Abstract
The toll-like receptors (TLR) and myocardial infarction (MI) promote NF-κB-dependent inflammatory transcription and oxidative injury in myocardium. The multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by oxidation and contributes to NF-κB-dependent transcription, myocardial hypertrophy and post-MI death. The myeloid differentiation protein 88 (MyD88) is an adapter protein critical for many TLR functions, but downstream targets for TLR/MyD88 signaling in MI are not well understood. We asked if CaMKII and TLR/MyD88 pathways are interconnected and if TLR/MyD88 contributes to adverse outcomes after MI. Here we show that TLR-4 activation by lipopolysaccharide (LPS) induces CaMKII oxidation (ox-CaMKII) in cardiomyocytes. MI enhances ox-CaMKII in wild type (WT) hearts but not in MyD88−/− hearts that are defective in MyD88-dependent TLR signaling. In post-MI WT hearts expression of pro-inflammatory genes TNF-α (Tnfa), complement factor B (Cfb), myocyte death and fibrosis were significatly increased, but increases were significantly less in MyD88−/− hearts after MI. MyD88−/− cardiomyocytes were defective in NF-κB activation by LPS but not by the MyD88-independent TLR agonist poly(I:C). In contrast, TNF-α induced Cfb gene expression was not deficient in MyD88−/− cardiomyocytes. Several hypertrophy marker genes were upregulated in both WT and MyD88−/− hearts after MI, but Acta1 was significantly attenuated in MyD88−/− hearts, suggesting that MyD88 selectively affects expression of hypertrophic genes. Post-MI cardiac hypertrophy, inflammation, apoptosis, ox-CaMKII expression and mortality were significantly reduced in MyD88−/− compared to WT littermates. These data suggest that MyD88 contributes to CaMKII oxidation and is important for adverse hypertrophic and inflammatory responses to LPS and MI.
Keywords: Myocardial infarction, Hypertrophy, Inflammation, Oxidant stress, CaMKII, Innate Immunity
1. Introduction
Myocardial infarction (MI) is marked by inflammatory gene expression [1–5] and is associated with increased reactive oxygen species (ROS) production, pathological myocardial hypertrophy, heart failure and increased mortality [6]. The multifunctional Ca2+/calmodulin dependent protein kinase II (CaMKII) has recently emerged as a MI and ROS-activated signaling molecule that regulates expression of inflammatory genes and affects adverse outcomes after MI [5, 7]. Cardiac-specific transgenic overexpression of CaMKII results in cardiac hypertrophy, heart failure and premature death [8, 9]. CaMKII becomes constitutively active by threonine 287 ‘autophosphorylation’ (phospho-CaMKII) and by oxidation of methionines 281/282 (ox-CaMKII) and constitutively active CaMKII appears to contribute to increased mortality in mice after MI [7, 10]. In contrast, inhibition of CaMKII activity can attenuate many of these MI-related adverse effects [7, 11]. We found that CaMKII modulates post-MI expression of genes involved in inflammation such as complement factor B (Cfb) [5]. Mice deficient in Cfb gene were protected from cardiac hypertrophy after an MI and had improved survival. Cfb and other proinflammatory genes, such as TNF-α are dependent on the NF-κB transcription factor and are induced through activation of toll-like receptors (TLRs), suggesting that TLR signaling may activate CaMKII and contribute to diverse adverse consequences to MI [5, 6].
TLRs are integral components of the innate immune system that recognize pathogens and sterile injury and elaborate the inflammatory response, including expression of cytokines, chemokines and complement pathways [12, 13]. TLR-4 deficient mice have reduced myocardial injury after ischemia-reperfusion [14, 15] and had reduced hypertrophy in pressure overload and MI models [16, 17]. Adenoviral infection with a dominant negative form of myeloid differentiation protein 88 (MyD88) was protective against hypertrophy, apoptosis and fibrosis in a rat model of aortic banding, [18] whereas in an ischemia-reperfusion model, MyD88 inhibition suppressed NF-κB induction and myocyte apoptosis [19]. Inhibition of NF-κB by cardiomyocyte-specific expression of a dominant-negative IκB mutant reduced cardiac hypertrophy in a myotrophin transgene mediated hypertrophy/heart failure model [20]. These studies suggest that following injury or stress, activation of the NF-κB pathway through TLR is pathologically important to the myocardium. Most TLRs function through an adapter MyD88 that has no known enzymatic activity, but is required for downstream signaling and activation of the NF-κB transcription factors [13]. TLR-3 does not require MyD88 for its signaling [21] whereas TLR-4, the predominant TLR isoform in myocardium [4], utilizes MyD88-dependent and MyD88-independent pathways for downstream signaling to NF-κB [22]. Loss of MyD88 severely compromises many TLR mediated inflammatory responses [23, 24]. MyD88 signaling is important in pathological responses to aortic banding and dilated cardiomyopathy [18, 19, 25], but little is known about the potential role of MyD88 in inflammatory responses after MI. Furthermore, potential molecular mechanisms underlying TLR/MyD88 signaling in post-MI hearts are not understood. Here we show a role of MyD88 in TLR-mediated inflammation and hypertrophy, while MyD88−/− mice are protected from MI-mediated increases in CaMKII oxidation and phosphorylation, myocardial hypertrophy, inflammation, cell death, fibrosis and mortality compared to wild type littermate controls.
2. Materials and methods
2.1 Animals
C57BL6/J mice were obtained from Jackson Laboratories. MyD88−/− mice were developed by Shizuo Akira’s group [23] in Japan and were kindly provided to us by Dr. M. Nedim Ince, University of Iowa. HLL mice were a generous gift from Drs. Timothy Blackwell and Fiona Yull, Vanderbilt University [5, 26]. All experiments with animals were done in accordance with the regulations put forth by the Institutional Animal Care and Use Committee of University of Iowa.
2.2 MI surgery and echocardiography
MI surgery and pre- and post-MI echocardiography were performed as described [5]. Briefly, hearts were infarcted by placing a permanent ligature on the left anterior descending coronary artery. Both male and female mice were used for MI surgery. Infarct size was determined by echocardiography [27]. Mice with comparable infarct size were included in the studies.
