Abstract
Anaplasma marginale is an obligate intraerythrocytic bacterium that infects ruminants, and notably causes severe economic losses in cattle worldwide. A. phagocytophilum infects neutrophils and causes disease in many mammals, including ruminants, dogs, cats, horses, and humans. Both bacteria cause persistent infection – infected cattle never clear A. marginale and A. phagocytophilum can also cause persistent infection in ruminants and other animals for several years. This review describes correlates of the protective immune response to these two pathogens as well as subversion and dysregulation of the immune response following infection that likely contribute to long-term persistence. I also compare the immune dysfunction observed with intraerythrocytic A. marginale to that observed in other models of chronic infection resulting in high antigen loads, including malaria, a disease caused by another intraerythrocytic pathogen.
1. Introduction
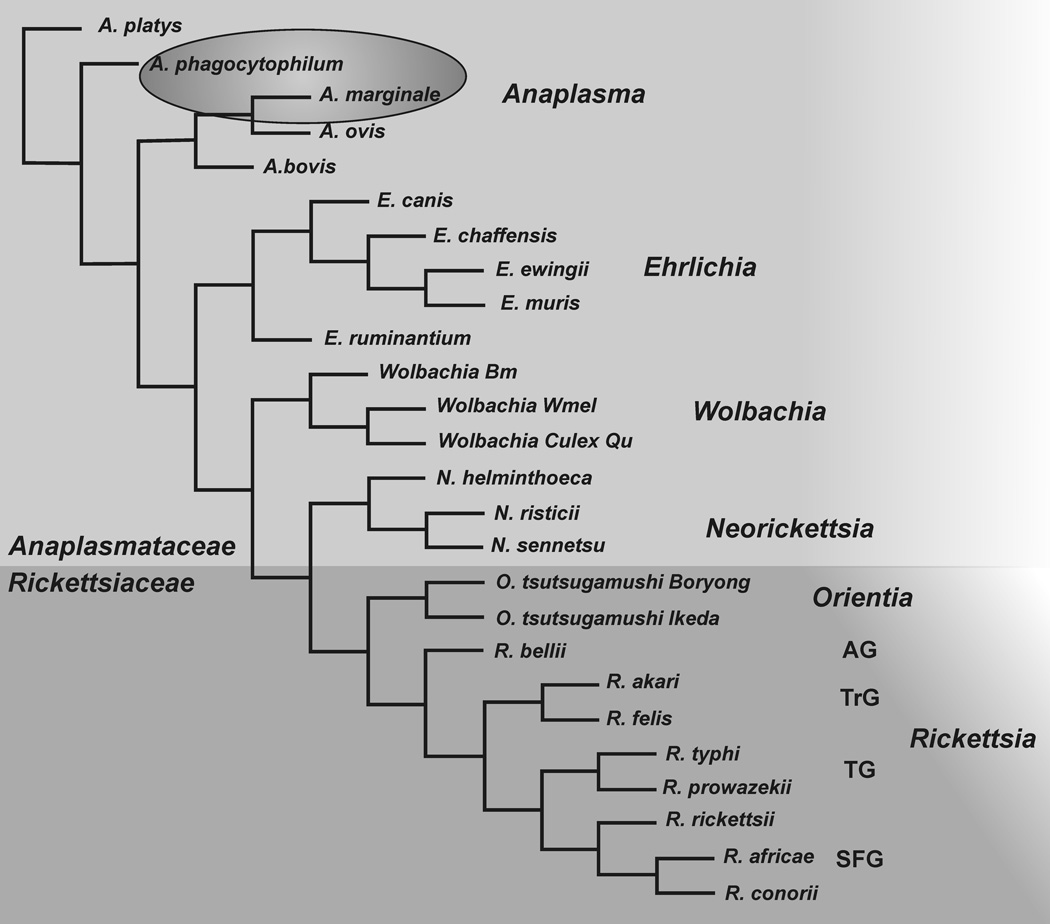
Anaplasma marginale and A. phagocytophilum are ixodid tick-transmitted gram-negative bacterial pathogens in the family Anaplasmatacae in the order Rickettsiales that notably cause disease in ruminants (both pathogens) and humans (A. phagocytophilum). Despite their evolutionary relatedness [1] and Fig. 1, these obligate intracellular bacteria have different host restrictions and infect and replicate in distinct blood cells (erythrocytes and neutrophils) causing different pathologies and immune responses.
