Abstract
Diabetes increases the risk of Alzheimer’s disease (AD). The pathological hallmarks for AD brains are extracellular amyloid plaques formed by β-amyloid peptide (Aβ) and intracellular neurofibrillary tangles consisting of hyperphosphorylated tau protein. This study was designed to determine AD-like brain changes in mice modeling for type 2 diabetes. The effects of metformin on these changes also were studied. Seven-week old male db/db mice received intraperitoneal injection of 200 mg kg−1 d−1 metformin for 18 weeks. They were subjected to Barnes maze at an age of 21 weeks and fear conditioning at an age of 24 weeks to assess their cognitive functions. Hippocampus was harvested after these tests for biochemical evaluation. The db/db mice had more tau phosphorylated at S396 and total tau in their hippocampi than their non-diabetic control db+ mice. Activated/phosphorylated c-jun N-terminal kinase (JNK), a tau kinase, was increased in the db/db mouse hippocampus. Metformin attenuated the increase of total tau, phospho-tau and activated JNK. The db/db mice had increased Aβ levels. Metformin attenuated the reduction of synaptophysin, a synaptic protein, in the db/db mouse hippocampus. Metformin did not attenuate the impairments of spatial learning and memory as well as long-term hyperglycemia in the db/db mice. Our results suggest that the db/db mice have multiple AD-like brain changes including impaired cognitive functions, increased phospho-tau and Aβ as well as decreased synaptic proteins. Activation of JNK may contribute to the increased phospho-tau in the db/db mice. Metformin attenuates AD-like biochemical changes in the brain of these mice.
Keywords: Aβ, Alzheimer disease, diabetes, metformin, mice, tau phosphorylation
1. Introduction
Diabetes mellitus (DM) affects more than 20 million Americans (Cherian et al., 2009; Kim et al., 2009). Studies have shown that DM increases the risk for Alzheimer’s disease (AD) (Brands et al., 2005; Ott et al., 1999; Strachan et al., 1997), the most common form of dementia in the elderly (Berg and Morris, 1994). The pathological hallmarks in the brain of a patient with AD are extracellular amyloid plaques formed by β-amyloid peptide (Aβ), an enzymatic product of amyloid precursor protein (APP) by β-secretase/β-amyloid converting enzyme 1 (BACE1) and γ-secretase (Vassar, 2004; Vassar and Citron, 2000), and intracellular neurofibrillary tangles consisting of hyperphosphorylated tau proteins (Selkoe, 2001). It has been suggested that Aβ and hyperphosphorylated tau proteins induce local neurotoxicity that ultimately results in AD.
There are at least two types of DM: type 1 and type 2. More patients suffer from type 2 DM (Cherian et al., 2009). Multiple animal models have been developed to facilitate DM research. It has been shown that animals with type 1 and type 2 DM have an increased phospho-tau expression in their brains (Jolivalt et al., 2008; Kim et al., 2009; Li et al., 2007; Planel et al., 2007b). These animals also have increased Aβ expression in the brains (Jolivalt et al., 2008; Li et al., 2007). Among animal models for DM, the db/db (BKS.Cg-Dock7m+/+Leprdb/J) mice are a common model for type 2 DM. These mice develop hyperglycemia and hyperinsulinemia and are obese, polyphagic, polydipsic and polyuric (Fujita et al., 2002; Kim et al., 2009).
Various treatments for DM have been developed. Many of them aim to control blood glucose levels (Del Prato, 2009). Oral hypoglycemic agents are often used in patients with type 2 DM. Glucophage (metformin) is the most commonly used oral hypoglycemic agent (Kirpichnikov et al., 2002; Knowler et al., 2002). Interestingly, it has been shown recently that metformin increases Aβ expression in neuronal cultures (Chen et al., 2009). However, the effects of metformin on Aβ expression in diabetic mice are not reported. Its effects on other AD-like brain features, such as tau phosphorylation as well as learning and memory functions, in diabetic animals are not known. Thus, we designed this study to evaluate the AD-like brain functional and pathological changes and the effects of metformin on these changes in the db/db mice.
2. Materials and methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996.
2.1. Animal groups
Six-week old male db/db mice and sex- and age-matched non diabetes-prone db+ control mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The db/db mice were divided into control, normal saline and metformin groups (11 mice for each group to provide an n ≥ 6 for each experimental condition in the assays described below). Animals in the metformin group received intraperitoneal injection of 200 mg kg−1 d−1 metformin in saline until they were killed. This method of metformin application has been used in previous studies (del Prete et al., 1999; Heishi et al., 2008). Mice in saline group received daily injection of normal saline at the same volume as for the metformin-treated mice. The db/db mice in the control group and the db+ control mice did not receive any injection. Metformin or saline injection was started when animals were 7 weeks old. Their body weights were measured every two weeks. The volumes/dosages of metformin/saline were adjusted according to the weights.
Fasting blood glucose levels were measured at 6 and 24 weeks of age using blood glucose test strips (Bayer, Mishawaka, IN) with blood from the tail. Random blood glucose levels were measured 90 min after metformin or saline injection at 7, 8, 9, and 11 weeks of age.
2.2. Barnes maze
When mice were 21-week old, they were tested with Barnes maze system equipped with ANY-Maze video tracking system (San Diego Instruments, San Diego, CA). The natural agoraphobia of mice was enhanced by an 85-db buzzer and bright light from a 250 W bulb that was 50 cm above the Barnes maze platform. Animals were encouraged to find the target hole that was linked to a hiding box. There were other 19 holes identical to the target hole but these holes were not linked to a hiding box. Mice learnt the location of the target hole by using spatial intra- and extra-maze references during training. Each trial was recorded and analyzed to determine the latency for the mouse to enter the target hole and the time spent in the zone of each hole (within 2 cm around the hole).
The test was performed as described before (Reiserer et al., 2007; Zheng et al., 2009) with minor modifications. Each animal was subjected to two protocols: cued-target and hidden-target. In cued-target protocol, the location of the target hole varied in each trial and was marked by a conspicuous polystyrene cone attached to the maze perimeter. In hidden-target protocol, the location of the target hole was fixed and was not marked by any intra-maze reference. Both protocols involved training sessions on 5 consecutive days, four 3-min training sessions with a 15-min inter-session interval on each day. The short-term probe trial was performed at 2 h after the last training session on the fifth day. The long-term probe trial was performed one week after the short-term probe trial in the hidden-target protocol. The hidden-target protocol was started at 2 days after the completion of the cued-target protocol.
2.3. Fear conditioning
Mice (24-week old) were subjected to fear conditioning test at 2 days after the completion of Barnes test. Fear conditioning test was performed as described previously (Stratmann et al., 2009). Briefly, a mouse was placed in a plexiglas training chamber whose floor had a stainless grid for shock delivery. After a 3-min baseline exploratory period in the chamber, mice received 3 tone (2000 Hz, 90 db)–shock (0.7 mA, 2 s) pairings separated by 1 min. Each training chamber was cleaned with 95% ethyl alcohol before the placement of a mouse and was illuminated only with a 10 W bulb in a dark experimental room.
