Abstract
The biosynthesis of androgens requires multiple steps and during the conversion of pregnenolone to 17α-hydroxypregnenolone and dehydroepiandrosterone (DHEA) by CYP17a1. Acetaldehyde is potentially formed as a by-product in theca cells during antral follicular development. In this study, acetaldehyde level was significantly increased after eCG stimulation and reached a maximum level at 36-hr post-eCG. By 48 hr, the level of acetaldehyde decreased in association with the induction of aldehyde dehydrogenase (ALDH) type 1 family members. When immature mice were co-injected with the ALDH inhibitor, cyanamide, and eCG, the expression of genes involved in the differentiations of granulosa cells was suppressed and the number of ovulated oocytes was reduced. The in vitro studies showed that ALDH inhibitors prevented FSH-induced granulosa cell differentiation. These results indicate that acetaldehyde is generated as a by-product during steroidogenesis and can exert toxic effects to impair the differentiation of granulosa cells, reduce ovulation and decrease oocyte quality.
Keywords: granulosa cells, steroidogenesis, metabolisms, testosterone, oocyte maturation
1. Introduction
The pituitary gonadotropins, FSH (follicular stimulating hormone) and LH (luteinizing hormone) are essential for the follicular development to preovulatory stage [1]. In growing follicles LH activates its cognate receptor (LHCGR) present in the theca cell layer external to the basement membrane and stimulates androgen biosynthesis from cholesterol [2, 3]. The androgens are then converted to estrogens by the aromatase (CYP19a1) enzyme that is induced in granulosa cells of antral follicles [1,4,5]. Estrogen, mainly estradiol 17β (E2) acts on ESR2 (estrogen receptor beta; ERβ) that is expressed in granulosa cells, and enhances FSH-mediated granulosa cell proliferation and differentiation (Lhcgr induction) and follicle growth to the preovulatory stage [4,6,7]. Elevated serum E2 activates neuronal estrogen receptor alpha (ERS1) inducing GnRH synthesis/release, leading to the LH surge and the initiation of ovulation and luteinization [8,9,10]. Esr2 [11] and Esr1 mutant mice [12] are subfertile or infertile due to impaired ovarian and hypothalamic functions.
The biosynthesis of androgens (C17) from cholesterol (C21) in theca cells depends on multiple steps and involves not only the generation of functional steroids but also specific catabolic by-products. For example, the generation of pregnenolone leads to the release of the by-product isocaproaldehyde [13]. The conversion of pregnenolone to 17α-hydroxypregnenolone and dehydroepiandrosterone (DHEA) or progesterone to 17α-hydroxyprogesterone and androstendione by CYP17a1 (Cytochrome P450 17α-hydroxylase/C17–20 lyase) [14,15] yields acetaldehyde [16]. Both ioscaproaldehyde and acetaldehyde can be toxic to cells and therefore are metabolized rapidly by specific reductase and dehydrogenase enzymes.
The toxic activity of isocaproaldehyde in steroidogenic cells was first reported by Lefrancois-Martinez et al. [13] who showed that an aldo-keto-reductase like protein (Akr1b7) is expressed in adrenal cells and catalyzes the breakdown of endogenous isocaproaldehyde. The transfection of Akr1b7 antisense cDNA to murine adrenocortical Y1 cells, reduced the number of living cells, and the toxicity was decreased by the treatment with aminoglutethimide, an inhibitor of CYP11A1, the cholesterol side chain cleavage enzyme. Therefore, in adrenal cells, ACTH increases the expression and activity of both CYP11A1 and AKR1B7, which produce and immediately catabolize the toxic isocaproaldehyde, respectively [17,18,19]. However, Akr1b7 knockout mice are fertile [20] suggesting that other enzyme(s) play a redundant role in metabolizing isocaproaldehyde [20]. Alternatively, the toxic activity may not be sufficient to alter granulosa cell functions or induce granulosa cell death during follicular development.
Although acetaldehyde is a potent cellular toxin that can act to suppress DNA repair, cell proliferation, adhesion and differentiation [21,22], most research is limited to understanding its actions in alcohol toxicity in the liver. Specifically, alcohol is metabolized to acetaldehyde by alcohol dehydrogenate (ADH) in liver [23,24,25]. The endogenous acetaldehyde is neutralized by aldehyde dehydrogenase (ALDH) to acetyl-CoA, and then to carbon dioxide and water [26]. Mutations of the Aldh gene in human is associated with decreased dehydrogenase activity, increased of acetaldehyde levels and cell toxicity [27]. Thus, this metabolic pathway is essential for the maintenance of viable cells and normal liver functions. However there is no report about endogenous acetaldehyde toxicity and the expression of ALDH family members during antral follicular development. In this study, we analyzed the levels of acetaldehyde present in the mouse ovary during antral follicular development when steroidogenesis is increased. Moreover, we detected the induction of specific ALDH family members in the ovary, and investigated the role of ALDH activity in follicular development by using a broad ALDH inhibitor, cyanamide [28] or specific ALDH type I inhibitor, disulfiram [29].
2. Materials and Methods
2.1. Materials
Equine and human chorionic gonadotropins (eCG and hCG) were purchased from Asuka Seiyaku (Tokyo, Japan). Ovine follicle stimulating hormone (FSH) and luteinizing hormone (LH) were a gift from the National Hormone and Pituitary Program (Rockville, MD). DMEM:F12 medium, penicillin-streptomycin were from Invitrogen (Carlsbad, CA, USA); fetal bovine serum (FBS) from Life Technologies Inc. (Grand Island, NY); oligonucleotide poly-(dT) from Invitrogen, and AMV reverse transcriptase, Taq polymerase from Promega (Madison, WI). Cyanamide, tetraethyltiuram disulfide and retinoic acid were purchased from Sigma Chemical Co. (Sigma; St. Louis, MO). Cyanamide was directly dissolved in medium or saline. Tetraethyltiuram disulfide was dissolved in dimethylsulfoxide (DMSO, Sigma) at 40 mM, and then diluted (1:1000) with the culture medium. Aminoglutethimide (AGT, Sigma) was dissolved in DMSO at 1 M, and was stored at −20 degrees C. Final concentrations were obtained by diluting in the culture medium. Routine chemicals and reagents were obtained from Nakarai Chemical Co (Osaka, Japan), or Sigma.
2.2. Animals
Immature female C57BL/6 mice were obtained from Clea Japan (Tokyo, Japan). On day 23 of age, female mice were injected intraperitoneally (IP) with 4 IU of eCG to stimulate follicular growth followed 48 hr later with 5 IU hCG to stimulate ovulation and luteinization. Animals were housed under a 16-hr light/8-hr dark schedule in the Experiment Animal Center at Hiroshima University and provided with food and water ad libitum. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Hiroshima University.