Fig. 1.
Phylogenetic tree of order Rickettsiales. Genera in the families Anaplasmataceae and Rickettsiaceae are shown in shaded areas. Species of interest are circled. The Rickettsia are subgrouped according to ancestral group (AG), transitional group (TrG), Typhus group (TG) and spotted fever group (SFG). The tree is based on a clustalW alignment of 16S ribosomal RNA gene sequences using POWER (http://power.nhri.org.tw/power/home.htm).
Ehrlichia phagocytophila and E. equi had long been known as veterinary pathogens [2]. The agent of human granulocytic ehrlichiosis (HGE) was more recently reported as an emerging pathogen for humans in 1990 and the agent isolated four years later [3]. All three were later reclassified as a single species in the genus Anaplasma [2]. A. phagocytophilum infects many mammals including ruminants, horses, humans, rodents, dogs, cats and deer [2, 4]. This bacterium replicates primarily within vacuoles of neutrophils, leukocytes designed to phagocytose and destroy blood-borne pathogens, although endothelial cells and bone marrow progenitors can also be infected [5,6]. Most infected humans are asymptomatic or experience mild clinical signs of human granulocytic anaplasmosis (HGA) [4]. However, when symptomatic, common clinical signs of HGA are fever, malaise, headache, myalgia, anemia, thrombocytopenia, leukopenia and elevated hepatic transaminase levels. Pathologic changes include hepatitis with occasional apoptosis, mild lymphoid depletion, mononuclear phagocyte hyperplasia in spleen and lymph nodes, and hemophagocytosis in bone marrow, liver, and spleen [4]. Human infection occurs in the US and many parts of Europe and Asia, where Ixodes ticks are prevalent [1, 4]. Acute and fatal cases comprise approximately five to seven percent of clinical cases, and death is caused by a toxic shock-like syndrome involving coagulopathy, atypical pneumonitis/acute respiratory distress syndrome (ARDS), acute abdominal syndrome, rhabdomyolysis, myocarditis, acute renal failure, hemorrhage, brachial plexopathy, demyelinating polyneuropathy, cranial nerve palsies, and opportunistic infections [4]. In clinically ill humans the disease appears to be largely immune-mediated. The white-footed mouse serves as a reservoir for A. phagocytophilum infection of humans in the US. Although deer and sheep can be persistently infected, there is no evidence for persistent infection in humans, white-footed mice or laboratory mice [4].
A. phagocytophilum has long been recognized as the causative agent for a disease called tick borne fever (TBF) in small ruminants in Europe, notably sheep [7]. Goats, cattle, horses and dogs can also be clinically affected by A. phagocytophilum infection [8–10]. In sheep, infection results in a high fever that lasts up to two weeks, and a leukopenia involving both granulocytes and lymphocytes, similar to what is observed in humans [9–11]. Secondary infections are common, and include Staphylococcus aureus mediated tick pyaemia, pasteurellosis, and septicemic listeriosis resulting from the immunosuppressed state. Thus unlike HGA, TBF is not an immune-mediated disease but results from immune suppression.
