Abstract
Objective
To determine and compare outcomes with accepted benchmarks in trauma care at seven academic Level I trauma centers in which patients were treated based on a series of standard operating procedures (SOPs).
Background
Injury remains the leading cause of death for those under 45 years of age. We describe the baseline patient characteristics and well-defined outcomes of persons hospitalized in the United States for severe blunt trauma.
Methods
We followed 1,637 trauma patients from 2003–2009 up to 28 hospital days using SOPs developed at the onset of the study. An extensive database on patient and injury characteristics, clinical treatment, and outcomes was created. These data were compared with existing trauma benchmarks.
Results
The study patients were critically injured and in shock. SOP compliance improved 10–40% during the study period. Multiple organ failure and mortality rates were 34.8% and 16.7% respectively. Time to recovery, defined as the time until the patient was free of organ failure for at least two consecutive days, was developed as a new outcome measure. There was a reduction in mortality rate in the cohort during the study that cannot be explained by changes in the patient population.
Conclusions
This study provides the current benchmark and the overall positive effect of implementing SOPs for severely injured patients. Over the course of the study, there were improvements in morbidity and mortality and increasing compliance with SOPs. Mortality was surprisingly low, given the degree of injury, and improved over the duration of the study, which correlated with improved SOP compliance.
Keywords: trauma, injury, mortality, benchmark
INTRODUCTION
Injuries continue to be the fifth leading cause of death overall and the leading cause of death for persons less than 45 years of age in the U.S.1 In 2006, injuries accounted for 179,065 deaths2 and nearly two million hospital discharges.3 The burden of trauma and its costs to society remain staggering.
Profound physiologic derangement is expected after major trauma and hemorrhagic shock. However, the clinical course following initial resuscitation varies considerably, and may be affected by the patient’s care. Patients may recover quickly while others experience complicated recoveries including organ dysfunction and/or infections. Studies from large medical centers record the incidence of such complications;4,5 however, these data reflect various supportive care protocols, or none at all, despite reports on the benefits of using protocols in the critically ill.6 Except for the Advanced Trauma Life Support course, standard algorithms are rarely used in resuscitation and trauma critical care, unlike in sepsis, cancer, and cardiovascular disease. Furthermore, trauma study patients are not categorized in a uniform manner to capture mechanism or physiology, thus making generalizations difficult.
Our primary goal was to define outcomes in high-risk, severe blunt trauma patients from seven Level I trauma centers that agreed to follow standard operating procedures (SOPs) for patient treatment.7–15 We subsequently compared our clinical outcomes with established prediction models, APACHE II and Trauma Score-Injury Severity Score (TRISS), and another large matched database cohort from the National Trauma Data Bank (NTDB). As a secondary goal, we also developed the concept of Multiple Organ Failure (MOF) time to recovery (TTR) as a novel improved metric to describe more completely the progression of the complex physiologic and anatomic derangements experienced by severely injured trauma patients. Contrary to current dogma, our data confirm that morbidity and mortality continue to exist in the severely traumatized patient. Thus, we hypothesized that through the implementation and quarterly auditing of SOPs, we would be able to document an improvement in clinical outcomes over time. In addition, we hypothesized that improvement in compliance with the use of these SOPs would result in an overall survival benefit that would be better than predicted by both APACHE II and TRISS scores, as well as an NTDB matched cohort. Further, we also set out to demonstrate that TTR correlated well with several demographic features and the clinical status of our cohort, and thus may prove to be a useful measure to evaluate the critically ill trauma patients.
METHODS
Study Design
The Inflammation and the Host Response to Injury is a NIGMS large-scale collaborative project (“Glue Grant”). In this observational, prospective study conducted from November 2003 to July 2009 at seven U.S. Level I trauma centers, patients were enrolled if they were critically injured by blunt mechanism, were ≥16 years of age, had shock due to hemorrhage defined as base deficit ≥6 meq/L or systolic blood pressure <90 mm Hg within 60 minutes of emergency department (ED) arrival, and required initiation of blood transfusion within six hours of injury (Supplemental Table 1). Patient treatment SOPs were developed through literature reviews and comprehensive, quarterly face-to-face meetings, in the year prior to and during the first year of the study. Processes for auditing compliance were developed to include regular compliance data reports and on-site medical record audits.
Definition of Outcomes and Data Collection
Demographic, clinical, physiological, and outcomes data were abstracted through the first 28 days or ICU discharge following injury and uploaded to TrialDB.16 Study investigators prospectively defined and subsequently published the data definitions17 that relied wherever possible on existing standards. Trained health care data abstractors participated in meetings and conference calls to ensure uniformity of data abstraction among centers. A dedicated team was responsible for data management, curation, and storage. A clinical data manager performed ongoing quality monitoring to ensure completeness and accuracy including queries submitted to the abstractors to eliminate internal inconsistencies, and missing or implausible values. A 5% random sample of records was selected for review during every site visit when the team reviewed the records for study eligibility, cause of death (if applicable), and the presence/absence of infections and other complications. A waiver of informed consent was granted by the IRBs as the data was a limited dataset and the SOPs were used for all patients in the center.
Organ dysfunction and hospital mortality were analyzed as outcomes measures. Organ dysfunction assessment was performed using two widely accepted post-injury multiple organ dysfunction/failure (MOD/MOF) scores, Marshall MOD18 and Denver MOF19. The first 48 hours after injury was not included in the assessment for organ failure to exclude the impact of the initial insult and resuscitation upon initial organ function. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) were defined according to the European-American consensus conference.20 MOF was defined by adapting the Marshall MOD score to exclude the neurological component. MOF was defined as a MOF score >6 using either score. Other non-infectious and infectious complications were assessed using standardized definitions. Specifically, definitions of nosocomial infections21 and surgical site infections22 were those used by the Centers for Disease Control with the exception that superficial surgical site infections and all infectious complications required the presence of a positive microbial culture.
SOP Compliance
The primary goal was to determine overall SOP compliance, and determine the relationship between SOP compliance and mortality. Compliance to the SOP’s was determined from the case report forms using simple rules based on physiologic measurements (described at http://www.gluegrant.org/commonlyreferencedpubs.htm). These measures could not capture the full extent of the SOP’s so the compliance estimates are approximate and the actual compliance may be better than the estimates. The change in the compliance measures over time should reflect the true changes in compliance over the study period.
