Abstract
Program Death-1 (PD-1) has been documented to negatively regulate immune responses. However, the cellular and molecular mechanisms for PD-1-mediated immune suppression have not been fully elucidated. In this study, we show that loss of PD-1 does not lead to defective induction of CD4+ T cell anergy in vitro and in vivo. Rather, the absence of PD-1 inhibits the development of inducible CD4+Foxp3+ regulatory T cells (iTregs) induced by TGF-β in vitro. In support of this finding, PD-1 deficiency impairs the generation of iTregs in vivo and leads to development of severe T cell-transfer-induced colitis. Mechanistically, defective iTreg generation in the absence of PD-1 was attributed to the heightened phosphorylation of Akt. Therefore, we first demonstrate that PD-1 controls peripheral T cell tolerance via an anergy-independent but iTreg-dependent mechanism.
Keywords: costimulation, tolerance/suppression/anergy, T cell
1. Introduction
Program death-1 (PD-1), is a type I transmembrane protein composed of one immunoglobulin (Ig) superfamily domain, and is encoded by the Pdcd1 gene on chromosome 1 in mice and chromosome 2 in humans. PD-1 is inducibly expressed on T cells, B cells, natural killer (NK) cells, monocytes, and dendritic cells (DCs) upon activation [1]. PD-1 is considered to play an important inhibitory role in immune responses as its deficiency causes different types of autoimmunity on different genetic backgrounds in mice [2–4]. PD-1 and its ligands also control self-reactive T cells in several mouse models of autoimmunity [5,6]. Therefore, PD-1 delivers inhibitory signals that regulate the balance between T cell activation, tolerance, and immunopathology.
It was reported that PD-1 may mediate CD8+ T cell anergy in vitro and in vivo [7,8], but whether it is true for CD4+ T cells remains to be determined. Furthermore, PD-L1 expressed in APC appears to regulate the development and maintenance of inducible regulatory T cells (iTregs) [9]. However, whether a T cell-intrinsic defect in PD-1 contributes to impaired iTreg development remains unknown. In addition, PD-L1 has also been shown to interact with B7-1 to inhibit T cell responses [1]. Therefore, the precise cellular mechanism for PD-1-mediated inhibitory effect has not been well characterized.
In this study, we show that although PD-1 does not regulate the induction of CD4+ T cell anergy, it potentiates peripheral conversion of CD4+CD25− T cells into CD4+Foxp3+ iTregs in vitro and in vivo. Therefore, we first discovered a previously-uncharacterized mechanism for PD-1-mediated peripheral tolerance which is independent of T cell anergy.
2. Materials and Methods
2.1. Mice
BALB/c mice were purchased from the National Cancer Institute (NCI) (Fredrick, MD). DO11.10 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Rag-1−/− BALB/c mice were obtained from Dr. Yang-Xin Fu (University of Chicago; Chicago, IL). PD-1−/− B6 mice were obtained from Dr. Tasuku Honjo (Kyoto University, Kyoto, Japan), and have been backcrossed onto BALB/c background for 12 generations. PD-1−/− BALB/c mice were bred with DO11.10 mice to generate DO11.10.PD-1−/− mice. All experiments were performed in accordance with protocols approved by the University of Chicago Institutional Animal Care and Use Committee.
2.2. Induction of T cell anergy in vitro
For CD28 blockade-induced T cell anergy, naïve WT and PD-1−/− CD4+ T cells were activated with anti-CD3 together with irradiated WT APCs in the presence or absence of hCTLA-4Ig fusion protein for 3 days. The preactivated T cells were then restimulated with anti-CD3 for 24 h. T cell proliferation was determined by [3H]thymidine incorporation, and IL-2 production was measured by ELISA. For ionomycin-induced T cell anergy, naïve CD4+ T cells from spleens and lymph nodes of WT and PD-1−/− mice were treated as the protocol as previously described [10]. T cell proliferation and IL-2 production were determined.
2.3. In vitro induction of CD4+CD25+Foxp3+ iTregs from naïve CD4+CD25− T cells
Naïve CD4+CD25− T cells from WT and PD-1−/− mice were plated in 96-well plates coated with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) in the presence of recombinant human IL-2 (100 U/ml), and TGF-β1 (2.5 ng/ml) (R&D Systems; Minneapolis, MN) for 72 h. Cells were harvested 72 h later and surface-stained with anti-CD4 and anti-CD25, and intracellularly stained with anti-Foxp3 (eBioscience; San Diego, CA).