A. marginale was discovered by Sir Arnold Theiler in 1910 in the red blood cells of infected cattle [12], and infection by this intraerythrocytic pathogen is restricted to ruminants. Anaplasmosis occurs in tropical and subtropical regions of the world, including the United States, Central and South America, southern Europe, Africa, Asia and Australia where the ixodid tick vectors are found. Extensive reviews of bovine anaplasmosis including life cycle, epidemiology, diagnostics, and control methods were recently published [13, 14]. The most effective means of inducing protective immunity are immunization with an attenuated vaccine strain derived from A. centrale, which is not licensed for use in the US, and immunization with an outer membrane fraction, which can completely protect a percentage of cattle against infection, as discussed in section 2.2. Clinical disease is most prevalent in cattle, although ruminants, such as bison, water buffalo, and some species of African antelope and deer can be persistently infected. A. marginale invades and replicates within mature erythrocytes in the bovine host, and although endothelial cells can be infected in vitro [15], convincing evidence for endothelial cell invasion in vivo is lacking [16, 17]. Acute infection of cattle is characterized by an ascending bacteremia (infected erythrocytes) peaking two to six weeks after infection and reaching levels of 109 or more bacteria per ml of blood, determined by quantitative PCR [18–20]. The infection results in anemia, with drops in packed erythrocyte cell volumes generally ranging from 30- to 50% that can be accompanied by fever and weight loss. However, if elevated, body temperatures generally remain less than 104°F [21]. Death can occur in up to 30% of clinical cases. Anemia is caused by removal of infected erythrocytes from the circulation by phagocytic cells of the spleen, and splenectomized animals do not control bacteremias. In spleen-intact animals, the acute infection is generally resolved, but never completely cleared, and animals remain persistently infected for life with fluctuating bacteremias ranging from 102 to > 107 bacteria per ml of blood [20]. Thus both acute and persistent infections are characterized by relatively high antigen loads.
This review will compare what is known about the subversion of the immune response by A. phagocytophilum and A. marginale in humans and ruminants, and will also discuss the immune dysfunction observed in intraerythrocytic A. marginale infection with that which occurs during infection with other chronic pathogens, including intraerythrocytic malarial parasites.
2. Immune Response to Infection or Immunization
2.1. Immune response to A. phagocytophilum
The mechanisms that lead to control of A. phagocytophilum infection in humans are not completely understood. Studies examining human T cell responses have not been performed, although during acute infection, high antibody titers occur in approximately 40% of patients [22]. In patient sera, IgG reacts with A. phagocytophilum proteins on immunoblots [23], indicating the requirement for CD4+ T cell help. Similarly, 44% of equine patients also make high titers of specific antibody [24], although in both humans and horses a protective role for antibody in clearing infection was not demonstrated.
Few studies have examined cytokine responses to infection. In humans infected with A. phagocytophilum, recovery from acute infection was associated temporally with elevated serum levels of IFN-γ and IL-10, whereas serum TNF-α, IL-1β, and IL-4 levels were not elevated compared with controls [25]. In contrast, horses experimentally infected with A. phagocytophilum that developed mild disease had undetectable or weak expression of IFN-γ and IL-10 mRNA, but more consistently upregulated IL-1β and TNF-α mRNA expression by peripheral blood leukocytes, suggesting a role for these latter proinflammatory cytokines in disease [26].
In sheep, antigen-specific immune responses have not been extensively studied during infection, in part because they are suppressed (see section 3.2.2). Lymphocytes in the blood of experimentally infected sheep were compared with those of normal sheep, analyzed by flow cytometry. There was a significant reduction in total numbers of circulating lymphocytes, six days after infection, which was associated with significant losses of both B cells and T cells [10]. T cell losses were further shown to include CD4+, CD8+ and CD4− CD8− T cells. Antibody responses in infected sheep persisted for six to ten weeks in one study using counter electrophoresis [27] and began to decline after 18 weeks in a second study using indirect immunofluorescence assay (IFA) [28]. More recently, serum antibody responses to A. phagocytophilum and recombinant major surface protein (MSP)5 were measured during the course of acute and persistent infection of sheep [7]. Specific antibody was detected by two weeks post-infection, but diminished over time and was not detectable by immunoblotting by 14 weeks, despite persisting infection. Titers evaluated by indirect immunofluorescence assay during acute infection ranged from 1,280–5,120 in two animals.
Experimental infection of mice with A. phagocytophilum causes a disease progression similar to that of immunocompetent humans, with transient infection lacking clinical signs but sharing histologic lesions found in humans, including hepatic lymphohistiocytic aggregates with apoptosis [29]. Thus, mice are useful for studying the immune mechanisms that lead to control and clearance of this pathogen. Resolution of infection by A. phagocytophilum infection is mediated by a combination of neutralizing antibody and inflammatory responses of activated macrophages and neutrophils, which are dependent on CD4+ T cells [29–33].