2.3. Acetaldehyde measurements
The levels of acetaldehyde in control and hormone-stimulated mouse ovaries were determined using f-kit acetaldehyde (Roche Diagnostics, GmbH, Mannheim, Germany). Acetaldehyde was oxidized to acetate by exogenous ALDH and NADH shown as follows “Acetaldehyde+NAD++H2O→Acetate+NADH+H+”. The amount of NADH was equivalent to the amount of acetaldehyde. NADH was determined by measurement of its absorbance at 340 nm. Collected tissues were weighed and then homogenized in distilled water (80 ml/1 g sample). The lysed samples were covered with mineral oil to prevent evaporation and then incubated for 15 minutes at 50 °C. After cooling on ice, 3.6 % (w/v) of K4[Fe(CN6)]·3H2O (Sigma), 7.2 % (w/v) of ZnSO4·7H2O (Sigma) and 1 M of NaOH (Nakarai tesque) were added to the reactions. The samples were then centrifuged and the cleared supernatant used for the measurement of absorbance by NanoDrop 2000 (Thermo scientific, Wilmington, DE). Levels of acetaldehyde were determined based on a standard curve generated using acetaldehyde (Sigma).
2.4. RNA extraction and validation of amplified products
Total RNA was obtained from mouse ovaries or positive control tissues (liver, testis, brain and uterus) using the RNAeasy Mini Kit (Qiagen Sciences, Germantown, MD, USA) according to the manufacturer’s instructions. RT-PCR analyses were performed as previously described (Shimada et al., 2008). Briefly, total RNA was reverse transcribed using 500 ng poly-dT (Amersham Pharmacia Biotech, Newark, NJ, USA) and 0.25 U avian myeloblastosis virus-reverse transcriptase (Promega Corp., Madison, WI, USA) at 42°C for 75 minutes and 95°C for 5 minutes. For RT-PCR analysis, specific primers pairs as shown in Table 1, dNTP (Promega), Taq polymerase and Thermocycle buffer (Promega) were added to PCR mixture. cDNA products were resolved on 2% (w/v) agarose gels.
Table 1.
primer list
| gene | Forward Primer | Reverse Primer | Size (bp) | anneling temperature |
|---|---|---|---|---|
| Aldh1a1 | 5′-CCCGGATTTTTGTTGAGGAG-3′ | 5′-GAGAACACTGTGGGCTGCAC-3′ | 244 | 64 |
| Aldh1a3 | 5′-CTGGATGCACTGAGCAGAGG-3′ | 5′-GTCCTGCCCTGGATTTTGTC-3′ | 194 | 62 |
| Aldh1a7 | 5′-CTGTCCCTGTCCAATGCCCA-3′ | 5′-GGTGACTGTATGAGATGTACAGC-3′ | 190 | 64 |
| Aldh1l2 | 5′-CCAAGGACGCCTTTGAGAAC-3′ | 5′-CCAGCCTGCGAAGTATCTGA-3′ | 206 | 62 |
| Aldh2 | 5′-GGACCGTGGCTACTTTATCCAG-3′ | 5′-AGAGGTTGGGACAGGTAATTGG-3′ | 213 | 64 |
| Aldh3a2 | 5′-TCAGTGAAAACACGGCCAAG-3′ | 5′-GCCACGTCCAGATCACAGTC-3′ | 259 | 64 |
| Aldh6a1 | 5′-GATCCAACCAGGCAGGAGAG-3′ | 5′-ACCAGTTCTGGCAGCCACTT-3′ | 229 | 64 |
| Aldh8a1 | 5′-GGGTGAGACACAAGGGCTTC-3′ | 5′-ATGAGCCACCTGGGCTGTAT-3′ | 275 | 64 |
| Aldh9a1 | 5′-CTTCACGCCTGTGTCTGCTC-3′ | 5′-AGGAGATTTGCCCCCAAGTT-3′ | 232 | 64 |
| Aldh16a1 | 5′-TTCTGCGACGGACTCTAGCA-3′ | 5′-CCATCCAGTCCTCTCCCACT-3′ | 244 | 64 |
| Aldh18a1 | 5′-TGGACTGATGGCCTTGTACG-3′ | 5′-GACGGCATCGTTTGTGTTGA-3′ | 175 | 64 |
| Bmp7 | 5′-ACCATCTGGGCTTCTGAGGA-3′ | 5′-CAGGGCCTCTTGGTTCTTTG-3′ | 297 | 62 |
| Ccnd2 | 5′-TCACCACGTGTTCCCAAGAC-3′ | 5′-AGCGAATTCCCTCCATCAGA-3′ | 243 | 60 |
| Cyp11a1 | : 5′-GGGAGACATGGCCAAGATGG-3′ | 5′-CAGCCAAAGCCCAAGTACCG-3′ | 279 | 60 |
| Cyp17a1 | 5′-GGCCCCAGATGGTGACTCT-3′ | 5′-GGGACTCCCCGTCGTATGTA-3′ | 205 | 64 |
| Cyp19a1 | 5′-TTGCACCCAAATGAGGACAG-3′ | 5′-CTTCACTGGTCCCCAACACA-3′ | 290 | 60 |
| Hsd3b1 | 5′-TGGGGAGAGAAGTCCATTCA-3′ | 5′-GGAGCCCCCATTCCTTACTA-3′ | 263 | 60 |
| Hsd3b6 | 5′-CGGTTGTACGGGCAAATTCT-3′ | 5′-GAAGCCCCACTCCTTGCTC-3′ | 199 | 64 |
| Hsd17b1 | 5′-GAAGGTTTGTGCGAGAGTCTG-3′ | 5′-AAGCGGTTCGTGGAGAAGTAG-3′ | 287 | 64 |
| Fshr | 5′-TGGCCATTACTGGGAACACC-3′ | 5′-TGCCAAAGATGGGGAAGAGA-3′ | 261 | 60 |
| L19 | 5′-GGCATAGGGAAGAGGAAGG-3′ | 5′-GGATGTGCTCCATGAGGATGC-3 | 199 | 60 |
| Lhcgr | 5′-ACTGGTGTGGTTTCAGGAATT-3′ | 5′-CCTAAGGAAGGCATAGCCCAT-3′ | 244 | 60 |
| Star | 5′-GCAGCAGGCAACCTGGTG-3′ | 5′-TGATTGTCTTCGGCAGCC-3′ | 249 | 60 |
2.5. Quantitative PCR analyses in hormone stimulated ovarian samples
Quantitative real-time PCR analyses were performed as previously [30]. Briefly, total RNA was extracted from hormone stimulated mouse ovaries at selected time intervals. The RNA was reverse transcribed using 500 ng poly-dT (Invitrogen) and 0.25 U avian myeloblastosis virus-reverse transcriptase (Promega) at 42°C for 75 minutes and 95°C for 5 minutes. cDNA and primers were added to 15 μl total reaction volume of the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). PCR reactions were then performed using the StepOne real time PCR system (Applied Biosystems). Conditions were set to the following parameters: 10 minutes at 95 °C followed by 45 cycles each of 15 seconds at 95 °C and 1 minute at 62 or 64 °C. Specific primers pairs were selected and analyzed as indicated in Table 1 and verified as above (2.5).
