Abstract
The Kv4.3 transient outward current (Ito) channel, which produces early repolarization in human cardiomyocytes, is downregulated with cardiac pathology. This is evident in cultured neonatal rat cardiomyocytes in which Angiotensin II (Ang II) acts via p38 mitogen-activated protein kinase (p38K) to increase apoptosis and induce Kv4.3 mRNA destabilization to downregulate the channel protein. However, it is not understood how p38K activation, which is activated transiently for minutes, induces downstream effects hours later. Here we show that there is a second phase of p38K activation. Inhibiting this delayed p38K activation eliminated Kv4.3 mRNA destabilization. Furthermore, inhibiting endosome generation left the transient activation of p38K intact, but blocked delayed p38K activation and the Kv4.3 effect. CamKII was also found to be required for delayed p38K activation and Kv4.3 mRNA destabilization. Finally, CamKII methionine oxidation and activation are biphasic, with the delayed phase requiring endosomes. Hence, in addition to participating in channel traffic, cardiomyocyte endosomes control channel mRNA expression by mediating delayed oxidative CamKII-p38K signaling.
1. Introduction
The Kv4.3 channel gene encodes the pore of the transient outward current (Ito) channel in human myocardium [1]. Cardiomyocyte Ito influences action potential repolarization, restitution and Ca2+ dynamics [2–4]. Interestingly, many cardiac pathologies are associated with suppression of Ito [1], suggesting that the signaling controlling Ito channel expression is relevant to the health of the heart. The linkage between Ito gene expression and cardiomyocyte pathology is evident in cultured cardiomyocytes, which respond to Angiotensin II (Ang II) by increasing apoptosis and decreasing Kv4.3 mRNA and protein expression [5,6]. Unlike many other examples of altered cardiac channel expression, the Ang II effect on Kv4.3 features destabilization of the 3’ untranslated region (UTR) of the channel mRNA, which begins to downregulate the channel mRNA within a few hours [6,7]. This effect is mediated by Ang II-induced upregulation and activation of AUF1, which binds to and destabilizes the Kv4.3 mRNA 3’ UTR [8]. The Kv4.3 effect, like the apoptotic response, is triggered by the AT1 receptor-rac-Nadph oxidase (Nox)-apoptosis signal-regulating kinase 1 (ASK1)-p38 mitogen-activated protein kinase (p38K) pathway [7–11].
Despite the above progress, there are puzzling features of Ang II-Nox-ASK1-p38K signaling in cardiomyocytes. First, while ASK1 and p38K are stimulated rapidly and transiently (i.e., peaking within 10 minutes) [12–14], apoptosis and Kv4.3 mRNA destabilization require hours of Ang II exposure. This apparent kinetic mismatch is suggestive of uncharacterized delayed signaling following rapid activation of ASK1 and p38K. Second, the sensor for Nox-derived reactive oxygen species that activates ASK1-p38K signaling to destabilize Kv4.3 mRNA has not been identified. Interestingly, insights into signaling suggest potential mechanisms for resolving the above issues. For example, Ang II acts via Nox to induce stimulatory Ca2+/calmodulin-dependent protein kinase II (CamKII) methionine oxidation in the heart [15]. Because CamKII directly activates ASK1 resulting in p38K activation [16], CamKII-mediated activation of p38K by Ang II [11] may be explained by CamKII acting as a redox sensor. CamKII oxidation is already implicated in cardiomyocyte apoptosis [15], but the role of this mechanism in Kv4.3 expression is unknown. Also potentially relevant, endosomes can induce delayed signaling [17]. Indeed, outside of the heart, endosomes participate in Nox signaling [18,19]. Thus, endosomes might be involved in the Ang II-Nox-Kv4.3 response. Yet, while endosomes are known to traffic cardiac Kv channels [20,21], the role of endosome-dependent signaling in Kv channel gene expression, as well as in other p38K effects in cardiomyocytes, has not been studied. Taken together, the above observations guided our investigation into the delayed signaling that regulates cardiomyocyte Kv4.3 mRNA stability.
Here it is shown that Ang II acts on cardiomyocytes to induce biphasic CamKII methionine oxidation and activation and downstream biphasic p38K activation. The novel delayed activation of the two kinases, which is evident after hours, requires endosomes. Furthermore, endosome-dependent delayed activation of p38K is required for destabilization of Kv4.3 mRNA. Thus, in addition to trafficking channels, endosomes induce delayed CamKII-p38K signaling that controls cardiac Kv4.3 mRNA expression.
2. Materials and Methods
2.1 Cardiomyocyte culture, transfection and real-time PCR measurements
Neonatal Sprague-Dawley rat ventricular myocyte cultures were generated by a protocol [7] approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Transfections of cultured cardiomyocytes, luciferase assays and real-time pcr measurements followed published methods [7].
2.2 Constructs and chemical reagents
The Kv4.3 3’ UTR (untranslated RNA) luciferase reporter has been described previously [7]. Clathrin-relevant plasmids [22,23] were kindly provided by Jeffrey Benovic (Thomas Jefferson University), the dynamin 2 shRNA vector [24] was kindly provided by Liangyi Chen (Chinese Academy of Science) and the dominant negative dynamin K44A plasmid [25] was kindly provided by Timothy Feinstein (University of Pittsburgh). All inhibitors were applied 1 hour prior to treatment with 100 nM Ang II. SB239063, dynasore, myristoylated dynamin inhibitory peptide (DIP), and its control (DIPC) were obtained for Tocris Bioscience. Apocynin and myristoylated Calmodulin Kinase II Ntide were obtained from Calbiochem.
