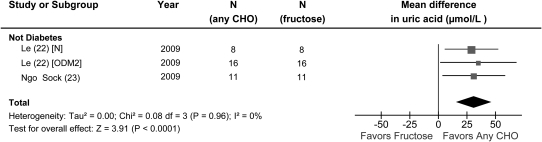
Hypercaloric feeding trials.
There was a significant effect of hypercaloric (+35% E) fructose on serum uric acid in participants without diabetes (Fig. 3). A large uric acid-raising effect [MD = 31.0 (95% CI: 15.4, 46.5); P < 0.05] of hypercaloric (+35% E) fructose was seen without any evidence of inter-study heterogeneity (I2 = 0%; P = 0.97). A priori subgroup analyses were not conducted owing to an insufficient number of trials.
FIGURE 3.
Forest plots of feeding trials investigating the effect of hypercaloric fructose feeding on serum uric acid under hypercaloric conditions, where a control diet was supplemented with excess energy from fructose, in people without diabetes. Data are for weighted MD with 95% CI in uric acid (μmol/L). Data are expressed as weighted MD with 95% CI using generic inverse variance random effects models. Inter-study heterogeneity was tested by Cochrane’s Q statistic (chi-square) at a significance level of P < 0.10 and quantified by I2, where I2 ≥ 50% is considered to be evidence of substantial heterogeneity and ≥75%, considerable heterogeneity. CHO, any carbohydrate comparator; MD, mean difference; N, normal healthy participants; ODM2, offspring of type 2 diabetes mellitus.
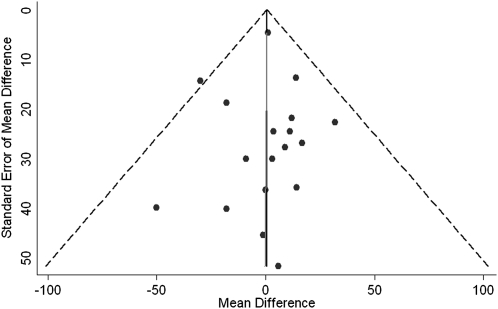
Publication bias.
Funnel plots were inspected for the presence of publication bias (Fig. 4). There was a suggestion of funnel plot asymmetry with a lack of small trials favoring a uric-acid raising effect of fructose; however, neither Egger nor Begg tests provided sufficient evidence of publication bias for uric acid (Egger test, P = 0.95; Begg test, P = 0.14).
FIGURE 4.
Funnel plots for the effect of fructose in isocaloric exchange for other carbohydrate on uric acid. The dashed lines represent the pooled effect estimate expressed as a MD. The solid fitted lines represent Egger regression test for funnel-plot asymmetry. MD, mean difference.
Discussion
The present pooled analyses of 21 controlled feeding trials in 425 prediabetic/diabetic and nondiabetic participants demonstrate that the uric acid response to fructose feeding differs between isocaloric and hypercaloric feeding conditions. Isocaloric fructose intake did not raise uric acid, whereas hypercaloric fructose intake did. The mean increase in the hypercaloric trials was 31.0 μmol/L.
The clinical and practical importance of the increase in uric acid in the hypercaloric trials is unclear. The 3 hypercaloric trials in this meta-analysis fed fructose at 35% excess E (852–876 kcal/d or 213–219 g/d) in fluid form in nondiabetic males for 1 wk, a level of exposure that is more than double the 95th percentile (87 g/d) of fructose intake in the US (46). At these tremendous doses, the resulting 31.0-μmol/L increase in uric acid has the potential to place many individuals at risk of hyperuricemia (>416 μmol/L in men or >339 μmol/L in women), where the mean serum urate concentrations in U.S. adults are 365 μmol/L in men and 290 μmol/L in women (4). Extreme fructose doses inducing hyperuricemia have also been studied in the past. Perez-Pozo et al. (10) conducted a randomized, 2-wk crossover trial in which participants were randomized to either allopurinol, a xanthine oxidase inhibitor which inhibits the production of uric acid, or placebo while consuming 200 g/d of fructose for 2 wk. Allopurinol was shown to decrease the fructose-induced rise in uric acid by 65 μmol/L. Although this increase was reported under weight-maintaining conditions, the evidence from observational studies suggests that an excess of energy may be a prerequisite for a sustainable uric acid-increasing effect of fructose. In models not adjusted for total energy or carbohydrate intake, high fructose intake increased uric acid in the NHANES III (32); however, in energy- and nonfructose carbohydrate-adjusted models, no effect of total fructose was found on either uric acid concentrations or risk of gout in NHANES 1999–2004 (32). Although these data taken together with our findings highlight possible confounding from excess energy at high doses, the small number of trials in our hypercaloric analysis limits the confidence in our estimates.