2.6. TUNEL assay
Analysis of apoptotic cells (containing fragmented DNA) in immature mouse ovaries treated with or without cyanamide was performed by the terminal deoxynucleotidyl transferase (Tdt)-mediated biotinylated deoxyuridine triphosphates (dUTP) nick end-labeling (TUNEL) method (In Situ Cell Death Detection Kit POD, Roche Diagnostics) according to the manufacturer’s instructions. Paraffin-embedded tissue sections (4 μm) of mouse ovary were de-paraffinized and treated with 30 % H2O2/methanol to block endogenous peroxidase activity. After washing with PBS, the slides were incubated with 20 μg/ml proteinase K (Sigma) in 10 mM Tris-HCl, pH 7.4, at room temperature for 30 min. After washing with PBS, the positive control sample (but not experimental samples) was treated with DNase (5 μg/ml) for 15 minutes at 4°C. Then, all tissue samples were rinsed in PBS and incubated with TUNEL reaction mixture at 37°C for 30 min. The tissue was treated in POD (Converter-peroxidase) for 30 min at 37°C. Subsequently, tissues were rinsed in PBS and incubated in DAB (POD substrate) for 10 min. From the next process, we performed by the same method as immunohistochemistry. The sections were observed under light microscope.
2.7. Ovulation rate analyses
Immature mice (Day 23) were co-injected (i.p.) with eCG (4IU) and/or the ALDH inhibitor, cyanamide (15 mg/kg) and/or retinoic acid (RA) (2.5 mg/kg). Cyanamide and retinoic acid were re-administrated 24-hr later. At 48 hr post-eCG, the mice were treated with hCG (5IU) and 16-hr later the ovulated cumulus cell-oocyte complexes (COCs) were collected from oviducts and the number of oocytes counted.
2.8. Three-dimensional follicle culture
Ovaries isolated from immature mice were cut and opened by forceps to form a sheet under the microscope. After washing with PBS, the separated follicles were placed into 0.5% (w/v) sodium alginate (WAKO, Osaka Japan)/PBS (Ca2+, Mg2+ free) and then dropped into 50 mM CaCl2 (Nakarai tesque)/saline to cross-link the alginate. Alginate beads, each containing ovaries ????, were rinsed and cultured for 48 hr in the medium (DMEM: F12 containing penicillin and streptomycin) containing in 500 ng/ml ovine FSH (NIDDK, Torrance, CA), 50 ng/ml of ovine LH (NIDDK) and 10 ng/ml testosterone (Sigma). These ovaries were further treated with the ALDH inhibitor, disulfiram. Retinoic acid (10 μM) was also added. To determine if the acetaldehyde was generated in the ovary was related to steroid biosynthesis, ovaries were also cultured with the CYP11A1 inhibitor, aminoglutethimide. The concentrations of tadded reagents is shown in Figure 4.
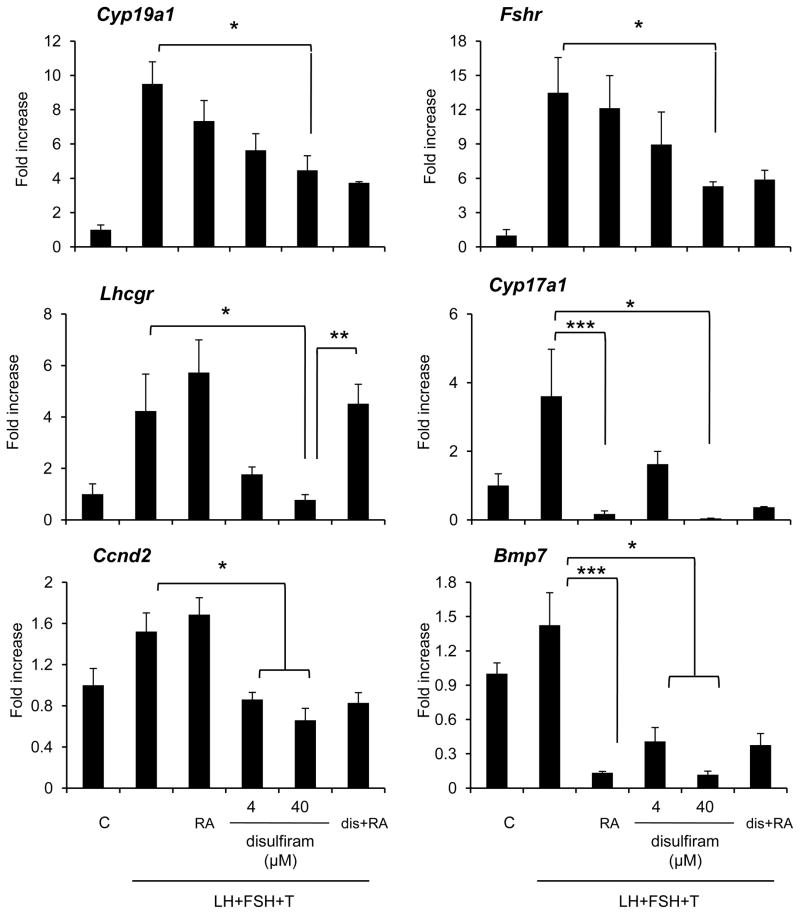
Fig. 4. The expression of FSH target genes was suppressed by disulfiram, a selective ALDH1 inhibitor, in in vitro cultured mice ovary.
The expression level of genes known as follicular development makers in granulosa cells, *; Significant differences were observed as compared with those in ovary cultured for 48 hr with 4 or 40 μM of disulfiram in the presence of LH, FSH and testosterone (LH+FSH+T) (P<0.05). **; The addition of retinoic acid (RA) to LH+FSH+T and 40 μM of disulfiram-containing medium significantly increased the expression of Lhcgr mRNA as compared with that in ovary cultured with LH+FSH+T and 40 μM of disulfiram. ***; The addition of RA significantly suppressed the expression of Bmp7 and Cyp17a1 as compared with those in ovary cultured with LH+FSH+T. Values are mean +/−SEM of three replicates. C; ovaries were embedded in alginate gels and then cultured for 48 hr. RA; ovaries were cultured with 10 μM of RA in the presence of LH+FSH+T for 48 hr. dis+RA; ovaries were cultured with 40 μM of disulfiram and 10 μM of RA in the presence of LH+FSH+T for 48 hr.
2.9. Fertilization and oocyte quality
Adult mice (8 weeks) were co-injected with eCG (5 IU) and/or ALDH inhibitor, cyanamide (15 mg/kg) by i.p. injection. After 48 hr, these mice were treated with hCG (5 IU). After hCG injection, each female mouse was mated with a proven fertile male mouse overnight. Successful mating was confirmed the following morning by the presence of a vaginal plug. Two days later from mating, the fertilized/unfertilized eggs or fragmented eggs were collected from oviducts by perfusion using PVP (Sigma)/DMEM (0.04 g/10ml). All 2 cell embryos were cultured in the developing medium (KSOM+Amino Acid, Millipore, Billerica, MA) for 3 days and the number of eggs which developed to the blastocyst stage was counted.
2.10. Statistics
Statistical analyses of all data from three or four replicates for comparison were carried out by one-way ANOVA followed by Duncan’s multiple-range test (Statview; Abacus Concepts, Inc., Berkeley, CA).