2.4. In vitro Th17 cell differentiation
Naïve CD4+ T cells isolated from WT and PD-1−/− mice were differentiated under Th17-polarized condition as described [11]. IL-17- producing cells were determined by intracellular staining.
2.5. Adoptive transfers, in vivo T cell anergy induction, and in vivo iTreg generation
For T cell adoptive transfer, lymph node and spleen cells from DO11.10 or DO11.10.PD-1−/− mice were collected. Naïve CD4+CD25−KJ1-26+ T cells were purified and then injected intravenously (5 × 106) into non-irradiated syngeneic BALB/c recipients. In vivo T cell anergy was induced as previously described [12]. In vivo iTreg development was performed according to the protocol described by Chen et al. [13].
2.6. Induction of colitis in Rag-1−/− mice and histological assessment of colitis
Naïve CD4+CD25− T cells from WT and PD-1−/− mice were adoptively transferred into 6–10-wk-old Rag-1−/− mice by i.v. injection. Mice were weighed daily. Colitis development was monitored by histology of colon as described [14]. Mesenteric lymph node (MLN) cells were surface-stained with anti-CD4, anti-CD25, and intracellularly stained with anti-mouse-Foxp3, anti-IFN-γ or anti-IL-17.
2.7. Immunoblotting
Naïve CD4+CD25− T cells from WT and PD-1−/− mice were stimulated with anti-CD3 for different time-points, and lysed. The cell lysates were blotted with phospho-Abs against Akt, ERK, JNK, and IκBα (Cell Signaling; Boston, MA). The membranes were reprobed with anti-Akt or anti-actin.
2.8. Statistical analysis
Data are expressed as the mean±SD. Differences between groups were determined using Student’s t test. Clinical scores were analyzed using the Mann–Whitney U-test. The level of significance was set as P<0.05.
3. Results and Discussion
3.1. PD-1 does not mediate CD4+ T cell anergy induction in vitro and in vivo
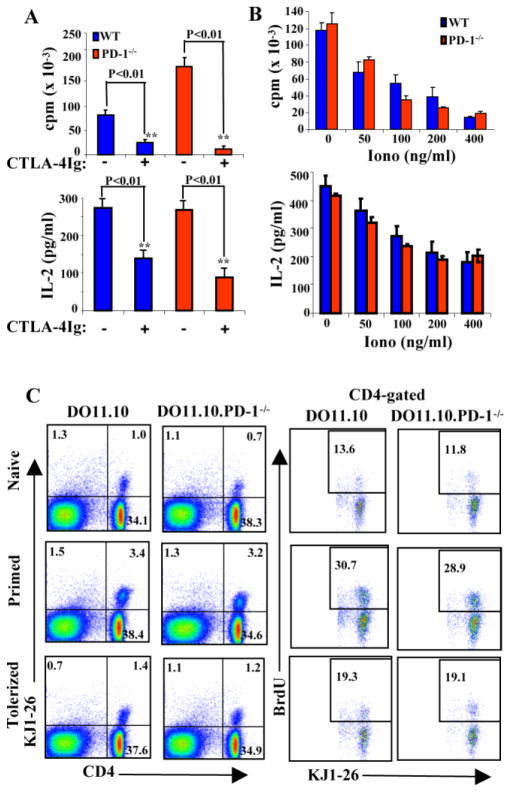
It is widely believed that PD-1 regulates peripheral T cell tolerance via an anergy-dependent mechanism [1]. However, this conclusion was based upon the data generated from CD8 TCR transgenic system [7,8]. It is not known whether it is true for CD4+ T cells. We then asked whether PD-1−/− CD4+ T cells could be anergized in vitro. It has been well documented that TCR ligation in the absence of CD28 costimulation leads to development of T cell anergy [15]. For in vitro anergy induction, we stimulated naïve wild-type (WT) or PD-1−/− CD4+CD25− T cells with soluble anti-CD3 and WT APCs in the presence or absence of CTLA4-Ig which blocks CD28-B7 interaction [11]. WT CD4+ T cells were unable to proliferate and produce significant quantities of IL-2 following initial stimulation under anergizing conditions (Fig. 1A). Unexpectedly, CD28 blockade also induced a state of unresponsiveness in PD-1−/− T cells (Fig. 1A). To confirm whether it is true, we also utilized a well-characterized ionomycin-induced T cell anergy protocol [10]. As shown in Figure 1A, ionomycin treatment induced an anergic state in both WT and PD-1−/− T cells (Fig. 1B).
