Abstract
Therapies using adult stem cells often require mechanical manipulation such as injection or incorporation into scaffolds. However, force-induced rupture and mechanosensitivity of cells during manipulation is largely ignored. Here, we image cell mechanical structures and perform a biophysical characterization of three different types of human adult stem cells: bone marrow CD34+ hematopoietic, bone marrow mesenchymal and perivascular mesenchymal stem cells. We use micropipette aspiration to characterize cell mechanics and quantify deformation of subcellular structures under force and its contribution to global cell deformation. Our results suggest that CD34+ cells are mechanically suitable for injection systems since cells transition from solid- to fluid-like at constant aspiration pressure, probably due to a poorly developed actin cytoskeleton. Conversely, mesenchymal stem cells from the bone marrow and perivascular niches are more suitable for seeding into biomaterial scaffolds since they are mechanically robust and have developed cytoskeletal structures that may allow cellular stable attachment and motility through solid porous environments. Among these, perivascular stem cells cultured in 6% oxygen show a developed cytoskeleton but a more compliant nucleus, which can facilitate the penetration into pores of tissues or scaffolds. We confirm the relevance of our measurements using cell motility and migration assays and measure survival of injected cells. Since different types of adult stem cells can be used for similar applications, we suggest considering mechanical properties of stem cells to match optimal mechanical characteristics of therapies.
1. Introduction
Advances in the fundamental biological understanding of adult stem cells have enabled a variety of therapeutic applications, primarily using bone marrow derived cells (Watt and Driskell, 2010). Injection of cells and embedding cells within implantable biomaterial scaffolds are the main currently used forms of introducing adult stem cells into patients (Li et al., 2009; Noth et al., 2010). Stem cells from niches away from sites of cardiac injury have been used in several clinical trials, mainly with bone marrow origin, where high amounts of death of implanted cells occur (Assmus et al., 2006; Cleland et al., 2006; Grogaard et al., 2010; Mansour et al., 2006; Meyer et al., 2006; Schachinger et al., 2006; Xu et al., 2009), mainly due to inflammatory and immune reactions, and the presence of apoptotic and necrotic factors preexistent in the injured site or caused by the delivery system. Among these factors, the clinical efficacy of these methods may be limited by a poor understanding of stem cell mechanical properties. In both technologies, stem cells are exposed to an altered mechanical environment, either with fluid shear during injection or with a stiff scaffold matrix. Both applied forces and the mechanical properties of cellular environments, in addition to chemical factors, can direct cell differentiation and alter cell function (Chowdhury et al., 2010; Engler et al., 2006).
The number of known stem cells available for therapies has increased in the last decade, and stem cells have been collected from different regions of the body and stages of human development (Teo and Vallier, 2010). The bone marrow offers rich sources of both hematopoietic and mesenchymal stem cells, and cells of mesenchymal origin have also been isolated from other regions of the body including the perivascular region (Crisan et al., 2009; Crisan et al., 2008). With the variety of available stem cell sources, we suggest that structural and related mechanical properties of stem cells, including cytoskeletal structures, stiffness and resilience, can be a factor to optimize the efficiency of stem cell delivery.
Mechanical characterization of stem cells also provides valuable information related to cell function. Cells are mechanoactive systems that sense, react to and match the force and mechanical properties of the surrounding environment (Dembo and Wang, 1999; Discher et al., 2005; Lo et al., 2000). Cells respond to mechanical stimulations by remodeling the cytoskeleton (Fletcher and Mullins, 2010), membrane (Charras et al., 2004) and cell nucleus (Dahl et al., 2008). Stem cells are sensitive to mechanical stimulation. Differentiation can be directed by the extracellular stiffness (Engler et al., 2006) and often involves the alteration of structural elements, such as the actin cytoskeleton (Maloney et al., 2010; McBeath et al., 2004), the membrane (Titushkin and Cho, 2006) and the nucleus (Dahl et al., 2005). However, mechanics and mechanical structures of stem cells isolated from different niches have not been compared, quantitatively or qualitatively.
Here, we characterized mechanics and mechanical structures of three different adult stem cell types: Human hematopoietic stem cells (CD34+ cells) are of bone marrow origin and are small single-nucleated cells expressing the CD34+ cell surface marker (Baum et al., 1992). Human mesenchymal stem cells (MSCs) are also of bone marrow origin, but are of a different lineage and are characterized by surface markers CD105+, CD166+, CD29+, CD44+, CD14−, CD34− and CD45− (Pittenger et al., 1999). Human perivascular stem cells (PSCs) are isolated from blood vessels in fetal muscle and express CD146, NG2, PDGF-Rβ, MSC markers, and are believed to be closely related to MSCs (Crisan et al., 2008). Cells types were chosen since they are all currently used for therapeutic applications, either via injection or incorporation into scaffolds.
2. Materials and Methods
A more detailed description for this section is located in supplemental materials.
Cells culture, labeling and imaging
CD34+ and MSCs were cultured according to vendor’s instructions (Stem Cell Technologies), and PSCs were cultured as reported (Crisan et al., 2008). For actin and lamin imaging, cells were fixed, permeabilized, blocked and labeled with Oregon green phalloidin (for F-actin), antibodies to lamin A/C and DAPI (nucleus).
Chemotaxis, scratch and injection assays
Already published methodologies were used to perform the scratch (Tottey et al., 2010) and chemotaxis (Liang et al., 2007) assays. Cells were mock-injected through needles (BD 18-gauge 1.5 inch needle) at 0.5 mL/sec. Cell recovery and viability was evaluated with a hemacytometer and Trypan blue.
Micropipette aspiration of cells
Cells were aspirated according to previously published methodologies (Dahl et al., 2005; Pajerowski et al., 2007) at 37°C, atmospheric oxygen levels in DMEM with 10% FBS and nocodazole and cytochalasin-D for cytoskeletal depolymerization. The membrane and nucleus was labeled with fluorescent conjugated wheat germ agglutinin and Hoechst 33342 DNA dye, respectively.