Twenty-four hours after the training session, mouse was placed again in the training chamber for 8 min without tone and foot shock. Each animal’s freezing behavior was scored every 8 s during the 8 min observation period. The percentage of time in freezing behavior was calculated using the formula of 100*f/n, where f was the number of freezing events in the 8 min and n was the total number of 8-s observation period in 8 min. One hour later, the mouse was placed in a new chamber without a stainless floor grid. This chamber was cleaned with the lemon fresh pine-sol each time after use and room light was turned on during the test. After a 3-min exploratory period in this new chamber, a 30-s tone (2000 Hertz, 90 db) was applied. The mouse was then left in the chamber for another 1 min. The percentage of time in freezing was calculated by the formula of 100*f/30, where f is the total freezing time during the 30-s observation period.
2.4. Brain tissue and blood sampling
After the completion of Barnes maze and fear conditioning tests, 24-week old mice were fasted overnight and then deeply anaesthetized with isoflurane. Blood was collected via direct cardiac puncture. The mice were immediately perfused transcardially with normal saline. After decapitation, hippocampus was dissected out quickly for Western blotting and measurement of Aβ1-42. Blood was collected for insulin, hemoglobin A1c (HbA1c) and lactate measurements. HbA1c was determined by the clinical laboratory of our hospital. Plasma insulin levels were determined by enzyme-linked immunosorbent assay (mouse insulin kit; Shibayagi, Gunma, Japan).
2.5. Western blotting
Total protein lysates were prepared from hippocampus in the lysis buffer (Cell Signaling Technology, Danvers, MA) containing protease inhibitors (Protease Inhibitor Cocktail; Sigma Aldrich, St Louis, MO) and phosphatase inhibitors (phosSTOP Phosphatase Inhibitor Cocktail Tablets; Roche, Nutley, NJ). They (30 – 50 μg/lane) were separated on sodium doecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). The primary antibodies used were rabbit polyclonal anti-tau [pS199/202] phosphospecific antibody (Invitrogen, Camarillo, CA; 1:1000); rabbit polyclonal anti-tau [pS396] phosphospecific antibody (Invitrogen; 1:1000); rabbit polyclonal anti-tau [pT231] phosphospecific antibody (BioSource International, Camarillo, CA, USA; 1:1000); mouse monoclonal anti-tau (tau5) antibody (Millipore,; 1:1000); mouse monoclonal anti-phospho-c-jun N-terminal kinase (JNK) (Thr183/Tyr185) (G9) antibody (Cell Signaling Technology; 1:2000); rabbit monoclonal anti- JNK (56G8) antibody (Cell Signaling Technology; 1:1000); rabbit polyclonal anti-phospho-glycogen synthase kinase-3β (GSK-3β) (Ser9) antibody (Cell Signaling Technology; 1:1000); rabbit polyclonal anti-GSK-3α [pY279]/β [pY216] phosphospecific antibody (Invitrogen; 1:1000); rabbit monoclonal anti-GSK-3β antibody (Cell Signaling Technology; 1:1000); rabbit polyclonal anti-cyclin-dependent kinase 5 (cdk5) antibody (Cell Signaling Technology; 1:1000); mouse monoclonal anti-protein phosphatase 2A (PP2A) C subunit antibody (Millipore; 1:2000); mouse monoclonal anti-synaptophysin antibody (Millipore; 1:10000); rabbit anti-drebrin antibody (Sigma Aldrich; 1:1000); rabbit polyclonal anti-neprilysin antibody (Millipore; 1:500); rabbit polyclonal anti-BACE1 C-terminal epitope (residues 485–501; Calbiochem, Darmstadt, Germany; 1:1000); rabbit polyclonal anti-insulin degrading enzyme (IDE) antibody (Abcam, Cambridge, MA, USA; 1:600); rabbit polyclonal anti-actin antibody (Sigma; 1:4000); rabbit monoclonal anti-APP antibody (Abcam; 1:5000); and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Sigma; 1:1000). After being incubated with horse radish peroxidase-conjugated secondary antibodies, protein bands were detected with the enhanced chemiluminescence methods (ECL plus kit; GE Healthcare, Sunnyvale, CA). The bands were visualized and quantified using G:Box equipped with Gene tools analysis software (Syngene, Frederick, MD). All protein band density was normalized by that of actin or GAPDH from the same sample.
2.6. Immunofluorescent staining
Hippocampus was fixed in 4% paraformaldehyde in phosphate buffered saline for 24 h at 40C. Hippocampal 14 μm-thick cryostat sections were obtained for immunofluorescent staining. Antigen retrieval with microwave heating for 15 min in 10 mM tri-sodium citrate buffer (pH = 6.0) containing 0.05% tween-20 was performed as described before (Erber et al., 1996; Huang and Zuo, 2005; Taylor et al., 1996). Sections were blocked with 5% donkey serum in phosphate buffered saline containing 0.1% triton-X 100 and 0.05% tween-20. The primary antibodies used were: mouse monoclonal anti-microtubule-associate protein 2 (MAP2) (Abcam, Cambridge, MA; 1:1000), rat monoclonal anti-cluster of differentiation molecule 11b (CD11b) (Abcam; 1:100), mouse monoclonal anti-glial fibrillary acidic protein (GFAP) (Millipore; 1:600) and mouse monoclonal anti-NeuN antibody (Millipore; 1:100 dilution). After being incubated with the primary antibodies at 40C overnight, the Cy3-conjugated goat polyclonal anti-rabbit serum (Abcam; 1:100) or Alexa Flour 488-labeled goat anti-mouse/rat serum secondary antibody (Invitrogen; 1:1000) was applied for 1 h at room temperature.
2.7. Aβ1-42 assay
Aβ was extracted from brain tissues as described before (Gravina et al., 1995). Briefly, hippocampus was homogenized in 8 x volume of 70% glass distilled formic acid. The homogenates were kept at 4°C for 15 min and then centrifuged at 100,000 g for 1 h. The formic acid extract layer between a thin overlying lipid layer and a small pellet was used for Aβ1-42 quantification by using an Aβ1-42 enzyme-linked immunosorbent assay kit (catalog number KMB3441; Invitrogen). The level of the Aβ1-42 in each brain sample was standardized to the brain tissue weight.
2.8. Serum lactate measurement
Serum lactate concentration was determined using a colorimetric method with a lactate assay kit (Lactate Assay Kit II, BioVision, Mountain View, CA) according to the manufacture’s protocol. The optical density of samples was read at 450 nm using a microplate reader. The final serum lactate concentrations are presented as mmol/l.
2.9. Statistical analysis
All results are presented as means ± S.D. or median with 95% confidence interval (n ≥ 6). Data from different groups of animals were analyzed by one way analysis of variance followed by the Student-Newman-Keuls test after confirmation of normal distribution of the data, by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test when the data are not normally distributed or by Student’s t test as appropriate. One way repeated measures analysis of variance or paired t test was used for the comparisons of the values from the same animals at different time-points. A P ≤ 0.05 was accepted as significant.