3. Results
3.1. Acetaldehyde levels in the ovary increase during hormonal stimulation of antral follicular development
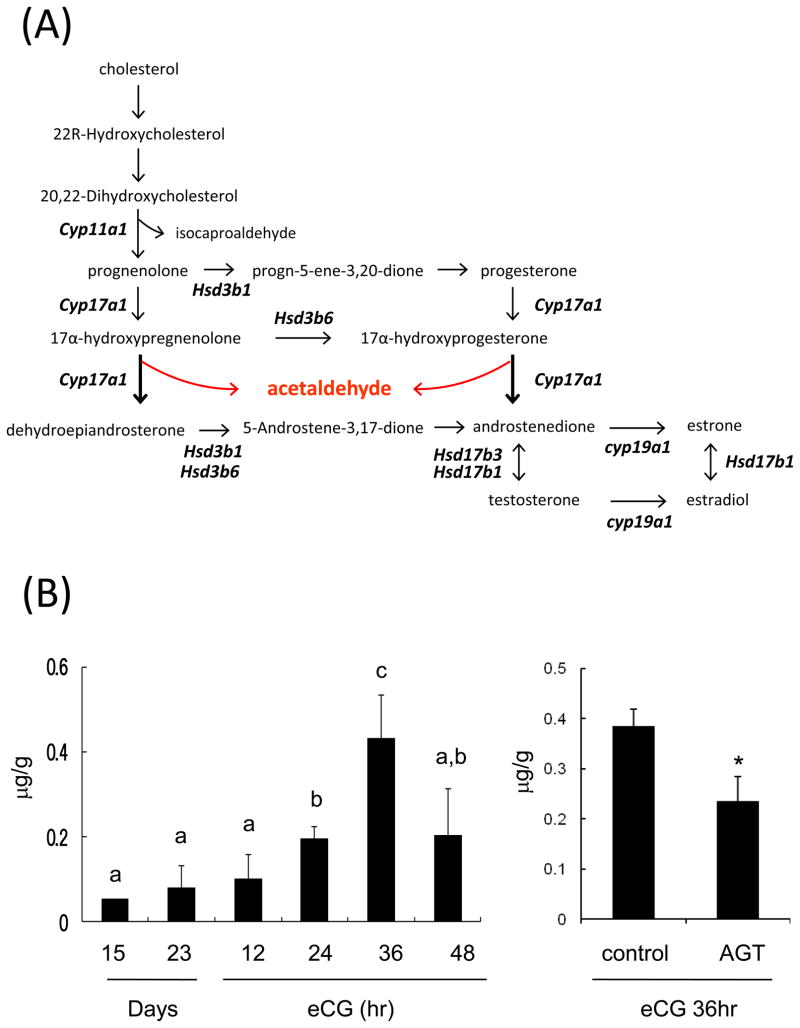
Acetaldehyde is potentially synthesized as a by-product of CYP17A1 (Fig. 1A). To determine the levels of acetaldehyde in ovary during follicular development, whole ovaries were collected from immature (day 15, 20, or 23) and d23 mice prior to and at 0, 12, 24, 36 or 48 hr after eCG. The ovaries were lysed to analyze the acetaldehyde concentrations using f-kit acetaldehyde (Roche Diagnostics). Levels of acetaldehyde were less than 0.1 mg/g at day 15 and 23 or at 12 hr after eCG stimulation. However, the levels were significantly increased at 24 hr and reached a maximum level at 36 hr after eCG (0.43+/−0.11 mg/ml, Fig. 1B). By 48 hr, however, the levels of acetaldehyde decreased to that observed at 24 hr after eCG injection.
Fig. 1. The production of acetaldehyde in mouse ovary during follicular development.
(A) Schematic pathway of acetaldehyde production from cholesterol in developing follicles
(B) Levels of acetaldehyde in ovaries of mice treated with or without eCG, a–c; Letters that are not common designated significance (p<0.05). Values are mean +/−SEM of three replicates. AGT; Immature mice (Day 23) were co-injected with eCG (4IU) and/or CYP11A1 inhibitor, aminoglutethimide (4mg/kg) by i.p. injection. After 36-hr injection, ovary was collected to use for detection of acetaldehyde level. *; Significant difference was observed as compared with those in ovaries of control mice (P<0.05).
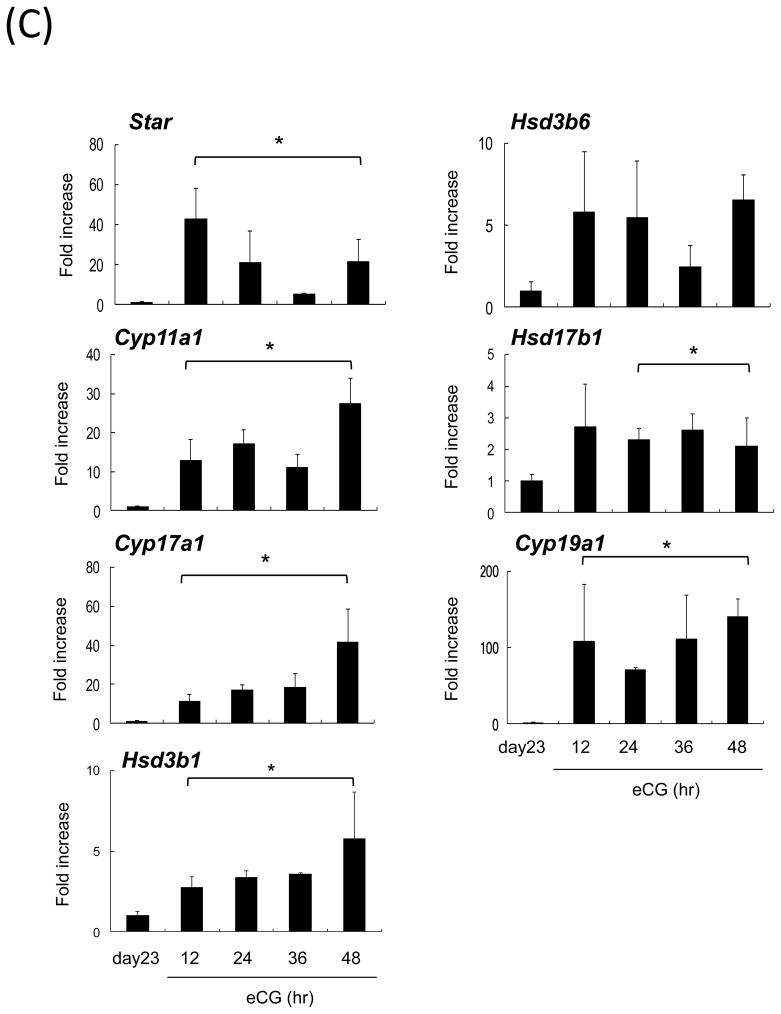
(C) Temporal changes in expression of steroidogenic genes ovaries of mice treated with or without eCG, For reference, the day 23 value was set as 1, and the data are presented as fold increase. Values are mean +/−SEM of three replicates. *; Significant differences were observed as compared with those in ovaries of untreated day23 mice (P<0.05). day23; day 23 immature female mice before eCG priming
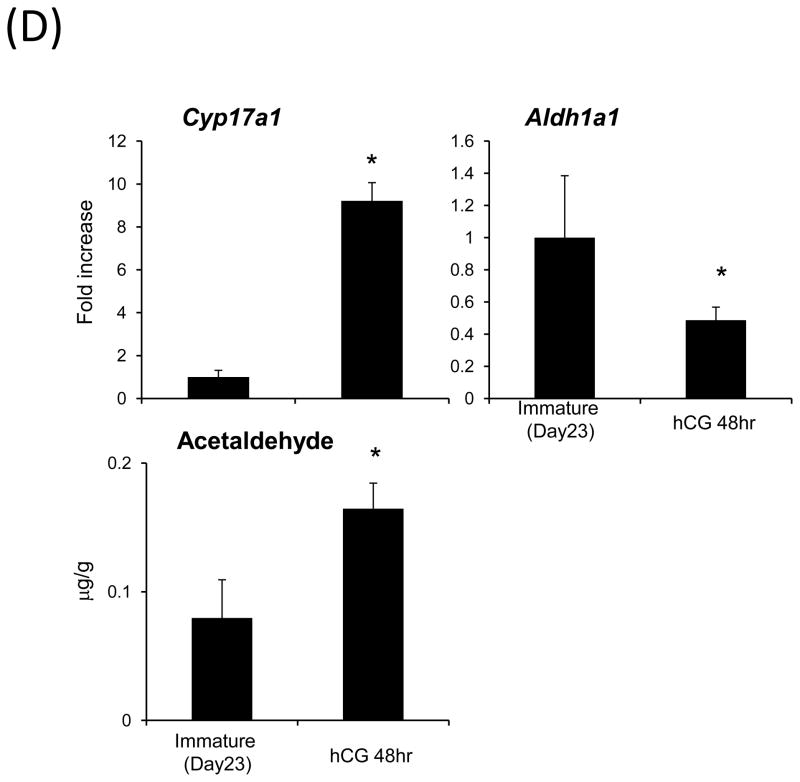
(D) The effects of hCG injection to immature mice without eCG priming on acetoaldehyde production. Immature mice (Day 23) were injected with hCG (5IU) by i.p. injection. After 48-hr injection, ovary was collected to use for detection of acetaldehyde level and analyze the gene expression levels. *; Significant difference was observed as compared with those in ovaries of immature mice (P<0.05).