Time to Recovery
A secondary goal was to identify organ recovery and to assess whether TTR was a valid measure of morbidity. Organ recovery is relatively straightforward to identify when a failing organ requires support, but less intuitive for more subtle degrees of organ dysfunction. Making the assumption that subtle degrees of organ dysfunction have little impact on mortality, we chose to consider an organ system to have recovered when the parameters reflecting normalization of function were present for at least two consecutive days (Supplemental Table 2). Patients who died during the 28-day period were assigned a time to recovery of >30 days while patients who were still in organ failure on day 28 were assigned a value of 29 days.
Statistical Analyses
Differences in baseline covariates between survivors and non-survivors were assessed using a Wilcoxon test, chi-square test, or Fisher’s exact test. Association of baseline covariates and MOF TTR was assessed using Spearman correlation. Differences between observed and predicted mortality for both TRISS23 and APACHE II24 and between observed and predicted multiple organ failure25 were assessed using Flora’s z-statistic.26 Logistic regression was used to correct the time trend in mortality for important baseline covariates.
We compared our mortality to that of a subset of the National Trauma Data Bank (NTDB) patients who, based on the available non-missing data, met the following criteria: known outcome (dead on hospital arrival excluded); Level I or II trauma center; blunt injury mechanism; ED arrival <24 hours of injury; ED disposition to the Intensive Care Unit (ICU) or Operating Room (OR); ED systolic blood pressure (SBP) <90 mmHg or ED base deficit >6 meq/L; maximum head Abbreviated Injury Scale27 (AIS) Score <6; maximum spine AIS Score <6; known age; known Injury Severity Score28 (ISS). This subset together with our study cohort was stratified into age and ISS quintiles (for a total of 25 strata) and log odds of death was compared between the study and NTDB patients using stratified logistic regression.
Survival and TTR plots through study day 28 were made using Kaplan-Meier estimates. To examine the relationship between TTR and MOF, we created a plot of the MOF score over time after injury for each of the 1,637 massively injured patients.
Analysis was performed in SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA) and two-sided p-value ≤0.05 were considered evidence of statistical significance.
RESULTS
Study Population
Table 1 shows patient characteristics upon ED presentation. The data confirmed critical injuries with a mean ISS of 32.1 ± 13.4 and more than half of the injuries due to motor vehicle crashes (MVC). Patients were predominantly male (66.3%), Caucasian (89.1%), and middle-aged with a mean (± S.D.) age of 42 ± 18 years. Approximately 2/3 of the patients presented with ED hypotension, and the degree of soft tissue injury and blood loss were indicative of significant metabolic dysfunction, as measured by mean base deficit of −10.4 ± 5.1 meq/L and lactate of 5.4 ± 3.2 mmol/L. On average, these patients were given three times their blood volume in a combination of crystalloid (11 liters) and packed red blood cells (8 units). These patients were also found to have severe underlying coagulopathy requiring additional blood products.
Table 1.
Baseline Characteristics of the Study Subjects
| Baseline and injury characteristics
| ||||
|---|---|---|---|---|
| Total Cohort n=1,637 | Alive n=1,363 | Dead n=274 | Probability | |
| Age | 42.4 ± 18.5 | 41.2 ± 17.7 | 48.3 ± 21.4 | <.0001 |
| Male Sex | 1,085 (66.3) | 901 (66.1) | 184 (67.2) | 0.7375 |
| Race | 0.1022 | |||
| African American | 107 (6.5) | 91 (6.7) | 16 (5.8) | |
| Asian | 38 (2.3) | 30 (2.2) | 8 (2.9) | |
| Caucasian | 1,459 (89.1) | 1,217 (89.2) | 242 (88.3) | |
| Native American | 16 (1.0) | 15 (1.1) | 1 (0.4) | |
| Pacific Islander | 6 (0.4) | 4 (0.3) | 2 (0.7) | |
| Other | 5 (0.3) | 3 (0.2) | 2 (0.7) | |
| Unknown | 6 (0.4) | 3 (0.2) | 3 (1.1) | |
| Hispanic Ethnicity | 197 (12.0) | 162 (11.9) | 35 (12.8) | 0.2611 |
| Comorbidities | 0.1057 | |||
| 1 | 460 (28.1) | 394 (28.9) | 66 (24.1) | |
| 2 or more | 560 (34.2) | 476 (34.9) | 84 (30.7) | |
| None | 541 (33.0) | 482 (35.4) | 59 (21.5) | |
| Unknown | 76 (4.6) | 11 (0.8) | 65 (23.7) | |
| APACHE II | 28.8 ± 7.4 | 27.5 ± 6.9 | 35.3 ± 6.2 | <.0001 |
| Maximum AIS† | 4.1 ± 0.8 | 4.0 ± 0.8 | 4.4 ± 0.8 | <.0001 |
| Head | 1.5 ± 1.9 | 1.4 ± 1.8 | 2.0 ± 2.1 | <.0001 |
| Thorax | 2.5 ± 1.8 | 2.4 ± 1.7 | 2.9 ± 1.8 | <.0001 |
| Abdomen | 2.1 ± 1.7 | 2.0 ± 1.7 | 2.2 ± 1.8 | 0.2798 |
| Extremity | 2.4 ± 1.7 | 2.4 ± 1.6 | 2.3 ± 1.8 | 0.1916 |
| ISS‡ | 32.1 ± 13.4 | 31.0 ± 2.9 | 37.5 ± 14.6 | <.0001 |
| NISS§ | 37.9 ± 14.1 | 36.6 ± 13.3 | 44.6 ± 15.5 | <.0001 |
| Mechanism of Injury | 0.0105 | |||
| MVC | 936 (57.1) | 767 (56.3) | 169 (61.7) | |
| MCC | 248 (15.1) | 219 (16.1) | 29 (10.6) | |
| Pedestrian-MVC | 216 (13.2) | 169 (12.4) | 47 (17.2) | |
| Fall | 139 (8.5) | 123 (9.0) | 16 (5.8) | |
| Other | 98 (6.0) | 85 (6.2) | 13 (4.7) | |
| Presenting Physiology and Underlying Shock Parameters | ||||
| Initial ER SBP, mmHg | 111.1 ± 31.7 | 112.9 ± 30.6 | 102.2 ± 35.4 | <.0001 |
| ER SBP < 90 mm Hg | 1,076 (65.7) | 857 (62.9) | 219 (80.0) | <.0001 |
| Total PRBC units in first 12 hours | 8.0 ± 9.5 | 6.4±6.8 | 15.8 ± 15.2 | <.0001 |
| Total Fresh Frozen Plasma in first 12 hours, ml | 1,067.8 ± 1,536.8 | 883.8 ± 1,288.4 | 1,982.3 ± 2,204.8 | <.0001 |
| Total Crystalloid in first 12 hours, ml | 11,000 ± 6289 | 10,568 ± 5761 | 13,148 ± 8116 | <.0001 |
| Worst Base Deficit in first 6 hours, meq/L | −10.4 ± 5.1 | −9.6 ± 4.4 | −14.2 ± 6.5 | <.0001 |
| Worst Lactate in first 6 hours, mmol/L | 5.4 ± 3.2 | 4.9 ± 2.8 | 8.0 ± 3.9 | <.0001 |
Values represent mean ± SD or N(%). Data were analyzed by Wilcoxon Signed Rank Test, Chi-Square Test, or Fisher’s Exact Test as appropriate.