Figure 1. PD-1−/− T cells are not resistant to anergy induction in vitro and in vivo.
A. Induction of naïve WT and PD-1−/− CD4+ T cell anergy by CD28 blockade was described in “Materials and Methods”. B. Induction of naïve WT and PD-1−/−CD4+ T cell anergy by ionomycin was described in “Materials and Methods”. C. Naïve CD4+KJ1-26+CD25− T cells from DO11.10 and DO11.10.PD-1−/− mice were adoptively transferred into BALB/c recipients, the mice were treated by either immunized or tolerized protocol, or left untreated. Ag-specific T cell expansion was detected by BrdU staining, and analyzed by flow cytometry. The figures are representative of at least four mice for each group. Data represent three or four independent experiments.
To directly evaluate the role of PD-1 in CD4+ T cell anergy in vivo, we used the well-established DO11.10 adoptive transfer model [12] in which T cells expressing a transgenic DO11.10 TCR, specific for a known peptide antigen, ovalbumin (OVA)323–339 plus I-Ad, were transferred into syngeneic recipients and exposed to the antigen in immunogenic and tolerogenic forms. To this end, naïve CD4+KJ1-26+CD25− T cells were purified from DO11.10 or DO11.10.PD-1−/− mice and transferred into syngeneic BALB/c recipients. Recipient mice were either not immunized (naive), given OVA323–339 in IFA s.c. (immunized), which induces a functional response in T cells in the draining lymph nodes, or treated with a large dose of OVA323–339 (i.p.) (tolerized), which induces tolerance in the lymph node cells [12]. All of the mice were injected with BrdU on day 10 after transfer every 12 h for 3 days. As shown in Figure 1C, DO11.10 and DO11.10.PD-1−/− T cells increased similarly in the BALB/c recipients receiving an immunogenic stimulus. DO11.10 and DO11.10.PD-1−/− T cells receiving a tolerogenic stimulus divided significantly less in the recipients to a similar degree (Fig. 1C). Therefore, our data collectively indicate that PD-1 does not regulate the induction of CD4+ T cell anergy. It is currently unknown why PD-1 mediates CD8+ but not CD4+ T cell anergy induction, but one possible explanation is that the requirement for T cell anergy induction in CD4+ vs. CD8+ T cells is different.
3.2. PD-1 deficiency impairs iTreg development in vitro and in vivo
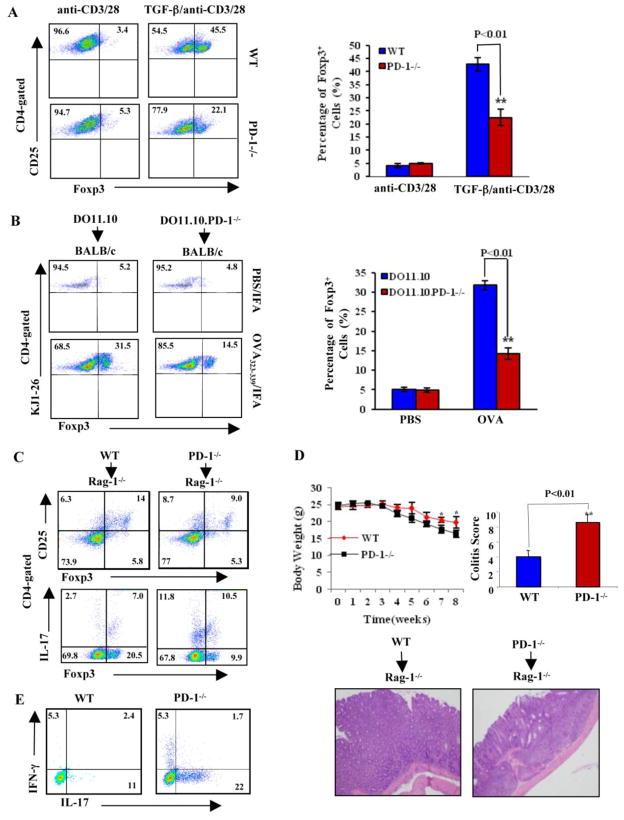
To search for the potential cellular mechanism by which PD-1 mediates peripheral T cell tolerance, we decided to look at whether PD-1 plays a role in the development of Tregs. As PD-1 deficiency did not alter the development and function of naturally-occurring Tregs (nTregs) in the thymus and periphery (Suppl. Fig. 1; data not shown), we determined whether the loss of PD-1 affects the peripheral conversion of conventional CD4+CD25− T cells into CD4+CD25+Foxp3+ iTregs in vitro induced by TGF-β in combination with TCR signaling [13]. We found that iTreg generation induced by TGF-β was significantly reduced in the absence of PD-1 (Fig. 2A). Our data together with those recently reported by Sharpe’s group using PD-L1-deficient mice [9] indicate that PD-1-PD-L1 interaction indeed regulates iTreg development. To demonstrate that PD-1 regulates iTreg development in vivo, we adoptively transferred naïve CD4+KJ1-26+CD25− T cells from DO11.10 or DO11.10.PD-1−/− mice into BALB/c mice. We then immunized the recipients with OVA323-339 peptide in IFA [13]. In support of in vitro data, peripheral conversion of iTregs during OVA immunization was impaired in BALB/c mice receiving CD4+KJ1-26+CD25− T cells from DO11.10.PD-1−/− mice (Fig. 2B).