To relate changes in acetaldehye with ovarian steroidogenic activity, the expression levels of genes involved in steroid biosynthesis, including Cyp17a1, were examined using RNA prepared from whole ovaries prior to or after eCG injections into mice on day 23 of age. With the exception of Hsd3b6, genes in the steroidogenic pathway increased significantly in whole ovarian samples within 12 hr after eCG treatment (Fig. 1C). When the female mice were co-injected with eCG and aminoglutethimide, the levels of acetaldehyde were significantly suppressed at 36h (Fig. 1B). To determine the association between the production of acetaldehyde and steroidogenesis in more detail, we injected hCG into immature female mice without prior eCG priming. The expression of Cyp17a1 was significantly increased by hCG alone whereas that of Aldh1a1 was significantly lower than that in immature control mice (Fig. 1D; Fig. 2A). As with eCG, the levels of acetaldehyde were also significantly increased by hCG alone (Fig. 1D) suggesting that acetaldehyde was produced as by product in the steroidogenic pathway in theca cells.
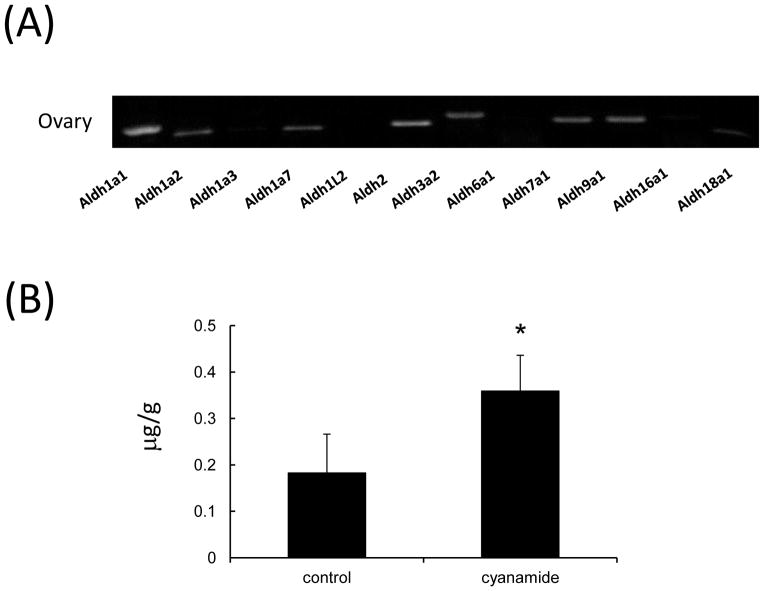
Fig. 2. The expression of acetaldehyde dehydrogenase family members in ovaries of mice treated with eCG for 48 hr.
(A) The expression patterns of Aldh family genes in ovaries of mice treated with eCG for 48 hr.
(B) Levels of acetaldehyde in ovaries of mice 48 hr after the treatment with eCG and/or ALDH inhibitor, cyanamide (15 mg/kg), Values are mean +/−SEM of three replicates. *; The injection of 15 mg/kg cyanamide with eCG significantly increased the level of acetaldehyde in ovary (P<0.05).
3.2. Specific acetaldehyde dehydrogenases are expressed in the mouse ovary during follicular development
Because acetaldehyde exerts potent cellular toxicity [21,22] and because it is increased transiently in ovaries after eCG stimulation, we predicted that the induction and activation of one or more members of the Aldh family would reduce acetaldehyde toxicity by 48 hr post-eCG. Using RT-PCR and specific primers for all family members, we detected PCR products for 7 ALDH family members (Fig. 2A). To determine the levels of ALDH activity in mouse ovary, the levels of ovarian acetaldehyde was measured in female mice treated with vehicle, eCG or eCG combined with the broad ALDH inhibitor, cynamide. The levels of acetaldehyde were significantly increased by the treatment with cyanamide as compared with those in ovaries recovered from eCG-primed mice (0.36+/−0.05 μg/g vs. 0.18+/−0.03 μg/g, Fig. 2B).
3.3. ALDH may promote survival and differentiation in granulosa cells during follicular development
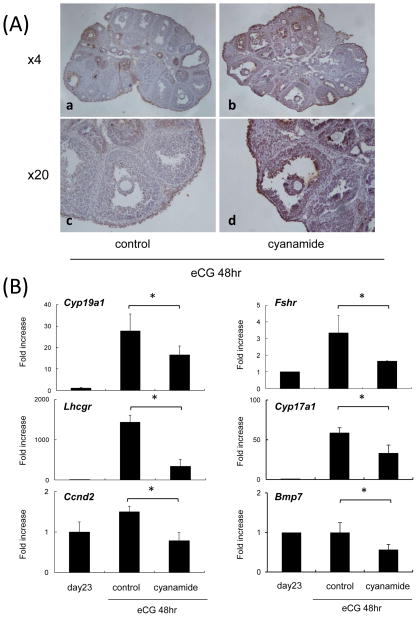
To elucidate the physiological role of acetaldehyde in the mouse ovary, immature mice were injected with eCG alone or co-injected with cyanamide (15 mg/kg) and eCG. Whereas eCG alone stimulated the development of antral follicles with healthy granulosa cells, eCG and cyanamide caused granulosa cells of large antral follicles to detach from the basal lamina and many non-adherent cells were TUNEL positive, indicative of apoptosis (Fig. 3A). The number of TUNEL positive cells in the liver was not increased by the injections of cyanamide and/or eCG (data not shown).