2.3 Cell cultures
Neonatal cardiomyocyte cultures were prepared from 1 to 3 day old mouse pups [5]. Following enzymatic digestion of the tissue matrix, adherent fibroblasts were removed by pre-plating on plastic Petri dishes. Non-adherent cardiomyocytes were cultured in growth medium supplemented with a mix of 5% of equine and fetal bovine serum and penicillin/streptomycin. After 24 hours, the culture medium was replaced with a defined medium to allow for cell differentiation. Cultures were supplemented with 5-bromo-2’-deoxyuridine (BrdU). For adenovirus transduction, cultured cells were challenged with virus particles for 48 h followed by LPS treatment.
2.4 RNA isolation and qRTPCR
Total RNA from mouse tissues and cardiomyocytes was isolated using mirVana RNA isolation kit (Ambion) and RNeasy RNA Isolation Kit (Qiagen), respectively. A 500 ng or 1 µg aliquot of cardiomyocytes or tissue RNA sample, respectively, was used to synthesize cDNA in 50 µl reactions using Oligo(dT) as primers and SuperScript III Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was performed on iQ5 cycler (BioRad) using SYBR green based PCR reactions, as described [5]. Quantifications were done using ΔΔCT method where Gapdh was used as a reference gene for normalization of RNA expression. The primers used in this study are listed in Table-1. Sequences for Myh6 and Myh7 primers were kindly provided by Dr. Leslie Leinwand.
Table-1.
Sequences of primers used for qRTPCR.
| Cfb For | 5’-AGCCTTCCTGCCAAGATTCC-3’ |
| Cfb Rev | 5’-CTTCCTCTTCTGCTGTTCTCC-3’ |
| Cxcl10 For | 5’-GCTGCCGTCATTTTCTGC-3’ |
| Cxcl10 Rev | 5’-TCTCACTGGCCCGTCATC-3’ |
| Tnfa For | 5’-TGCCTATGTCTCAGCCTCTTC-3’ |
| Tnfa Rev | 5’-GAGGCCATTTGGGAACTTCT-3’ |
| Acta1 For | 5’-AATGAGCGTTTCCGTTGC-3’ |
| Acta1 Rev | 5’-ATCCCCGCAGACTCCATAC-3’ |
| Nppa For | 5’-GTCTTGGCCTTTTGGCTTC-3’ |
| Nppa Rev | 5’-TTCCTCAGTCTGCTCACTC-3’ |
2.5 Luciferase activity assays
HLL mice harbor a transgenic construct of firefly luciferase cDNA fused to the proximal 5’ human immunodeficiency virus (HIV-1) long terminal repeat [26]. This promoter is highly responsive to NF-κB activation. NF-κB activity in the reporter HLL mouse tissues was determined by measuring luciferase activity at indicated time points after surgery using Luciferase assay reagent (Promega). Luciferase activity from equal volumes of lysates was normalized to the total protein content of the samples.
Cardiomyocytes were transfected with an NF-κB-luciferase reporter plasmid (Clontech) using Fugene 6 transfection reagent (Roche). An expression plasmid construct for Renilla luciferase (pRL-TK, Promega) was also added as a control for transfection efficiency. Cells were treated with TNF-α (400 pg per ml), LPS (E. coli, Sigma, 10 µg per ml) or poly(I:C) (4 µg per ml, Sigma). Cells were harvested in passive lysis buffer (Promega) and luciferase activity was measured in a luminometer (Berthold Technologies) using the Dual Luciferase Assay (DLR, Promega). Firefly luciferase measurements were normalized with Renilla luciferase.
2.6 Myeloperoxidase activity assay
Myeloperoxidase activity assays were performed as described by Bradley et al. [28]. Hearts were rinsed to remove blood and homogenized in 0.5% hexadecyltrimethylammonium bromide, HTAB) in 50 mM potassium phosphate buffer (pH 6.0). Homogenates were cleared by centrifugation and MPO activity was determined using O-dianisidine dihydrochloride (0.167 mg/ml, Sigma) as a substrate and a Nanodrop 2000c spectrophotometer (460 nm; Thermo Scientific). MPO activity measured as absorbance (460 nm) in each heart sample was normalized to its protein content determined by BCA method (Thermo Scientific). A time-course of each reaction was measured and results were calculated when the reaction was in a linear range for all the samples.
2.7 Immunoblotting
Tissue samples were homogenized in modified RIPA buffer (50 mM Hepes, pH 7.5; 150 mM NaCl; 5 mM EDTA; 1% v/v NP-40 and 0.5% w/v deoxycholate) or MPO lysis buffer containing a mixture of protease- and phosphatase inhibitors. Equal amounts of protein were fractionated on NuPAGE gels (Invitrogen) and transferred onto PVDF membranes (BioRad). After blocking non-specific binding with 10% w/v non-fat milk powder in TBS-T (50 mM Tris-HCl, pH 7.6; 150 mM NaCl and 0.1% v/v Tween-20), blots were incubated in primary antibody (rabbit anti-Ox-CaMKII) [7]. Blots were washed in TBS-T and incubated with appropriate HRP-conjugated secondary antibodies. Protein bands were detected using ECL reagent (Lumi-Light, Roche) and after stripping, the blots were reprobed with an anti-CaMKII antibody raised against the C-terminal domain of CaMKIIδ. For detection of HA-Rac1DN, mouse monoclonal antibody Mab 16B12 (Covance) was used. Sample loading in lanes were routinely monitored after antibody probing by Coomassie staining of the blots. For quantification of immunoblots we used Quantity One software (BioRad).
2.8 Statistics
Statistical analyses were performed using an unpaired Student’s t-test (two-tailed; paired t-test for pre- and post-MI echocardiography data) or by ANOVA, as shown in respective figures. Post-hoc analyses on multiple group data were performed using Bonferroni’s test. A P value of less than 0.05 was considered to be statistically significant. All results are presented as mean± SEM.