2.3 Biochemical samples
For CamKII immunoprecipitation, lysates were generated from cultures (3×106 cardiomyocytes per 100 mm plate or 1×106 per 60 mm plate) that were briefly rinsed with ice-cold PBS and then extracted in NP40 cell lysis buffer (50 mM Tris, pH7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4) supplemented with freshly made 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail (Roche). After gently scraping lysate from the plate, debris was pelleted by centrifugation on 10,000 g at 4°C for 15 minutes and the remaining extract was used immediately or stored at −80°C. Equal amounts of total protein (100 to150 µg) from each extract were pre-cleared with 30 µl of protein A/G plus agarose (Calbiochem) for 2 hours at 4°C before they were incubated with 5 µl of goat polyclonal antibody against total CamKIIδ (Santa Cruz Biotechnology) overnight at 4°C with gentle agitation. Antibody-protein complexes were then precipitated with 60 µl of protein A/G plus-agarose for 3 hours at 4°C. Beads were then washed with five times of 1× PBS and dissolved in 2× reducing electrophoresis sample buffer. After brief centrifugation, the supernatants of protein samples were collected and boiled for 5 minutes at 100°C and then subjected to gel electrophoresis.
For detection of p38K autophosphorylation (i.e., p-p38K), cell extracts were prepared similarly as above except for the specific cell extraction buffer (10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate) supplemented with fresh PMSF and protease inhibitor cocktail. Each gel loading sample contained 15–20 µg of protein.
The p38 MAP Kinase Assay Kit (Nonradioactive) from Cell Signaling was used according the manufacturer’s protocol. Briefly, cells were lysed with the provided buffer supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) for 5 minutes. Cell lysates (200 µl) were mixed with 20 µl of 50% (v/v) immobilized agarose hydrazide beads conjugated to phospho-p38 MAPK(Thr180/Tyr182) monoclonal antibody and incubated overnight at 4°C with gentle agitation. Beads were spun down and washed twice each with lysis buffer and kinase buffer. Then pellets were suspended in 50 µl of kinase buffer containing 200 µM ATP and 1 µg of Activating Transcription Factor 2 (ATF-2) fusion protein. After 30 minutes incubation at 30°C, the kinase reaction was terminated with 3× SDS sample buffer so that immunoblots could be used to detect phospho-ATF-2 (pATF2).
2.4 Immunoblots
For SDS-PAGE and immunoblotting, protein concentrations were measured by the BCA (bicinchoninic acid) protein Assay Kit (Thermo Scientific) according to the manufacturer's protocol with bovine serum albumin as a standard. Samples containing equivalent amounts of protein were loaded on an 8.5% SDS-PAGE gel for electrophoresis and then transferred onto a Trans-Blot Transfer Medium Pure Nitrocellulose Membrane (0.2 µm) (Bio-Rad). After blocking with 1% (w/v) BSA in PBST (0.1% Tween 20 in PBS) for 1 hour at room temperature, the membrane was incubated either overnight at 4°C or 1 hour at room temperature with mouse monoclonal against phospho-CamKII antibody (1:500) (Santa Cruz Biotechnology), rabbit polyclonal against oxidized-CamKII antibody (1:20,000)(kindly provided by Mark Anderson, University of Iowa), goat polyclonal antibody against CamKIIδ antibody (1:1000) (Santa Cruz Biotechnology), rabbit polyclonal against phospho-p38 MAPK (Thr180/Tyr182) antibody (1:1000) (Cell Signaling), or rabbit polyclonal against p38 MAPK antibody (1:1000) (Cell Signaling) diluted into 1% (w/v) BSA in PBST. Membranes were washed 3 times and then incubated with peroxidase-conjugated secondary affinipure antibodies from Jackson ImmunoResearch. Specifically, we used goat anti-rabbit IgG (H+L) antibody, goat anti-mouse IgG (H+L) and donkey anti-goat IgG (H+L). Blots were visualized with a SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). The amplified signals were then detected using Kodak X-OMAT AR film.
Results are expressed with error bars representing the standard error of the mean from at least three independent experiments. Statistical significance was determined by Student’s t-test for pair-wise comparisons or one-way ANOVA followed by Dunnett’s post-test.
3. Results
3.1 Delayed p38K activation regulates Kv4.3 mRNA in cardiomyocytes
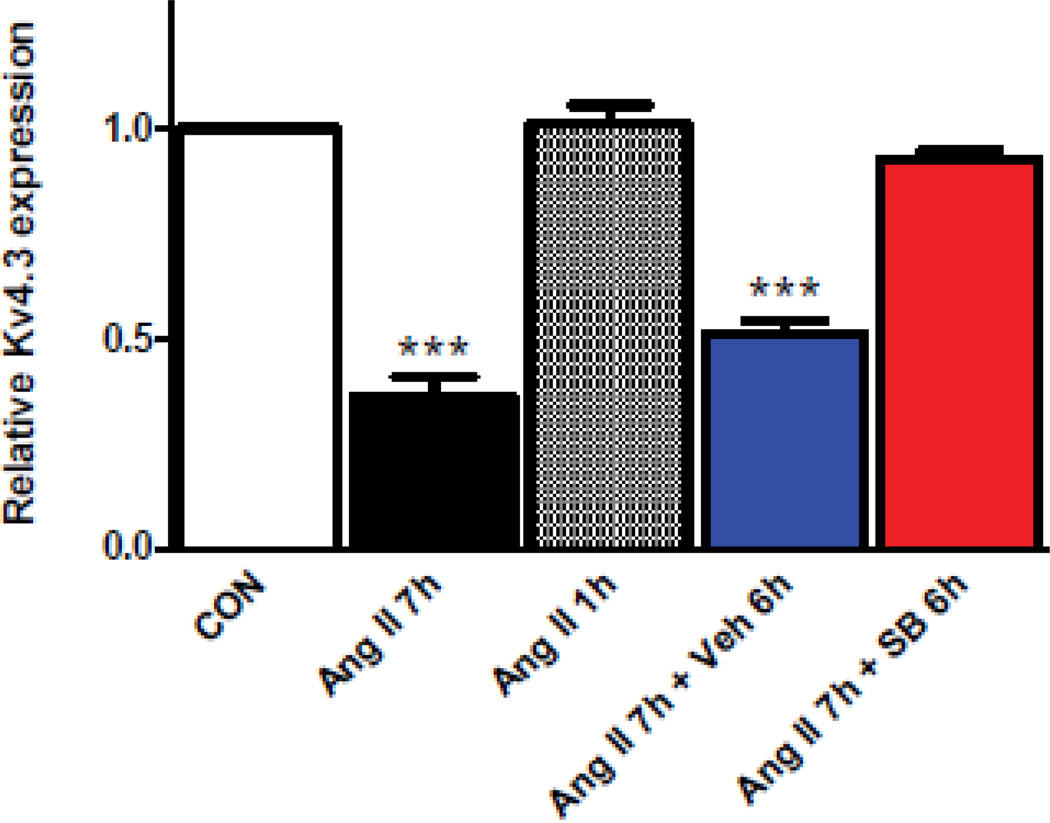
To explore whether rapid and transient p38K activation is sufficient for the delayed effect on Kv4.3 mRNA, the effects of treating cardiomyocytes with 100 nM Ang II for 1 and 7 hours were compared at the same time point (i.e., 7 hours after the initial addition of Ang II). Real-time pcr showed that Kv4.3 mRNA was only affected by the longer treatment (Fig. 1, compare Ang II 7h to Ang II 1h). Hence, the known transient activation of p38K that occurs in minutes is not sufficient for the delayed Ang II effect.