A role for lymphocytes in mediating pathogen clearance was shown in studies using T cell- and B cell-deficient SCID mice, which remained persistently infected [34–36]. In the murine A. phagocytophilum model of HGA, passively administered immune serum protected 63% of experimentally challenged animals from developing detectable infection measured by PCR amplification of A. phagocytophilum DNA, whereas 88% of control mice developed infection [30]. In mice experimentally infected with A. phagocytophilum, bacterial burdens in blood peaked on day 7, and IFN-γ produced by splenic leukocytes peaked later, on day 10, whereas maximal tissue pathology was observed on day 14 [29, 33]. A decrease in organisms within the tissues was paralleled by later declines in IFN-γ levels and hepatic pathology three and seven days later, respectively, suggesting a causal role of IFN-γ in both controlling infection and mediating tissue damage. Further studies confirmed a role of IFN-γ in both protection against infection and immunopathology. In IFN-γ knockout mice, increased bacterial levels in blood and tissues occurred during early infection, but bacteria were eventually cleared [31, 33]. Interestingly, in IFN-γ knock out mice, histopathologic lesions were absent, indicating pathology is mediated by this cytokine [33]. However, in the same studies when IL-10 knockout mice were used, mice had similar levels of IFN-γ as wild type mice but had increased histopathologic lesions that were not resolved in the face of controlled bacterial burdens [33]. IL-10, which acts to suppress macrophage proinflammatory cytokine responses, likely decreased tissue injury otherwise mediated by cytokines that are induced by IFN-γ. Later studies showed that IFN-γ produced by NK and possibly NKT cells was important for controlling early infection, but again, not crucial for complete elimination of the pathogen [37, 38]. Together, these studies confirm a role for IFN-γ in mediating both pathology and early bacterial control, although it is not essential for bacterial clearance.
IFN-γ may also help reduce bacterial burdens by inducing reactive nitrogen intermediates, which are toxic for intracellular bacteria [32]. When NOS2-deficient mice were infected with A. phagocytophilum, bacteria were still readily detected at 12 days, which in wild type mice are cleared. A role for IFN-γ in the NOS2-mediated bacterial clearance was suggested, as NOS2 levels were lower in IFN-©R knockout mice, which also still had detectable bacteria at 12 days. Nevertheless, in NOS2 deficient mice, bacteria were cleared at 20 days, showing that reactive nitrogen intermediates are not necessary for clearing infection. Subsequent studies using mice deficient in TLR2 and TLR4, MyD88, TNF, iNOS, or phagocyte NADPH oxidase showed that these mice were also completely capable of clearing the infection [36]. Thus, innate immune mediators used to activate phagocytes to kill other intracellular bacteria did not appear to play a major role in A. phagocytophilum clearance. These same mediators did, however, contribute to the observed pathology [39].
CD4+ T cells were more recently found to be required for complete A. phagocytophilum elimination in the mouse model [38]. However unlike the paradigm for controlling other intracellular pathogens, studies using mice deficient in perforin, Fas/FasL, Th1 cytokines IL-12 and IFN-γ, or MCP-1 showed that these mice were able to clear bacteria [38]. Thus, the precise mechanisms by which CD4+ T cells mediate pathogen clearance are still not understood.
2.2 Immune response to A. marginale
Because A. marginale does not replicate within cells that express MHC molecules, it is hypothesized that protective immunity to infection requires activation of both CD4+ T cell responses and antibody [40]. CD4+ T cells are required for B cell isotype switching to high affinity IgG isotypes that promote opsonization of bacteria, and through IFN-γ secretion, play a role in activating macrophages to produce bactericidal molecules such as nitric oxide (NO). In cattle, IFN-γ secreted by antigen-stimulated CD4+ T cells provides help to B cells for IgG2 production [41, 42].