In the isocaloric setting, there was no effect of fructose substitution for other carbohydrate on uric acid concentrations. This lack of effect contradicts data from the Health Professionals Follow-up study that found that sweetened soft drink intake (equivalent to ~ ≥100 g/d of fructose as high fructose corn syrup) was associated with a 29.1-μmol/L increase in uric acid concentrations (29). Similarly, an analysis of NHANES III found that isocaloric substitution of sugar-sweetened beverages (equivalent to ~≥16% E as fructose) for other energy was associated with a 25.0-μmol/L increase in uric acid and a multivariate OR of 1.82 for hyperuricemia (29). The reason for the discordance between these observational data and our meta-analysis of controlled feeding trials is unclear. It is possible that there may be a threshold for fructose-mediated ATP depletion/AMP production, which is thought to lead to increased uric acid concentrations. The mean fructose dose in the isocaloric trials (93.4 g/d) was below the level of exposure associated with higher uric acid concentrations in the observational studies, which may also have incompletely adjusted for energy compensation. It was also well below the mean dose used in our included hypercaloric trials (215 g/d) used to induce higher uric acid concentrations and the animal models (60% E as fructose, which is equivalent to 300 g/d on a 2000-kcal diet) used to elucidate the mechanism of fructose-induced uric acid production (9). A fructose dose threshold has been observed in clinical trials, below which the effects on other metabolic biomarkers are lost, e.g., ≤60 g/d in type 2 diabetes (36) and <100 g/d across all subject types (47) for TG. Additionally, there is evidence of fructose intolerance and incomplete absorption at higher doses and concentrations, further confounding results from trials at high doses (48). Population-level intakes of fructose, where the 50th percentile of fructose intake in the US is 49 g/d (46), may be unlikely to elicit this mechanism in a clinically significant way. Both dose alone and dose in relation to excess energy, therefore, are important considerations in assessing adverse effects of fructose on uric acid.
The strength of the present analyses is the lack of both statistical heterogeneity and clinical heterogeneity. Neither stratification of the data nor a priori subgroup analyses altered the significance of the effect estimates or heterogeneity. The lack of an effect of fructose in isocaloric exchange for carbohydrate on uric acid was robust to stratification by diabetes status. It was also not modified by dose at a lower threshold (≤ or >60 g/d), comparator (starch, sucrose, and glucose), follow-up (< or ≥4 wk), design (crossover, parallel), study quality (MQS ≥ or <8), randomization (yes, no), or fructose form (solid, liquid, mixed). Although not tested formally in subgroup analyses, composition of the background diet in the trials also did not appear to have an effect. One of the included isocaloric trials, Forster et al. (17), had a background diet devoid of fat. This study was included, because it met all eligibility criteria. Removal of this trial during sensitivity analyses did not alter the conclusions, although this trial should be interpreted cautiously. The consistency across these different trial conditions helps to strengthen the generalizability of our conclusions.
Our analyses, nevertheless, have several limitations. First, our literature search would not have identified trials that measured uric acid and displayed uric acid data but was only discussed within a table or figure. Second, the hypercaloric studies recruited only men; previous studies have shown both sex and hormone effects on uric acid concentrations. Men generally have higher concentrations of uric acid than women and hormones play a role. Estrogen can reduce uric acid concentrations, mitigating many of the metabolic effects of uric acid (49), whereas testosterone has been associated with increased uric acid (50). Although the data support increased uric acid concentrations in men, we could not determine from the existing data the effects of hypercaloric fructose on women. Third, only 5 of the included isocaloric trials and none of the hypercaloric trials were longer than 12 wk. Although we found no evidence of effect according to follow-up, it is unclear whether the lack of effect of isocaloric fructose exchange for other carbohydrates on uric acid would be sustainable over the long term. It is also unclear whether isocaloric exchange conditions can be maintained over the long term. Longer-term trials would be helpful in separating out the short-term from the longer-term effects of fructose. Fourth, study quality was poor (MQS <8) in 50% of the included trials. Complicating this issue was the incomplete reporting in many publications requiring a fair degree of imputations (7/18 trials required imputations). There was, however, no effect (P = 0.97) of MQS (<8 vs. ≥8) in subgroup analyses. Although the use of a quality scoring method, such as the MQS, may have been inadequate (51), the consistent lack of effect of all a priori subgroups (Fig. 2) supports the lack of a uric acid–raising effect (P > 0.2 for all comparisons) of isocaloric fructose exchange for other carbohydrates. Fifth, only two-thirds of the isocaloric trials used crossover designs. However, Lathyris et al. (52) performed a systematic review of Cochrane Reviews and found generally good agreement between parallel and crossover trials. Finally, publication bias was a possibility in both the isocaloric and hypercaloric trials. Visual inspection of funnel plots for both sets of trials along with Egger or Begg tests found limited evidence of funnel plot asymmetry and publication bias.