Figure 2. PD-1 potentiates iTreg generation in vitro and in vivo.
A. Naïve CD4+CD25− T cells from WT and PD-1−/− mice were cultured under iTreg differentiation protocol. The expression of Foxp3 was determined on CD4+CD25+ population (n=3). The figures are representative of three experiments repeated with similar results. B. CD4+KJ1-26+CD25− T cells from DO11.10 and DO11.10.PD-1−/− mice were adoptively transferred into BALB/c recipients, immunized with OVA323–339 in IFA by i.p, and Foxp3 expression in CD4+KJ1-26+ population was determined. The dot plot figures are representative of three experiments repeated with similar results. C. Rag-1−/− mice (n=5) were adoptively transferred i.v. with naïve WT or PD-1−/− CD4+CD25− T cells (5 × 106/mouse). The expression of Foxp3, IFN-γ, and IL-17 within CD4+ T cells in draining lymph nodes collected from the recipients 8 weeks after transfer was determined by intracellular staining. D. The severity of colitis of the recipients was determined by body weight loss (upper panel) and H&E-stained paraffin sections of colons obtained 8 weeks after transfer of naïve CD4+CD25− T cells (lower panel). E. Naïve CD4+ T cells isolated from WT and PD-1−/− mice were stimulated under Th17 condition for 4 days. The cells were then collected and restimulated with PMA plus Ionomycin. IL-17-producing cells were determined by intracellular staining. These data are representative of at least four mice for each group, from three independent experiments with similar results.
It has been shown that adoptive transfer of CD4+CD25−CD45RBhi cells into immunodeficient hosts results in the development of colitis, which can be prevented by cotransfer of nTregs [16,17]. It has also been documented that CD4+Foxp3+ Tregs can spontaneously develop from naïve CD4+CD25− T cells in a lymphopenic environment [9]. One would expect that if PD-1 deficiency impairs iTreg development in vivo, severe colitis would develop in Rag-1−/− mice receiving naïve PD-1−/−CD4+CD25− T cells. To test whether this is the case, we adoptively transferred naïve CD4+CD25− T cells from WT and PD-1−/− mice to Rag-1−/− mice and monitored the mice for 8 weeks. As expected, there was a significant defect in de novo iTreg development in Rag-1−/− mice adoptively transferred with naïve CD4+CD25− T cells from PD-1−/− mice (Fig. 2C) which was associated with severe lymphocyte infiltration, crypt drop-out, epithelial regeneration, overall crypt architectural alteration in the colons and body weight loss (Fig. 2D). As both Th1 and Th17 cells have been shown to mediate T cell transfer-induced colitis [18,19], we also examined the Th1 and Th17 cells in the draining lymph nodes. We noticed a significant increase in CD4+IL-17+ Th17 cells in Rag-1−/− mice receiving naïve PD-1−/− CD4+CD25− T cells (Fig. 2C), but there was no difference in CD4+IFN-γ+ Th1 cells from Rag-1−/− mice receiving naïve WT and PD-1−/− CD4+CD25− T cells (data not shown). This observation is further supported by the fact that PD-1 deficiency facilitated Th17 cell differentiation (Fig. 2E).