Figure 1.
Delayed p38K signaling regulates cardiomyocyte Kv4.3 mRNA expression. Real-time pcr assays of cardiomyocyte Kv4.3 mRNA normalized to controls (CON). While application of 100 nM for 7 h (Ang II 7h) induces downregulation, a 1 h application is ineffective (Ang II 1h). Furthermore, this regulation is disrupted by addition of 20 µM SB239063 1 h after Ang II (i.e. for a total of 6h, Ang II 7h+SB 6h), but not by the vehicle control (Ang II 7h+Veh 6h). n=3. ***p<0.001.
One potential explanation for this result is that the transient p38K activation synergizes with delayed p38K-independent signaling induced by Ang II. According to this scenario, addition of a p38K inhibitor (20 µM SB239063) an hour after Ang II should not affect the downstream destabilization of Kv4.3 mRNA. However, the delayed addition of the p38K inhibitor still effectively inhibited the Ang II-induced downregulation of cardiomyocyte Kv4.3 mRNA measured by real-time pcr (Fig. 1, compare the right two columns). In contrast, adding the inhibitor during the last hour of the Ang II treatment was ineffective (data not shown). The block of the Ang II response by p38K inhibition between 1 and 6 hours suggests that regulation of cardiomyocyte Kv4.3 mRNA requires a previously unknown secondary phase of p38K activation.
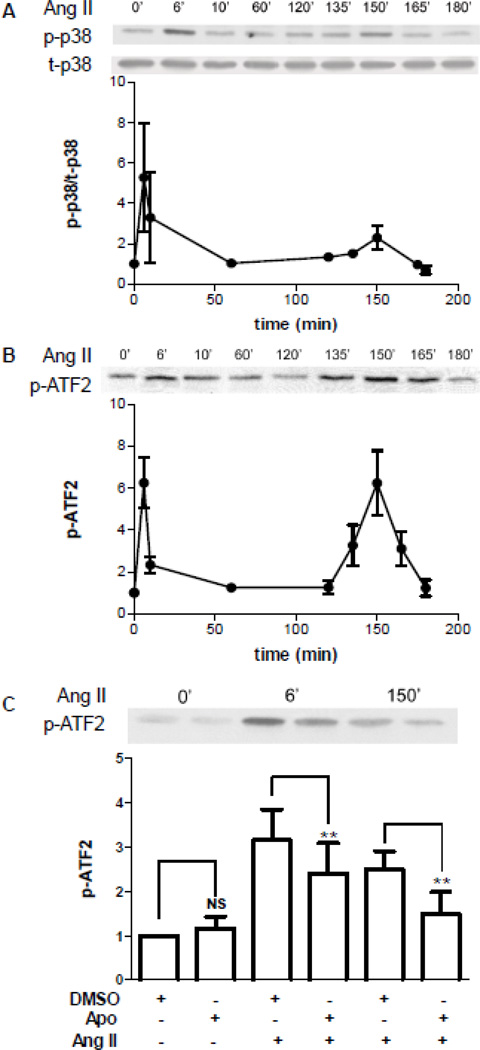
To directly test whether there is delayed activation of p38K, the time course of Ang II-induced p38K activation was determined by measuring autophosphorylation (Fig. 2A) and enzymatic activity, which was assayed in vitro by measuring phosphorylation of the substrate ATF2 by immunoprecipitated p38K (Fig. 2B). In all 7 sets of measurements, the known transient p38K response seen at 6–10 minutes was followed hours later by a second phase of activation (Fig. 2A,B). At both 6 and 150 minutes (i.e., the peaks of biphasic p38K signaling), p38K activation was attenuated by the Nox inhibitor apocynin (Fig. 2C). Thus, Ang II stimulates Nox-dependent biphasic p38K activation. Together with the above inhibitor results, these experiments show that a delayed phase of p38K activation is responsible for the Ang II effect on Kv4.3 mRNA in cardiomyocytes.
Figure 2.