3. RESULTS
3.1. Effects of metformin on the characteristics of type 2 DM in the db/db mice
The db/db mice start to have hyperglycemia at 3 – 4 weeks of age and develop a full spectrum of type 2 DM characteristics including obesity, hyperglycemia, hyperinsulinemia and insulin resistance by 8 weeks of age (Kim et al., 2009; Lin et al., 2000). As shown in Table 1, the db/db mice had a higher fasting blood glucose level and were heavier than the lean control mice when they were 6 weeks old (P < 0.001 and t(33) = 12.227 for the body weight comparison, P = 0.025 and t(44) = 2.326 for blood glucose comparison) (Table 1). These differences continued when the mice were 24 weeks old. Metformin treatment for 18 weeks did not decrease the fasting blood glucose levels in the db/db mice (Table 1). This ineffectiveness of metformin to reduce hyperglycemia was similar to the finding in a previous study (Lin et al., 2000). Consistent with the glucose results, metformin treatment for 18 weeks did not affect the HbA1c levels (Table 2). The db/db mice had hyperinsulinemia that was not affected by metformin treatment. Although reduction in weight gain was reported in the db/db mice treated with metformin (Fujita et al., 2005; Lee and Morley, 1998), there were no differences in the body weights among the three groups of db/db mice (Table 1). The db/db mice had higher serum lactate concentrations than the db+ mice (P = 0.005, t(14) = 3.309). Treatment with metformin or saline did not affect the increased serum lactate concentrations in the db/db mice (Table 2).
Table 1.
Body weights and blood glucose levels
| Weight (g) | Glucose (mg/dl)
|
|||
|---|---|---|---|---|
| 6 weeks | 24 weeks | 6 weeks | 24 weeks | |
| db+ | 23.3 ± 1.7 | 29.7 ± 2.2^ | 172 ± 35 | 111 ± 8^ |
| db/db | 35.5 ± 3.2* | 57.3 ± 3.7*^ | 212 ± 75* | 160 ± 32* |
| db/db + S | 52.7 ± 4.1*^ | 151 ±35* | ||
| db/db + M | 58.8 ± 5.3*^ | 203 ± 104* | ||
Data are means ± S.D. (n = 6 – 23). Data of the 6-week old db/db mice represent the pooled data from the three groups of db/db mice with an n = 23. The glucose results were obtained after animals were fasted overnight.
P < 0.05 compared with the age-matched db+ mice;
P <0.05 compared with the values of the same animals at an age of 6-weeks. Statistical analysis was performed by Student’s t test for the results of 6-week old animals and by one way analysis of variance for the results of 24-week old mice when comparisons were performed among groups. Paired t-test was used to compare the values of the same animals at ages of 6-weeks and 24-weeks. S: normal saline; M: metformin.
Table 2.
Blood biochemical results (24-week old mice)
| Insulin (ng/ml) | HbA1c (%) | Lactate (mM) | |
|---|---|---|---|
| db+ | 1.46 ± 1.3 | 4.2 ± 0.7 | 10.9 ± 2.6 |
| db/db | 66.5 ± 27.7* | 6.5 ±0.7* | 14.9 ± 2.2* |
| db/db + S | 50.5 ± 17.4* | 6.1 ±0.6* | 16.5 ± 3.5* |
| db/db + M | 57.8 ± 16.1* | 6.8 ±1.1* | 18.4 ± 2.5* |
Data are means ± SD (n = 6 – 11). These data were obtained after animals were fasted overnight.
P < 0.05 compared with db+ mice. Statistical analysis was performed by one way analysis of variance. S: normal saline; M: metformin.
3.2. The db/db mice had impaired cognitive functions
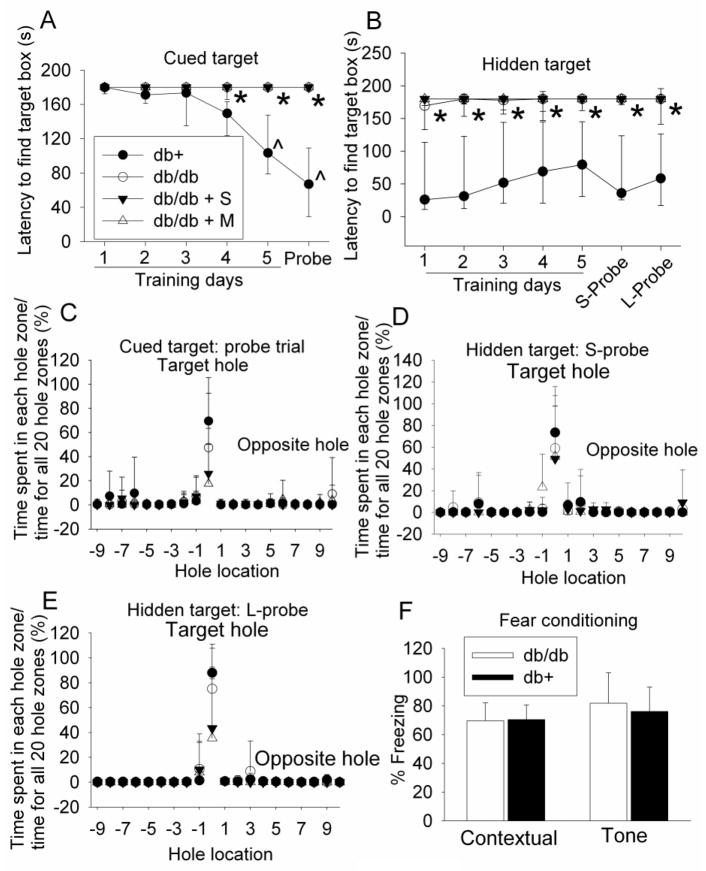
The performance of db+ mice during the cued-target training sessions was improved significantly over the sessions (P < 0.001, F(5, 10) = 16.872), suggesting that the db+ mice develop spatial learning (Fig. 1). These mice quickly identified the target box in the probe trial (Fig. 1), suggesting that they have good short-term spatial memory. The performance of db/db mice was not improved with increase in training sessions during the cued-target and hidden-target tests. They took more time than the db+ mice to find the target box in the probe trials (Fig. 1) [P < 0.001 and t(20) = 6.098 for probe trial in the cued target test, P < 0.001 and t(20) = −4.795 for the short-term probe trial in the hidden target test, and P = 0.002 and t(14) = −3.742 for the long-term probe trial in the hidden target test]. These results suggested that the db/db mice had significant impairments in spatial learning and memory. However, the db/db mice spent more time in the target hole zone than other zones in the probe trials (Fig. 1), suggesting that these mice developed some spatial learning and memory. Interestingly, metformin and saline application did not affect the performance of db/db mice in the Barnes maze (Fig. 1).
Fig. 1. Performance of mice on Barnes maze and fear conditioning tests.
Seven-week old male db/db mice received or did not receive normal saline or 200 mg kg−1 d−1 metformin in saline for 18 weeks. They were then tested with Barnes maze and fear conditioning tasks. Two forms of Barnes maze tests, cued target and hidden target, were performed. The time for animals to find the target hole during the training sessions and the probe trials is shown in panels A and B. The percentage of the time spent in the zone of each hole in the total time spent in the zones of all 20 holes during the probe trial is shown in panels C, D and E. The percentage of the time with freezing behavior in the total observation time during the fear conditioning tests is shown in panel F. Results are median with 95% confidence interval (panels A and B) or means ± SD (panels C, D, E and F) (n = 8 – 11). * P < 0.05 compared with db+ mice; ^ P < 0.05 compared with the values of the same animals on the first training day. Statistical analysis was performed by Kruskal-Wallis analysis of variance on ranks for data presented in panels A and B and by one way analysis of variance for the fear conditioning data when comparisons were performed among groups. One way repeated measures analysis of variance was used to compare values of the same animals on different training days. db/db + S: db/db mice treated with saline; db/db + M: db/db mice treated with metformin; S-Probe: short-term probe trial; L-Probe: long-term probe trial.