3. Results
3.1 Mechanics and deformation of stem cells
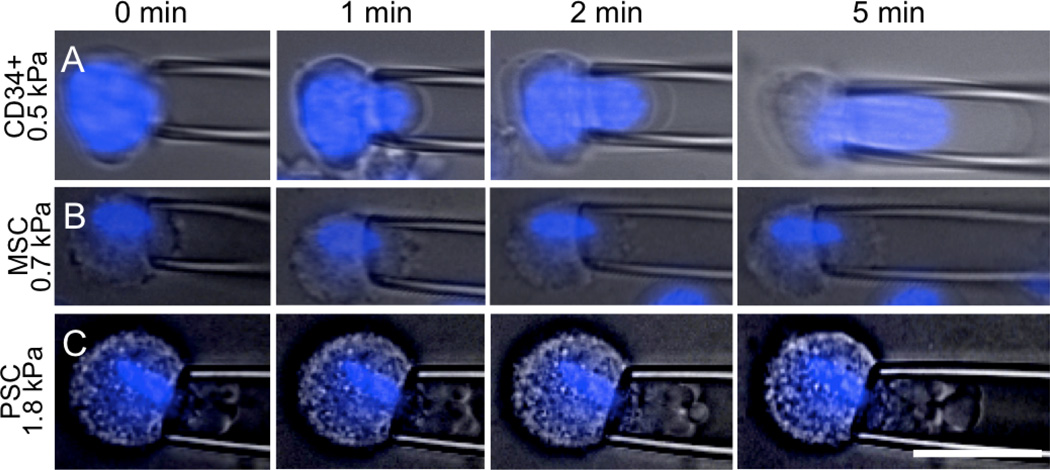
To determine mechanical properties of stem cells while visualizing deformation of subcellular structures, we performed micropipette aspiration (Hochmuth, 2000). All cells show viscoelastic character as observed by time-dependent deformation under constant aspiration pressure (Fig. 1). CD34+ cells were the most deformable of the tested cell types and could be aspirated completely into the small diameter micropipette. The nucleus, which represents a large portion of the cell, also deforms under aspiration. At early aspiration times the cell deforms but flows slowly. After 5 minutes of constant applied aspiration, CD34+ cells appear to transition and flows into the pipette. A subset of CD34+ cells rupture before complete deformation into the pipette, but these cells are mechanically stiffer than the rest (Supplemental Fig. 2).
Figure 1. Micropipette aspiration of CD34+ cells, MSCs and PSCs.
Aspiration of the different cell types shows dramatically different response to force in magnitude of stiffness, viscoelastic deformation and contributions of cell components. Plasma membrane and nucleus (blue) deformations were followed over time after applying a constant aspiration pressure (in kPa, to the left of each set of images). (A) CD34+ cells showed significant viscous deformation with a strong contribution from the nucleus. (B) MSCs were less fluid, and showed slight contributions from a more condensed nucleus. (C) PSCs were stiff and showed minimal contributions from the nucleus. Scale is 25 µm.
MSCs and PSCs were both stiffer and presented a dramatically different mechanical behavior compared to CD34+ cells. MSCs (Fig. 1B) and PSCs (Fig. 1C) deformed viscoelastically under applied aspiration pressure until they reached a pseudo-equilibrium length. Neither cell type was completely aspirated into the pipette in the considered time frame. Nuclei of both MSCs and PSCs were elongated, occupied a smaller fraction of the cytoplasm when compared to CD34+ cells (Fig. 1B, 1C) and reflected a smaller cell fraction (Supplemental Fig. 3).
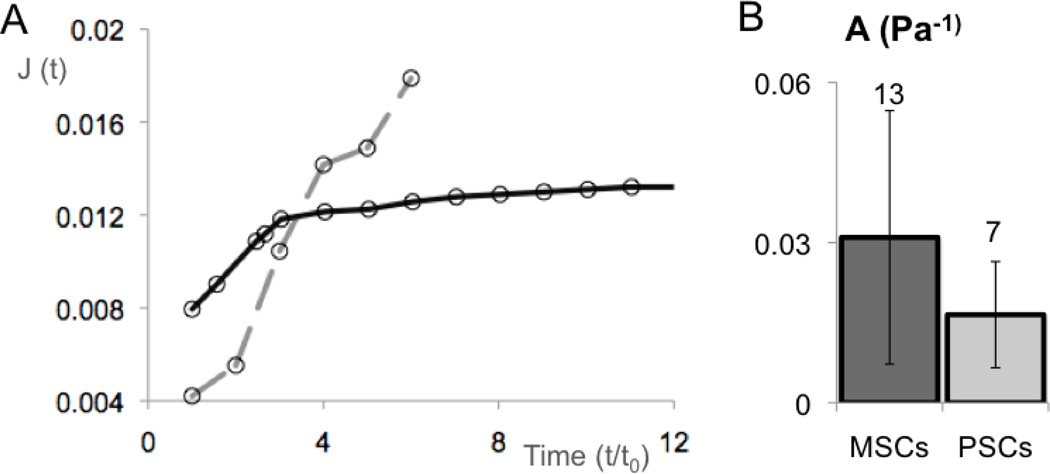
The deformation of CD34+ cells was consistent with the observed fluid transition (Fig. 2A; Supplemental Fig. 2). This mode of deformation has been observed in other non-adherent cell types (including leukocytes) and is consistent with a mostly cortical resistance to deformation (Evans and Kukan, 1984). Conversely, PSCs and MSCs were modeled as power law materials. Both A and α (Supplemental Methods; equation 5) were calculated for PSCs and MSCs by fitting regressions to log-log plots of J as a function of t. Values of α were similar for both cell types, between 0.2 and 0.3, as expected for materials dominated by cytoskeletal structures (Hoffman et al., 2006; Pajerowski et al., 2007), but there was a difference in the deformability A (Fig. 2B). PSCs were stiffer (A = 0.01 ± 0.003 Pa−1) than MSCs (A= 0.03 ± 0.006 Pa−1). These values were obtained from initial aspiration until 200 seconds, after which the membrane deformed substantially more than the remaining cell structures.
Figure 2. Quantification of micropipette aspiration shows different types of deformation and flexibility of cells of mesenchymal origin.
(A) Proposed general profile curve for creep compliance, J (Pressure−1) (equation 4 in supplemental material), as a function of time highlight the different deformation behavior of CD34+ cells relatively to PSCs and MSCs. These differences in profile can be better visualized in the raw data represented in the supplemental figures 2 (for CD34+ cells) and 5 (for PSCs and MSCs) (B) Both PSCs and MSCs deform as viscoelastic solids. Comparisons of the creep prefactor A shows that MSCs are significantly more deformable than PSCs (t-test test p-value < 0.03). Error bars are SEM, and numbers above the bars are n of independently tested cells.