Nox-dependent biphasic activation of p38K in cardiomyocytes. A. Time course of Ang II-induced p38K autophosphorylation. Top, immunoblot showing from phosphorylated p38K (p-p38K) and total p38K (t-p38K) from one experiment. Bottom, normalized values of p-p38K/t-p38K (n=3). B. Time course of Ang II-induced p38K activity. Enzymatic activity was assayed in vitro with ATF2 as the substrate. Top, immunoblot showing activity from extracts isolated at the indicated time after addition of Ang II. Bottom, normalized quantification of activity (n=3–4). C. The Nox inhibitor apocyin (Apo, 100 µM) reduces p38K activation at 6 and 150 minutes. DMSO is the vehicle control. **p<0.01. NS, not significant. (n=3–4)
3.2 Endosomes are required for delayed p38K activation and the Kv4.3 effect
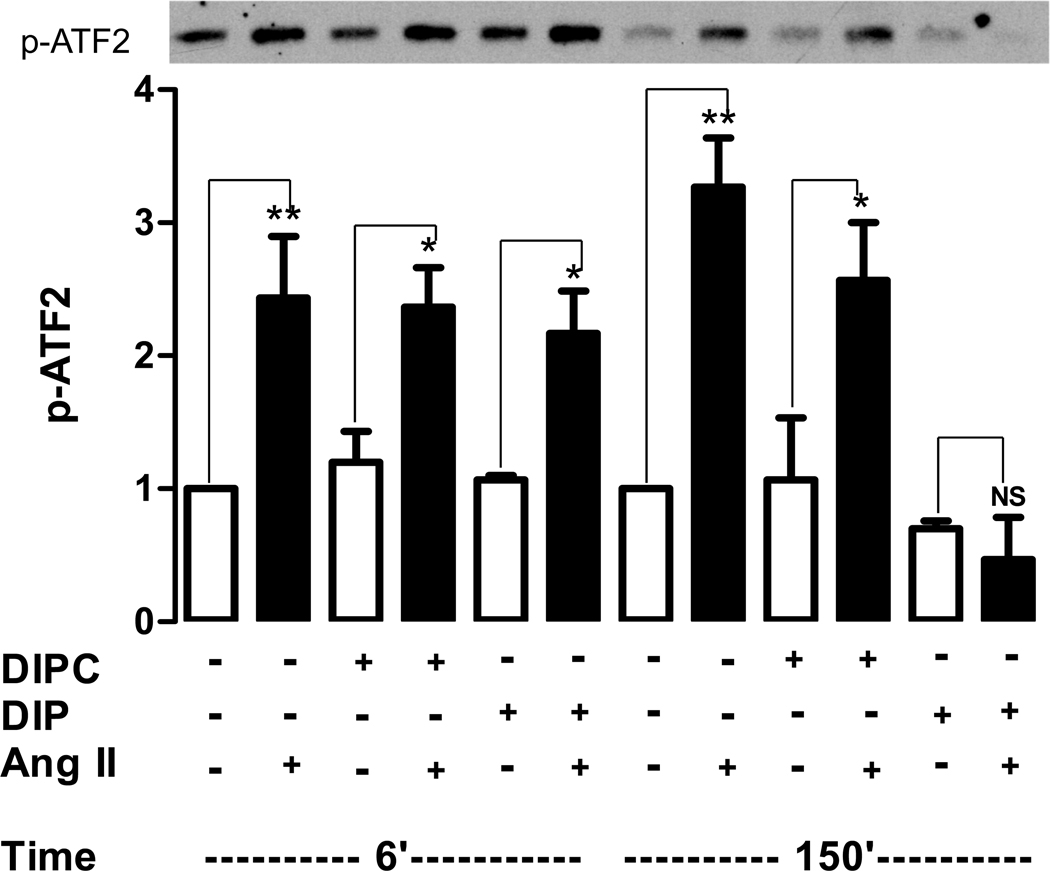
Because endosomes can produce delayed signaling, the effect of inhibiting endosome production on the biphasic activation of p38K was examined. Specifically, cardiomyocytes were treated with the myristoylated dynamin inhibitory peptide (DIP) or its control (DIPC) and then p38K activity was measured at 6 minutes to assay transient activation and 150 minutes to assay delayed activation. Both phases of the Ang II response were unaffected by DIPC. Likewise, DIP did not alter the initial phase of p38K activation. However, DIP eliminated the delayed phase of p38K activation (Fig. 3, right). Hence, dynamin is required specifically for the second phase of p38K activation.
Figure 3.
Endosomes are required for the delayed phase of p38K activation. Top, immunoblot showing effect of Ang II on p38K activity after 6 and 150 minutes measured by abundance of p-ATF2. Bottom, quantification from 3 experiments. DIPC does not affect biphasic activation, while DIP specifically blocks the delayed phase of activation. Ang II bars are filled. *p<0.05, **p<0.01, NS, not significant.