3. Results
3.1 MyD88 activates NF-κB and proinflammatory gene expression in post-MI hearts
NF-κB activation is a major determinant of inflammation after infection and injury [29]. MI causes increased NF-κB-mediated expression of pro-inflammatory genes. We used a transgenic NF-κB-luciferase reporter mouse [5, 26] to test activation of the NF-κB pathway in post-MI hearts. Post-MI induction of NF-κB was determined 1 and 7 days after MI (Figure 1A). We observed an increase in the NF-κB activity in hearts at day 1 after MI that persisted through 7 days post-MI. Luciferase activity at these time points was significantly higher than in sham-operated controls (day 1), consistent with the previously established NF-κB proinflammatory response to MI.
Figure 1.
Post-MI induction of NF-κB and inflammatory gene expression in MyD88−/− hearts. (A) Schematic of transgenically expressed NF-κB activity reporter construct (upper panel). Luciferase activity from heart lysates from NF-κB reporter mice 1 and 7 days after MI were determined. Luciferase activity was normalized to the protein content of the lysates (n≥3 mice/group). (B) RNA from control (Ctr) and post-MI (1week and 3 week) WT and MyD88−/− hearts was isolated and mRNA for (B) complement factor B (Cfb), (C) TNF-α (Tnfa), and (D) chemokine Cxcl10 (Cxcl10) were quantified using qPCR (ΔΔCT method). Gapdh mRNA expression was used as a loading control (n=3 hearts per group). One-way ANOVA with Bonferroni’s post-test (*** P ≤ 0.0001, * P ≤ 0.01, n.s. = not significant).
TLRs are pathogen and injury-sensing receptors of the innate immune system that signal inflammatory response through NF-κB activation. Several TLRs are expressed on cardiomyocytes that participate in MI induced inflammation and gene expression [3]. MyD88 is a common adapter for downstream signaling of TLRs. We asked if MyD88-dependent NF-κB activation is important for post-MI proinflammatory gene expression. We measured expression of two proinflammatory genes TNF-α (Tnfa) and complement factor B (Cfb), because the expression of these genes is dependent on NF-κB activation in post-MI hearts [5, 30]. In WT hearts, we measured increased expression of Tnfa (Figure 1B) and Cfb (Figure 1C) at 1 week after MI. However, in MyD88−/− hearts, expression of these genes was significantly reduced compared to WT hearts (Tnfa P < 0.05, Cfb P<0.0001). Interestingly, post-MI expression of Cxcl10, a reparative chemokine-encoding gene that is induced by MI [31] and whose expression does not depend on MyD88 signaling [22], was unaffected in MyD88−/− hearts (Figure 1D). These results suggest that MI induced expression of specific pro-inflammatory genes relies upon MyD88-dependent activation of NF-κB.
3.2 Reduced expression of Cfb and TNF-α in MyD88−/− cardiomyocytes
We previously demonstrated that CaMKII in cardiomyocytes activates TLR-4 induced NF-κB mediated transcription of Cfb [5]. Therefore, we tested whether reduced proinflammatory gene expression in MyD88−/− post-MI hearts was due to reduced gene expression in cardiomyocytes. We measured mRNA expression of Cfb in WT and MyD88−/− cultured neonatal cardiomyocytes. Expression of Cfb mRNA was strongly induced by LPS in WT cardiomyocytes (Figure 2A). However, LPS treated MyD88−/− cardiomyocytes had significantly (P < 0.001) reduced Cfb mRNA. To ensure that reduced expression of Cfb in MyD88−/− cardiomyocytes was attributable to MyD88 signaling and not to a general defect in the NF-κB pathway, we measured the expression of Cfb in WT and MyD88−/− cardiomyocytes after treatment with TNF-α, which also induces Cfb expression by a MyD88-independent, NF-κB-dependent pathway [5]. Both WT and MyD88−/− cardiomyocytes strongly induced Cfb expression upon TNF-α treatment (Figure 2B). These results suggest a selective upstream defect in the TLR/MyD88 -NF-κB pro-inflammatory gene expression pathway of MyD88−/− cardiomyocytes.
Figure 2.
NF-κB induction in MyD88−/− cardiomyocytes. Cfb RNA was quantified from WT and MyD88−/− cultured neonatal cardiomyocytes after 12 h treatment with LPS (A) or TNF-α (B). Expression was compared by ΔΔCT method and statistical analyses performed using One-way ANOVA and Bonferroni’s post-test. For testing role of MyD88 in NF-κB induction, cultured neonatal cardiomyocytes from WT (C) and MyD88−/− (C and D) mice were transfected with an NF-κB-luciferase reporter plasmid. After 48 h of transfection, cells were treated with 10 µg per ml LPS (C) or 4 µg per ml poly(I:C) (D) for indicated time. Controls (Ctr) were left untreated. Luciferase activity from a Renilla luciferase reporter was used as a control for transfection efficiency.
The MyD88 adapter is utilized by most TLRs, with the notable exception of TLR-3, for signaling to activate the NF-κB transcription factor. To determine if the reduction in pro-inflammatory gene expression in MyD88−/− cardiomyocytes was restricted to MyD88 mediated signaling, we tested the induction of NF-κB by LPS (a TLR-4 agonist) and poly(I:C) (a TLR-3 agonist). WT and MyD88−/− cultured cardiomyocytes were transfected with an NF-κB –luciferase reporter plasmid and treated with LPS for different durations (1.5 h, 6 h, 12h). WT cardiomyocytes readily induced luciferase activity within 6 hours after LPS treatment and luciferase activity continued to increase 12 hours after the treatment (Figure 2C). In contrast, MyD88−/− cardiomyocytes did not induce NF-κB activity upon LPS treatment. However, poly(I:C) did induce NF-κB mediated luciferase activity in MyD88−/− cardiomyocytes (Figure 2D). Thus, loss of MyD88 expression leads to circumscribed defects in in MyD88−/− cardiomyocytes. Taken together, these results show that MyD88 knockout did not prevent induction of the NF-κB pathway in response to TLR-3 agonist stimulation. We interpret these data to confirm that reduced pro-inflammatory gene expression in post-MI MyD88−/− hearts is due to defective MyD88-dependent signaling rather than to a generalized defect in NF-κB signaling.