3.2 Contribution of the nucleus to cell deformability
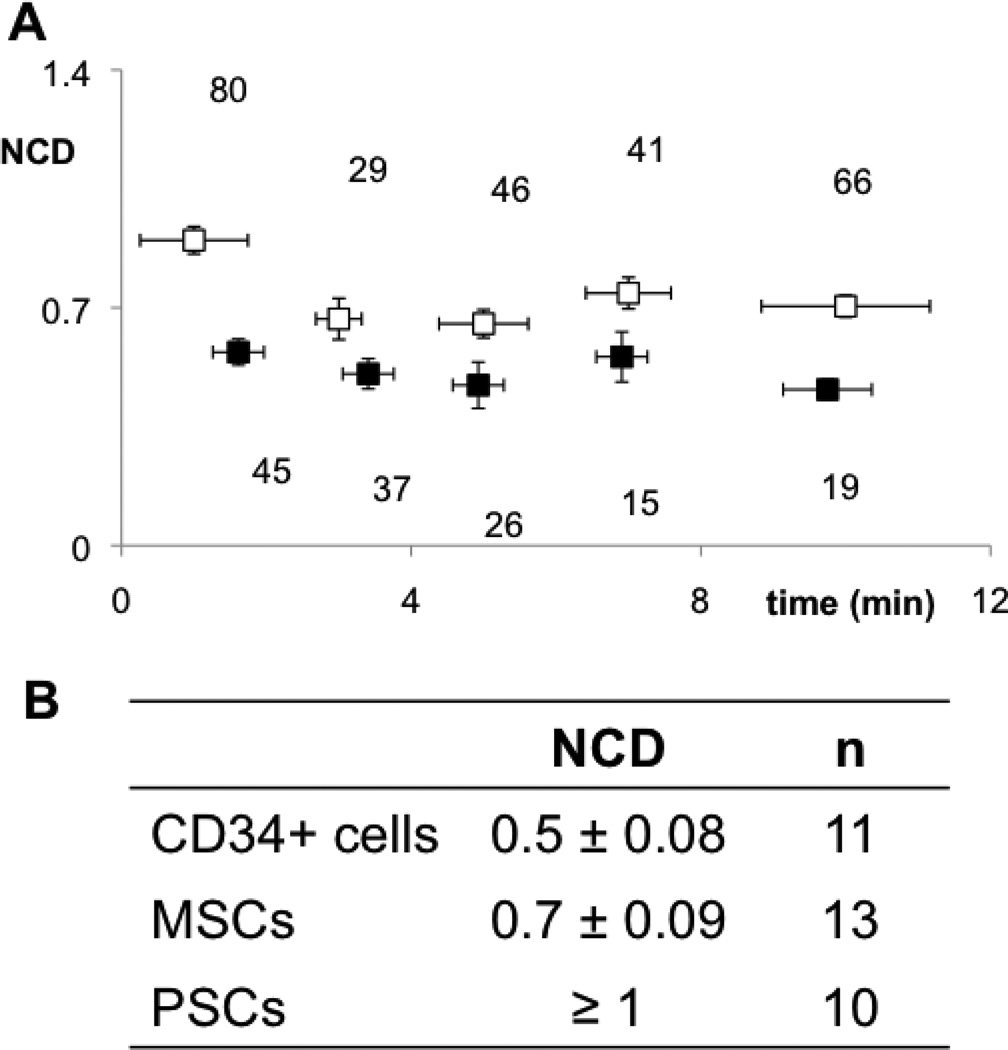
During deformation, nuclei in CD34+ cells showed morphological changes similar to the whole cell (Fig. 1A), suggesting that the nucleus contributed significantly to cell mechanics. Elongated nuclei in MSCs deformed less and moved slowly through the cytoplasm during aspiration (Fig. 1B). Nuclei in aspirated PSCs remained undeformed and immobile, even at late stages of aspiration (Fig. 1C), suggesting that the nucleus was either stiffer or shielded by denser mechanical structures inside the cell. To discriminate between the two scenarios, we analyzed nuclear translocation during deformation. We calculated the nuclear contribution to deformation (NCD) from images (Supplemental Fig. 1B) to quantify subcellular movement of the nucleus. Both average NCD and time-dependent NCD values were lowest for CD34+ cells since CD34+ nuclei readily deformed and moved inside the cell towards the pipette tip (Fig. 3). Conversely, the NCD of MSCs was considerably higher over the aspiration timeline (Fig. 3B). Also stochastic failure of cellular structures may occur as manifested by fluctuations in the NCD of MSCs during time. No nuclear deformation is observed for PSCs and the NCD >1, implying a higher intracellular or cytoskeletal stiffness, which could explain its higher stiffness relatively to MSCs (Fig. 2B). Plots of NCD versus time for CD34+ cells (black) show that the nucleus deforms evenly and in sync with the cell. For MSCs (white) the higher NCD values with time suggest considerable stochastic reorganization of structures inside the cell under stress. This suggests that cytoskeletal structures within MSCs and PSCs play a stronger role in defining overall cell and nuclear mechanics compared with CD34+ cells (Supplemental Fig. 3), where mechanics is dominated by the nucleus.
Figure 3. Nuclear contribution to deformation (NCD) in aspirated stem cells is a quantitative measure of how the cell deforms as a unified structure.
(A) Black points in plots of NCD versus time represent CD34+ cells and white dots represent MSCs. Error bars are SEM. (B) Average values of NCD for each cell type show that the nucleus of CD34+ cells contributes more to cell deformation, less in MSCs, but not at all in PSCs, where NCD is always above 1 due to absence of nuclear mobility during aspiration. (p-value < 10−5 between cell types).
3.3 Actin and actin-nucleoskeleton connections in stem cell
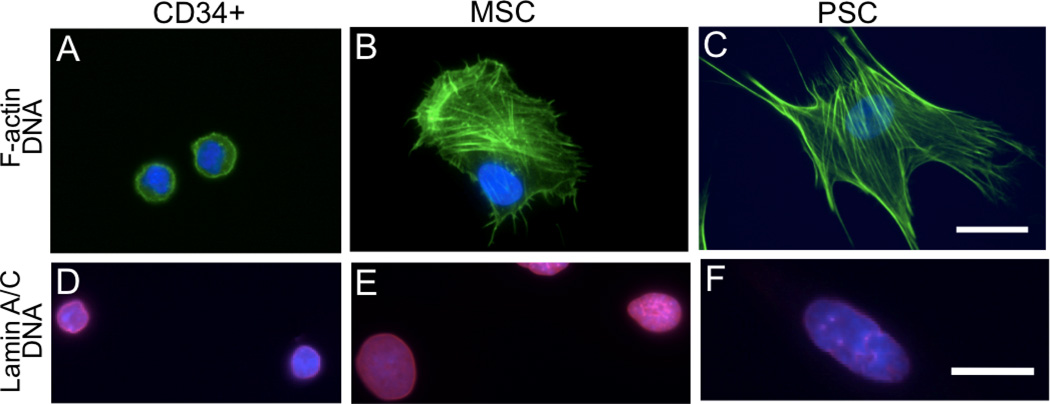
The power-law deformation of MSCs and PSCs was similar to other adherent cell types and could be modeled as a viscoelastic solid. However, CD34+ cells deformed in a manner similar to non-adherent cells, including neutrophils. We visualized actin structures of these cell types in culture conditions, since the actin cytoskeleton is the primary mechanical structure in most adherent mammalian cells (Van Citters et al., 2006). Actin fibers connect to the extracellular matrix via focal adhesions and to other cells via adherens junctions, connecting also to the nucleoskeleton via the LINC complex (Dahl et al., 2010; Haque et al., 2010).