There were no differences between the db+ and db/db mice in the freezing behavior during the fear conditioning test (Fig. 1). Fear conditioning tests emotion-related learning and memory. These results suggest that the db/db mice did not have impairments in this type of learning and memory.
3.3. Metformin treatment partially reduced the increase of tau phosphorylation in the db/db mouse hippocampus
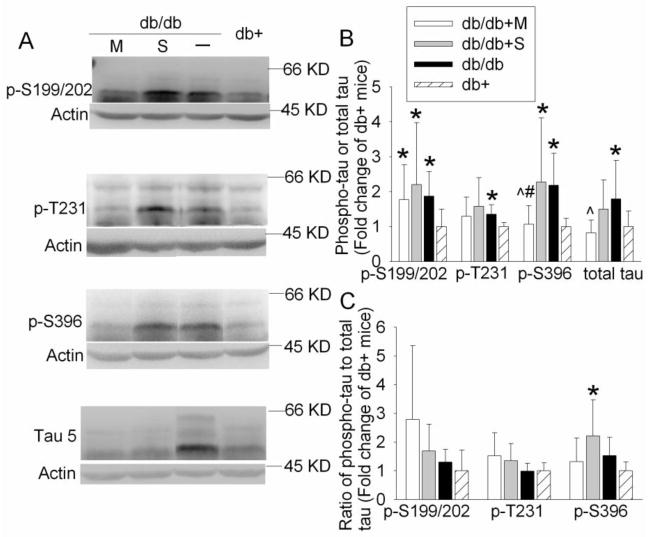
Since tau is a family of closely related proteins at 55 – 62 KD (Hirokawa et al., 1988), at least three protein bands in this molecular weight range were detected with antibodies that were developed to specifically bind to tau or phosphorylated tau (Fig. 2). The quantification results described in this report were the sum of all tau protein bands. Phosphorylation at all three examined sites, Ser199/202, Ser396 and Thr231, of the tau proteins and the total tau levels in the hippocampi of db/db mice at 24 weeks of age were significantly higher than these in db+ mice [P = 0.005 and t(18) = 3.175 for Ser199/202, P = 0.014 and t(10) = 2.977 for Thr 231, P = 0.003 and t(14) = 3.530 for Ser396, and P = 0.033 and t(18) = 2.288 for total tau]. Metformin-treated db/db mice had reduced total tau protein levels and phosphorylated tau at Ser396 when compared to the control db/db mice [P = 0.017 and t(18) = −2.618 for total tau and P = 0.01 and t(14) = −2.976 for Ser396]. The expression of phosphorylated tau at Ser396 in the metformin-treated db/db mice was lower than that in the saline-injected db/db mice [P = 0.036 and t(14) = −2.326]. Saline injection in the db/db mice did not cause any significant changes in the total tau and phosphorylated tau expression (Fig. 2). When phosphorylated tau was normalized by total tau, phosphorylation at Ser396 in the hippocampi of db/db mice trended to be higher than that in the db+ mice [P = 0.055, t(14) = 2.098]. The phosphorylated tau at Ser396 in the db/db mice injected with saline was significantly higher than that in the db+ mice [P = 0.019, t(14) = 2.642] (Fig. 2).
Fig. 2. Expression of phosphorylated tau and total tau in mouse hippocampus.
A representative Western blot is shown in panel A and the graphic presentation of protein abundance quantified by integrating the volume of autoradiograms from 6 – 10 mice for each experimental condition is shown in panels B and C. In panel B, the results of phosphorylated tau and total tau were normalized by the corresponding actin data and then expressed as fold change over the mean value of the db+ control mice in the same experiment. In panel C, the results of phosphorylated tau were normalized by the corresponding actin data. These results were then normalized by the total tau. The final results are expressed as fold change over the mean value of the db+ control mice in the same experiment. All results are presented as means ± SD. * P < 0.05 compared with db+ mice; ^ P < 0.05 compared with db/db mice; # P < 0.05 compared with db/db mice injected with saline. Statistical analysis was performed by one way analysis of variance or Kruskal-Wallis analysis of variance on ranks. db/db + S: db/db mice treated with saline; db/db + M: db/db mice treated with metformin.
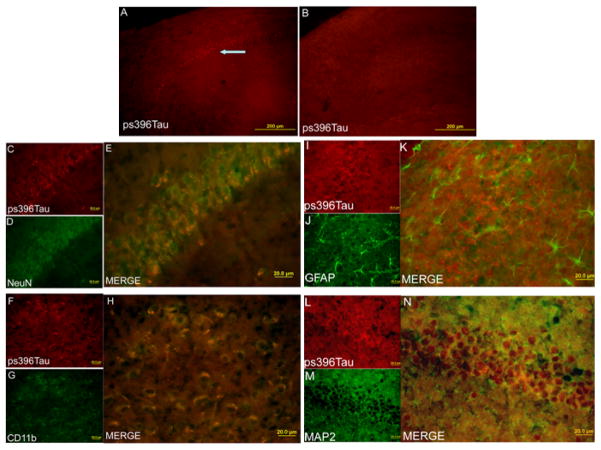
Similar to the results from Western analysis, there was a significant increase in the phospho-tau at Ser396 in the hippocampus of db/db mice as assessed by immunofluorescent study. These phosphorylated proteins were co-localized with NeuN and MAP2, two neuronal specific proteins, as well as CD11b, a microglial protein marker, but were not co-localized with GFAP, an astrocytic protein. The phospho-tau appeared to be mainly in the cytoplasm of neurons and microglia as the positive staining for the phospho-tau was mostly in the areas outside the nuclei (Fig. 3).
Fig. 3. Expression of tau phosphorylated at S396 in mouse hippocampus.
Hippocampus from 24-week old male db+ (panel B) and db/db (all other panels) mice was harvested for immunofluorescent staining of tau phosphorylated at S396 (red), NeuN (green), CD11b (green), GFAP (green) and MAP2 (green). Both db+ and db/db mice did not receive any injections. NeuN and MAP2 are proteins expressed in neurons. CD11b and GFAP are proteins expressed in microglia and astrocytes, respectively. An arrow in panel A indicates positive staining for tau phosphorylated at S396. The bar represents 200 μm in panels A and B and 20 μm in other panels (panels C to N).