3.3 Reduced immune cell infiltration and cell death in post-MI MyD88−/− hearts
MI results in infiltration of inflammatory immune cells and tissue death at the site of injury. Injured tissue releases cytokines and chemokines to attract circulating neutrophils to the site of injury. Cardiomyocytes produce inflammatory molecules and cytokines in response to MI [5, 32]. Therefore, we tested if TLR/MyD88 signaling influences infiltration of neutrophils in post-MI hearts (Figure 3A). Sections from post-MI hearts (day 1) were labeled with antibody against S100a9, a neutrophil marker. WT hearts had significantly more infiltrating cells than MyD88−/− (Figure 3A and 3B; WT average 24 cells per frame vs. MyD88−/− average 15 cells; P= 0.0008). No infiltration of S100a9 positive cells was observed in sham-operated hearts. (Figure 3A). Neither WT nor MyD88−/− hearts showed infiltrating S100a9 positive cells in the tissue remote from the infarct zone (not shown). To confirm these results, we also performed a myeloperoxidase (MPO) enzyme assay on 1day post-MI heart lysates from WT and MyD88−/− mice (Figure 3C). Sham operated hearts from WT and MyD88−/− mice had similarly low basal MPO enzyme activity. However, post-MI WT hearts showed a 10-fold increase in MPO activity after MI. In contrast, post-MI MyD88−/− hearts had only 4-fold increase that was not statistically significant. Thus, post-MI MyD88−/− hearts had reduced MPO enzyme activity compared to post-MI WT hearts (approximately 2-fold, unpaired t-test P= 0.04). These results show that TLR/MyD88 signaling is an important determinant in MI related immune response at the site of injury.
Figure 3.
Immune cell infiltration in infarcted myocardium. (A) Tissue sections from post-MI or sham operated (1 day) hearts from WT and MyD88−/− mice were labeled with neutrophil and granulocytes marker S100a9 (green fluorescence). Nuclei were stained with DAPI (Blue). Scale bars represent 50 µm. (B) Average number of infiltrating cells were counted in multiple sections from 3 hearts. MyD88−/− hearts had significantly less infiltration (unpaired t-test, ***P= 0.0008). (C) Myeloperoxidase (MPO) activity in sham operated or MI hearts (1-day post surgery) was performed as described in the text. MyD88−/− hearts showed significant reduction in MPO activity (One way ANOVA with Bonferroni’s post-test; n=3 each, *P≤ 0.01).
We determined the extent of apoptotic cell death by TUNEL staining in the heart at 1day post-MI. In both WT and MyD88−/− hearts, almost all the TUNEL positive nuclei were confined to the tissue region affected by MI. WT hearts showed a large number of nuclei that were TUNEL positive (53% TUNEL positive nuclei, Figure 4A and 4B). In contrast, MyD88−/− hearts had significantly less TUNEL positive nuclei (31% TUNEL positive nuclei, P< 0.01). These results show that MyD88 deficiency in MyD88−/− hearts resulted in significantly reduced apoptotic cell death.
Figure 4.
TUNEL staining in infarcted (1 day post-MI) WT and MyD88−/− hearts. (A) Green fluorescence shows TUNEL positive nuclei whereas blue fluorescence represents total stained nuclei with DAPI. Scale bars= 50 µm. (B) Post-MI MyD88−/− hearts had significantly less TUNEL positive nuclei (3 hearts each group, *P ≤ 0.01).
3.4 Reduced post-MI hypertrophy in post-MI MyD88−/− hearts
We measured MI-induced cardiac hypertrophy in WT and MyD88−/− mice 3 weeks after MI. Compared to the WT hearts, MyD88−/− hearts had significantly reduced cardiac hypertrophy (P= 0.02; Figure 5A). To test if the observed cardiac hypertrophy was due to increased size of cardiomyocytes, we measured the cross-section area of cardiomyocytes in 3-week post-MI heart sections (Figure 5B). Compared to the WT cells (n= 276, area 450 µm2) MyD88−/− cardiomyocytes had significantly reduced size (Figure 5C; n= 296, area 402 µm2, P< 0.0001).
Figure 5.
Reduced cardiac hypertrophy in MyD88−/− hearts after MI. (A) Cardiac hypertrophy was measured as ratio of heart weight and tibia length 3 weeks after MI in WT and MyD88−/− (n= 16 hearts each group; *P = 0.02). (B) H & E staining of tissue sections from WT and MyD88−/− hearts 3 weeks after MI (Scale bars= 50 µm). Cross-section area from WT (n= 276 cells) and MyD88−/− (n=296 cells) cardiomyocytes was determined (C). MyD88−/− had significantly less hypertrophy (***P < 0.0001). Expression of hypertrophy marker genes for α-MHC (Myh6; D), β-MHC (Myh7; E), atrial natriuretic factor (Nppa; F), and skeletal muscle α-actin 1 (Acta1; G) was determined by qPCR in control (Ctr) and after 1 week or 3 week of MI. In each group, 3 hearts were used and expression was compared by ΔΔCT method using Gapdh as ‘loading control’. Statistical analyses were performed by ANOVA with Bonferroni’s post test. ***P ≤ 0.0001, n.s= not significant.
MI induced myocardial hypertrophy involves expression of a fetal gene program [33]. Inhibition of inflammation and activation of NF-κB have been shown to reduce cardiac hypertrophy in animal models of cardiac diseases [20], suggesting that MyD88 could participate in hypertrophic signaling. Increased CaMKII activates hypertrophic gene expression [34, 35]. Since we have shown that CaMKII regulates NF-κB mediated inflammatory gene expression in post-MI heart, we decided to measure the expression of a group of hypertrophic marker genes in post-MI WT and MyD88−/− hearts by qRTPCR. WT hearts subjected to MI showed a significant increase in expression of β-myosin heavy chain gene (Myh7, Figure 5E), α-skeletal actin (Acta1, Figure 5G), and atrial natriuretic peptide (Nppa, Figure 5F) gene expression compared to the sham-operated controls. In addition, the α-MHC (Myh6) gene expression was reduced after MI (Figure 5D), consistent with its reciprocity with Myh7 expression. Interestingly, MyD88−/− hearts also displayed similar MI-mediated induction in expression of Myh7 and Nppa genes and reduced expression of Myh6 after MI. However, in contrast to post-MI WT hearts, post-MI MyD88−/− hearts had greatly reduced expression of Acta1 (Figure 5G). These results show that MyD88−/− hearts undergo incomplete reprogramming toward a fetal gene expression pattern after MI, suggesting that MyD88 selectively activates hypertrophic genes.
