Summary
Prospective analysis was performed of self-reported and biochemically confirmed tobacco use in 50 head and neck cancer patients during treatment. With 93.5% compliance to complete weekly self-report and biochemical confirmatory tests, 29.4% of smokers required biochemical assessment for identification. Accuracy increased by 14.9% with weekly vs. baseline self-reported assessments. Data confirm that head and neck cancer patients misrepresent true tobacco use during treatment.
Keywords: tobacco, smoking, head/neck, radiotherapy, cotinine
Introduction
Tobacco use is a recognized risk factor for the development of several cancers and increasing evidence suggests that continued tobacco use in head and neck cancer patients is associated with decreased survival (1-2). Integrating accurate tobacco assessments and cessation into clinical care has been advocated for several years (3) and are advocated according to National Comprehensive Cancer Network (NCCN) Guidelines, the American Society of Clinical Oncology (ASCO), and Joint Commission for the Accreditation of Hospital Organizations (JCAHO) (4-6). Accurate assessments of tobacco use are necessary to effectively implement cessation strategies and may require biochemical confirmation to overcome inaccuracies of self-reported tobacco use (7). Some authors have questioned the accuracy of self-reported tobacco assessments in head and neck cancer patients (8) and the importance of biochemical confirmation is exemplified by Marin et al. demonstrating poor cancer treatment outcomes in surgical head and neck cancer patients as correlated with biochemically confirmed tobacco use, but with no correlation using self-reported assessments (9).
Materials and Methods
Beginning in 2007, patients with non-metastatic squamous cell carcinoma of the head and neck were enrolled in an Institutional Review Board approved study to evaluate the effect of tobacco exposure on therapeutic outcome. Eligible patients included patients with measurable disease on pretreatment imaging and who were candidates for treatment with definitive radiotherapy or platinum-based chemoradiotherapy. Standard clinical interventions were administered for all patients, clinical data were recorded, and patients were informed that tobacco use would be evaluated by standardized weekly self-reported assessments. Self-reported assessments were administered and recorded by nurses. Detailed tobacco use histories were obtained at study entry and current self-reported tobacco exposure were obtained during treatment through the following questions: Do you currently smoke cigarettes or cigars?, Do you currently use smokeless tobacco?, Do you currently use nicotine replacement?, Are you exposed to people who smoke on a regular basis? Questions used for self-reported assessments are provided in the supplementary materials. Current tobacco use was considered by self-report at the time of assessment (i.e., current tobacco use was considered a dynamic point of contact variable that could change weekly over the course of treatment). No monetary compensation or incentive was provided for participation in this study.
Biochemical confirmation of tobacco use was performed using serum samples. Serum was obtained weekly to analyze biochemical tobacco exposure using quantitative cotinine (7-8) measured with a commercially available solid-phase competitive chemiluminescent immunoassay (Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA). Cotinine values > 10 ng/mL were considered positive for biochemical confirmation of tobacco use.
An interim analysis of study accrual was performed and tobacco use behaviors in the first 50 patients were performed. Descriptive analyses consisted of the accuracy, specificity, sensitivity, positive predictive value, and negative predictive value of self-report with serum cotinine analysis used as the “gold standard” for accurate assessment.
Results and Discussion
Patient demographics and tobacco use history are shown in Table 1. Data are presented for all 50 patients during treatment. Only 2 patients (4%) did not complete treatment and entered hospice care: one with a cerebrovascular accident at week 2, one with hepatic encephalopathy at week 3. However, the weekly self-reported assessment and serum cotinine values were included in this analysis.
Table 1.
Patient Characteristics and Tobacco Use History.
| Number of patients (%) | |
|---|---|
| Gender | |
| Male | 40 (80%) |
| Female | 10 (20%) |
| Median Age (range) | 56 (39-75) |
| Race | |
| Caucasian | 46 (92%) |
| African-American | 4 (8%) |
| Tumor Site | |
| Oropharynx | 32 (64%) |
| Larynx | 14 (28%) |
| Other | 4 (8%) |
| Overall Stage | |
| I | 1 (2%) |
| II | 3 (6%) |
| III | 11 (22%) |
| IVA | 30 (60%) |
| IVB | 5 (10%) |
| Treatment | |
| Radiotherapy (RT) | 8 (16%) |
| Concurrent chemoradiotherapy (CRT) | 42 (84%) |
| Self-Reported Tobacco Use History | |
| Yes, including smokeless tobacco | 41 (82%) |
| Yes, excluding smokeless tobacco | 40 (80%) |
| Median years of tobacco use (range) | 30 (3-55) |
| Median pack years (range) | 30 (3-138) |
| No | 9 (18%) |
| Current Tobacco Use | 20 (40%) |
| Yes to cigarettes/cigar use | 19 (38%) |
| Median packs per day (range) | 0.8 (0.5-1.5) |
| Median average lifetime packs per day (range) | 1 (0.5-3) |
| Median years of tobacco use (range) | 35 (16-55) |
| Median pack years of tobacco (range) | 35 (16-120) |
| Yes to smokeless tobacco | 1 (2%) |
| Former Tobacco Use | 24 (48%) |
| Median duration of successful cessation (range) | 1.5-3 yrs (2 wk – 40 yr) |
| Median years of tobacco use before quit (range) | 27 (3-50) |
| Baseline Self-Reported Pharmacologic Cessation Agent | |
| Nicotine replacement | 5 (10%) |
| Chantix | 3 (6%) |
Weekly data are presented in patients who completed both the self-reported assessment and biochemical confirmation in a given week. Not all patients completed 8 visits including patients who entered hospice care (as noted earlier) and some patients who would only have 7 potential visits due to radiotherapy scheduling and treatment duration. Of 367 total opportunities to capture both serum and self-reported tobacco use, 343 were completed resulting in a 93.5% compliance rate. Cotinine was detected in 35.1% of samples with the following characteristics (in ng/mL): average 261 (235 standard deviation), range 10-1248, median 191, upper quartile 379 ng/mL, and lower quartile 73. Nicotine replacement was reported in 8% of cotinine positive assessments; however, cotinine values (range 51.8 - 654 ng/mL) supported active tobacco use rather than nicotine replacement alone (7, 12).