Actin fibers in CD34+ cells were poorly developed in culture conditions and were located mainly in the cell periphery (Fig. 4 A,D). The nucleus of CD34+ cells was spherical, (Fig. 4A) and the nucleoskeleton, labeled by lamin A/C, was homogeneously distributed at the nuclear periphery (Fig. 4D). Cultured MSCs and PSCs adhered to the substrate and developed actin fibers (Fig. 4 B,E). In PSCs, nuclei were centrally located and colocalized with regions of dense actin fibers (Fig. 4C,F). PSC nuclei were larger, more elongated and had heterogeneously organized nucleoskeletal lamin A/C within punctuate, filamentous structures (Fig. 4F). This altered nuclear structure may result from strains applied on the nucleus by direct or indirect interactions with actin fibers (Dahl et al., 2008). The nucleus of MSCs was often located at the periphery of the cell and did not colocalize with regions of dense actin fibers (Fig. 4B,C). Nuclei of MSCs were rounder and showed homogeneous lamin A/C distribution (Fig. 4E). This differential spatial localization could imply higher nuclear-cytoskeleton connectivity in PSCs compared to MSCs, which supports the hypothesis of a higher nuclear shielding by cytoskeletal structures to applied stress. The significant differences in cell and nuclear structures between MSCs and PSCs suggest that structural and mechanical properties may strongly depend on the tissue origin of stem cells.
Figure 4. Actin, lamins and actin-nucleus connections are different among the studied cell types in adherent cultures.
(A) CD34+ cells show poorly developed F-actin (green) structures located around a central nucleus (blue), which occupies most of the cell volume. (B) MSCs show regions of higher development of F-actin stress fibers not coincident with nucleus. (C) PSCs show the most developed actin stress fibers colocalized with the nucleus. (D) A-type lamins (red) are homogeneously distributed in CD34+ cells, but are heterogeneous in MSCs (E) and PSCs (F). Scale is 25 µm; A–C and E–F scaled collectively.
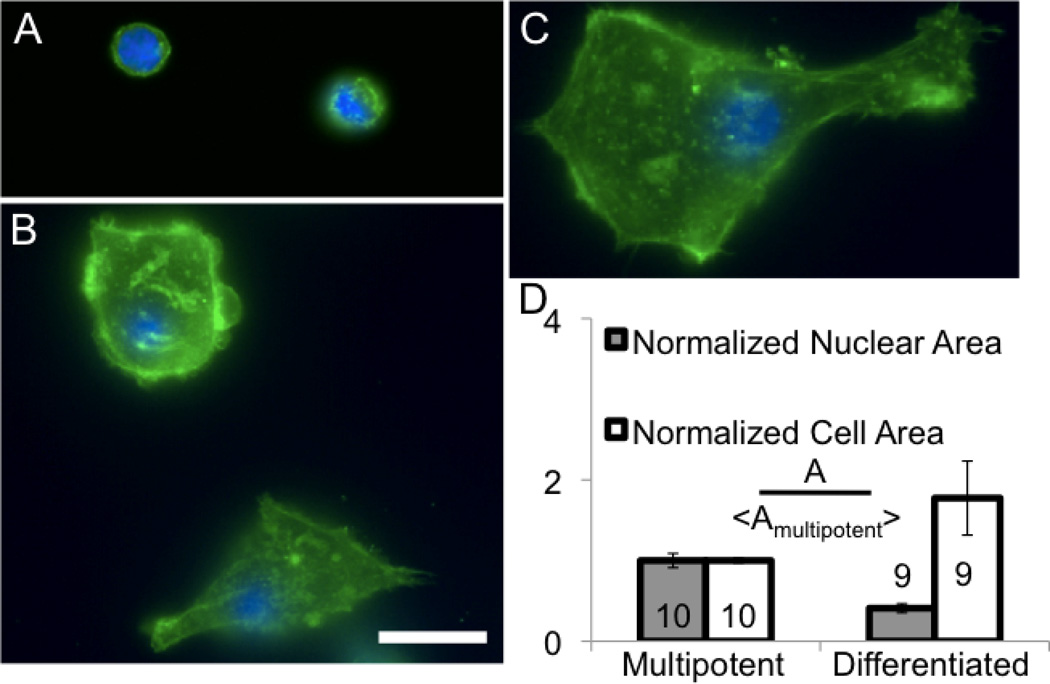
CD34+ cells were differentiated to test the potential of actin fibers or nuclear structures to develop (Supplemental Fig. 4). Given the similarity of CD34+ cells to leukocytes, differentiated cells could still maintain a spherical and fluid phenotype. However, actin fibers and substrate adhesions are formed in differentiated CD34+ cells (Fig. 5 A–C), which were correlated to changes in morphology and cell area (Fig. 5D). However, projected nuclear area decreased with differentiation (Fig. 5D) probably due to nuclear compaction observed with the formation of heterochromatin, as observed in stem cell differentiation (Meshorer and Misteli, 2006). A minority of cells after 9 days of differentiation showed larger nuclei than the pre-differentiated CD34+ cells, but these were present in highly spread cells with large cell area and highly extended fibers (Fig. 5C).
Figure 5. Morphology, area and actin structures change significantly in CD34+ cells changes with differentiation.
(A) Multipotent CD34+ cells show poorly organized actin (green) and a nucleus (blue) which occupies most of the cell volume. (B–C) With differentiation, cells show developed actin stress fibers and enlargement. (D) With differentiation the cell area increases, but the nuclear area decreases (p<0.002), suggesting cytoskeletal reorganization and stiffening of the cell. Scale is 25 µm. Errors are SEM.