3.4. Metformin attenuated JNK activation but did not block the reduction of PP2A C subunit expression in the db/db mice
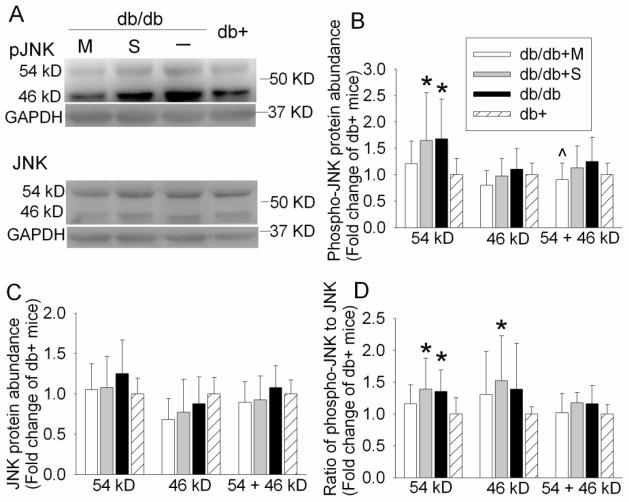
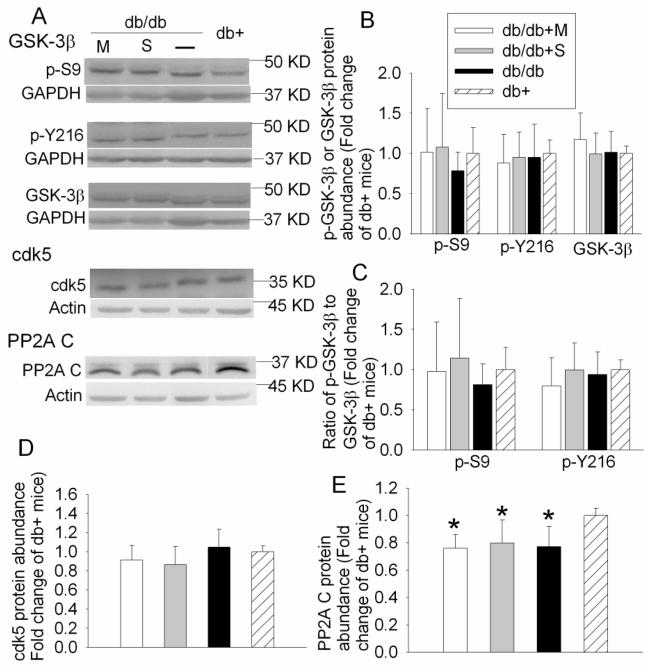
JNK, GSK-3β, CaMKII and cdk5 are considered as the major tau kinases (Litersky et al., 1996; Maccioni et al., 2001; Planel et al., 2001). We observed an increase in tau phosphorylation at Ser199/202, Ser396 and Thr231, potential phosphorylation sites by JNK, GSK-3β and cdk5 (Litersky et al., 1996; Maccioni et al., 2001; Planel et al., 2001). JNK is activated by double phosphorylation at Thr183/Tyr185. Phosphorylation of GSK-3β at Tyr216 is essential for its activity; whereas phosphorylation at Ser9 leads to partial inhibition (Planel et al., 2007a; Planel et al., 2007b). As shown in Fig. 4, the phosphorylated/activated 54 KD JNK was increased in the control and saline-injected db/db mice when compared with db+ mice. This increase was attenuated by metformin [P = 0.046, F(3, 36) = 2.948]. There was no difference in the expression of phosphorylated GSK-3β at Tyr216 or Ser9 among the four groups of mice (Fig. 5). Cdk5 protein level also was not different among the animals from various groups (Fig. 5).
Fig. 4. Expression of JNK in mouse hippocampus.
A representative Western blot is shown in panel A and the graphic presentation of protein abundance quantified by integrating the volume of autoradiograms from 6 – 10 mice for each experimental condition is shown in panels B, C and D. In panels B and C, the results of phosphorylated JNK and total JNK were normalized by the corresponding GAPDH data and then expressed as fold change over the mean value of the db+ control mice in the same experiment. In panel D, the results of phosphorylated JNK were normalized by the corresponding GAPDH data. These results were then normalized by the total JNK. The final results are expressed as fold change over the mean value of the db+ control mice in the same experiment. All results are presented as means ± SD. * P < 0.05 compared with db+ mice; ^ P < 0.05 compared with db/db mice. Statistical analysis was performed by one way analysis of variance or Kruskal-Wallis analysis of variance on ranks. db/db + S: db/db mice treated with saline; db/db + M: db/db mice treated with metformin.
Fig. 5. Expression of GSK-3β, cdk5 and PP2A C subunit in mouse hippocampus.
A representative Western blot is shown in panel A and the graphic presentation of protein abundance quantified by integrating the volume of autoradiograms from 6 – 10 mice for each experimental condition is shown in panels B, C, D and E. In panels B, D and E, the results of phosphorylated GSK-3β, cdk5 and PP2A C subunit were normalized by the corresponding GAPDH or actin data and then expressed as fold change over the mean value of the db+ control mice in the same experiment. In panel C, the results of phosphorylated GSK-3β were normalized by the corresponding GAPDH data. These results were then normalized by the total GSK-3β. The final results are expressed as fold change over the mean value of the control db+ mice in the same experiment. All results are presented as means ± SD. * P < 0.05 compared with db+ mice. Statistical analysis was performed by one way analysis of variance. db/db + S: db/db mice treated with saline; db/db + M: db/db mice treated with metformin.
PP2A is one of the tau Ser/Thr phosphatases. PP2A is a heterotrimeric enzyme consisting of a catalytic subunit (C subunit), scaffolding subunit (A subunit) and regulatory subunit (B subunit). As shown in Fig. 5, the db/db mice had significantly less PP2A C subunit in their hippocampi than the db+ mice. Metformin or saline application did not attenuate this decrease [P = 0.002, F(3, 28) = 6.407).
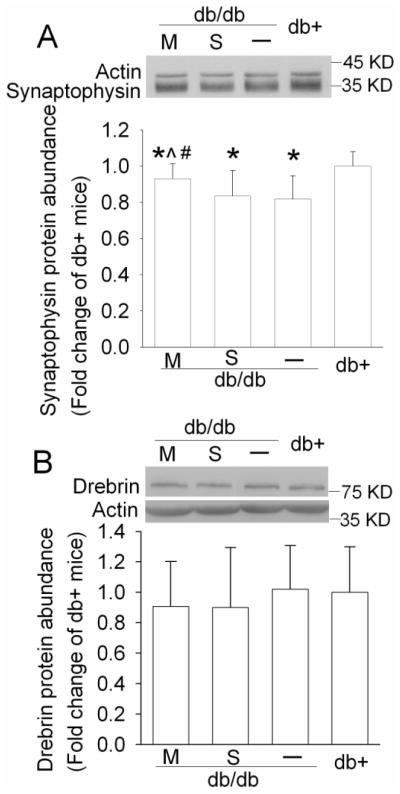
3.5. Metformin attenuated the reduction of synaptophysin expression in db/db mice
Tau is a microtubule-binding protein. This binding contributes to the stability of microtubules. Hyperphosphorylation of tau affects this binding and form intracellular neurofibrillary tangles, which may lead to dysfunction of synapses, degeneration of neurons and cognitive impairment (Avila and Diaz-Nido, 2004; Johnson and Stoothoff, 2004). As shown in Fig. 6, the db/db mice had a significant lower level of synaptophysin, a synaptic protein (Julien et al., 2010), in their hippocampi than the db+ mice. This reduction was attenuated by metformin and was not affected by saline application. Metformin-treated animals had a higher level of synaptophysin than saline-injected mice [P = 0.007, H(3) = 12.224]. The expression of drebrin, a dendritic spine protein (Julien et al., 2010), was not different among the four groups of mice (Fig. 6).
Fig. 6. Expression of neuronal proteins in mouse hippocampus.