Fig. 3. The broad ALDH inhibitor, cyanamide, impaired follicular development in eCG-primed mice.
(A) Cyanamide induced apoptosis in granulosa cells as detected by TUNEL assay
(B) The expression genes known to be markers of granulosa cell differentiation and follicular development, *; Significant differences were observed as compared with those in granulosa cells recovered from mice coinjected with eCG and cyanamide (P<0.05). Values are mean +/−SEM of three replicates. immature: immature mice before eCG priming, control: 48 hr after eCG priming, cyanamide: coinjection with eCG and 15 mg/kg of cyanamide for 48 hr
In addition, cyanamide treatment negatively affected the expression of genes in both granulosa cells and theca cells. The cell proliferation maker, Ccnd2 mRNA was significantly lower in the cyanamide treatment group as compared with that in the eCG controls. Moreover, markers (Lhcgr, Fshr and Cyp19a1) of differentiated granulosa cells present in preovulatory follicles were dramatically increased 48 hr after eCG alone but were decreased by cyanamide (Fig. 3B). The expression of genes selectively expressed in theca cells (Cyp17a1 and Bmp7) was also suppressed by cyanamide (Fig. 3B).
3.4. The ALDH type I family inhibitor, disulfiram, altered granulosa cell functions in 3-D ovary cultures
The ALDH family is not only expressed in the ovary but also liver and other tissues [29]. To eliminate the possibility that the effects of acetaldehyde produced by the liver might alter ovarian functions, we cultured follicles from immature mice using a 3-D culture system [30]. The follicles were embedded in alginate gels and cultured with FSH, LH and testosterone. The levels of acetaldehyde in the cultured ovares were significantly increased by FSH, LH and testosterone (83.29+/−9.48 μg/ml) as compared with that in ovary without any hormone (10.41+/−3.22 μg/ml). Addition of the CYP11A1 inhibitor, aminoglutethimide (500 μM), significantly reduced the levels of acetaldehyde (5.21+/−1.36 μg/ml) and this was associated with increased expression of the genes known to be makers of LH+FSH+testosterone-mediated granulosa cell differentiation. By contrast, the expression of these same genes was suppressed by treatment with 40 μM of disulfiram, a highly specific ALDH type I (not type II) family inhibitor [29] (Fig. 4). LH+FSH+testosterone also increased the expression of genes selectively expressed in theca cells (Cyp17a1 and Bmp7)) and the expression of these genes was also significantly suppressed by the treatment with 40 μM of disulfiram (Fig. 4).
The ALDH family is also involved in retinoic acid metabolism [31]. Therefore, we determined if co-treatment with retinoic acid (RA) would overcome the inhibitory effects of disulfiram on granulosa cell functions. The reduced expression of Cyp19a1, Ccnd2, Fshr, Cyp17a1 and Bmp7 mRNA that occurred in response to disulfiram was not reversed by the addition of RA to the inhibitor-containing medium (Fig. 4). However, the expression of Lhcgr was significantly increased by RA treatment as compared with that in disulfiram treatment group (Fig. 4). On the other hand, the addition of RA to the LH+FSH+testosterone cocktail did not change the gene expression level of granulosa cell markers including Lhcgr (Fig. 4). However, the expression of genes selectively expressed in theca cell (Cyp17a1 and Bmp7) was significantly decreased by RA (Fig. 4). These results suggest that Lhcgr, unlike other genes, is not a target of acetaldehyde toxicity but rather is regulated by a RA-dependent mechanism(s).
3.5. ALDH activity is required for the development of small follicles to the preovulatory stage
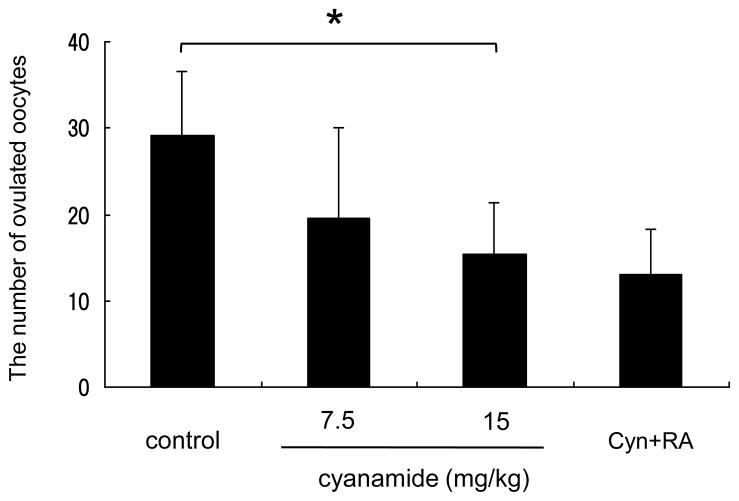
The present in vivo and in vitro data provide data that the suppression of ALDH activity abolishes the induction of eCG-target genes that are essential for ovulation. To test this hypothesis, mice were injected with eCG and cyanamide, followed by hCG for 48 hr. At16 hr after hCG, the ovulated COCs were collected from oviducts and the oocytes counted. The number of ovulated oocytes was significantly decreased by cyanamide in a dose-dependent manner (Fig. 5). Co-injection of cyanamide with RA did not increase the number of ovulated oocytes (Fig. 5).
Figure 5.
Ovulation was also suppressed by ALDH inhibitor, Immature mice were co-injected with eCG and/or ALDH inhibitor, cyanamide and/or retinoic acid (RA). After 48 hr, these mice were treated with hCG. 16-hr post-hCG injection, the ovulated oocytes were collected from oviducts and counted. *; The injection of 15 mg/kg cyanamide with eCG significantly reduced the number of ovulated oocytes (P<0.05). Values are mean +/−SEM of 9 mice. Cyn+RA; 15 mg/kg cyanamide and RA were co-injected with eCG.
When the hormonaly-primed, cyanamide-treated mice were mated with adult male mice, the fertilization rate of ovulated oocytes in vivo was significantly lower as compared with that in control mice (Table 2). When fertilized eggs (2 cell embryos) were further cultured for 3 days, the majority of the embryos from control mice developed to blastocyst stage. However, blastocyst formation was significantly decreased in the cyanamide treated group (Table 2).
Table 2.
The effects of cyanamide (ALDH inhibitor) injection to eCG-treated mice on the fertilization and oocyte developmental compartment
| Total number of ovulated oocytes1 | Rate of fertilization (%)2 | Rate of blastocyst stage (%)3 | |
|---|---|---|---|
| Control (n=4) | 91 | 78.0+/−8.1 | 85.9+/−8.7 |
| Cynamide (n=4) | 64a | 41.4+/−4.8b | 39.7+/−12.9c |
ovulated oocytes were recovered from oviducts of 4 female mice in each treatment group
Fertilization rate was 2cell embryo/ovulated oocytes
Rate of blastocyst stage was blastocyst stage/2 cell embryo
The significant difference was observed between the treatment groups.