In conclusion, our work suggests that contrary to concerns, isocaloric fructose exchange for other sources of carbohydrate does not raise uric acid concentrations and this lack of effect holds across different experimental conditions. These conclusions, however, are limited by the short follow-up of the majority of trials and poor quality of one-half of the trials included in the meta-analysis. On the other hand, high fructose intake (213–219 g/d) under hypercaloric feeding conditions (+35% E) does raise serum uric acid concentrations, although confounding from excess energy cannot be ruled out in these trials. These data highlight the need for larger and longer fructose feeding trials conducted under free-living conditions to assess whether fructose consumption leads to excess energy intake and whether in turn the effects on uric acid are dependent on excess energy.
Acknowledgments
D.D.W., J.L.S., R.J.d.S., A.M., A.J.C., M.D., A.L.J., L.A.L., T.M.S.W., J.B., C.W.C.K., and D.J.A.J. designed the research; D.D.W., J.L.S., R.J.d.S., L.C., V.H., A.I.C., and A.M. conducted the research; D.D.W., J.L.S., L.C., V.H., A.I.C., and R.J.d.S. performed the statistical analysis; D.D.W. and J.L.S. wrote the paper; and D.J.A.J. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by a Canadian Institutes of Health Research Knowledge Synthesis (CIHR) to J.L.S., R.J.d.S, A.M., A.J.C., M.D., A.L.J., L.A.L., T.M.S.W, J.B., C.W.C.K., and D.J.A. and an unrestricted research grant from the Calorie Control Council to J.L.S., R.J.D, J.B., C.W.C.K, D.J.A.J., and J.L.S. was supported in the initial stages of this work by a Province of Ontario Postdoctoral Fellowship and the Edie Steinberg Scholarship Fund and the Edward Christie Stevens Fellowship in Medicine. R.J.d.S. was funded by a CIHR Postdoctoral Fellowship Award and A.M. by a CIHR Canada Graduate Scholarship Master's award. D.J.A.J. was funded by the Government of Canada through the Canada Research Chair Endowment. None of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
This systematic review was registered at clinicaltrials.gov as NCT01363791.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: E, energy; MD, mean difference; MQS, methodological quality score.
Literature Cited
- 1.Ardern CI, Katzmarzyk PT. Geographic and demographic variation in the prevalence of the metabolic syndrome in Canada. Can J Diab. 2007;31:34–46 [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9 [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41 [DOI] [PubMed] [Google Scholar]
- 5.Becker MA, Jolly M. Hyperuricemia and associated diseases. Rheum Dis Clin North Am. 2006;32:275–93 [DOI] [PubMed] [Google Scholar]
- 6.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RJ, Sautin YY, Oliver WJ, Roncal C, Mu W, Gabriela Sanchez-Lozada L, Rodriguez-Iturbe B, Nakagawa T, Benner SA. Lessons from comparative physiology: could uric acid represent a physiologic alarm signal gone awry in western society? J Comp Physiol B. 2009;179:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillinger MH, Abeles AM. Such sweet sorrow: fructose and the incidence of gout. Curr Rheumatol Rep. 2010;12:77–9 [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–31 [DOI] [PubMed] [Google Scholar]
- 10.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010;34:454–61 [DOI] [PubMed] [Google Scholar]
- 11.Stirpe F, Della Corte E, Bonetti E, Abbondanza A, Abbati A, De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1 [DOI] [PubMed] [Google Scholar]
- 12.Anderson JW, Story LJ, Zettwoch NC, Gustafson NJ, Jefferson BS. Metabolic effects of fructose supplementation in diabetic individuals. Diabetes Care. 1989;12:337–44 [DOI] [PubMed] [Google Scholar]
- 13.Bantle JP, Laine DC, Thomas JW. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA. 1986;256:3241–6 [PubMed] [Google Scholar]
- 14.Blayo A, Fontveille A, Rizkalla S, Bruzzo F, Slama G. Effets métaboliques de la consommation quotidienne pendant un an de saccharose ou de fructose par des diabétiques. Médecine et nutrition 1990;26:11–4 [Google Scholar]
- 15.Crapo PA, Kolterman OG. The metabolic effects of 2-week fructose feeding in normal subjects. Am J Clin Nutr. 1984;39:525–34 [DOI] [PubMed] [Google Scholar]
- 16.Crapo PA, Kolterman OG, Henry RR. Metabolic consequence of two-week fructose feeding in diabetic subjects. Diabetes Care. 1986;9:111–9 [DOI] [PubMed] [Google Scholar]
- 17.Förster H, Heller G. [Studies on the significance of carbohydrates in a fully synthetic fat-free diet]. Dtsch Med Wochenschr. 1973;98:1156–63 [DOI] [PubMed] [Google Scholar]
- 18.Grigoresco C, Rizkalla SW, Halfon P, Bornet F, Fontvieille AM, Bros M, Dauchy F, Tchobroutsky G, Slama G. Lack of detectable deleterious effects on metabolic control of daily fructose ingestion for 2 mo in NIDDM patients. Diabetes Care. 1988;11:546–50 [DOI] [PubMed] [Google Scholar]