A representative Western blot is shown in the top panel and the graphic presentation of protein abundance quantified by integrating the volume of autoradiograms from 6 – 8 mice for each experimental condition is shown in the bottom panel. Values in graphs are expressed as fold change over the mean value of the db+ mice in the same experiment and presented as means ± SD. * P < 0.05 compared with db+ mice; ^ P < 0.05 compared with db/db mice; # P < 0.05 compared with db/db mice injected with saline. Statistical analysis was performed by one way analysis of variance or Kruskal-Wallis analysis of variance on ranks. S: db/db mice treated with saline; M: db/db mice treated with metformin.
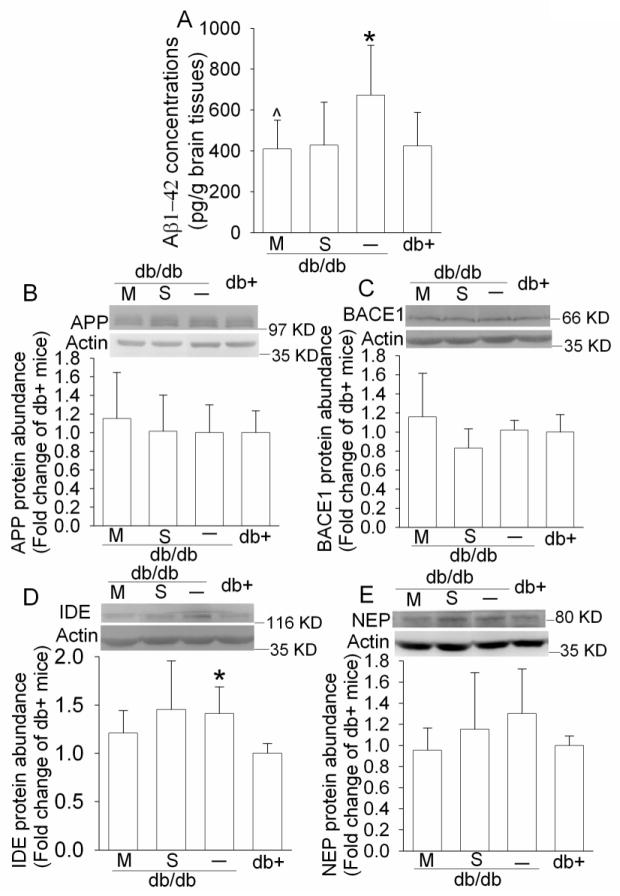
3.6. Aβ levels were increased in the hippocampi of db/db mice
There are two forms of Aβ: Aβ1-42 and Aβ1-40. Aβ1-42 is generally considered as the key and more neurotoxic amyloidogenic Aβ species than Aβ1-40 (Finder et al., 2010). As shown in Fig. 7, the Aβ1-42 levels in the hippocampi of db/db mice were significantly higher than those of the db+ mice. This increase appeared to be attenuated by metformin treatment and saline injection [P = 0.031, F(3, 28) = 3.41]. There were no differences in the expression of APP, BACE1 and neprilysin in the hippocampi of the four groups of animals. However, the expression of IDE in the db/db mice was significantly higher than that in the db+ mice [P = 0.031, t(6) = 2.807] (Fig. 7).
Fig. 7. Expression of Aβ1-42, APP, BACE1, NEP and IDE in mouse hippocampus.
For panel A, results are means ± SD (n = 6 – 8). For panels B, C, D, and E, a representative Western blot is shown in the top panel and the graphic presentation of protein abundance quantified by integrating the volume of autoradiograms from 6 – 8 mice for each experimental condition is shown in the bottom panel. Values in graphs are expressed as fold change over the mean value of the db+ mice in the same experiment and presented as means ± SD. * P < 0.05 compared with db+ mice; ^ P < 0.05 compared with db/db mice. Statistical analysis was performed by one way analysis of variance. M: db/db mice treated with metformin; NEP, neprilysin; S: db/db mice treated with saline.
4. Discussion
It has been shown that there is a significant increase in the phospho-tau in the brains of mice and rats with type 1 and type 2 DM (Clodfelder-Miller et al., 2006; Kim et al., 2009; Li et al., 2007; Planel et al., 2007b). This phosphorylation occurs at multiple sites including Ser199, Ser202, Thr212, Thr231, Ser262 and Ser396 (Clodfelder-Miller et al., 2006; Kim et al., 2009; Li et al., 2007; Planel et al., 2007b). Obvious phosphorylation of tau starts as early as 10 days after streptozotocin injection in wild type mice and 8 weeks of age in the db/db mice (Kim et al., 2009; Planel et al., 2007b). Increase of phospho-tau in the streptozotocin injected mice may be due to inhibition of PP2A activity (Planel et al., 2007a; Planel et al., 2007b). The increase of phospho-tau in these mice can be attenuated by administration of insulin (Jolivalt et al., 2008). However, there is very limited information on how phospho-tau is increased in the brains of animals with type 2 DM. It is not yet known the mechanisms for the increased phospho-tau in the db/db mice and the effects of DM therapies on the expression of phospho-tau expression in these animals.
Our results showed that phospho-tau was increased in the hippocampus of the db/db mice. The increased phospho-tau at Ser396 was attenuated by metformin. Associated with this tau phosphorylation change, the db/db mice had an increase in phosphorylated/activated 54 KD JNK, a major tau protein kinase (Planel et al., 2007b). The increased JNK activation was attenuated by metformin. There were no differences among the four groups of animals in the phosphorylated/activated GSK-3β and cdk5, the other two tau kinases determined in this study (Maccioni et al., 2001; Planel et al., 2007b). These results suggest that the 54 KD JNK is involved in tau phosphorylation in the db/db mouse brains and that attenuation of JNK activation may be a mechanism for metformin to decrease tau phosphorylation in these mice. Consistent with our results, a recent study has shown that JNK may be involved in tau phosphorylation in a rat model of type 2 DM (Yoon et al., 2010). Also, Neuro-2a cells, a mouse neuroblastoma cell line, express more phospho-tau at Ser396 if these cells are induced to become insulin-resistant by chronic incubation with insulin. This increase of phospho-tau at Ser396 is decreased by metformin. However, the protein kinase(s) that may be responsible for this phosphorylation and the metformin effects are not clearly determined in this in vitro study (Gupta et al., 2011)
In addition to the increased phospho-tau at Ser396, there was also an increase in phospho-tau at Ser199/202 and Thr231 in the db/db mice. In vitro studies have suggested that Ser199/202 and Thr231 are phosphorylation sites for GSK-3β and cdk5 (Reynolds et al., 2000). However, our study showed that the expression of activated GSK-3β, total GSK-3β and cdk5 was not different between the db/db and db+ mice. A previous study indicates no difference in the expression of activated GSK-3β and cdk5 in the hippocampi of rats with type 2 DM (Yoon et al., 2010). These results suggest that these protein kinases are not involved in the increased phospho-tau at Ser199/202 and Thr231 in the brains of animals with type 2 DM.