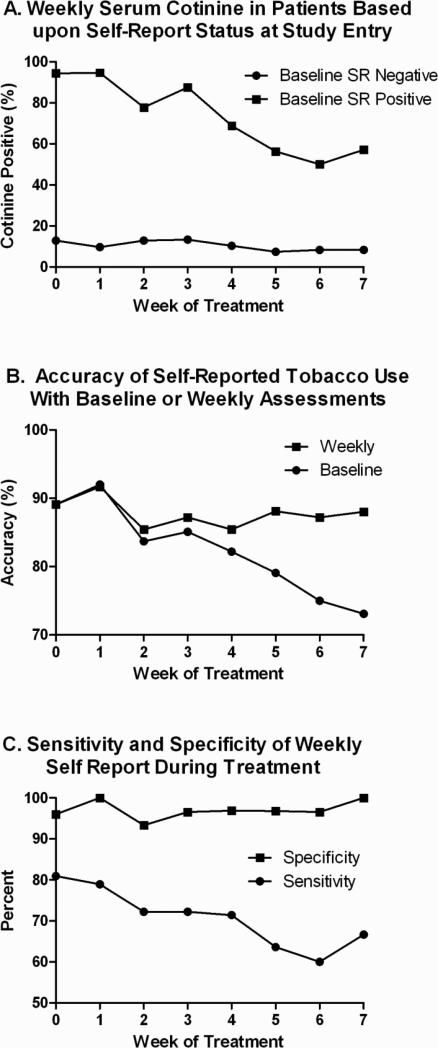
Weekly cotinine values stratified according to baseline self-reported tobacco use are shown in Fig. 1A demonstrating that baseline assessments alone do not accurately characterize patient behavior during treatment. The decreasing trend of cotinine positivity noted in Baseline Self-Reported (SR) Positive patients during treatment is associated with one of two apparent behaviors in patients: decreased tobacco use over the course of treatment (in one patient group) or a consistent rate of misrepresentation throughout treatment (in a separate patient group). The characteristics of these subgroups are shown in Table 2 and further discussed in the following paragraph. As shown in Fig. 1B, weekly self-reported assessments increased the absolute accuracy of identifying true tobacco use by 14.9% as compared with baseline assessments alone. However, biochemical confirmation was necessary to identify an average of 29.4% (weekly range 14.3 – 40.0%) of tobacco users who denied any tobacco use by self report suggesting that biochemical confirmation further enhanced accurate identification of true tobacco use over repeated weekly self-reported tobacco assessments. Figure 1C demonstrates that the sensitivity of weekly self-report followed a downward trend during treatment (average 70.8%, range 60.0-81.0%), but specificity remained stable throughout treatment (average 97.0%, range 93.3-100%). The positive predictive value of weekly self-report averaged 92.3% (range 85.7-100%) and the negative predictive value averaged 86.5% (range 84.2-88.6%).
Figure 1. The Accuracy of Baseline and Weekly Self-Reported Tobacco Assessments During Cancer Treatment.
Baseline and weekly self-reported tobacco assessments were administered using a standardized questionnaire and weekly serum was collected to assess biochemically confirmed tobacco use with cotinine analysis. Patients testing positive for biochemically confirmed tobacco use (serum cotinine > 10 ng/mL) were reported according to baseline self-reported (SR) status (A). The accuracy of self-report was generated by including the total percentage of patients who self-report current tobacco use and have positive cotinine combined with patients who self-report no current tobacco use and have negative serum cotinine (B). A positive serum cotinine assessment is used as the “gold standard” for reporting the sensitivity and specificity of weekly self-reported tobacco use (C).
Table 2.