3.4 Oxygen dependent changes in PSC mechanics
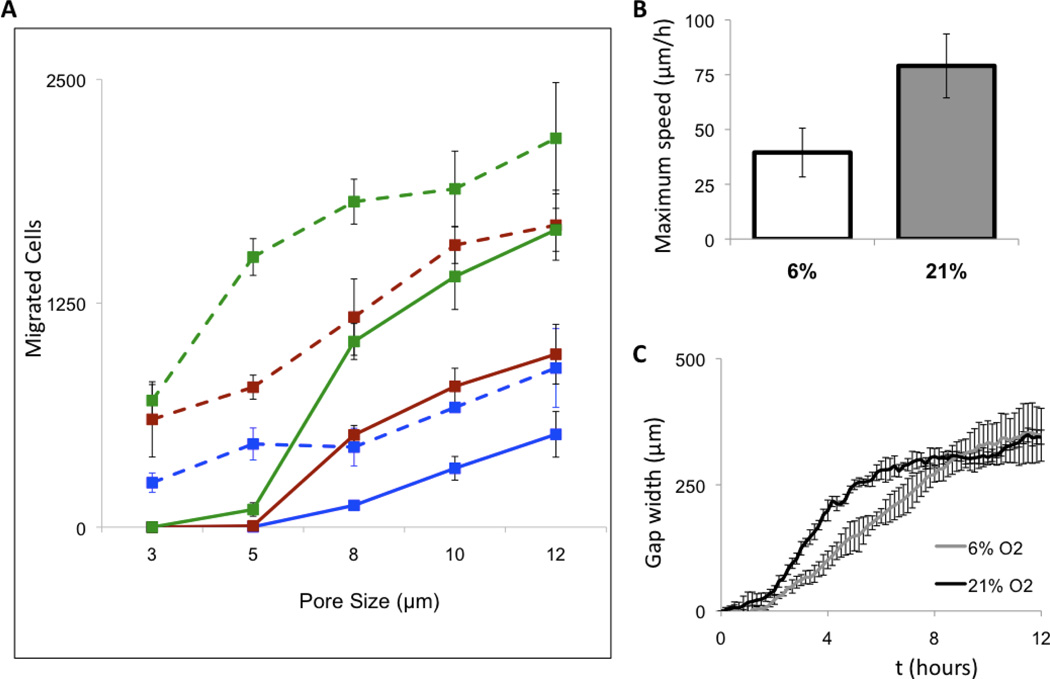
While similar to MSCs, PSCs originate from a more rigid and mechanically active niche in the body. Increased stiffness may result from a higher degree of connectivity between the actin and nucleus. However, being too stiff to enter into scaffolds may be a concern with this cell type. We tested the migration of cells through pores, and no cell migration was detected through 3µm pores (Fig. 6A). Stem cells originate from niches, where low oxygen tension plays a role in the maintenance of pluripotency and cell propagation that have been replicated in vitro (Lekli et al., 2009; Piccoli et al., 2007; Szablowska-Gadomska et al., 2011), including in PSCs (Tottey et al., 2010). All tissues of the body have oxygen concentrations lower than atmospheric levels (21% oxygen), and the concentration of oxygen is below 5% in the bone marrow (Hao et al., 2011). Since adult stem cells move from their niches into sites of injury or development (Hess and Allan, 2011), we hypothesized that PSCs in lower oxygen would be able to migrate through smaller diameter openings. Generally, higher migration was observed in cells cultured in 6% than in cells cultured in 21% oxygen, and importantly PSCs in 6% oxygen were able to translocate through 3µm pores (Fig. 6A).
Figure 6. Oxygen levels increase PSC migration through pores but decrease migration on surfaces.
(A) Migration of PSCs through small pores in 6% oxygen (dashed lines) is always significantly higher than in 21% oxygen (solid lines) independent of the type of chemoattractant. Extracellular matrix digests (green) showed the best migration. Serum (red) also attracted cells but there was a significant increase in migration with no attractants (blue). (B) On a surface, cells grown in 6% oxygen migrate slower and take longer to close a scratched wound (C). Error bars are standard deviation.
We also tested the effects of low oxygen on translocation of cells on two-dimensional surfaces. We also performed an in vitro scratch assay with induced 500 µm scratches on a monolayer of PSCs and monitored both individual and group cell movement (Fig. 6B,C). PSCs in 21% oxygen migrated quicker than PSCs in 6% oxygen, up to double the maximum speed (Fig. 6B). Scratch closure in 21% oxygen was also quicker than in 6% oxygen (Fig. 6C). Thus, PSCs at 21% oxygen are faster moving individually and in groups, but at 6% oxygen are able to better deform and translocate through pores.
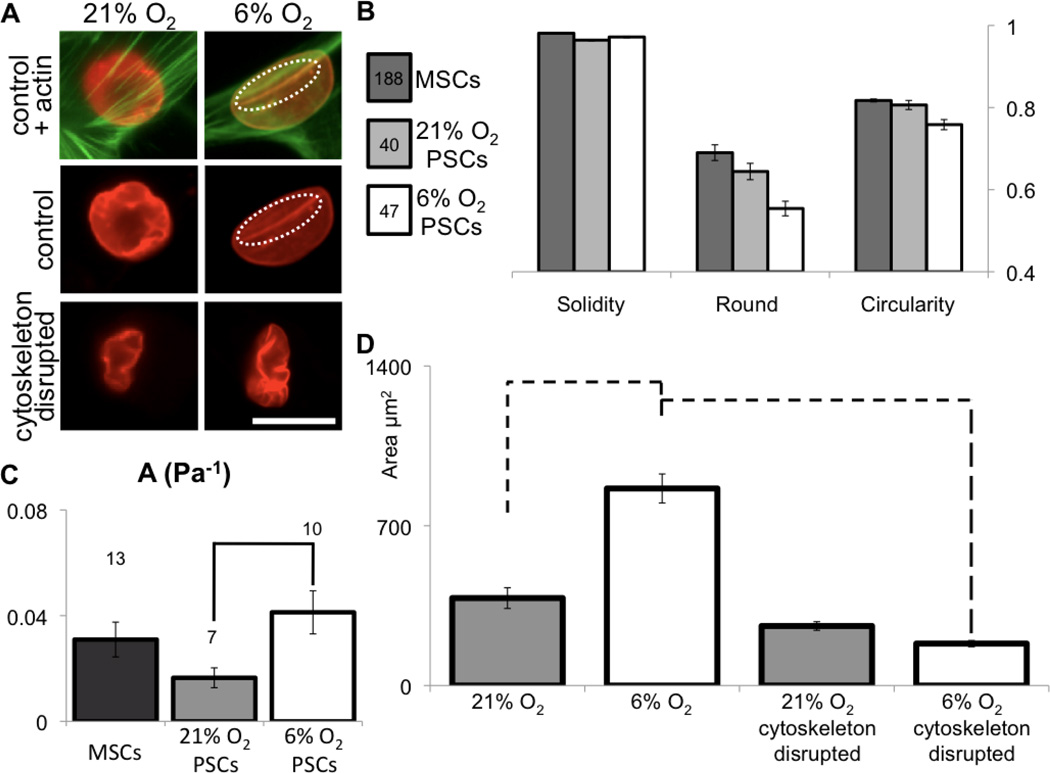
It appeared that increased pore translocation of PSCs in 6% oxygen was not a function of cell motility, but rather was dependent on cell deformability. A more deformable cell can result from lower actin myosin connectivity (Van Citters et al., 2006) and a differently organized cytoskeleton (Maloney et al., 2010). The organization of actin fibers and actin-nucleus interactions seem similar for PSCs in 6% and 21% oxygen (Fig. 7A). However, actin fibers colocalized with nucleoskeletal invaginations in 6% oxygen (Fig. 7A – dashed region). Statistical analysis of nuclear shape parameters, such as solidity, roundness, and circularity, showed that MSCs have the roundest nuclei and PSCs in 6% oxygen present the most elongated (Fig. 7B). More elongated nuclei can result from an increase in compliance or stronger strains from actin cytoskeleton (Houben et al., 2007).