Increased tau phosphorylation also can be due to reduction of tau dephosphorylation. We showed here that the catalytic subunit of PP2A was significantly reduced in the db/db mice, suggesting that PP2A may be involved in the tau phosphorylation in the db/db mouse brains. However, metformin attenuated tau phosphorylation at Ser396 and Thr231 but did not attenuate the decrease of PP2A C subunit expression in the hippocampus of db/db mice. These results suggest that the reduced PP2A C subunit may not be responsible for the increased tau phosphorylated at these sites. Consistent with our results, a recent study showed that dephosphorylation of tau at Ser202 but not at Ser396 was mediated by PP2A (Kickstein et al., 2010). This finding is consistent with our results that metformin did not reduce the amount of tau phosphorylated at Ser199/202 because metformin also did not attenuate the decrease of PP2A C expression in the db/db mice. We also observed a significant increase of total tau expression. This increase may contribute to the increased tau phosphorylated at Ser199/202 and Thr231 because phosphorylation at these two sites in the db/db mice was not higher than that in the db+ mice if these phosphorylated tau proteins were normalized by the total tau. Interestingly, metformin also attenuated the increase of total tau expression in the db/db mice, further supporting that increased total tau as a mechanism for the increased phospho-tau in the db/db mouse brains.
In addition to the intracellular neurofibrillary tangles formed by phosphorylated tau, extracellular amyloid plaques formed by Aβ in the brain is another pathological hallmark of AD (Selkoe, 2001). The Aβ expression in mouse brain is not changed at 10 days after streptozotocin-induced type 1 DM but is significantly increased when mice have type 1 DM for 4 weeks or longer (Jolivalt et al., 2008; Planel et al., 2007b; Shuli et al., 2001). Similarly, there is an increased Aβ expression in the brains of the BB/Wor rats and BBZDR/Wor rats that develop spontaneous diabetes because of gene mutation (Li et al., 2007). The BB/Wor rats and BBZDR/Wor rats are considered to model type 1 and type 2 DM, respectively. However, there is no report yet on whether Aβ level is changed in the db/db mouse brain. Our results showed that the Aβ1-42 levels in the hippocampus of the db/db mice were significantly higher than that in the db+ mice. To determine the mechanisms for this increased Aβ1-42, we measured the expression of APP, BACE1, neprilysin and IDE in the hippocampus. APP is the substrate for Aβ production (Selkoe, 2001). BACE1 is the rate-limiting enzyme for Aβ production (Vassar, 2004; Vassar and Citron, 2000). Neprilysin and IDE are the major enzymes to degrade Aβ (Miners et al., 2008). Unlike in the case of BBZDR/Wor rats that have a significant increase of APP and BACE1 in their brains (Li et al., 2007), our results showed that there were no differences in the expression of APP, BACE1 and neprilysin in the hippocampi of db/db and db+ mice. IDE expression in the db/db mice was higher than that in the db+ mice. This increase may be due to a feedback mechanism from the increased insulin and Aβ1-42, substrates of IDE, in the db/db mice.
A recent study shows that metformin increases Aβ production in neuronal cultures (Chen et al., 2009). Although the effects of metformin on Aβ production have not been studied in a more clinically relevant model, such as diabetic animals, these results warn us of the possibility that a commonly used medication for type 2 DM may induce AD-like neuropathology. Interestingly, the same study also shows that insulin reduces Aβ production in the N2a695 cells, a neuroblastoma cell line expressing human APP, and metformin enhances this reduction (Chen et al., 2009). Another study has shown that mouse neuroblastoma cells that have been chronically incubated with insulin to induce insulin resistance have increased Aβ production. This increase is attenuated by metformin (Gupta et al., 2011). Our study showed that the metformin- and saline-injected db/db mice that have increased plasma insulin levels appeared to have reduced contents of Aβ1-42 compared with the control db/db mice. These results suggest that metformin does not increase Aβ1-42 accumulation in diabetic brains. Thus, our in vivo data, along with the evidence from cell culture models in the two previous studies (Chen et al., 2009; Gupta et al., 2011), indicate that metformin does not increase Aβ in the presence of high insulin levels.
Neuronal and synaptic loss is a characteristic of AD brains (Selkoe, 2002). These features were shown in the brains of rats with type 2 DM (Li et al., 2007). Although there was no difference in the drebrin expression among the four groups of animals, the db/db mice had significantly less synaptophysin, a synaptic protein, in their hippocampi. This result suggests that the db/db mice have decreased synapses. Interestingly, metformin attenuated the decrease of synaptophysin in the db/db mice, suggesting that metformin preserves neuronal structures in these mice.
It is now recognized that DM and AD share many abnormalities including impaired glucose metabolism and insulin signaling, increased oxidative stress and inflammation, as well as insulin resistance (Zhao and Townsend, 2009). It has been firmly established that DM increases the risk of developing AD (Brands et al., 2005; Ott et al., 1999; Strachan et al., 1997). In parallel, up to 80% AD patients have DM (Janson et al., 2004). Consistent with these phenomena, hyperglycemia impairs cognitive performance in animals and humans (Kawasaki et al., 2005; Mohapatra et al., 2009) and patients with DM have impaired learning and memory functions (Brands et al., 2005; Strachan et al., 1997). Our study showed that the db/db mice did not have impairments in emotion-related learning and memory as tested by fear conditioning. Although they developed some degrees of spatial learning and memory in the Barnes maze test, these mice had a significant impairment in spatial learning and memory compared with the db+ mice. Consistent with our results, a previous study showed that db/db mice had impaired spatial memory assessed by Morris water maze (Li et al., 2002). Interestingly, metformin did not improve the spatial learning and memory as assessed by Barnes maze in our study. This finding is seemingly surprising because metformin attenuated the increase of tau phosphorylation and preserved the expression of synaptophysin. However, metformin did not improve long-term hyperglycemia as assessed by HbA1c. Since hyperglycemia can impair the learning and memory functions (Kawasaki et al., 2005; Mohapatra et al., 2009), it is possible that hyperglycemia in the db/db mice treated with metformin contributes to the failure for metformin to improve the cognitive functions in these mice. In addition, leptin is known to facilitate spatial learning and memory (Oomura et al., 2006) and the db/db mice have a defect in leptin signaling. Finally, insulin resistance can impair spatial learning and memory (Stranahan et al., 2008) and metformin may not significantly attenuate insulin resistance of the db/db mice as indicated by similar levels of hyperglycemia and hyperinsulinemia in the db/db mice treated with or without metformin. All of these factors may contribute to our findings that metformin improved AD-like biochemical changes in the brain but did not improve the learning and memory impairments assessed by Barnes maze in the db/db mice.
The db/db mice had increased serum lactate concentrations, which is consistent with the theme that diabetes disrupts normal metabolism (Zhao and Townsend, 2009). Metformin is previously considered to increase the risk of lactic acidosis in diabetic patients (Price, 2003). It is now controversial whether metformin indeed increases this risk (Bodmer et al., 2008; Salpeter, 2010). In agreement with this controversy, our results showed that metformin did not further increase the serum lactate concentrations in the db/db mice. Metformin has an anorectic effect that can lead to weight loss (Fujita et al., 2005; Lee and Morley, 1998), although acute increase of food intake within 4 h after intraperitoneal injection of metformin has been reported (del Prete et al., 1999). We did not specifically study the food intake in our animals. However, metformin did not affect the weights of these rats, suggesting a possible lack of significant effects of chronic application of metformin on food intake.