Characteristics of Patients who Misrepresented True Tobacco Use During Treatment.
| Patient | Week of First Misreport | Cessation Agent at Any Time During Treatment | BC Tobacco Use at Last Week of Treatment | Behavior Pattern for Tobacco Use |
|---|---|---|---|---|
| 1 | Baseline | NRT | Yes | Continue |
| 6 | Baseline | None | No | Quit |
| 20 | Baseline | Chantix, NRT | No | Quit |
| 38 | 2 | None | Yes | Restart |
| 40 | 1 | Chantix | No | Quit |
| 41 | 3 | NRT | Yes | Continue |
| 45 | 3 | None | No | Quit |
| 50 | 6 | None | Yes | Continue |
The characteristics of patients who misreported tobacco use are presented in Table 2. In patients with biochemically confirmed tobacco use at least once during treatment, 33% (8 of 24 patients) misrepresented true tobacco use at least twice during treatment based upon self-reported assessments. In 4 patients (patients 6, 20, 40, and 45), misrepresentation was associated with significant decreases in tobacco use during treatment resulting in biochemically confirmed cessation suggesting misrepresentation was associated with efforts to stop tobacco use. In 4 patients (patients 1, 38, 41, and 50), misrepresentation was associated with continued tobacco use or restarting tobacco use during treatment.
The most significant finding from this study is that among patients who are aware that both self-reported assessments (using questionnaires) and biochemically confirmed tobacco assessments (using serum cotinine analysis), a substantial proportion of biochemically confirmed tobacco users did not accurately self-report tobacco use during cancer treatment. Data further demonstrate that tobacco use among cancer patients can vary substantially during treatment and a single assessment of tobacco use at diagnosis can be highly inaccurate over the course of cancer treatment. Repeated self-reported assessments increase the accuracy of identifying tobacco use during cancer treatment, but data demonstrate that repeated self-reported tobacco assessments during cancer treatment still underestimate the true tobacco use behavior of patients. Importantly, this study also demonstrates a high compliance rate for repeated self-reported and biochemically confirmed assessments in a head and neck cancer treatment population.
Though limited by small patient size, this study represents the largest assessment of tobacco use using weekly self-reported and biochemically confirmed assessments in any clinical cancer treatment population. In this head and neck cancer population, patients should have a higher tobacco burden than in other non-tobacco related disease sites such as colorectal, breast, or prostate cancer. Observed inaccuracies associated with self-report could be influenced by the possibility that in tobacco related cancers (head/neck or lung cancer), patients may be reluctant to report tobacco use due to a “guilt by association” phenomenon. Unfortunately, self-reported accuracy during cancer treatment for cancers traditionally associated with tobacco use has not been compared to those cancers that are traditionally not associated with tobacco use. Notably, similar self-reported accuracy assessments have also been observed in non-cancer clinical populations (7).
Published data increasingly demonstrate that tobacco products are associated with decreased therapeutic efficacy and poor outcome in cancer patients (1-2, 9, 13-15). Though advocated in several national guidelines, tobacco cessation efforts are not well integrated into the management of cancer patients (3). Data presented herein support consideration of changes to tobacco use assessments for clinical oncology practice and research beyond single self-reported assessments only at the time of diagnosis. Incorporation of accurate tobacco use assessments will provide a better platform to understand the true impact of tobacco on clinical cancer treatment and may provide a cost effective mechanism of improving treatment outcomes (15-16).
Supplementary Material
Acknowledgements
This work was supported by the University of Kentucky General Clinical Research Center (M01-RR02602). The authors wish to acknowledge Jackie Sims and Laura Reichel for their assistance in conducting research interviews and collecting serum specimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 3.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services, Public Health Service; Rockville (MD): May, 2008. http://www.nccn.com/component/content/article/55/155-smoking-and-cancer.html.. NCCN guidelines support smoking assessment and cessation according to Public Health Service guidelines (ref. Treating tobacco use and dependence: 2008 update. 257)
- 5.Joseph AM, Knapp JM, Nichol KL, Pirie PL. Determinants of compliance with a national smoke-free hospital standard. 1995;274:491–4. [PubMed] [Google Scholar]
- 6.Tobacco Cessation and Quality Cancer Care J Oncol Pract. 2009;5:2–5. doi: 10.1200/JOP.0913501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benowitz NL, Schultz KE, Haller CA, et al. Prevalence of Smoking Assessed Biochemically in an Urban Public Hospital: A Rationale for Routine Cotinine Screening. Am J Epidemiol. 2009;170:885–891. doi: 10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hald J, Overgaard J, Grau C. Evaluation of objective measures of smoking status--a prospective clinical study in a group of head and neck cancer patients treated with radiotherapy. Acta Oncol. 2003;42:154–9. doi: 10.1080/02841860310005020. [DOI] [PubMed] [Google Scholar]
- 9.Marin VP, Pytynia KB, Langstein HN, Dahlstrom KR, Wei Q, Sturgis EM. Serum cotinine concentration and wound complications in head and neck reconstruction. Plast Reconstr Surg. 2008;121:451–457. doi: 10.1097/01.prs.0000297833.53794.27. [DOI] [PubMed] [Google Scholar]
- 10.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Tutka P, Mosiewicz J, Wielosz M. Pharmacokinetics and metabolism of nicotine. Pharmacol Rep. 2005;57:143–153. [PubMed] [Google Scholar]
- 12.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 13.Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79:414–419. doi: 10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 340:b5569. doi: 10.1136/bmj.b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gritz ER, Fingeret MC, Vidrine DJ, et al. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.