4. Discussion
The biosynthesis of steroids not only generates critical hormones required for reproductive success but also specific catabolic by-products, such as isocaproaldehyde (derived during the conversion of cholesterol to pregnenolone by CYP11A1) and acetaldehyde (CYP17A1 dependent conversions) that are potentially cytotoxic to gonadal cells. In this study, we document that acetaldehyde is produced in the mouse ovary in response to gonadotropin (eCG or hCG) stimulation, most likely by theca cell derived CYP17A1 that converts 17α-hydroxypregnenolone to dehydriepiandrosterone, or 17α-hydroxyprogesterone to androstendione (Fig. 1). Furthermore, we document that acetaldehyde altered not only theca cell but also granulosa cell functions.
The increased levels of acetaldehyde in the eCG-primed mouse ovaries reached a maximum at 36 hr after eCG stimulation and then decreased significantly by 48 hr. Because acetaldehyde has toxic activity and can induce apoptosis or inflammatory-like responses in the liver [32], we hypothesized that aldehyde dehydrogenase enzymes capable of metabolizing acetaldehyde during follicular development would be induced to remove the cytotoxic metabolites. Strikingly, two members of Aldh type I family (Aldh1a1 and Aldh1a7) were expressed in the mouse ovary and increased significantly in response to eCG. However, eCG did not increase the expression of Aldh type II, a form dominantly expressed and functional in liver tissue [26,33].
Cyanamide is a well-known broad ALDH inhibitor that reduces the activity of both type I and type II enzymes and has been used to study the effects of alcohol in vivo [26]. Therefore, to determine the role of ALDH enzymes during follicular development, we co-injected cyanamide with eCG into immature female mice. Whereas ovaries of control mice contained healthy preovulatory follicles at 48-hr post injection, ovaries of the cyanamide treated mice exhibited altered morphology and function associated with increased levels of acetaldehyde. Specifically, the mural granulosa cell layer was perturbed and some of non-adherent cells were apoptotic (TUNEL positive). Although the precise targets of acetaldehyde in the granulosa cells are not yet known, acetaldehyde may alter the functions of protein tyrosine phospathase 1B that normally complexes with E-cadherin to prevent apoptosis and maintain epithelial cell-cell adhesion [33–36].
Because the decreased the expression of granulosa cell specific genes by the administration of cyanamide in vivo could have been due to direct as well as indirect effects of increased acetaldehyde and/or altered metabolic functons in liver or other organs, we cultured follicles with hormones (LH-FSH-Testosterone) alone to stimulate follicle cell differentiation or combined the hormones with disulfiram, a drug that dominantly suppresses ALDH type I but not ALDH type II activity [29]. The differentiationa hormone treatment increased the expression of marker genes in control ovaries, whereas the addition of disulfiram significantly decreased the level of Lhcgr, Cyp19a1, Ccnd2, Fshr, Cyp17a1 and Bmp7 mRNAs. Thus, acetaldehyde negatively regulates not only theca cell functions but also granulosa cell differentiation. Although we presume that most of the acetaldehyde is produced in theca cells, this has not been directly tested.
In addition, it is known that ALDH1 is involved in the retinoic acid (RA) synthetic pathway [31], that RA receptor (RAR) is expressed in granulosa cells of eCG-primed rat ovary [37] and that RA can impact FSH regulation of granulosa cell differentiation [38,39,40,41]. Therefore, to determine if RA could overcome the inhibitory effects of disulfiram, ovaries were cultured with RA and disulfiram alone or in the presence of LH, FSH and testosterone. Although RA facilitated the expression of Lhcgr mRNA, other marker genes were not increased by treatment with RA in the presence of disulfiram. Thus, ALDH appears to have dual roles in follicular development; one is to synthesize RA, and the other is to metabolize and inactivate cytotoxic acetaldehyde. The latter pathway may be most critical because RA did not prevent the decreased number of ovulated oocytes in mice treated with cyanamide and because the addition of RA to LH+FSH+testosterone hormone cocktail did not enhance the expression genes, including Lhcgr. By contrast, Bagavandoss and Midgley [38] cultured granulosa cells with FSH and showed that RA significantly suppressed Lhcgr expression in a dose-dependent manner. In the present study, follicles were cultured in the presence of not only FSH but also LH, testostereone and serum. Because serum contains high level of retinol, it is possible that the synthesis of RA from retinol may occur in the ovary and that it plays multiple roles in a context specific manner. Therefore, the roles of RA during follicular development are under further investigation.
Lastly, the reduction of acetaldehyde toxicity during follicular development appears to be critical not only for granulosa cell differentiation but also for proper oocyte development. Specifically, the cyanamide-treated mice showed decreased numbers of ovulated oocytes after hCG, the ovulated oocytes were of poor quality and the fertilized eggs exhibited poor growth in culture. These results indicate that acetaldehyde generated during steroidogenesis can have toxic effects that impair proper granulosa cell differentiation and reduce oocyte viability either as consequence of granulosa cell malfunctions or by direct effects. Thus, ALDH activity is critical to metabolize acetaldehyde and prevent its accumulation to toxic levels that disrupt not only granulosa cell and theca cell functions but also proper oocyte development.
Research Highlights.
We analyzed the kinetic changes of acetaldehyde level in ovary during follicular development.
The level was transiently increased as a byproduct in a steroidogenic pathway.
However, the level was decreased in an ALDH dependent manner.
The reduction of acetaldehyde level was required for granulosa cell differentiation during follicular development.
Acknowledgments
FSH and LH ware kindly provided by Dr. A.F. Parlow, the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Disease, USA. The authors are grateful to Dr. Z. Liu, Baylor College of Medicine and Mr. N. Noma, Hiroshima University, for technical assistance, and Dr. Y. Kuwabara, Nihon Medical University for giving technical suggestions to culture mouse ovary.
Grant
This work was supported in part, by Grant-in-Aid for Scientific Research (No. 21688019, No.21028014, No. 21248032) from the Japan Society for the Promotion of Science (JSPS) (to M.S.), Young Research Fellowship from JSPS (No. 09J04118) (to I.K.) and and National Institute of Health (NIH)-HD-16229 and HD-07495 (Project III, Specialized Cooperative Centers Program in Reproduction and Infertility Research, SCCPIR) (to J.S.R).