MVC denotes motor vehicle crash, MCC motor cycle crash, ER emergency department, SBP systolic blood pressure, and PRBC packed red blood cells.
Scores for the Abbreviated Injury Scale (AIS) can range from 1 to 6, with higher scores indicating more severe injury.
The Injury Severity Score (ISS) can range from 1 to 75, with higher scores indicating more severe injury.
The New Injury Severity Score (NISS) can range from 1 to 75, with higher scores indicating more severe injury.
The study population experienced significant complications including ARDS (24.9%), ventilator-associated pneumonia (VAP) (26.5%), and MOF (34.8%) (Table 2). Outcomes for patients who died reflect most deaths occurring within the first 7 study days. Overall mortality was 16.7% with only 29.2% of the patients discharged home without assistance; the majority of the surviving patients were transferred to rehabilitation or skilled nursing facilities.
Table 2.
Patient Outcomes and Complications of the 1,637 Massively Injured Patients
| Total Cohort n=1,637 | Alive n=1,363 | Dead n=274 | |
|---|---|---|---|
| Survival (%) | 1,363 (83.3) | ||
| Length of Stay | |||
| Hospital | 23.8 ± 22.4 | 27.0 ± 22.6 | 7.4 ± 11.7 |
| ICU | 12.8 ± 12.6 | 14.0 ± 12.9 | 6.7 ± 8.4 |
| Ventilator days | 9.7 ± 10.7 | 10.3 ± 11.1 | 6.3 ± 7.8 |
| Disposition | |||
| Home | 478 (29.2) | ||
| Skilled Nursing Center | 374 (22.9) | ||
| Rehabilitation Center | 390 (23.8) | ||
| Complications | |||
| DVT | 76 (4.6) | 74 (5.4) | 2 (0.7) |
| PE | 58 (3.5) | 48 (3.5) | 10 (3.6) |
| Multiple Organ Failure | 570 (34.8) | 427 (31.3) | 143 (52.2) |
| Maximum Denver Score | 2.4 ± 2.2 | 1.9 ± 1.8 | 4.7 ± 2.5 |
| ARDS | 407 (24.9) | 333 (24.4) | 74 (27.0) |
| Maximum Organ Failure Score | 5.3 ± 2.8 | 5.0 ± 2.3 | 6.9 ± 3.9 |
| Cardiac | 2.4 ± 1.2 | 2.5 ± 1.1 | 2.3 ± 1.6 |
| Pulmonary | 1.6 ± 1.1 | 1.5 ± 1.1 | 2.3 ± 1.1 |
| Hepatic | 0.6 ± 1.0 | 0.6 ± 0.9 | 0.7 ± 1.2 |
| Renal | 1.0 ± 0.6 | 1.0 ± 0.5 | 1.4 ± 0.8 |
| Nosocomial Infections | |||
| BSI | 229 (14.0) | 192 (14.1) | 37 (13.5) |
| SSI | 244 (14.9) | 218 (16.0) | 26 (9.4) |
| VAP | 434 (26.5) | 386 (8.3) | 48 (17.5) |
Values represent mean ± SD, N(%) or, frequency.
Abbreviations: ICU = intensive care unit, DVT = deep venous thrombosis, PE = pulmonary embolism, ARDS = acute respiratory distress syndrome, BSI = blood stream infection, SSI = surgical site infection, VAP = ventilator associated pneumonia
Multiple Organ Failure is defined as Maximum Marshall Score >=6
SOP Compliance
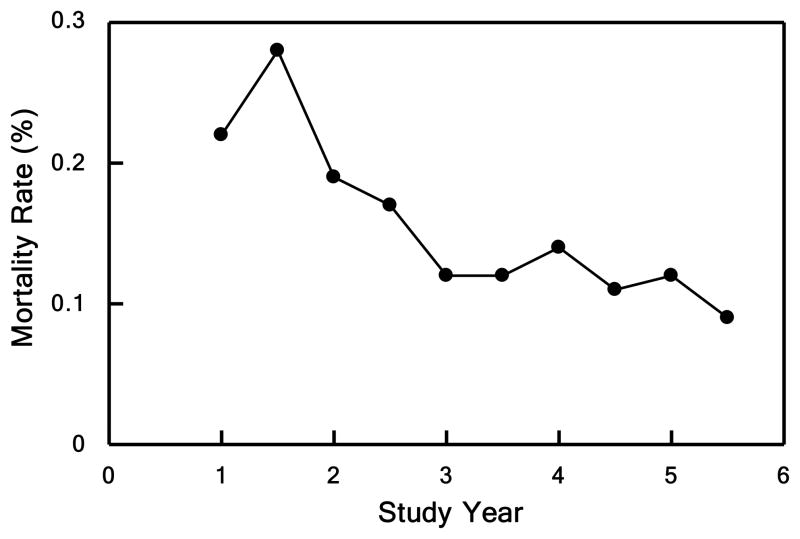
A primary study goal was to implement resuscitation and clinical management SOPs to standardize patient care across the trauma centers, and subsequently to examine the impact of these SOPs on clinical outcomes. Initially, protocol compliance within each institution varied (Table 3); however, mean compliance improved over time. Significant improvements in compliance included a decrease in tidal volumes in patients with ALI/ARDS (7.5 to 6.8 ml/kg), a decrease in inappropriate vascular thrombo-embolic (VTE) prophylaxis (34% to 8%), and use of bronchoalveolar lavage for the diagnosis of VAP (72% to 100%) (all p<0.05). Compliance with certain protocols showed little significant improvement despite regular reviews and site visits (e.g. central venous pressure (CVP) monitoring in early resuscitation (70% to 77%) and the underfeeding of patients one week after injury (46% to 42%)). Importantly, our overall mortality decreased over the study from 22% in the first two years to 11% in the last two years (p<0.01), as demonstrated in Figure 1a. This decrease was significant even if we corrected for baseline age, APACHE II, AIS, New Injury Severity Score (NISS), systolic blood pressure, and base deficit.