3.5 Post-MI fibrosis is reduced in MyD88−/− hearts
We determined fibrosis in WT and MyD88 hearts 3 weeks after surgery by quantifying Masson’s trichrome staining of heart sections. We obtained heart sections from apical, middle and base regions of the heart (Figure 6A). Fibrosis was measured as the fraction of blue staining (collagen) compared to the total staining (blue plus red) in the entire section including both the ventricle chambers (Figure 6B). The values from multiple heart sections (60 WT and 45 MyD88−/−) from 3 hearts each were averaged. MyD88−/− hearts showed less collagen staining compared to WT hearts (Figure 6C). On average, in MyD88−/− heart sections, blue collagen stain was 20% of total stained tissue compared to 32% in WT (P= 0.003). Thus, MyD88−/− hearts have significantly reduced cardiac fibrosis after MI.
Figure 6.
Reduced post-MI fibrosis in MyD88−/− hearts. After MI (3 weeks) WT and MyD88−/− multiple heart sections were obtained spanning apical, middle and basal region (A). Sections were stained with Masson’s trichrome and collagen staining (blue) was isolated from the total staining in these images (B). Fibrosis was determined as percentage of the blue staining (collagen) of the total staining (blue+red). Average values from WT (60 sections) and MyD88−/− (45 sections) were determined that showed significant reduction in fibrosis in MyD88−/− (C; unpaired t-test, **P = 0.003).
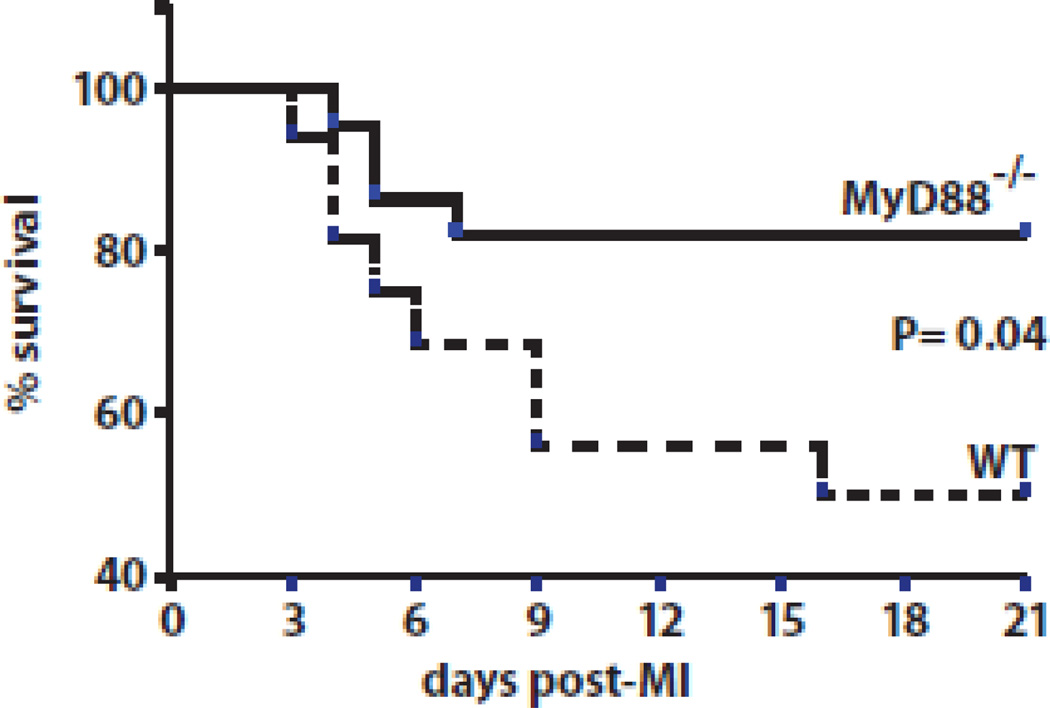
3.6 Reduced Post-MI mortality in MyD88−/− mice
We tested whether MyD88 affected post-MI survival. MyD88−/− mice displayed a significantly improved survival compared to WT mice (Figure 7A). Whereas WT mice had 50% survival (n= 16) 3 weeks after MI, MyD88−/− mice had 82% survival (n= 22; P= 0.04). MyD88−/− and WT mice that underwent sham surgery, which included thoracotomy but no ligation of the coronary artery, had 100% survival for both WT and MyD88−/− (data not shown). Thus, MyD88 deletion significantly improved post-MI survival. We studied mice with echocardiography, and did not detect a difference in post-MI left ventricular ejection fraction between WT and MyD88−/− mice (Table 2). Mice with comparable infarct size were used in these studies. Infarct size was determined by echocardiography as described in Materials and Methods section and confirmed by measurements on representative histological sections (Suppl Figure 1). Taken together, these results suggest that MyD88-dependent signaling significantly affects mortality without improving post-MI systolic function.
Figure 7.
Improved survival of MyD88−/− mice 3 weeks after MI. Kaplan-Meier graph of survival of MyD88−/− and WT mice after inducing MI. MyD88−/− mice had a significantly greater survival (*P = 0.04; 82% survival, n=22) compared with WT mice (50% survival, n=16).
Table-2.