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–51. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 2.Guigon CJ, Mazaud S, Forest MG, Brailly-Tabard S, Coudouel N, Magre S. Unaltered development of the initial follicular waves and normal pubertal onset in female rats after neonatal deletion of the follicular reserve. Endocrinology. 2003;144:3651–62. doi: 10.1210/en.2003-0072. [DOI] [PubMed] [Google Scholar]
- 3.Leung PC, Armstrong DT. Interactions of steroids and gonadotropins in the control of steroidogenesis in the ovarian follicle. Annu Rev Physiol. 1980;42:71–82. doi: 10.1146/annurev.ph.42.030180.000443. [DOI] [PubMed] [Google Scholar]
- 4.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–66. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–21. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 6.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–40. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- 8.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–34. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbison AE. Multimodal influence of estrogen upon gonadotropinreleasing hormone neurons. Endocrinology. 1998;19:302–30. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 10.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology. 2005;146:2817–26. doi: 10.1210/en.2004-1108. [DOI] [PubMed] [Google Scholar]
- 12.Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology. 1999;140:5855–65. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- 13.Lefrançois-Martinez AM, Tournaire C, Martinez A, Berger M, Daoudal S, Tritsch D, et al. Product of side-chain cleavage of cholesterol, isocaproaldehyde, is an endogenous specific substrate of mouse vas deferens protein, an aldose reductase-like protein in adrenocortical cells. J Biol Chem. 1999;274:32875–80. doi: 10.1074/jbc.274.46.32875. [DOI] [PubMed] [Google Scholar]
- 14.Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–99. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 15.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100:51–4. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 16.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–70. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 17.Aigueperse C, Martinez A, Lefrançois-Martinez AM, Veyssière G, Jean CI. Cyclic AMP regulates expression of the gene coding for a mouse vas deferens protein related to the aldo-keto reductase superfamily in human and murine adrenocortical cells. J Endocrinol. 1999;160:147–54. doi: 10.1677/joe.0.1600147. [DOI] [PubMed] [Google Scholar]
- 18.Brockstedt E, Peters-Kottig M, Badock V, Hegele-Hartung C, Lessl M. Luteinizing hormone induces mouse vas deferens protein expression in the murine ovary. Endocrinology. 2000;141:2574–81. doi: 10.1210/endo.141.7.7569. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A, Aigueperse C, Val P, Dussault M, Tournaire C, Berger M, et al. Physiological functions and hormonal regulation of mouse vas deferens protein (AKR1B7) in steroidogenic tissues. Chem Biol Interact. 2001;130–132:903–17. doi: 10.1016/s0009-2797(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 20.Baumann C, Davies B, Peters M, Kaufmann-Reiche U, Lessl M, Theuring F. AKR1B7 (mouse vas deferens protein) is dispensable for mouse development and reproductive success. Reproduction. 2007;134:97–109. doi: 10.1530/REP-07-0022. [DOI] [PubMed] [Google Scholar]
- 21.Koivisto T, Salaspuro M. Acetaldehyde alters proliferation, differentiation and adhesion properties of human colon adenocarcinoma cell line Caco-2. Carcinogenesis. 1998;19:2031–36. doi: 10.1093/carcin/19.11.2031. [DOI] [PubMed] [Google Scholar]
- 22.Shveiky D, Shushan A, Ben Bassat H, Klein BY, Ben Meir A, Levitzky R, et al. Acetaldehyde differentially affects the growth of uterine leiomyomata and myometrial cells in tissue cultures. Fertil Steril. 2009;91:575–79. doi: 10.1016/j.fertnstert.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Lieber CS. Metabolic consequences of ethanol. Endocrinologist. 1994;4:127–39. [Google Scholar]
- 24.Rivera-Meza M, Quintanilla ME, Tampier L, Mura CV, Sapag A, Israel Y. Mechanism of protection against alcoholism by an alcohol dehydrogenase polymorphism: development of an animal model. FASEB J. 2010;24:266–74. doi: 10.1096/fj.09-132563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–54. [PMC free article] [PubMed] [Google Scholar]
- 26.Klyosov AA, Rashkovetsky LG, Tahir MK, Keung WM. Possible role of liver cytosolic and mitochondrial aldehyde dehydrogenases in acetaldehyde metabolism. Biochemistry. 1996;35:4445–56. doi: 10.1021/bi9521093. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Q, Weiner H, Crabb DW. The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion. J Clin Invest. 1996;98:2027–32. doi: 10.1172/JCI119007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair JD, Lindros KO, Terho K. Aldehyde dehydrogenase inhibitors and voluntary ethanol drinking by rats. Adv Exp Med Biol. 1980;132:481–87. doi: 10.1007/978-1-4757-1419-7_49. [DOI] [PubMed] [Google Scholar]
- 29.Moore SA, Baker HM, Blythe TJ, Kitson KE, Kitson TM, Baker EN. Sheep liver cytosolic aldehyde dehydrogenase: the structure reveals the basis for the retinal specificity of class 1 aldehyde dehydrogenases. Structure. 1998;6:1541–51. doi: 10.1016/s0969-2126(98)00152-x. [DOI] [PubMed] [Google Scholar]
- 30.Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, et al. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–502. doi: 10.1210/me.2007-0042. [DOI] [PubMed] [Google Scholar]
- 31.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–24. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 32.Baroni GS, Marucci L, Benedetti A, Mancini R, Jezequel AM, Orlandi F. Chronic ethanol feeding increases apoptosis and cell proliferation in rat liver. J Hepatol. 1994;20:508–13. doi: 10.1016/s0168-8278(05)80498-2. [DOI] [PubMed] [Google Scholar]
- 33.Peluso JJ, Pappalardo A, Fernandez G. E-cadherin-mediated cell contact prevents apoptosis of spontaneously immortalized granulosa cells by regulating Akt kinase activity. Biol Reprod. 2001;64:1183–1190. doi: 10.1095/biolreprod64.4.1183. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:1280–88. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 35.Machell NH, Farookhi R. E-and N-cadherin expression and distribution during luteinization in the rat ovary. Reproduction. 2003;125:791–800. doi: 10.1530/rep.0.1250791. [DOI] [PubMed] [Google Scholar]
- 36.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, et al. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang YH, Ylikomi T, Lindfors M, Piippo S, Tuohimaa P. Immunolocalization of retinoic acid receptors in rat, mouse and human ovary and uterus. J Steroid Biochem Mol Biol. 1994;48:61–8. doi: 10.1016/0960-0760(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 38.Bagavandoss P, Midgley AR., Jr Biphasic action of retinoids on gonadotropin receptor induction in rat granulosa cells in vitro. Life Sci. 1988;43:1607–14. doi: 10.1016/0024-3205(88)90532-2. [DOI] [PubMed] [Google Scholar]
- 39.Hattori M, Takesue K, Nishida N, Kato Y, Fujihara N. Inhibitory effect of retinoic acid on the development of immature porcine granulosa cells to mature cells. J Mol Endocrinol. 2000;25:53–61. doi: 10.1677/jme.0.0250053. [DOI] [PubMed] [Google Scholar]
- 40.Minegishi T, Hirakawa T, Kishi H, Abe K, Ibuki Y, Miyamoto K. Retinoic acid (RA) represses follicle stimulating hormone (FSH)-induced luteinizing hormone (LH) receptor in rat granulosa cells. Arch Biochem Biophys. 371–373:203–10. doi: 10.1006/abbi.1999.1528. [DOI] [PubMed] [Google Scholar]
- 41.Minegishi T, Hirakawa T, Kishi H, Abe K, Tano M, Abe Y, Miyamoto K. The mechanisms of retinoic acid-induced regulation on the follicle-stimulating hormone receptor in rat granulosa cells. Biochim Biophys Acta. 2000;1495:203–11. doi: 10.1016/s0167-4889(00)00003-3. [DOI] [PubMed] [Google Scholar]