Table 3.
SOP Compliance: First Study Year and Last Year
| Protocol | First Year (percentage) | Last Year (percentage) | Relative Improvement |
|---|---|---|---|
| ARDS | 36 | 46 | 1.3 |
| Nutrition | 54 | 69 | 1.3 |
| Resuscitation | 70 | 77 | 1.1 |
| Insulin | 56 | 82 | 1.5 |
| VTE | 66 | 96 | 1.6 |
| Pneumonia | 73 | 96 | 1.3 |
Values listed as percentage of patients treated by specific protocol
Abbreviations: ARDS = acute respiratory distress syndrome, VTE = venous thromboembolic event
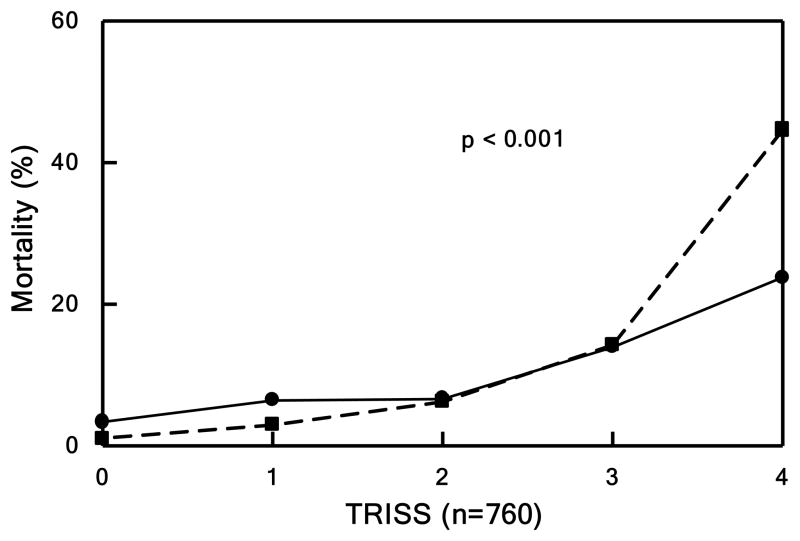
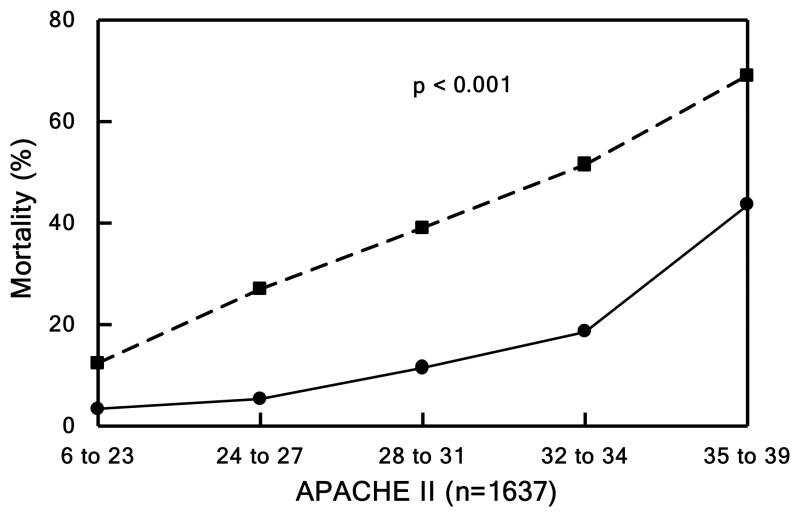
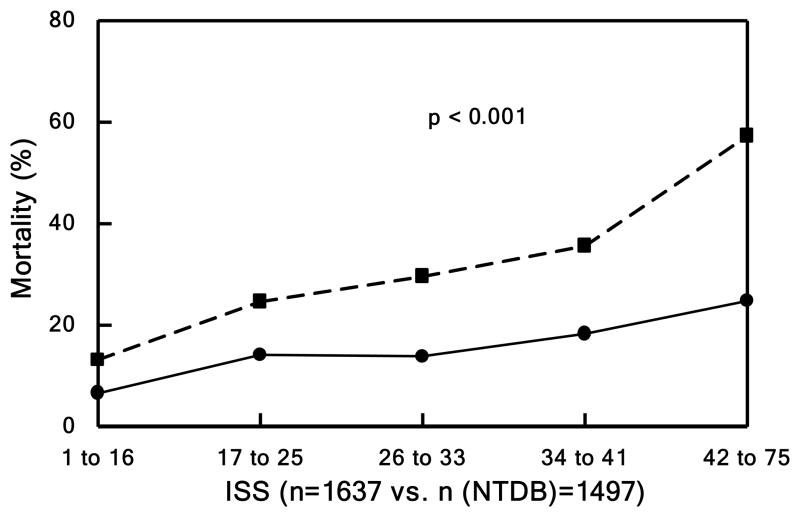
Figure 1. Mortality.