- 19.Hallfrisch J, Ellwood K, Michaelis O, Reiser S, Prather E. Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J Am Coll Nutr. 1986;5:61–8 [DOI] [PubMed] [Google Scholar]
- 20.Huttunen JK, Makinen KK, Scheinin A. Turku sugar studies XI. Effects of sucrose, fructose and xylitol diets on glucose, lipid and urate metabolism. Acta Odontol Scand. 1976;34:345–51 [DOI] [PubMed] [Google Scholar]
- 21.Koh ET, Ard NF, Mendoza F. Effects of fructose feeding on blood parameters and blood pressure in impaired glucose-tolerant subjects. J Am Diet Assoc. 1988;88:932–8 [PubMed] [Google Scholar]
- 22.Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–5 [DOI] [PubMed] [Google Scholar]
- 23.Ngo Sock ET, Le KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103:939–43 [DOI] [PubMed] [Google Scholar]
- 24.Osei K, Bossetti B. Dietary fructose as a natural sweetener in poorly controlled type 2 diabetes: a 12-month crossover study of effects on glucose, lipoprotein and apolipoprotein metabolism. Diabet Med. 1989;6:506–11 [DOI] [PubMed] [Google Scholar]
- 25.Osei K, Falko J, Bossetti BM, Holland GC. Metabolic effects of fructose as a natural sweetener in the physiologic meals of ambulatory obese patients with type II diabetes. Am J Med. 1987;83:249–55 [DOI] [PubMed] [Google Scholar]
- 26.Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr. 1989;49:832–9 [DOI] [PubMed] [Google Scholar]
- 27.Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Pérez-Méndez O, Vázquez A, Ruiz A, Lanaspa MA, Jimenez CR, et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism. 2011;60:1551–9 [DOI] [PubMed] [Google Scholar]
- 28.Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, Kshirsagar AV. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010;77:609–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi JWJ, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–16 [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, Ascherio A. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50:306–12 [DOI] [PubMed] [Google Scholar]
- 32.Sun SZ, Flickinger B, Williamson-Hughes P, Empie M. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab (Lond). 2010;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Diabetes Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, et al. Nutrition recommendations and interventions for diabetes. Diabetes Care. 2008;2008:S61–78 [DOI] [PubMed] [Google Scholar]
- 34.Mann JI, De Leeuw I, Hermansen K, Karamanos B, Karlström B, Katsilambros N, Riccardi G, Rivellese AA, Rizkalla S, Slama G, et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14:373–94 [DOI] [PubMed] [Google Scholar]
- 35.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333 [DOI] [PubMed] [Google Scholar]
- 36.Sievenpiper JL, Carleton AJ, Chatha S, Jiang HY, de Souza RJ, Beyene J, Kendall CW, Jenkins DJ. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care. 2009;32:1930–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011 2008 [cited January 30, 2012]. Available from: www.cochrane-handbook.org
- 38.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–900 [DOI] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–53 [DOI] [PubMed] [Google Scholar]
- 41.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–9 [DOI] [PubMed] [Google Scholar]
- 42.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10 [DOI] [PubMed] [Google Scholar]
- 43.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diab. 2008:S1–201 [DOI] [PubMed] [Google Scholar]
- 44.Begg CB. A measure to aid in the interpretation of published clinical trials. Stat Med. 1985;4:1–9 [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:S1228–35 [DOI] [PubMed] [Google Scholar]
- 47.Livesey G. Fructose ingestion: dose-dependent responses in health research. J Nutr. 2009;139:S1246–52 [DOI] [PubMed] [Google Scholar]
- 48.Kneepkens CM, Vonk RJ, Fernandes J. Incomplete intestinal absorption of fructose. Arch Dis Child. 1984;59:735–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354:650. [DOI] [PubMed] [Google Scholar]
- 50.Denzer C, Muche R, Mayer H, Heinze E, Debatin K-M, Wabitsch M. Serum uric acid levels in obese children and adolescents: linkage to testosterone levels and pre-metabolic syndrome. J Pediatr Endocrinol Metab. 2003;16:1225–32 [DOI] [PubMed] [Google Scholar]
- 51.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60 [DOI] [PubMed] [Google Scholar]
- 52.Lathyris DN, Trikalinos TA, Ioannidis JP. Evidence from crossover trials: empirical evaluation and comparison against parallel arm trials. Int J Epidemiol. 2007;36:422–30 [DOI] [PubMed] [Google Scholar]