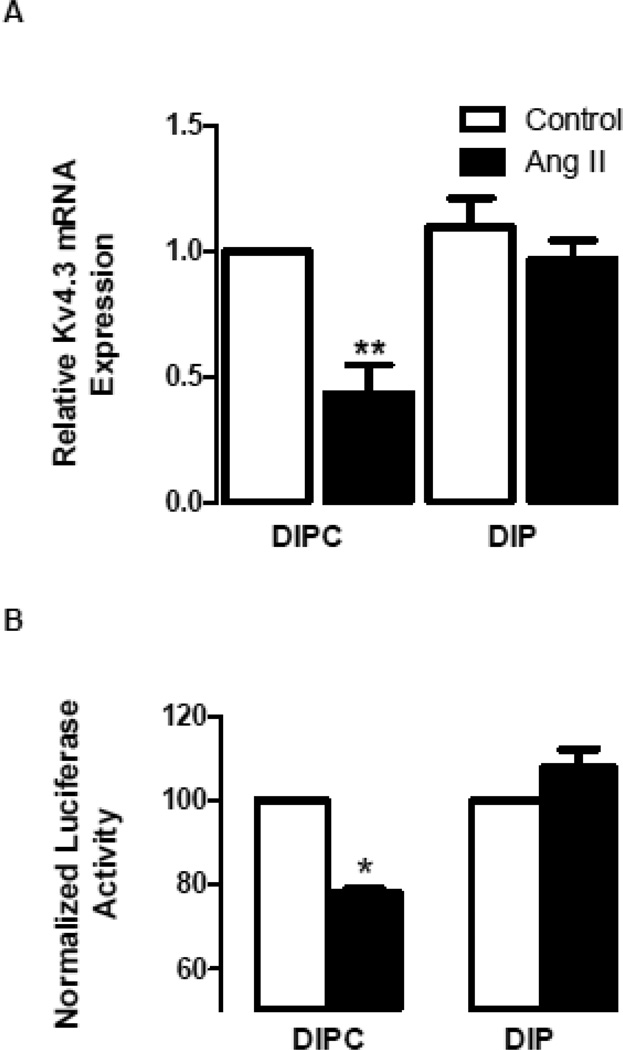
Because delayed p38K activation is required for the Kv4.3 effect (Figs. 1 and 2), the role of endosomes in the regulation of Kv4.3 mRNA was examined. First, cardiomyocytes were treated with DIP or DIPC for an hour prior to addition of 100 nM Ang II for 7 hours. Real-time pcr assays then revealed the expected downregulation of Kv4.3 mRNA in the presence of DIPC, but DIP abolished the Ang II effect (Fig. 4A). This trend was also evident using the Kv4.3 3’ UTR luciferase construct, which reports Kv4.3 mRNA destabilization [7]: in the presence of DIPC Ang II decreased the reporter signal, but DIP blocked the Ang II effect (Fig. 4B). These results suggest that dynamin is required for Kv4.3 mRNA destabilization.
Figure 4.
A dynamin inhibitor blocks Ang II regulation of Kv4.3 mRNA stability in cardiomyocytes. A. Real-time pcr quantification shows that myristoylated dynamin inhibitory peptide (DIP, 10 µM) blocks the Ang II-induced downregulation of Kv4.3 mRNA, while the myristoylated control peptide (DIPC, 10 µM) leaves the effect intact (n=3). B. DIP, but not DIPC, blocks the Ang II-induced destabilization of Kv4.3 mRNA revealed with the channel 3’ UTR luciferase reporter (n=3). *p<0.05, **p<0.01.
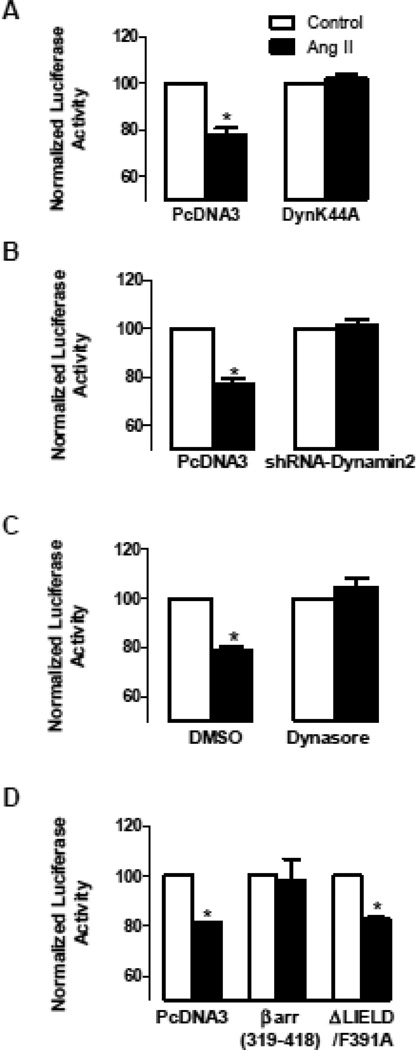
Follow-up experiments with the reporter construct, which allows cotransfection of molecular reagents, verified the requirement for dynamin. Specifically, the Ang II effect on the Kv4.3 reporter was inhibited with expression of a dominant negative dynamin (DynK44A) (Fig. 5A) or a short hairpin RNAi construct targeting dynamin 2 (Fig. 5B), the isoform in cardiomyocytes. Likewise, the chemical dynamin inhibitor dynasore blocked the Ang II effect (Fig. 5C). Thus, four independent approaches established that dynamin is required for the Ang II effect on Kv4.3 mRNA stability.
Figure 5.
Endosome generation is required for the Ang II effect on the Kv4.3 3’ UTR reporter. A. Inhibition of the Ang II effect by expression of dominant negative dynamin mutant (DynK44A), but not by the empty expression plasmid PcDNA3 (n=3). B. Inhibition of the Ang II effect by expression of a short hairpin RNAi construct targeting dynamin 2 (shRNA-dynamin2) (n=3). C. Inhibition of the Ang II effect by 100 µM dynasore, but not by the vehicle DMSO (n=3). D. The clathrin binding domain (βarr(319-318)) blocks the Ang II effect (n=3), but βarr(ΔLIELD/F391A) has no effect even though it prevents arrestin-mediated receptor interaction with clathrin and β2-adaptin (n=3). *p<0.05.
This regulation was also blocked by a clathrin-binding construct derived from arrestin2 that does not interact with G-protein coupled receptors (GPCRs) [22] (Fig. 5D, βarr(319-418)). This inhibition was found to be independent of native arrestin because the Kv4.3 effect was not altered by a dominant negative arrestin2 mutant that binds GPCRs and prevents their interaction with clathrin and β2-adaptin [23] (Fig. 5D, ΔLIELD/F391A). Furthermore, agonists that specifically activate AT1 receptor-dependent arrestin signaling in cardiomyocytes [26] or β1-adrenergic receptors, which induce endosome-dependent arrestin signaling in cardiomyocytes [27], did not affect the Kv4.3 reporter (data not shown). Thus, while arrestin is not be involved, clathrin is required. Together with the above dynamin results, the implication of clathrin shows that cardiomyocyte endosomes are required for the Ang II effect on Kv4.3 mRNA stability.