Patients divided into quintiles in Panels B, C, and D based underlying score or injury severity. Panel A shows mortality over the entire study period. Observed (solid lines) vs. expected (dashed line) outcome for Panel B) mortality by TRISS (p<0.001), Panel C) mortality by APACHE II (p<0.001), and Panel D) mortality by NTDB (p<0.001).
Outcomes Compared to Standardized Benchmarks
To further assess the mortality rates in the context of injury severity, survival was evaluated using several published measures of mortality propensity including TRISS (Figure 1b) and APACHE II (Figure 1c), as well as the observed mortality within a matched cohort from the NTDB during the same period of time (Figure 1d). Of the 2,433,507 NTDB patients, 410,419 with known outcome following blunt trauma arrived at Level I or II trauma centers within 24 hours of injury and were sent to the intensive care unit or operating room. Of these, 21,447 had SBP <90 or base deficit >6 while 345,755 were excluded with SBP ≥90 and unknown base deficit. Of these, 2,466 had head AIS codes of 1 to 5, but 18,969 were excluded with missing head AIS code. The remaining 2,408 NTDB patients with known age and ISS were compared to our study cohort.
Based on TRISS, our outcomes were markedly improved in those patients from the most critically ill quintile. In addition, the observed mortality in our cohort was dramatically better than predicted by APACHE II across all quintiles. Our cohort mortality matched to NTDB patients demonstrated a significant improvement in ISS-related mortality, odds ratio of 0.370 (95% CI 0.314–0.437). In addition, the matched cohort in NTDB, unlike our study population, was found to have a minimal non-significant improvement in survival.
Organ Dysfunction and TTR
Although multiple established measures of clinical outcome in critically ill patients exist, they often lack granularity, are narrow in focus, and ineffectively capture multiple physiologic features in complex disorders such as traumatic injury. The concept of TTR from organ dysfunction was examined to develop a more comprehensive parameter. TTR is associated with demographic characteristics including sex and advanced age (Table 4) demonstrated in studies to have poorer outcomes.29,30 TTR varied directly with increasing severity of anatomic injury, whether assessed by ISS or overall maximum AIS score. Additionally, prolonged TTR was associated with the presence of shock based on initial base deficit or lactate, increasing transfusion requirements, and elevated day 1 glucose levels. We concluded that MOF TTR correlated well with a wide range of features easily ascribed to severely injured, critically ill patients.
Table 4.
Patient Demographic, Injury and Shock Characteristics and Their Association with Time to Recovery
| Variable | N | Median Days (IQ 25–75%) | p-value |
|---|---|---|---|
| Cohort | 1,637 | 10 (4–25) | |
| Sex | 0.0168 | ||
| Male | 1,085 | 11 (4–26) | |
| Female | 552 | 9 (4–21) | |
| Age | <.0001 | ||
| <55 | 1,224 | 9 (4–21) | |
| >=55 | 413 | 16 (6–29) | |
| ISS | <.0001 | ||
| <15 | 154 | 4 (2–10) | |
| 15–24 | 328 | 6 (3–19) | |
| >=25 | 1,155 | 13 (5–28) | |
| Maximum AIS | 0.0008 | ||
| <3 | 26 | 4 (2–11) | |
| >=3 | 1,611 | 10 (4–25) | |
| Base Deficit | <.0001 | ||
| <6 | 237 | 6 (4–15) | |
| 6–10 | 626 | 7 (3–16) | |
| >=10 | 706 | 16 (6–30) | |
| Lactate | <.0001 | ||
| <2 | 97 | 5 (3–13) | |
| 2–4 | 386 | 7 (4–14) | |
| >4 | 768 | 14 (6–29) | |
| Transfusion | <.0001 | ||
| <6 units | 908 | 6 (3–15) | |
| >=6 units | 729 | 16 (7–30) | |
| Glucose | <.0001 | ||
| <200 | 896 | 7 (3–17) | |
| >=200 | 738 | 15 (6–29) |
Spearman correlation or Wilcoxon test p-values.
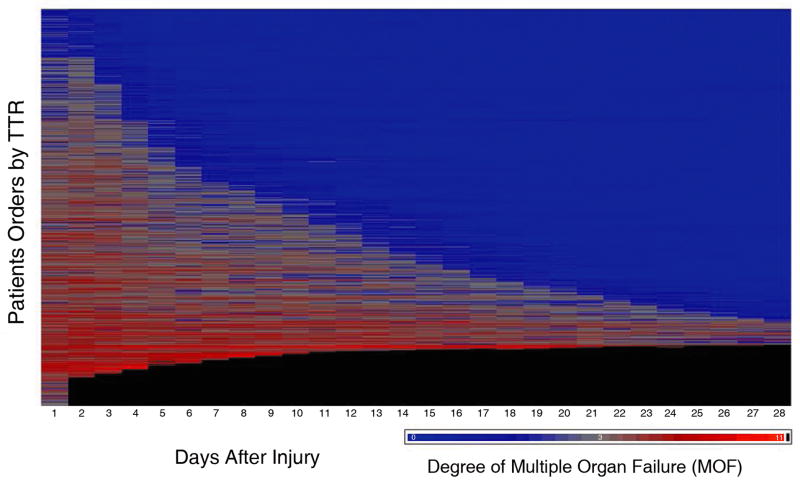
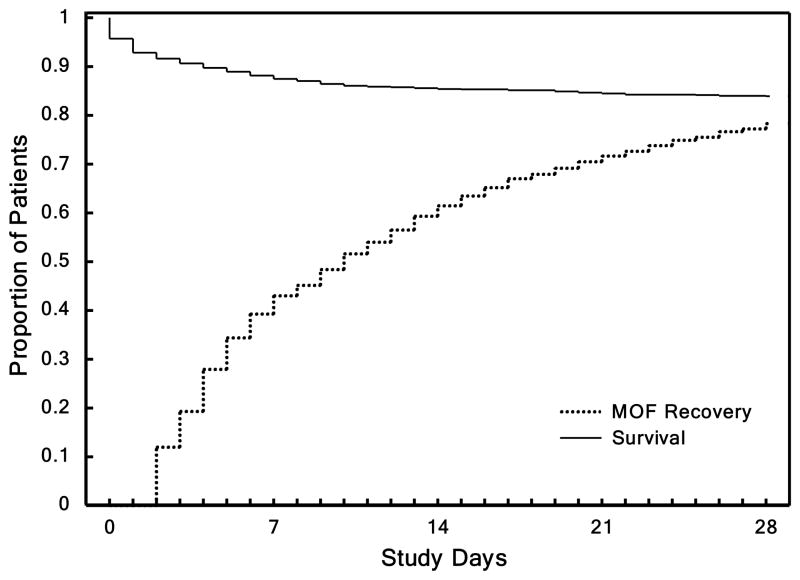
As demonstrated, TTR reflects recovery of organ dysfunction independent of the first seven-day mortality. In Figure 2a from top to bottom, the patients are ordered by their TTR. The patients who never had organ dysfunction or recovered rapidly are nearest the top while patients with prolonged TTR are towards the bottom. Shades of red represent degree of MOF, blue represents the absence of MOF, and black represents death. Figure 2b is a graphical representation of the same data. Here, we show the proportion of patients who either died, recovered, or remained with organ failure on any given day. As shown, most deaths occur within the first seven days after injury, whereas the proportion of patients who recovered from MOF continued to increase over time without evidence of recurrent episodes of organ dysfunction during the entire 28 days post-injury with only a small number of patients not recovered by 28 days.