Our study showed that metformin did not persistently reduce hyperglycemia in the db/db mice. Consistent with our finding, 350 mg kg−1 d−1 metformin did not decrease blood glucose levels of the ob/ob mice when measurements were performed at 4 weeks after the initiation of metformin therapy in a previous study (Lin et al., 2000). That study also showed that metformin improved the disturbance of fatty metabolism, suggesting that metformin can still affect important biochemical processes of diabetic animals in the absence of obvious anti-hyperglycemic effect, a pattern that is similar to our findings here. Consistent with this pattern, it is known that metformin affects multiple intracellular signaling pathways and that some of these effects are not necessarily involved in reducing hyperglycemia (Viollet et al., 2012). Our study and those previous studies (Lin et al., 2000; Viollet et al., 2012) suggest that metformin can induce beneficial effects that are independent on its effects on glucose metabolism.
One novelty of our study is that we administered metformin to diabetic animals for a long-time (18 weeks) to study the effects of metformin on AD-like brain biochemical and cognitive changes. These effects under in vivo conditions have not been reported. Chronic use of metformin is a common practice in patients with type 2 diabetes. Thus, our study closely simulates clinical situations. We showed here that metformin attenuated the increase of phospho-tau and reduction of synaptophysin in the db/db mouse brain, suggesting a beneficial effect in these diabetic animals.
Research highlight.
The db/db mice have Alzheimer’s disease-like brain changes.
The increased phospho-tau at 396 may be due to JNK activation in the db/db mouse brain.
Metformin attenuates the Alzheimer’s disease-like brain biochemical changes in the db/db mice.
Acknowledgments
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, Charlottesville, VA22908, USA.
This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, the Robert M. Epstein Professorship endowment, University of Virginia and a grant (81070912 to W Sheng) from National Natural Science Foundation of China, Beijing, China.
Footnotes
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avila J, Diaz-Nido J. Tangling with hypothermia. Nat Med. 2004;10:460–1. doi: 10.1038/nm0504-460. [DOI] [PubMed] [Google Scholar]
- Berg L, Morris JC. Diagnosis. In: RDT, Katzman R, Bick K, editors. Alzheimer Disease. New York: Raven Press; 1994. pp. 197–229. [Google Scholar]
- Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31:2086–91. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–35. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–12. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian B, Meka N, Katragadda S, Arora R. Therapeutic implications of diabetes in cardiovascular disease. Am J Ther. 2009;16:e51–9. doi: 10.1097/MJT.0b013e31815db924. [DOI] [PubMed] [Google Scholar]
- Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–5. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia. 2009;52:1219–26. doi: 10.1007/s00125-009-1352-5. [DOI] [PubMed] [Google Scholar]
- del Prete E, Lutz TA, Scharrer E. Acute increase in food intake after intraperitoneal injection of metformin in rats. Physiol Behav. 1999;67:685–9. doi: 10.1016/s0031-9384(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Erber WN, Gibbs TA, Ivey JG. Antigen retrieval by microwave oven heating for immunohistochemical analysis of bone marrow trephine biopsies. Pathology (Phila) 1996;28:45–50. doi: 10.1080/00313029600169513. [DOI] [PubMed] [Google Scholar]
- Finder VH, Vodopivec I, Nitsch RM, Glockshuber R. The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42. J Mol Biol. 2010;396:9–18. doi: 10.1016/j.jmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Fujita H, Fujishima H, Koshimura J, Hosoba M, Yoshioka N, Shimotomai T, et al. Effects of antidiabetic treatment with metformin and insulin on serum and adipose tissue adiponectin levels in db/db mice. Endocr J. 2005;52:427–33. doi: 10.1507/endocrj.52.427. [DOI] [PubMed] [Google Scholar]
- Fujita H, Fujishima H, Morii T, Koshimura J, Narita T, Kakei M, et al. Effect of metformin on adipose tissue resistin expression in db/db mice. Biochem Biophys Res Commun. 2002;298:345–9. doi: 10.1016/s0006-291x(02)02464-6. [DOI] [PubMed] [Google Scholar]
- Gravina S, Ho L, Eckman C, Long K, Otvos L, Jr, Younkin L, et al. Amyloid beta protein in Alzheimer’s disease brain. J Biol Chem. 1995;270:7013-7-16. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60:910–20. doi: 10.1016/j.neuropharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Heishi M, Hayashi K, Ichihara J, Ishikawa H, Kawamura T, Kanaoka M, et al. Comparison of gene expression changes induced by biguanides in db/db mice liver. J Toxicol Sci. 2008;33:339–47. doi: 10.2131/jts.33.339. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Shiomura Y, Okabe S. Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol. 1988;107:1449–59. doi: 10.1083/jcb.107.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell surface protein level and activity of glutamate transporter type 3. Mol Pharmacol. 2005;67:1522–33. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–81. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–9. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, et al. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res. 2008;86:3265–74. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–31. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Matsuda M, Kanda Y, Inoue H, Kaku K. Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab. 2005;288:E510–8. doi: 10.1152/ajpendo.00128.2004. [DOI] [PubMed] [Google Scholar]
- Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107:21830–5. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–24. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Litersky JM, Johnson GV, Jakes R, Goedert M, Lee M, Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem J. 1996;316 (Pt 2):655–60. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Otth C, Concha, Munoz JP. The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer’s pathology. Eur J Biochem. 2001;268:1518–27. doi: 10.1046/j.1432-1033.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–52. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra J, Sharma M, Singh S, Chatterjee A, Swain P, Balaraman R, et al. Subtherapeutic dose of pioglitazone reduces expression of inflammatory adipokines in db/db mice. Pharmacology. 2009;84:203–10. doi: 10.1159/000235996. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–49. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007a;27:3090–7. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007b;27:13635–48. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Yasutake K, Fujita SC, Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001;276:34298–306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- Price G. Metformin lactic acidosis, acute renal failure and rofecoxib. Br J Anaesth. 2003;91:909–10. doi: 10.1093/bja/aeg255. [DOI] [PubMed] [Google Scholar]
- Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem. 2000;74:1587–95. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Salpeter S. Cochrane Database Syst Rev. 2010 Apr 14;(4):CD002967. doi: 10.1002/14651858.CD002967.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shuli S, Yongmei Z, Zhiwei Z, Zhijuan J. beta-Amyloid and its binding protein in the hippocampus of diabetic mice: effect of APP17 peptide. Neuroreport. 2001;12:3317–9. doi: 10.1097/00001756-200110290-00034. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–45. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Shi SR, Chen C, Young L, Yang C, Cote RJ. Comparative study of antigen retrieval heating methods: microwave, microwave and pressure cooker, autoclave, and steamer. Biotech Histochem. 1996;71:263–70. doi: 10.3109/10520299609117171. [DOI] [PubMed] [Google Scholar]
- Vassar R. BACE1: the beta-secretase enzyme in Alzheimer’s disease. J Mol Neurosci. 2004;23:105–14. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- Vassar R, Citron M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron. 2000;27:419–22. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechansims of metformin: an overview. Clinical Science. 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Park JS, Choi JE, Choi JM, Lee WJ, Kim SW, et al. Rosiglitazone reduces tau phosphorylation via JNK inhibition in the hippocampus of rats with type 2 diabetes and tau transfected SH-SY5Y cells. Neurobiol Dis. 2010;40:449–55. doi: 10.1016/j.nbd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792:482–96. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Zheng JF, Patil SS, Chen WQ, An W, He JQ, Hoger H, et al. Hippocampal protein levels related to spatial memory are different in the Barnes maze and in the multiple T-maze. J Proteome Res. 2009;8:4479–86. doi: 10.1021/pr9002596. [DOI] [PubMed] [Google Scholar]