3.3 CamKII is required for the p38K-mediated Kv4.3 effect
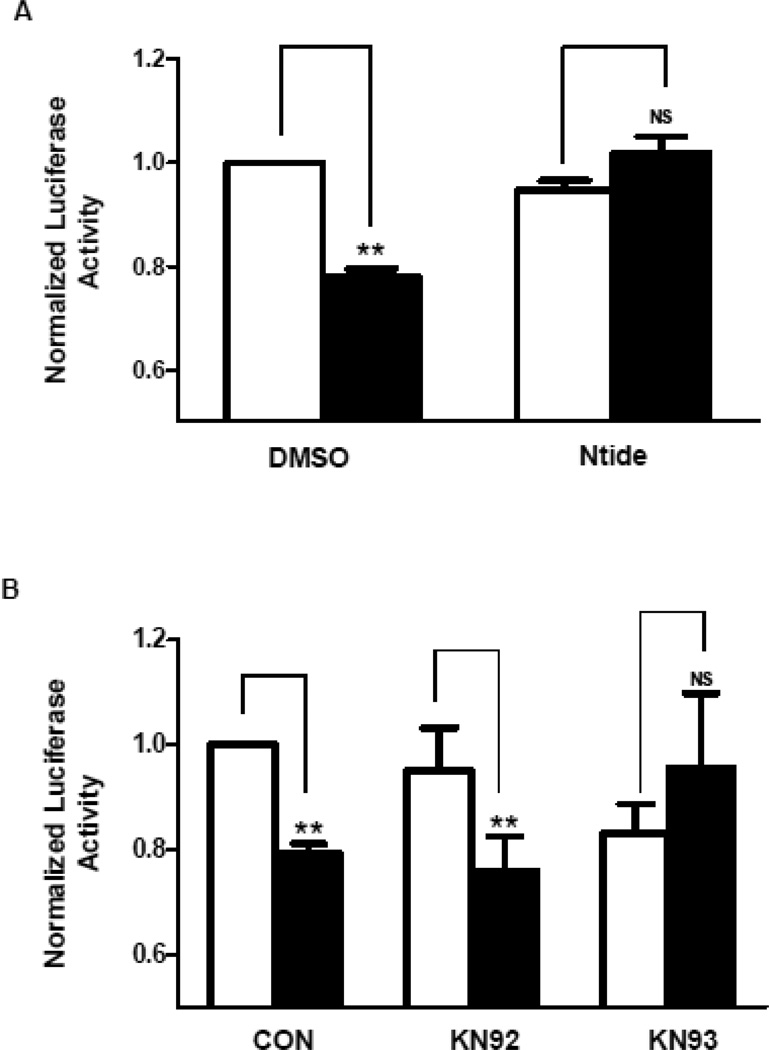
To explore the potential role of CamKII in the delayed Ang II effect, the Kv4.3 3’ UTR luciferase reporter was assayed after treatment of cardiomyocytes with myristoylated Ntide (a CamKII inhibitory peptide), KN93 (a specific chemical inhibitor of CamKII), or KN92 (an inactive analog control). Myristolyated Ntide and KN93 each blocked the decrease in reporter activity induced by Ang II, while KN92 had no effect (Fig. 6A,B). Thus, CamKII is required for the Ang II-induced destabilization of cardiomyocyte Kv4.3 mRNA.
Figure 6.
CamKII is required for the Ang II effect on the Kv4.3 3’ UTR. A. Ang II-dependent inhibition of Kv4.3 3’ UTR reporter activity is blocked by 1 µM myristoylated Ntide (n=3). B. Ang II-dependent inhibition of reporter activity is blocked by 1 µM KN93, but not KN92 (n=3). **p<0.01.
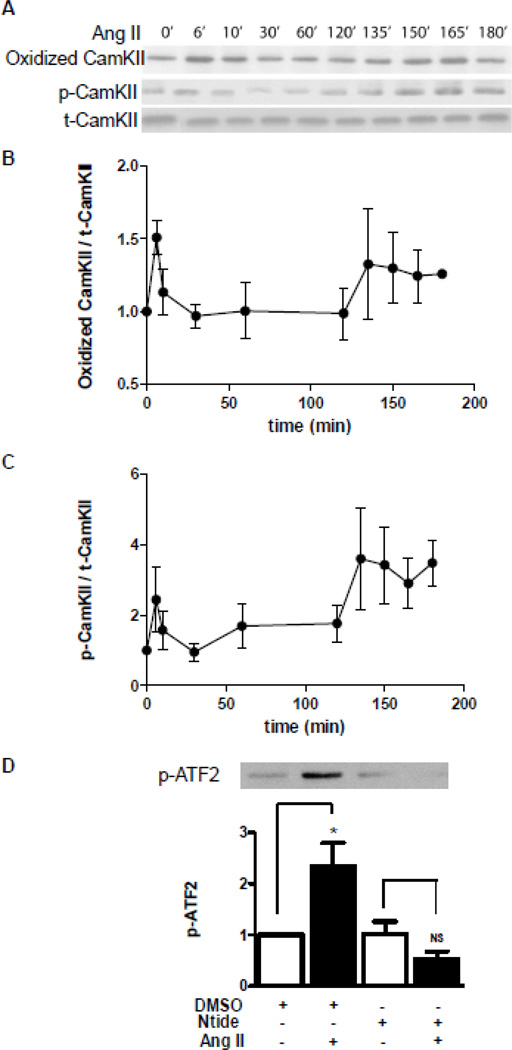
Ang II induces methionine oxidation and activation of CamKII in cardiomyocytes [15], but the kinetics of these effects has not been fully characterized. Therefore, CamKII was isolated by immunoprecipitation after different periods of Ang II exposure and immunoblots were used to measure CamKII methionine oxidation and autophosphorylation (Fig. 7A–C), with the latter assay being indicative of activation. Within 6 minutes, Ang II induced CamKII methionine oxidation and activation. However, in all experiment this response decayed until a second delayed phase of CamKII methionine oxidation and activation became apparent ~150 minutes later (Fig. 7A–C). Thus, CamKII methionine oxidation and resultant activation are biphasic.