Figure 2. Time to Recovery.
Panel A shows time to recovery from MOF. Heat map of the MOF scores over time after injury up to 28 days for each of the 1,637 massive injured patients. From top to bottom, patients are ordered according to their TTR days. Panel B shows mortality and time to recovery following severe injury. Kaplan-Meier survival curve and proportion of patients fully recovered from organ dysfunction in the first 28 days following blunt traumatic injury.
DISCUSSION
This study represents one of the first comprehensive, multi-institutional assessments of patient and injury characteristics, anatomic and physiological derangement, and outcomes from severe blunt trauma to date using evidence-based patient treatment protocols. Through development of detailed methodologies for study inclusion, data collection, and clinical care, we were able to amass a well-characterized set of 1,637 severely injured patients. In addition, our data set is web-accessible to any investigator at www.gluegrant.org/ and should serve as a rich and novel data resource in critically injured patients.
Current dogma suggests that multiple organ dysfunction or mortality rarely occurs after severe blunt trauma in the U.S. because prehospital, field transportation, and in-hospital trauma care has improved substantially over past decades. While it is true that the majority of blunt injury patients leave the hospital after an uneventful course, this study describes a cohort that was critically injured, as measured by ISS, and in shock as measured by systolic blood pressure, base deficit, and lactate levels. By virtue of our inclusion criteria, this cohort represents those individuals at the highest risk for complications and death. These patients exhibited significant mortality and MOF with 39.7% of the patients either dying or having a TTR of >14 days. If we are to achieve further advances to reduce morbidity and mortality, then attention must be paid to those most severely injured with shock. Interestingly in Figure 2, patients who developed MOF did so rapidly without apparent recovery followed by worsening, subsequent organ dysfunction as classically ascribed to the “two-hit hypothesis” of MOF.
A strength of the study is that clinical SOPs were developed, implemented, and audited in areas where data might suggest that practice variations could influence patient outcomes, e.g. infections and mortality. In recognition that SOPs have not been widely published or routinely practiced in major U.S. trauma centers, the Journal of Trauma Injury, Infection and Critical Care published these as a series, and they have been adopted for utilization in large federally-funded clinical trials.7–15 SOP implementation was incremental with increasing compliance for most; only a few protocols persisted without improvement (Table 3). While full compliance was not achievable, 30 – 50% improvements were shown. Moreover, while improvement in protocol compliance is considered essential for outcome improvement, minor absolute changes in compliance have been shown to have a significant clinical benefit, as previously demonstrated for other clinical disorders, such as sepsis.31–33
For example in our ARDS SOP, although the observed compliance with ventilator volume protocols increased from only 36% to 46% of patients (Table 3), actual average tidal volumes decreased in a more clinically relevant manner (7.5 to 6.8 ml/kg). Of interest, adherence to the SOPs improved with time most likely as a result of compliance report reviews at our quarterly face-to-face investigators’ meetings, the site visit audit program, and dashboard compliance monitoring on our website. Compliance measures cannot be associated with outcome on an individual patient basis because the most severe patients are most likely to be out of compliance and in fact, overruling the SOP, is allowed in extreme situations. Therefore, an ecologic analysis based on group data is the only analysis possible. It cannot substitute for a clinical trial comparing SOP’s versus no SOP’s, but since the SOP’s are evidence-based, such a trial might be unethical. While mortality improvement may indicate a Hawthorne effect, as demonstrated in Figure 1a, the duration of the impact over five years with continued improvement after five years strongly suggests a direct effect of the SOPs, along with continued implementation and compliance. Thus, this is one of the first studies in severely injured patients demonstrating improved mortality with implementation of a “trauma bundle.”
We studied critically ill patients at high risk for complications and death. Despite their massive injuries, patient mortality was only 16.7% compared to a 10% mortality rate reported from the only other prospective outcomes study in trauma, and one that did not require massive injuries, transfusions, or a base deficit for inclusion.30 In that study, the adjusted mortality to patients that presented with shock, as defined as a SBP <90 mmHg, or significant injury, defined as AIS >3, ranged between 8.4% and 29.4%. However, unlike our study, the occurrence of complications, such as MOF, was not evaluated. Thus, our study provides not only a better benchmark for mortality, but also for the independent development and recovery of MOF in these patients.
Comparing our mortality or organ failure rates with APACHE II and TRISS predicted rates or with NTDB patients was difficult. One issue was that in the NTDB, there was very little physiological data demonstrating shock or its sequelae (base deficit and lactate level). In fact, base deficit, which is an NTDB data element, was recorded in only a very small proportion (~4%) of the nearly one million patients for the years 2002–2007. Although ISS is known to be a strong predictor of mortality when viewed over the entire dynamic range of ISS, it was interesting to note that when limiting comparisons to those with high injury scores, physiological parameters become critically important discriminators. At high ISS values, the presence of shock upon hospital presentation had a profound impact upon outcome. While physiological parameters and the degree of initial shock have not been included in traditional benchmarks, based on our observations, they should be. This influence of physiology has not been appreciated in previous studies and we believe physiological parameters greatly empower the predictive nature of these models or datasets at high ISS values, rather than merely relying on the severity of injury. In fact, this may be partly the reason for the differences in expected vs. observed mortality within NTDB and our current cohort.