Echocardiography measurements on pre- and post-MI hearts.
| WT | MyD88−/− | |||
|---|---|---|---|---|
| Pre-MI | Post-MI | Pre-MI | Post-MI | |
| LV EDV ± SEM (µl) | 37.55 ± 3.052 | 115.5 ± 16.64 | 38.26 ± 3.130 | 118.2 ± 17.49 |
| LV ESV ± SEM (µl) | 8.907 ± 1.283 | 91.19 ± 16.57 | 13.43 ± 1.510 | 84.03 ± 10.86 |
| LV mass ± SEM (mg) | 68.80 ± 4.389 | 89.25 ± 10.09 | 75.70 ± 4.265 | 79.00 ± 4.578 |
| LV Vol/mass ± SEM | 0.55 ± 0.03 | 1.36 ± 0.16 | 0.51 ± 0.04 | 1.67 ± 0.37 |
| LV EF ± SEM | 0.77 ± 0.02 | 0.29 ± 0.06 | 0.65 ± 0.034 | 0.31 ± 0.043 |
Note: There was no statistically significant difference between WT and MyD88−/− echocardiographic data before or after MI (Paired t-test).
3.7 MyD88 signaling pathway is involved in ROS mediated CaMKII activation
Because TLR-4 activation by LPS induces ROS [36], we asked whether LPS causes CaMKII oxidation. We treated cultured neonatal cardiomyocytes with LPS before cell lysates were prepared and subjected the lysates to non-reducing NuPAGE and immunoblotting with antibodies raised against oxidized CaMKII (ox-CaMKII). The ox-CaMKII band was readily detected in cardiomyocyte lysates 30 minutes after LPS treatment (Figure 8A). Stripping and reprobing the same blot with an antibody to CaMKII (CaMKII) showed that comparable amounts of CaMKII were present in these samples. Thus, LPS mediated TLR-4 induction in cardiomyocytes induces ox-CaMKII.
Figure 8.
Oxidative activation of CaMKII by LPS or MI requires MyD88. (A) LPS treatment induces oxidation of CaMKII. Cultured neonatal cardiomyocytes were untreated (lanes 1 to 3) or treated with LPS (10 µg/ml) for 30 min (lanes 4 to 6). Cell lysates were prepared and immunoblotted under non-reducing conditions for oxidized CaMKII (Ox-CaMKII). Blots were stripped and reprobed for CaMKII (CaMKII). (B) Reduced CaMKII oxidation in post-MI MyD88−/− hearts. WT or MyD88−/− mice underwent sham or MI surgery and heart lysates were prepared 1-day post-MI. Equal amounts of protein samples were fractionated by non-reducing NuPAGE gel electrophoresis and immunoblotted for ox-CaMKII. Blots were stripped and reprobed for CaMKII. (C) Relative abundance of ox-CaMKII was quantified from scanned autoradiographs and presented as the mean of the ratios of ox-CaMKII to CaMKII. One-way ANOVA with Bonferroni’s post-test (*P ≤ 0.01). (D) Reduced phosphorylation of a target protein (phospholamban, PLN) by CaMKII in MyD88−/− hearts after MI. Lysates from 1d post-MI hearts of WT (lanes 1 and 2) and MyD88−/− (lanes 3 and 4) mice were subjected to immunoblotting using antibody to the phosphorylated Thr-17 epitope of phospholamban (PLN-T17). Blots were then stripped and reprobed with antibody specific to total phospholamban (PLN). (E) Band intensity of PLN-T17 immunoblots were normalized to PLN blots. Statistical significance was determined by unpaired t-test (***P = 0.0002). (F) Cultured neonatal cardiomyocytes were transduced with adenovirus with empty expression vector or with a Rac1 dominant negative construct (Rac1DN) and treated with LPS. Rac1DN expressing cells had significantly reduced oxCaMKII (n=3 each, ***P = 0.0005).
Ox-CaMKII is increased in post-MI hearts [7, 10]. We investigated whether post-MI ox-CaMKII is affected by MyD88-mediated TLR signaling. We performed non-reducing NuPAGE and immunoblot analyses on post-MI (day 1) heart lysates from WT and MyD88−/− mice for ox-CaMKII and CaMKII (Figure 8B). Post-MI WT hearts had significantly increased ox-CaMKII (ox-CaMKII/CaMKII ratio) compared to sham-operated hearts. However, MyD88−/− hearts did not display increased ox-CaMKII after MI (Figure 8C). Thus, MyD88 appears to play an important, but previously unrecognized, role in MI mediated increases in ox-CaMKII.
CaMKII regulates intracellular Ca2+ homeostasis in cardiomyocytes through phosphorylation of calcium handling proteins [37]. Phospholamban (PLN) is a 5 kDa protein that constrains the sarcoplasmic reticulum Ca2+ pump (SERCA2). CaMKII phosphorylates PLN at the Thr-17 residue [38]. We asked whether ox-CaMKII had an effect on phosphorylation of PLN-T17 in post-MI heart lysates from WT and MyD88−/− mice. PLNT17 phosphorylation was significantly reduced in MyD88−/− post-MI hearts, while total PLN protein levels were not significantly different in these hearts (Figure 8D and 8E). Upon activation, CaMKII can be autophosphorylated at threonine-287 (T287). Therefore, we compared phospho-CaMKII in post-MI WT and MyD88−/− hearts by immunoblotting. Although a phospho-CaMKII band could be detected in both WT and MyD88−/− heart lysates 1day after MI, the phospho-CaMKII/total CaMKII ratio was significantly less in MyD88−/− compared to WT hearts (Suppl Figure 2). These results suggest that activation of TLR/MyD88 signaling in cultured cardiomyocytes by LPS and in hearts after MI induce ROS and ox-CaMKII. In addition, these results show that MyD88-deficient hearts have reduced ox-CaMKII and CaMKII activity.