Figure 7. Oxygen levels affect mechanics and organization of subcellular structures in PSCs.
(A) In low oxygen, nuclei become longer and lamins (red) appear smoothed at the nuclear periphery and interact with actin (green; dashed circle). Cytoskeleton depolymerization causes nuclear collapse and “wrinkling” of the nuclear lamina, suggesting that the intact cytoskeleton had been producing outward stress on the nucleus. Scale is 25 µm. (B) Quantification of nuclear morphology shows that PSC nuclei are more elongated than in MSCs, and low oxygen enhances this difference (ANOVA p < 0.001), (C) While PSCs are normally less deformable than MSCs (as in Figure 2), culture in low oxygen shows higher deformability (t-test p < 0.005 – line). The three values for A (Pa−1) are statistically distinct (ANOVA p < 0.04). (D) Changes in nuclear area with depolymerization of the cytoskeleton are related to how deformed the nucleus is inside the cell by mechanoactive structures. Projected nuclear area is larger in cells cultured at 6% O2 than at 21% O2, but nuclei dramatically retract with cytoskeletal disruption. Nuclear area in cells cultured at 21% O2 show no statistical change with cytoskeletal disruption. Dashed lines are p < 0.001 (t-test between samples). Errors are SEM.
We measured mechanical properties of PSCs using micropipette aspiration. In contrast to the PSCs at 21% oxygen, which were the stiffest cells, PSCs at 6% oxygen were the softest among tested cells of mesenchymal origin (Fig. 7C). To test the dependence of nuclear mechanics in actin-nuclear connections without suspending cells, PSCs were incubated in cytochalasin D and nocodazole (Fig. 7A) to disrupt the cytoskeleton. As observed previously in cells of mesenchymal lineage (Mazumder and Shivashankar, 2010), the nuclear envelope wrinkled, the nuclear area was reduced and nuclear shape evolved towards an elongated structure similar to when cells are detached from the substrate (Fig. 1).
With incubation in cytoskeleton disrupting agents, the nuclear size in 6% oxygen is significantly reduced compared to cells in 21% oxygen (Fig. 7D). Thus, the deformability of the cell (Fig. 7C) appears to be related to the deformability and collapsibility of the nucleus (Fig. 7D). Cell flexibility may be manipulated by altering oxygen conditions, allowing better experimental design as well as a switch in cell phenotype upon implantation.
4. Discussion
Tissues are responsive mechanical structures and cells respond to both external chemical and mechanical cues. Matching the mechanics of the tissues of origin is optimal for ex vivo cellular function and in directing stem cell differentiation (Chowdhury et al., 2010; Dimmeler et al., 2005; Engler et al., 2006). The mechanical differences of stem cells from two different niches (bone marrow and perivascular), or of two different origins (mesenchymal and hematopoietic), or in different oxygen culture conditions (6% and 21%) reveals the importance of these different factors in governing cell structures and mechanics. In general, organization of the cytoskeleton and nucleoskeleton can be affected by extracellular force, intracellular force and changes in signaling pathways (Dahl et al., 2008) – these may all be affected by low oxygen conditions.
Cell migration within hematopoietic microenvironments is increased in low oxygen conditions (Jing et al., 2011). Low oxygen cell culture conditions also help maintain pluripotency (Szablowska-Gadomska et al., 2011) in part due to the activation of hypoxia-inducible transcription factor (HIF) pathways (Nakayama, 2009). HIF1-α also alters MSC differentiation pathways (Jandial et al., 2011). Stimulation of HIF by low oxygen alters levels of vascular endothelial growth factor (VEGF) (Semenza, 2003) and αVβ3 integrin (Cowden Dahl et al., 2005), which are mechanotransducing elements (Roca-Cusachs et al., 2009). Thus, there are many indirect pathways activated by low oxygen conditions that alter cell phenotype and mechanics.
4.1 Optimizing the choice of stem cells for different usages based on their origins
MSCs have been the most widely studied for stem cell mechanics. Previous studies have characterized hMSCs to be viscoelastic solids with an instantaneous Young’s modulus of 890 +/− 200 Pa and an equilibrium Young’s modulus of 300 +/− 100 Pa (Tan et al., 2008; Yu et al., 2010) using a 3 component model or an elastic modulus of 1900 Pa (Darling et al., 2008). Different assays usually provide different results depending on the way forces are applied and on the tested length scales (Kasza et al., 2007). A power-law viscoleastic model was here used after qualitative evaluation of deformation of cells during aspiration (Supplemental figure 5). A direct comparison of data from different viscoelastic models is difficult, especially with large uncertainties in the parameters. From fits to our power-law analytical model, we observe an effective stiffness of ~900 Pa at 0.01 to 0.1 sec and an effective stiffness of 100 Pa at 1–10 sec. Generally, our results suggest a slightly softer MSC than previously reported possibly due to smaller aspiration pressures needed to fully observe the power-law deformation.
The methodology used in the presented study allows the comparison of three different types of adult stem cells as viscoleastic structures considering potential therapeutic applications. Hematopoietic, mesenchymal and perivascular stem cells present a high level of plasticity, allowing differentiation into several cell types (Graf, 2002). Selection of stem cells for the development of therapeutic devices has been traditionally based on biological criteria considering chemical regulatory phenomena. In potential therapies, implanted stem cells or progenitor cells may not only differentiate towards specific tissue cells, but also secrete paracrine factors that sustain regeneration (Dimmeler et al., 2005; Malliaras and Marban, 2011). We suggest that selection criteria for stem cell use for therapeutic applications should include consideration of mechanical and structural properties. Cells are sensitive to the mechanical environment, which can affect in situ localization. Extracellular mechanical environment can also impact stem cell differentiation (Engler et al., 2006); matching mechanical properties of biomaterial scaffolds to cell mechanical properties will enhance efficacy of hybrid materials. Similarly, injection of stem cells requires survival of suspended cells in flow conditions.