This ongoing challenge of inadequate benchmarks for these complex processes is one of the reasons why we have introduced the concept of TTR as a novel outcome benchmark. There are at least two potential advantages of TTR: (1) it is more meaningful clinically at the bedside, and (2) in our view, it is easier to recognize multi-system organ recovery signs than arbitrary grades of organ dysfunction. In our study, TTR showed strong correlations with parameters that can comprehensively and accurately describe critically ill patients with severe blunt trauma. A third potential advantage is increased power to detect treatment effects in randomized trials. TTR is similar to ventilator-free days for acute pulmonary dysfunction in that it combines information on both mortality and speed of recovery in survivors. Ventilator-free days has been shown to be a more powerful outcome measure than mortality alone.35 TTR appears to be a more comprehensive and appropriate monitor for the complexity of the disease response.
Mortality and multiorgan failure remain problematic in patients with severe traumatic injury. This study underscores the importance of two critical facets for improving patient care: (1) the implementation of standardized protocols based on best available evidence to enhance clinical practice and (2) use of relevant endpoints for patient outcomes that are not simply defined as lived or died, but integrate complications and organ failure. The morbidity and mortality rates in this study represent improvements over established benchmarks, and thus should set a high standard for the care of these challenging patients. In addition, by making our unique dataset publicly available, we hope to enable other clinicians to investigate their own hypothesis and/or questions to continue to improve current practice for critically injured patients.
Supplementary Material
Acknowledgments
Study concept and design: Tompkins, Maier, Cuschieri, Johnson, Sperry, West, Moore, Minei, Bankey, Nathens, Cuenca, Efron, Hennessy, Xiao, Mindrinos, McDonald-Smith, Billiar, Schoenfeld, Warren, Cobb, Moldawer, Davis
Acquisition of data: Maier, Cuschieri, Hennessy, Nathens, Minei, Moore, Johnson, Bankey, Billiar, Sperry, Bankey, Schoenfeld, Cobb
Analysis and interpretation of data: Schoenfeld, Xiao, Tompkins, Maier, Cuschieri, Johnson, Moore, Minei, West, Moldawer, Cuenca, Efron, Warren, Mason, Cobb
Drafting of the manuscript: Cuschieri, Johnson, Sperry, West, Moore, Minei, Bankey, Nathens, Cuenca, Hennessy, McDonald-Smith, Billiar, Schoenfeld, Moldawer, Maier, Tompkins
Critical revision of the manuscript for important intellectual content: Cuschieri, Maier, Tompkins, Warren, Moldawer, Cuenca, McDonald-Smith, Efron, West, Mason, Xiao, Mindrinos, Davis, Moore, Johnson, Cobb
Statistical analysis: Schoenfeld, Xiao, Mason
Obtained funding: Tompkins, Moldawer, Warren
Administrative, technical, or material support: Mindrinos, Hennessy, Cuenca, McDonald-Smith, Warren, Moldawer, Cobb
Study Supervision: Tompkins, Maier, Cuschieri, Nathens, Minei, Moore, Johnson, Bankey, Billiar, Sperry, Efron, Cobb, West, Davis, Cobb
Funding/Support: This study was supported by The Inflammation and the Host Response to Injury Large Scale Collaborative Research Grant from the National Institute of General Medical Sciences, 2 U54 GM 062119.
Funding: NIH, U54GM062119
The participants of the Inflammation and Hospital Response to Injury Large Scale Collaborative Research Program
Lily Altstein, Ph.D., Henry V. Baker, Ph.D., Ulysses G.J. Balis, M.D., Bernard H. Brownstein, Ph.D., Steven E. Calvano, Ph.D., David G. Camp II, Ph.D., Asit K. De, Ph.D., Celeste C. Finnerty, Ph.D., Richard L. Gamelli, M.D., Nicole S. Gibran, M.D., Brian G. Harbrecht, M.D., David N. Herndon, M.D., Shari E. Honari, R.N., Marc G. Jeschke, M.D., Ph.D., Matthew B. Klein, M.D., Stephen F. Lowry, M.D., Bruce A. McKinley, Ph.D., Frederick A. Moore, M.D., Carol L. Miller-Graziano, Ph.D., Grant E. O’Keefe, M.D., M.P.H., Laurence G. Rahme, Ph.D., Daniel G. Remick, M.D., Michael B. Shapiro, M.D., Richard D. Smith, Ph.D., John D. Storey, Ph.D., Robert Tibshirani, Ph.D., Mehmet Toner, Ph.D., Bram Wispelwey, M.S., Wing H. Wong, Ph.D.
Footnotes
Trial Registration clinicalTrials.gov Identifier NCT00257231
Author Contributions: Dr. Tompkins had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial disclosures: None reported
Contributor Information
Joseph Cuschieri, University of Washington School of Medicine and Harborview Medical Center, Seattle, WA
Jeffrey L. Johnson, University of Colorado Health Sciences Center and Denver Health Medical Center, Denver, CO
Jason Sperry, University of Pittsburgh Medical Center and Presbyterian University Hospital, Pittsburgh, PA
Michael A. West, University of California San Francisco and San Francisco General Hospital, San Francisco, CA
Ernest E. Moore, University of Colorado Health Sciences Center and Denver Health Medical Center, Denver, CO
Joseph P. Minei, University of Texas Southwestern Medical Center and Parkland Health and Hospital System, Dallas
Paul E. Bankey, University of Rochester Medical Center and Strong Memorial Hospital, Rochester, NY
Avery B. Nathens, University of Toronto and St. Michael’s Hospital, Toronto, Ontario, Canada
Alex G. Cuenca, University of Florida College of Medicine and Shands Hospital at the, Gainesville, FL
Philip A. Efron, University of Florida College of Medicine and Shands Hospital at the, Gainesville, FL
Laura Hennessy, Harborview Medical Center, Seattle, WA
Wenzhong Xiao, Massachusetts General Hospital, Boston, MA
Michael N. Mindrinos, Stanford Genome Technology Center, Palo Alto, CA
Grace P. McDonald-Smith, Massachusetts General Hospital, Boston, MA
Philip H. Mason, Massachusetts General Hospital, Boston, MA
Timothy R. Billiar, University of Pittsburgh Medical Center and Presbyterian University Hospital, Pittsburgh, PA.