Figure 7.
Biphasic oxidation and autophosphorylation of CamKII acts upstream of p38K. A. Immunoblot showing single time course of Ang II-dependent formation of methionine oxidized CamKII and phospho-CamKII (p-CamKII) compared to total CamKII (t-CamKII). B. Normalized time course of CamKII methionine oxidation (n=3). C. Normalized time course of CamKII autophosphorylation (n=3). D. p38K enzymatic activity after 150 minutes of Ang II is blocked by myristoylated Ntide, but not the vehicle DMSO. Top, representative immunoblot; Bottom, normalized quantification (n=5). *p<0.05; NS, not significant.
CamKII activates p38K [22], but this has not been examined in the context of biphasic signaling. Therefore, we tested whether inhibiting CamKII affects Ang II-dependent p38K activation at 150 minutes in cardiomyocytes. In fact, myristoylated Ntide blocked delayed p38K activation (Fig. 7D). Thus, the second phase of p38K activation, which is necessary for Kv4.3 mRNA destabilization, requires activation of CamKII.
3.4 Endosome-dependent activation and oxidation of CamKII
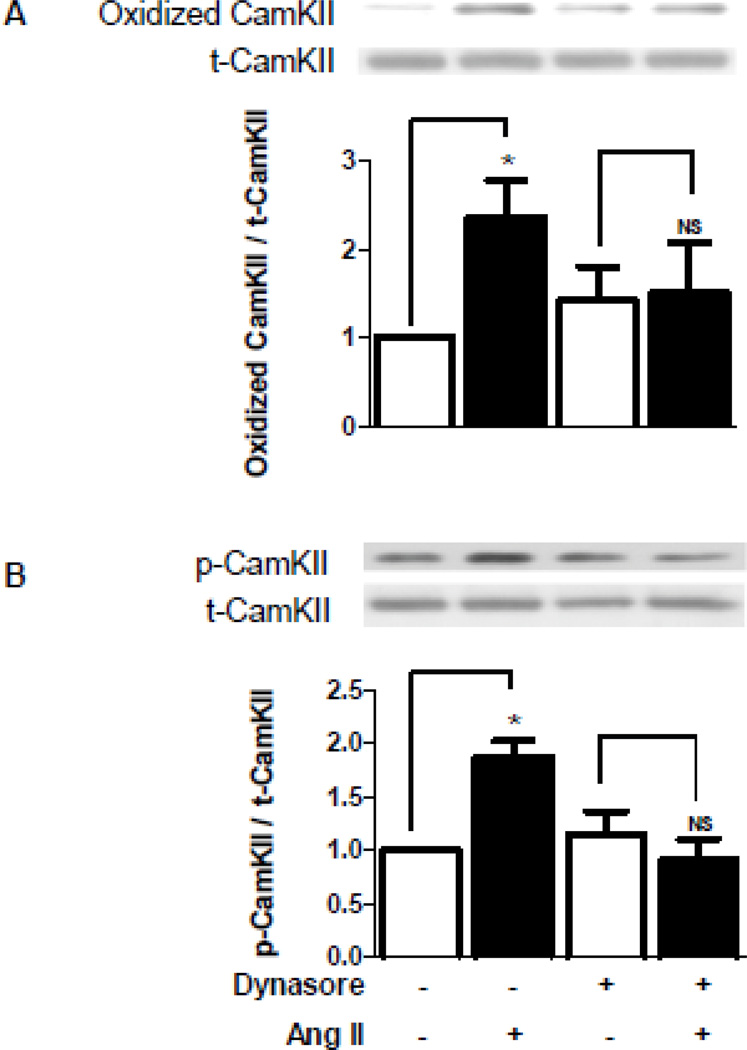
Given that the delayed p38K-mediated Kv4.3 effect requires endosomes as well as upstream biphasic CamKII activation, the dependence of the delayed phase of CamKII methionine oxidation and activation on endosomes was investigated. Immunoblots were performed on cardiomyocyte samples collected 150 minutes after Ang II in the presence or absence of the dynamin inhibitor dynasore, which was used because the DIPC control affected baseline CamKII (data not shown). The inhibition of endosome formation eliminated the delayed increases in CamKII methionine oxidation and activation (Fig. 8A,B). Thus, cardiomyocyte endosomes are required for delayed CamKII methionine oxidation and activation, which produces the delayed phase of p38K activation that is necessary for Kv4.3 mRNA destabilization.
Figure 8.
Endosomes induce delayed CamKII oxidation and activation. A. Dynasore (100 µM) blocks the delayed phase of Ang II-induced CamKII oxidation. Top, sample immunoblot showing oxidized and total CamKII at 150 minutes from one experiment. Bottom, normalized quantification of CamKII oxidation (n=4). B. Dynasore blocks the delayed phase of CamKII activation. Top, immunoblot showing CamKII autophosphorylation at 150 minutes from one experiment. Bottom, normalized quantification of CamKII autophosphorylation (n=4). *p<0.05.