Next, to gain a mechanistic insight into TLR mediated oxidation of CaMKII, we determined the source of ROS that oxidizes CaMKII in cardiomyocytes. NADPH oxidase is a major source of ROS. Indeed, oxidation of CaMKII is coupled to NADPH oxidation in Angiotensin II [7] and aldosterone [10]cardiac myocytes. To directly test the role of NADPH oxidase in TLR induced CaMKII oxidation, we used adenovirus transduction of cultured neonatal cardiomyocytes to express a dominant negative form of Rac1 (Rac1DN). Rac1 is a small GTP binding protein required for assembly of functional NADPH oxidase. Control cardiomyocyte cultures were infected with adenovirus with empty vector. Cells were then challenged with LPS to induce TLR-4 and lysates were first immunoblotted for oxCaMKII followed by stripping the blots and reprobing for CaMKII. We also confirmed expression of Rac1DN in these cultures by immunoblotting. Cardiomyocytes that expressed Rac1DN had significantly less ox-CaMKII after LPS treatment (18% reduction, P=0.0005, n=3; Figure 8F). These results show that TLR-4 activation leads to increases in ROS production and oxidized CaMKII. To our knowledge, this is a novel finding that suggests a mechanistic relationship between CaMKII and an innate immune pathway observed in our previous study.
4. Discussion
4.1 CaMKII in post-MI hypertrophic and inflammatory signaling
MI causes profound changes in cardiac gene expression, including induction of pro-hypertrophic and pro-inflammatory genes. MI and heart failure are associated with increased CaMKII activity, ROS production, cardiac hypertrophy and inflammation [6]. We have previously shown that CaMKII regulates TLR regulated inflammatory gene expression in LPS treated cardiomyocytes and in post-MI hearts [5]. Inhibition of CaMKII [11]or ablation of its target gene complement factor B (Cfb) [5]improves post-MI survival and cardiac hypertrophy in mouse models. CaMKII can be activated by oxidation (ox-CaMKII) and phosphorylation (phospho-CaMKII) in response to MI [7] and inhibiting CaMKII activity attenuates inflammatory gene expression in post-MI hearts and in LPS treated cardiomyocytes [5]. Thus, CaMKII may be a common link between hypertrophic and inflammatory gene expression programs [6]. Excessive ROS production after MI results in maladaptive myocardial responses in patients and in animal models [10, 39]. The TLR-4 agonist, LPS, and tissue inflammation are known to induce ROS production [40, 41], but were not known to activate the CaMKII pathway by oxidation. We observed increased ox-CaMKII in LPS treated cardiomyocytes as well as in post-MI hearts (Figure 8A and 8B). Interestingly, there was significantly reduced phospho-CaMKII and ox-CaMKII in post-MI MyD88−/− hearts. MyD88 is a key component of inflammatory pathways initiated from multiple TLRs that are activated upon MI. All known TLRs signal to activate NF-κB transcription factors. We have previously shown that CaMKII enhances NF-κB mediated inflammatory signaling in cardiomyocytes [5]. Several laboratories also have reported similar findings in other cells [42–44]. Although phospho-CaMKII and ox-CaMKII can be activated by independent pathways [7], ox-CaMKII mediated activation of the kinase can lead to its autophosphorylation [45]. In the future, it will be of importance to determine the interdependence of phospho-CaMKII and ox-CaMKII in post-traumatic cardiac tissue. Taken together with earlier findings, our new results suggest that TLR stimulation activates CaMKII by increasing ROS in post-MI hearts, leading to increased expression of proinflammatory and pro-hypertrophic genes. It may be that the CaMKII is also important for connecting elevated ROS with inflammation in other cardiovascular and noncardiovascular diseases.
Our results show that MyD88−/− mice are protected from mortality after MI. While we do not know the cause of death in these mice, it is likely that the excess mortality in wild type control mice relates to the greater increases in an oxidized CaMKII, inflammation, fibrosis and hypertrophy. Further studies will be necessary to determine if MyD88 ablation protects against specific outcomes that may plausibly cause post-MI mortality, such as arrhythmias and cardiac rupture [10].
4.2 Role of MyD88-dependent pathway in MI related disease
Our results show that a TLR/MyD88-dependent pathway is involved in a diverse set of events that may negatively impact post-MI survival. We found that MyD88−/− mice had significantly improved survival and reduced cardiac hypertrophy (Figure 7). In addition, the MyD88−/− hearts had reduced expression of MyD88-dependent inflammatory genes, while expression of a MyD88-independent gene (Cxcl10) was unaltered (Figure 1). These effects of MyD88 deficiency appeared to be specific for established downstream elements of the MyD88 pathway, because activation of TLR-3, a TLR that does not require MyD88 for signaling, readily induced the NF-κB pathway in poly(I:C) treated MyD88−/− cultured cardiomyocytes (Figure 2). Since TLR-3 is an intracellular receptor, our results also suggest that TLRs expressed on the surface may be more important than intracellular TLRs after MI. A role of TLRs in sensing sterile injury, including ischemia, has been proposed in other tissues [46, 47]. These findings may also be pertinent to cardiac injury [4]. Our results support a model where post-MI inflammatory signaling is initiated by endogenous TLR agonists, likely released by necrotic cells at the site of injury, and suggest that TLR agonists require MyD88 for pathological responses and CaMKII oxidation.
4.3 CaMKII may connect MyD88 to cardiac hypertrophy
We found that MyD88−/− cardiomyocytes surviving MI were resistant to hypertrophy, while conventional markers of cardiac hypertrophy, Myh7 and Nppa, were not reduced in post-MI MyD88−/− hearts compared to WT controls. However, another hypertrophy–associated gene, Acta1 showed a significantly reduced response to MI in MyD88−/− hearts compared to the increase in WT controls. These results suggest that in MI genes that are affected by a MyD88-dependent pathway may be involved in cardiac hypertrophy. Another possibility is that the expression of pro-hypertrophic genes is indirectly supported by a MyD88-dependent inflammatory response [6, 29]. In either scenario, CaMKII is a plausible linking signal between inflammation and hypertrophy [6].
Conclusions
The TLR/MyD88 pathway is important for adverse responses to MI and contributes to increasing myocardial ox-CaMKII. These data suggest MyD88 contributes to clinically important pro-inflammatory and pro-oxidant responses after MI.
Highlights.
MyD88−/− mice have improved post-MI survival.
MI induces TLR/MyD88 dependent inflammatory gene expression.
MyD88−/− mice have reduced cardiac hypertrophy and fibrosis.