David A. Schoenfeld, Harvard Medical School and Massachusetts General Hospital, Boston, MA
H. Shaw Warren, Harvard Medical School and Massachusetts General Hospital, Boston, MA
J. Perren Cobb, Harvard Medical School and Massachusetts General Hospital, Boston, MA
Lyle L. Moldawer, University of Florida College of Medicine and Shands Hospital at the, Gainesville, FL
Ronald W. Davis, Stanford Genome Technology Center, Palo Alto, CA
Ronald V. Maier, University of Washington School of Medicine and Harborview Medical Center, Seattle, WA
Ronald G. Tompkins, Harvard Medical School and Massachusetts General Hospital, Boston, MA.
References
- 1.Health, United States, 2008 with Chartbook. Hyattsville, Md: National Center for Health Statistics; 2009. [Google Scholar]
- 2.Heron M, Hoyert DL, Murphy SL, Xu J. National vital statistics reports 2009. 14. Vol. 57. Hyattsville, Md: National Center for Health Statistics; 2009. Deaths: Final data for 2006. [PubMed] [Google Scholar]
- 3.Bergen G, Chen LH, Warner M, Fingerhut LA. Injury in the United States: 2007 Chartbook. Hyattsville, Md: National Center for Health Statistics; 2007. [Google Scholar]
- 4.Evans JA, Van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010 Jan;34(1):158–63. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 5.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009 Sep;40(9):912–8. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos-Ortega A, Subeviola B, Garcia-Astudillo LA, Holanda MS, Ortiz F, Liorca J, Delgado-Rodriguez M. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010 Apr;38(4):1036–43. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 7.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005 Sep;59(3):764–9. Foreward:762–3. [PubMed] [Google Scholar]
- 8.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis, and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006 May;60(5):1106–13. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 9.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006 Jul;61(1):82–9. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 10.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006 Aug;61(2):436–9. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro MB, West MA, Nathens AB, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core—Standard Operating Procedures for Clinical Care. V Guidelines for Sedation and Analgesia During Mechanical Ventilation General Overview-CHECK. J Trauma. 2007;63(4):945–50. [Google Scholar]
- 12.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core—Standard Operating Procedures for Clinical Care. VI. Blood Glucose Control in the Critically Ill Trauma Patient. J Trauma. 2007 Sep;63(3):703–8. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 13.West MA, Moore EE, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VII. Guidelines for antibiotic administration in severely injured patients. J Trauma. 2008 Dec;65(6):1511–9. doi: 10.1097/TA.0b013e318184ee35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Keefe GE, Shelton M, Cuschieri J, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VIII--Nutritional support of the trauma patient. J Trauma. 2008 Dec;65(6):1520–8. doi: 10.1097/TA.0b013e3181904b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuschieri J, Freeman B, O’Keefe G, et al. Inflammation and the host response to injury a large-scale collaborative project: patient-oriented research core standard operating procedure for clinical care X. Guidelines for venous thromboembolism prophylaxis in the trauma patient. J Trauma. 2008 Oct;65(4):944–50. doi: 10.1097/TA.0b013e3181826df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yale Center for Medical Informatics. TrialDB – A Clinical Studies Data Management System. ( http://ycmi.med.yale.edu/trialdb/index.shtm)
- 17.Evans HL, Cuschieri J, Moore EE, et al. Inflammation and the host response to injury, a Large-Scale Collaborative Project: patient-oriented research core standard operating procedures for clinical care IX. Definitions for complications of clinical care of critically injured patients. J Trauma. 2009 Aug;67(2):384–8. doi: 10.1097/TA.0b013e3181ad66a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference of ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 21.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. Philadelphia, Pa: Lippincott Williams and Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 22.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guidelines for prevention of surgical site infections, 1999. Infect Control Hosp Epidemiol. 1999;20:248–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 23.Champion HR, Sacco WJ, Copes WS. Injury severity scoring again. J Trauma. 1995;38(1):94–5. doi: 10.1097/00005373-199501000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 25.Sauaia A, Moore FA, Moore EE, Norris JM, Lezotte DC, Hamman RF. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45(2):291–303. doi: 10.1097/00005373-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Flora JD. A Method for Comparing Survival of Burn Patients to a Standard Survival Curve. J Trauma. 1978;18(10):701–705. doi: 10.1097/00005373-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 27.The Abbreviated Injury Scale 1998 Revision, American Association for Automobile Medicine (now Association for the Advancement of Automotive Medicine), Des Plaines, IL.
- 28.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 29.Sperry JL, Nathens AB, Frankel HL, Vanek SL, Moore EE, Maier RV, Minei JP Inflammation and the Host Response to Injury Investigators. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008 Jun;36(6):1838–45. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie, Ellen J, Rivara Frederick P, Jurkovich Gregory J, Nathens Avery B, Frey Katherine P, Egleston Brian L, Salkever David S, Scharfstein Daniel O. A National Evaluation of the Effect of Trauma-Center Care on Mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 31.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and shock. Crit Care med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 32.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008 [published correction appears in Crit Care Med 2008; 36:1394–1396] Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer R, Artigas A, Levy MM, Blanco J, González-Díaz G, Garnacho-Montero J, Ibáñez J, Palencia E, Quintana M, de la Torre-Prados MV for the Edusepsis Study Group. Improvement in Process of Care and Outcome After a Multicenter Severe Sepsis Educational Program in Spain. JAMA. 2008;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 34.DuBose JJ, Browder T, Inaba K, Teixeira PG, Chan LS, Demetriades D. Effect of trauma center designation on outcome in patients with severe traumatic brain injury. Archives of Surgery. 2008 Dec;143(12):1213–7. doi: 10.1001/archsurg.143.12.1213. discussion 1217. UI: 19075174. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfeld DA, Bernard GR for the ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Critical Care Medicine. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.