To define the molecular mechanism by which PD-1 facilitates iTreg development, we first extensively screened the potential pathways that PD-1 might affect. To this end, WT and PD-1−/− CD4+CD25− T cells were stimulated with anti-CD3, and lysed. The cell lysates were blotted with phospho-antibodies against ERK, JNK, IκBα, and Akt. Although the absence of PD-1 did not affect ERK, JNK, and IκBα phosphorylation, it did significantly augmented Akt phosphorylation (Suppl. Fig. 2). It is possible that defective development of iTregs in the absence of PD-1 may be due to heightened Akt activation. To test whether this is the case, we treated naïve PD-1−/− CD4+CD25− T cells with LY294002, a specific PI3K inhibitor, and then cultured them with anti-CD3 and anti-CD28 together with TGF-β. Inhibition of PI3K completely rescued defective iTreg development caused by the absence of PD-1 (Suppl. Fig. 2), suggesting that PD-1 facilitates iTreg development via an Akt-dependent mechanism.
In conclusion, we show here that PD-1 does not seem to control the induction of CD4+ T cell anergy, but rather facilitates the development of iTregs which is essential for establishing and maintaining peripheral T cell tolerance. Therefore, our data first demonstrate that PD-1 may regulate peripheral tolerance via an anergy-independent but iTreg-dependent mechanism.
Supplementary Material
A. The splenocytes were surface stained with APC-anti-CD4 and then intracellular staining with PE-anti-Foxp3. B. CD4+CD25+ T cells were isolated from WT and PD-1−/− spleens and lymph nodes, and were co-cultured with WT CD4+CD25− T cells at different ratios in the presence of anti-CD3 and T-depleted APCs for 72 h. T cell proliferation was determined by [3H]thymidine incorporation.
A. WT and PD-1−/− CD4+CD25− T cells were stimulated with anti-CD3. The cell lysates were blotted with phospho-Abs against ERK, JNK, IκBα, and Akt. The membranes were stripped and reprobed with anti-actin. B. Naïve CD4+CD25−CD44lowCD62L+ T cells from PD-1−/− mice were stimulated with plate-bound anti-CD3 and anti-CD28, IL-2, and TGF-β in the presence or absence of LY294002 for 72 h, and the cells were surface-stained with anti-CD4, anti-CD25, and intracellularly stained with anti-Foxp3. Data represent one of three to five independent experiments.
Highlights.
Loss of PD-1 does not lead to defective induction of CD4+ T cell anergy
PD-1 deficiency facilitates the development of iTregs in vitro and in vivo
The facilitation of iTreg development by PD-1 seemed to be mediated by Akt
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH) (R01 AI090901 to JZ) and from the American Heart Association (09GRNT2010084 to JZ). LY was supported by a Scholarship from China Scholarship Council of the Ministry of Education of P.R. China ([2007] 3020). JZ was an American Lung Association Career Investigator. We thank Drs. Tasuku Honjo and Yang-Xin Fu for providing PD-1−/− mice and Rag-1−/− BALB/c mice, respectively.
Footnotes
Conflict-of-interest disclosure:
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182:6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 8.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, Xie D, Zhang J. Cutting edge: CTLA-4--B7 interaction suppresses Th17 cell differentiation. J Immunol. 2010;185:1375–1378. doi: 10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otten GR, Germain RN. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991;251:1228–1231. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- 16.Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 17.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 18.Powrie F, Coffman RL. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 19.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. The splenocytes were surface stained with APC-anti-CD4 and then intracellular staining with PE-anti-Foxp3. B. CD4+CD25+ T cells were isolated from WT and PD-1−/− spleens and lymph nodes, and were co-cultured with WT CD4+CD25− T cells at different ratios in the presence of anti-CD3 and T-depleted APCs for 72 h. T cell proliferation was determined by [3H]thymidine incorporation.
A. WT and PD-1−/− CD4+CD25− T cells were stimulated with anti-CD3. The cell lysates were blotted with phospho-Abs against ERK, JNK, IκBα, and Akt. The membranes were stripped and reprobed with anti-actin. B. Naïve CD4+CD25−CD44lowCD62L+ T cells from PD-1−/− mice were stimulated with plate-bound anti-CD3 and anti-CD28, IL-2, and TGF-β in the presence or absence of LY294002 for 72 h, and the cells were surface-stained with anti-CD4, anti-CD25, and intracellularly stained with anti-Foxp3. Data represent one of three to five independent experiments.