4. Discussion
Past studies had not resolved how Ang II-induced p38K signaling, which was thought to peak in minutes, affects cardiomyocytes hours later. Here we showed that Ang II activation of the p38K pathway in cardiomyocytes is biphasic, with a novel delayed phase occurring after hours. CamKII was found to mediate delayed activation of p38K and the downstream effect on Kv4.3 mRNA stability. Given the biphasic methionine oxidation of CamKII also documented here, this may reflect CamKII’s sensitivity to Nox-generated reactive oxygen species resulting in activation of ASK1 and downstream p38K [15,16]. Endosomes were also shown to be required for delayed activation of CamKII and p38K and the Kv4.3 effect. Thus, endocytosis is important for both trafficking cardiac Kv channels [20,21] and controlling their gene expression. Although our study focused on cardiac Kv4.3 mRNA stability, delayed endosome-dependent activation of CamKII and p38K could be relevant to Ang II regulation of Kv4.3 expression in neurons [28] and more generally to the participation of these kinases in cardiac pathology.
Why isn’t the initial endosome-independent phase of CamKII and p38K activation sufficient for the downstream effect on Kv4.3? One possibility is that the first phase of signaling occurs at the plasma membrane and so is not well positioned to induce upregulation and activation of cytoplasmic AUF1, the mRNA binding protein responsible for the Ang II effect on Kv4.3 mRNA stability [8]. Alternatively, the initial phase of signaling may be too transient to induce a sustained change in gene expression. Transient signaling at the cell surface may be well suited for acute and dynamic regulation of cardiac excitability and contractility, but not for prolonged changes in channel expression.
The results presented here pose the question of how endosomes induce oxidative activation of CamKII in cardiomyocytes. One possibility is that endosomes support AT1 receptor activation of rac, which is required for Nox-dependent control of Kv4.3 mRNA [7]. Alternatively, endosomes might act as a scaffold for Nox signaling by delivering reactive oxygen species (i.e., superoxide, hydrogen peroxide and other derivatives) [18,19] to cytoplasmic CamKII. Of course, other functions of endosomes are also possible. Regardless of the specific mechanism, for the first time endosomes have been shown to be important for sustained CamKII-p38K signaling and Kv channel mRNA expression in cardiomyocytes.
Highlights.
Angiotensin II induces biphasic CamKII and p38 kinase activation in cardiomyocytes.
Delayed oxidative activation of these kinases requires endosomes.
Endosome-dependent kinase signaling regulates K+ channel mRNA expression.
Acknowledgments
This work was supported by the National Institutes of Health grant R01 HL80632 to E.S.L. We thank Dr. Mark Anderson (University of Iowa) for the methionine oxidized CamKII antibody, Dr. Jeffrey Benovic (Thomas Jefferson University) for the clathrin-related constructs, Dr. Liangyi Chen (Chinese Academy of Science) for the dynamin 2 shRNA construct, and Dr. Timothy Feinstein (University of Pittsburgh) for the dynamin dominant negative construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 2.Greenstein JL, Wu R, Po S, Tomaselli GF, Winslow RL. Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circ Res. 2000;87:1026–1033. doi: 10.1161/01.res.87.11.1026. [DOI] [PubMed] [Google Scholar]
- 3.Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res. 2005;96:543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- 4.Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium. Am J Physiol Heart Circ Physiol. 2009;296:H1017–H1026. doi: 10.1152/ajpheart.01216.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 6.Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circ Res. 2001;88:476–482. doi: 10.1161/01.res.88.5.476. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Ziegler C, Birder LA, Stewart AF, Levitan ES. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res. 2006;98:1040–1047. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C, Vignere CZ, Levitan ES. AUF1 is upregulated by angiotensin II to destabilize cardiac Kv4.3 channel mRNA. J Mol Cell Cardiol. 2008;45:832–838. doi: 10.1016/j.yjmcc.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida K, Otsu K. The role of apoptosis signal-regulating kinase 1 in cardiomyocyte apoptosis. Antioxid Redox Signal. 2006;8:1729–1736. doi: 10.1089/ars.2006.8.1729. [DOI] [PubMed] [Google Scholar]
- 10.Kajstura J, Bolli R, Sonnenblick EH, Anversa P, Leri A. Cause of death: suicide. J Mol Cell Cardiol. 2006;40:425–437. doi: 10.1016/j.yjmcc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 12.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, Sato Y, Kawanishi T, Inoue R, Nagao T, Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem. 2005;280:18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 13.Aoki H, Richmond M, Izumo S, Sadoshima J. Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem J. 2000;347:275–284. [PMC free article] [PubMed] [Google Scholar]
- 14.Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 15.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, Matsumoto K, Ichijo H. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004;5:161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 19.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific K+ channel Kv1.5. Circ Res. 2009;104:1390–1398. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. J Biol Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Benovic JL. Differential Roles of Arrestin-2 Interaction with Clathrin and Adaptor Protein 2 in G Protein-coupled Receptor Trafficking. J Biol Chem. 2002;277:30760–30768. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, He Z, Fan J, Xu P, Chen L. Overlapping functions of different dynamin isoforms in clathrin-dependent and -independent endocytosis in pancreatic beta cells. Biochem Biophys Res Commun. 2008;371:315–319. doi: 10.1016/j.bbrc.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 25.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisco C, Marrone C, Galeotti J, Shao D, Vatner DE, Vatner SF, Sadoshima J. Endocytosis machinery is required for beta1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes. Cardiovasc Res. 2008;78:36–44. doi: 10.1093/cvr/cvn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Li Y, Schultz HD, Wang WZ, Wang W, Finch M, Smith LM, Zucker IH. Downregulated Kv4.3 expression in the RVLM as a potential mechanism for sympathoexcitation in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H945–H955. doi: 10.1152/ajpheart.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]