Stem cell injection systems have been successfully used for the treatment of cell damaging heart diseases in humans (Ghodsizad et al., 2009), treatment of autoimmunity (Locascio et al., 2011), induction of immune tolerance (Kline et al., 2008) and cancer treatment (Jeltsch et al., 2011). Our results suggest that CD34+ cells would be more tolerant to stem cell injection. At flow rates of 0.5 mL/s, 16.7 ± 3% of CD34+ cells survived injection through needles used clinically (see Supplemental Methods), but only 1.0 ± 0.2% of MSCs survived the same injection. For injection, cells must be suspended without altered biological properties, sufficiently deformable in fluid flow, and resist rupture. CD34+ cells were the most compliant under deformation without rupture. In addition, these cells transitioned to more fluid-like character when aspirated. Upon differentiation, CD34+ cells formed cell structures appropriate for adherent cell function.
Transplantation of porous biomaterials with seeded stem cells has presented promising outcomes in the treatment of several pathologies, as for example spinal cord injury (Sykova et al., 2006), osteogenesis (Ben-David et al., 2011) and diabetic foot ulcer (Mansbridge et al., 1998). For populating biomaterial scaffolds, cells must be flexible and interact with the solid substrate. Cells of mesenchymal lineage are soft but show stable adhesions, highly developed cytoskeletal and nucleoskeletal structures and may be the most suitable for implantation in solid materials for transplantation.
PSCs appear to be the most rigid and organized mechanical cells, but altering oxygen culture conditions increases flexibility. PSCs in 6% oxygen show optimal potential for seeding and penetration into porous materials. Previously, an increase in cell proliferation and migration was observed in cultures of PSCs under 6% oxygen (Tottey et al., 2010). This already suggested that tissue-engineered structures produced in vitro might present different mechanical and biological behaviors when implanted inside the body where oxygen conditions may significantly change. Probably engineering of tissues should focus on the influences of low oxygen environments to model more in vivo-like behavior.
4.2 Load bearing structures inside stem cells
Adult stem cells, including MSCs, can penetrate tight tissue matrices and travel long distances away from their original niches (Ferrari et al., 1998). Cell translocation is increased with actin force generation, but the nucleus is a resistive mechanical element that limits motility and migration through tissues (Friedl et al., 2011). Here, we found that organization of structural elements and cell mechanics in stem cells depend on the stem cell lineage, tissue origin and oxygen culture conditions. The region of cell isolation may be a useful metric for therapeutic usage. Only three different types of stem cells were here tested, which leaves a large domain for future discovery. Similar properties may be observed in the remaining variety of stem cells, such as stem cells isolated from the blood, presenting a high potential for the modeling and developing of several transplantation methods towards several tissue homing sites.
Supplementary Material
Acknowledgements
We gratefully acknowledge funding from the Portuguese Government - Ministry of Science and Higher Education (44299 FCT to A.R.), the NSF (CBET 0954421 to K.D.) and the NIH (5R01 AR054940 to S.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
AJS Ribeiro designed experiments, collected, analyzed and interpreted data, and wrote the manuscript. S Tottey designed experiments, collected, analyzed and interpreted data. RWE Taylor and R Bise collected and analyzed data. T Kanade designed experiments and approved the manuscript. SF Badylak designed experiments and provided financial support. KN Dahl designed experiments, provided financial support, and wrote part of the manuscript.
Conflict of Interest Disclosures
None of the authors have a conflict of interest
References
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. New English Journal of Medicine. 2006:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proceedings of the National Academy of Sciences U S A. 1992:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David D, Kizhner TA, Kohler T, Muller R, Livne E, Srouji S. Cell-scaffold transplant of hydrogel seeded with rat bone marrow progenitors for bone regeneration. Journal of Craniomaxillofacial Surgery. 2011:364–371. doi: 10.1016/j.jcms.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Charras GT, Williams BA, Sims SM, Horton MA. Estimating the sensitivity of mechanosensitive ion channels to membrane strain and tension. Biophysical Journal. 2004:2870–2884. doi: 10.1529/biophysj.104.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature Materials. 2010:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association. European Journal of Heart Failure. 2006:105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Molecular Biology of the Cell. 2005:1901–1912. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Peault B. Perivascular multipotent progenitor cells in human organs. Annals of the New York Academy of Sciences. 2009:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophysical Journal. 2005:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Kalinowski A, Pekkan K. Mechanobiology and the microcirculation: cellular, nuclear and fluid mechanics. Microcirculation. 2010:179–191. doi: 10.1111/j.1549-8719.2009.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circulation Research. 2008:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. Journal of Biomechics. 2008:454–464. doi: 10.1016/j.jbiomech.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal. 1999:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. Journal of Clinical Investigation. 2005:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evans E, Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984:1028–1035. [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Current Opinion in Cell Biology. 2011:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodsizad A, Niehaus M, Kogler G, Martin U, Wernet P, Bara C, Khaladj N, Loos A, Makoui M, Thiele J, Mengel M, Karck M, Klein HM, Haverich A, Ruhparwar A. Transplanted human cord blood-derived unrestricted somatic stem cells improve left-ventricular function and prevent left-ventricular dilation and scar formation after acute myocardial infarction. Heart. 2009:27–35. doi: 10.1136/hrt.2007.139329. [DOI] [PubMed] [Google Scholar]
- Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002:3089–3101. doi: 10.1182/blood.v99.9.3089. [DOI] [PubMed] [Google Scholar]
- Grogaard HK, Seljeflot I, Lunde K, Solheim S, Aakhus S, Forfang K, Arnesen H, Ilebekk A. Cell treatment after acute myocardial infarction prevents early decline in circulating IGF-1. Scandanavian Cardiovascular Journal. 2010:267–272. doi: 10.3109/14017431.2010.490949. [DOI] [PubMed] [Google Scholar]