MyD88−/− mice have reduced post-MI oxidation-dependent CaMKII activation.
Acknowledgments
We thank Kathy Zimmerman for expert technical help on mouse echocardiographic data acquisition and analyses and Jinying Yang for animal help. University of Iowa Central Microscopy Core Facility provided technical support and Virus Vector Core Facility provided Rac1DN adenovirus. We also thank Shari Lifson (Columbia University) for help with image analyses.
Source of funding:
This study was funded by the NIH grants R01 HL 079031, R01 HL 096652, and R01 HL 070250 to MEA, and NIH RR026293 to RMW. This work was also supported by the University of Iowa Research Foundation and, in part, by the Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Madhu V. Singh and Mark E. Anderson are named inventors on a patent application claiming to treat inflammatory heart disease by CaMKII inhibition.
References
- 1.Entman ML, Smith CW. Postreperfusion inflammation: a model for reaction to injury in cardiovascular disease. Cardiovascular Research. 1994;28:1301–1311. doi: 10.1093/cvr/28.9.1301. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, Ertl G, Bauersachs J. Toll-like receptor signaling in the ischemic heart. Front Biosci. 2008;13:5772–5779. doi: 10.2741/3114. [DOI] [PubMed] [Google Scholar]
- 4.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circulation Research. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, et al. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. The Journal of clinical investigation. 2009;119:986–996. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh MV, Anderson ME. Is CaMKII a link between inflammation and hypertrophy in heart? Journal of Molecular Medicine. 2011;89:537–543. doi: 10.1007/s00109-011-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson JR, Joiner M-lA, Guan X, Kutschke W, Yang J, Oddis CV, et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell. 2008;133:462. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, et al. The Cardiac-specific Nuclear delta B Isoform of Ca2+/Calmodulin-dependent Protein Kinase II Induces Hypertrophy and Dilated Cardiomyopathy Associated with Increased Protein Phosphatase 2A Activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 10.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nature Medicine. 2011 doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. NatMed. 2005;11:409. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, et al. Tolllike receptor 4 mediates ischemia/reperfusion injury of the heart. The Journal of thoracic and cardiovascular surgery. 2004;128:170–179. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Oyama J-i, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, et al. Reduced Myocardial Ischemia-Reperfusion Injury in Toll-Like Receptor 4-Deficient Mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 16.Ha T, Li Y, Hua F, Ma J, Gao X, Kelley J, et al. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res. 2005;68:224–234. doi: 10.1016/j.cardiores.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–6961. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 18.Ha T, Hua F, Li Y, Ma J, Gao X, Kelley J, et al. Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol. 2006;290:H985–H994. doi: 10.1152/ajpheart.00720.2005. [DOI] [PubMed] [Google Scholar]
- 19.Hua F, Ha T, Ma J, Gao X, Kelley J, Williams DL, et al. Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem Biophys Res Commun. 2005;338:1118–1125. doi: 10.1016/j.bbrc.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 20.Young D, Popovic ZB, Jones WK, Gupta S. Blockade of NF-kappaB using IkappaB alpha dominant-negative mice ameliorates cardiac hypertrophy in myotrophin-overexpressed transgenic mice. J Mol Biol. 2008;381:559–568. doi: 10.1016/j.jmb.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 23.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 25.Blyszczuk P, Kania G, Dieterle T, Marty RR, Valaperti A, Berthonneche C, et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res. 2009;105:912–920. doi: 10.1161/CIRCRESAHA.109.199802. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell TS, Yull FE, Chen C-L, Venkatakrishnan A, Blackwell TR, Hicks DJ, et al. Multiorgan Nuclear Factor Kappa B Activation in a Transgenic Mouse Model of Systemic Inflammation. Am J Respir Crit Care Med. 2000;162:1095–1101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- 27.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 28.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 29.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple Facets of NF-{kappa}B in the Heart: To Be or Not to NF-{kappa}B. Circulation Research. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 30.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 31.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, et al. Induction of the CXC Chemokine Interferon-{gamma}-Inducible Protein 10 Regulates the Reparative Response Following Myocardial Infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T, Hanawa H, Toba K, Watanabe H, Watanabe R, Yoshida K, et al. Expression of immunological molecules by cardiomyocytes and inflammatory and interstitial cells in rat autoimmune myocarditis. Cardiovascular Research. 2005;68:278–288. doi: 10.1016/j.cardiores.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz K, Boheler KR, de la Bastie D, Lompre AM, Mercadier JJ. Switches in cardiac muscle gene expression as a result of pressure and volume overload. The American Journal of physiology. 1992;262:R364–R369. doi: 10.1152/ajpregu.1992.262.3.R364. [DOI] [PubMed] [Google Scholar]
- 34.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. The {delta}C Isoform of CaMKII Is Activated in Cardiac Hypertrophy and Induces Dilated Cardiomyopathy and Heart Failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 36.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochemical pharmacology. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 38.Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989;264:11468–11474. [PubMed] [Google Scholar]
- 39.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. The Journal of clinical investigation. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res. 2004;64:279–288. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. American journal of physiology Heart and circulatory physiology. 2009;296:H470–H479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes K, Edin S, Antonsson A, Grundstrom T. Calmodulin-dependent kinase II mediates T cell receptor/CD3- and phorbol ester-induced activation of IkappaB kinase. J Biol Chem. 2001;276:36008–36013. doi: 10.1074/jbc.M106125200. [DOI] [PubMed] [Google Scholar]
- 43.Jang MK, Goo YH, Sohn YC, Kim YS, Lee SK, Kang H, et al. Ca2+/calmodulin-dependent protein kinase IV stimulates nuclear factor-kB transactivation via phosphorylation of the p65 subunit. J Biol Chem. 2001;276:20005–20010. doi: 10.1074/jbc.M010211200. [DOI] [PubMed] [Google Scholar]
- 44.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kB functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 45.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, et al. Angiotensin IIinduced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 46.Evankovich J, Billiar T, Tsung A. Toll-like receptors in hepatic ischemia/reperfusion and transplantation. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/537263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]