- Hao Y, Cheng D, Ma Y, Zhou W, Wang Y. The relationship between oxygen concentration, reactive oxygen species and the biological characteristics of human bone marrow hematopoietic stem cells. Transplantation Proceedings. 2011:2755–2761. doi: 10.1016/j.transproceed.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. Journal of Biological Chemistry. 2010:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DA, Allan AL. Migratory strategies of normal and malignant stem cells. Methods in Molecular Biology. 2011:25–44. doi: 10.1007/978-1-61779-145-1_2. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. Journal of Biomechanics. 2000:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Hoffman BD, Massiera G, Van Citters KM, Crocker JC. The consensus mechanics of cultured mammalian cells. Proceedings of the National Academy of Science U S A. 2006:10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben F, Ramaekers FC, Snoeckx LH, Broers JL. Role of nuclear lamina-cytoskeleton interactions in the maintenance of cellular strength. Biochimica Biophysica Acta. 2007:675–686. doi: 10.1016/j.bbamcr.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Jandial R, Chen MY, Ciacci J. HIF-1alpha potentiates mesenchymal stem cell mediated osteogenesis by coupling to angiogenesis. Neurosurgery. 2011:N13–NN14. doi: 10.1227/01.neu.0000405591.47966.a1. [DOI] [PubMed] [Google Scholar]
- Jeltsch KS, Radke TF, Laufs S, Giordano FA, Allgayer H, Wenz F, Zeller WJ, Kogler G, Fruehauf S, Maier P. Unrestricted somatic stem cells: interaction with CD34(+) cells in vitro and in vivo, expression of homing genes and exclusion of tumorigenic potential. Cytotherapy. 2011:357–365. doi: 10.3109/14653249.2010.523076. [DOI] [PubMed] [Google Scholar]
- Jing D, Wobus M, Poitz DM, Bornhauser M, Ehninger G, Ordemann R. Oxygen tension plays a critical role in the hematopoietic microenvironment in vitro. Haematologica. 2011 doi: 10.3324/haematol.2011.050815. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, Weitz DA. The cell as a material. Current Opinions in Cell Biology. 2007:101–107. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Kline J, Subbiah S, Lazarus HM, van Besien K. Autologous graft-versus-host disease: harnessing anti-tumor immunity through impaired self-tolerance. Bone Marrow Transplant. 2008:505–513. doi: 10.1038/sj.bmt.1705931. [DOI] [PubMed] [Google Scholar]
- Lekli I, Gurusamy N, Ray D, Tosaki A, Das DK. Redox regulation of stem cell mobilization. Canadian Journal of Physiology Pharmacology. 2009:989–995. doi: 10.1139/Y09-102. [DOI] [PubMed] [Google Scholar]
- Li SC, Wang L, Jiang H, Acevedo J, Chang AC, Loudon WG. Stem cell engineering for treatment of heart diseases: potentials and challenges. Cell Biology International. 2009:255–267. doi: 10.1016/j.cellbi.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocol. 2007:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio S, Spinelli J, Kurtz J. Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmunity in Type 1 Diabetes. Current Stem Cell Research Therapy. 2011:29–37. doi: 10.2174/157488811794480681. [DOI] [PubMed] [Google Scholar]
- Malliaras K, Marban E. Cardiac cell therapy: where we've been, where we are, where we should be headed. British Medical Bulletin. 2011:161–185. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney JM, Nikova D, Lautenschlager F, Clarke E, Langer R, Guck J, Van Vliet KJ. Mesenchymal stem cell mechanics from the attached to the suspended state. Biophysical Journal. 2010:2479–2487. doi: 10.1016/j.bpj.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbridge J, Liu K, Patch R, Symons K, Pinney E. Three-dimensional fibroblast culture implant for the treatment of diabetic foot ulcers: metabolic activity and therapeutic range. Tissue Engineering. 1998:403–414. doi: 10.1089/ten.1998.4.403. [DOI] [PubMed] [Google Scholar]
- Mansour S, Vanderheyden M, De Bruyne B, Vandekerckhove B, Delrue L, Van Haute I, Heyndrickx G, Carlier S, Rodriguez-Granillo G, Wijns W, Bartunek J. Intracoronary delivery of hematopoietic bone marrow stem cells and luminal loss of the infarct-related artery in patients with recent myocardial infarction. Journal of the Americal College of Cardiology. 2006:1727–1730. doi: 10.1016/j.jacc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Mazumder A, Shivashankar GV. Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. Journal of the Royal Society: Interface. 2010:S321–S330. doi: 10.1098/rsif.2010.0039.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nature Reviews Molecular Cell Biology. 2006:540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months'follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Cellular signal transduction of the hypoxia response. Journal of Biochemistry. 2009:757–765. doi: 10.1093/jb/mvp167. [DOI] [PubMed] [Google Scholar]
- Noth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Advanced Drug Delivery Reviews. 2010:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proceedings of the National Academy of Science U S A. 2007:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C, D'Aprile A, Scrima R, Ripoli M, Boffoli D, Tabilio A, Capitanio N. Role of reactive oxygen species as signal molecules in the pre-commitment phase of adult stem cells. Italian Journal of Biochemistry. 2007:295–301. [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proceedings of the National Academy of Science U S A. 2009:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. New England Journal Medicine. 2006:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sykova E, Jendelova P, Urdzikova L, Lesny P, Hejcl A. Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cellular and Molecular Neurobiology. 2006:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szablowska-Gadomska I, Zayat V, Buzanska L. Influence of low oxygen tensions on expression of pluripotency genes in stem cells. Acta Neurobiologiae Expperimentalis. 2011:86–93. doi: 10.55782/ane-2011-1825. [DOI] [PubMed] [Google Scholar]
- Tan SC, Pan WX, Ma G, Cai N, Leong KW, Liao K. Viscoelastic behaviour of human mesenchymal stem cells. BMC Cell Biology. 2008:40. doi: 10.1186/1471-2121-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo AK, Vallier L. Emerging use of stem cells in regenerative medicine. Biochemical Journal. 2010:11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- Titushkin I, Cho M. Distinct membrane mechanical properties of human mesenchymal stem cells determined using laser optical tweezers. Biophysical Journal. 2006:2582–2591. doi: 10.1529/biophysj.105.073775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Engineering Part A. 2010:37–44. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Citters KM, Hoffman BD, Massiera G, Crocker JC. The role of F-actin and myosin in epithelial cell rheology. Biophysical Journal. 2006:3946–3956. doi: 10.1529/biophysj.106.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Driskell RR. The therapeutic potential of stem cells. Philosophical Transactions of the Royal Society B: Biolical Sciences. 2010:155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Xu X, Guo Y, Gao W. Inflammatory responses after intracoronary mononuclear BM cell therapy in swine. Bone Marrow Transplant. 2009:427–431. doi: 10.1038/bmt.2009.52. [DOI] [PubMed] [Google Scholar]
- Yu H, Tay CY, Leong WS, Tan SC, Liao K, Tan LP. Mechanical behavior of human mesenchymal stem cells during adipogenic and osteogenic differentiation. Biochemical and Biophysical Research Communications. 2010:150–155. doi: 10.1016/j.bbrc.2010.01.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.