Monitoring the immune response in cattle to infection with A. marginale has documented a strong IgG1 and IgG2 response that fluctuates over the course of acute and persistent infection, maintaining titers of 3,000 to 100,000 during persistence [20, 43, 44]. The IgG response is primarily directed against the immunodominant and antigenically variable outer membrane protein MSP2 [43, 45] (described in section 3.2.1), although other MSPs, notably antigenically variable MSP3 (which shares the conserved C-terminal part of the MSP2 protein), MSP1, MSP4, and MSP5 can also be detected on immunoblots [44–47]. It is presumed that antibody (IgG2 was the only isotype investigated) to predominant variants of MSP2 is responsible for controlling the initial acute bacteremia, as well as subsequent peaks of bacteremia that arise during persistent infection from new antigenic variants [48, 49]. Antibody is hypothesized to act by either neutralizing extracellular bacteria in the process of invading new erythrocytes or by opsonizing bacteria that are then targeted for macrophage phagocytosis. Antibodies directed against the surface of infected erythrocytes have not been detected, so phagocytosis of infected erythrocytes probably occurs by other mechanisms. Nevertheless, antibody to continually emerging MSP2 variants is not sufficient to clear infection and cattle remain persistently infected for life. The inability to passively protect cattle against A. marginale infection with immune sera from either infected or immunized cattle [50] suggested mechanisms other than antibody also contribute to protection or that the antibody to the dominant MSP2 variants in sera could not prevent emergence of new antigenic variants.
Early studies on cellular immune responses elicited during infection with A. marginale established the correlation between protection and macrophage activation in the control of acute bacteremia [51, 52]. Further support of this was shown in studies where supernatants of proliferating blood leukocytes cultured with A. marginale killed the bacteria, but the toxic molecule(s) were not identified [53]. When monoclonal antibody that neutralized IFN-γ or an inhibitor of iNOS (aminoguanidine) was used to treat cattle during acute infection, only aminoguanidine had an effect, which contrary to expectations, actually improved the clinical response, suggesting that if induced during infection, iNOS may exacerbate both anemia and bacteremia [54]. Although plasma levels of these reagents were measured and detected, the effect on in vivo levels of NO and IFN-γ were not determined.
Few studies have actually examined the antigen-specific T cell response to A. marginale during infection. Gale et al. [55] measured T-lymphocyte proliferation in blood and spleens of cattle during infection. Responses were not detected in PBMC of all cattle that had recovered from infection, whereas responses by splenic lymphocytes were detected, although the responses were inconsistent between time points for individual animals. A caveat is that negative control antigens were not included in this study. A more thorough study was recently conducted where cattle were infected with either fresh blood derived from the Florida strain or by tick transmission of the South Idaho strain of A. marginale, and PBMC and spleen biopsy lymphocytes were tested for antigen-specific proliferation and IFN-γ production [20]. Frequent sampling of peripheral blood from the two Florida strain infected cattle revealed that significant antigen-specific T cell responses against A. marginale outer membranes (compared with negative control antigens) were not detected until five to seven weeks after infection, after bacteremias had peaked at approximately four weeks. Thereafter responses were sporadic and transient and observed only once or twice during the 120-day sampling period. Responses in the spleens of the same cattle sampled one to two times per week were first significant at weeks 7 and 15, and only for a total of two and three times during the sampling period. A longer lag period was observed in tick-transmitted South Idaho strain infected cattle sampled weekly for nearly one year, with significant proliferative responses observed first at 15 and 16 weeks post infection, well after infection had peaked at four to five weeks and was resolved (but not cleared). Responding cells were determined to be CD4+ cells, and not CD8+ or γδ T cells. Paradoxically, all cattle developed high titers (30,000) of A. marginale-specific IgG1 and IgG2 by two-three weeks of infection, when bacteremias were ascending, and titersl remained high throughout persistent infection. This suggests isotype switching occurred through T-dependent mechanisms. However, antibody, which was directed predominantly against MSP2, was not sufficient to clear infection. The dysfunctional CD4 T cell response will be discussed in depth in section 3.2.2.
Research on A. marginale in cattle immunized with protective vaccines is consistent in identifying CD4+ T cells, IFN-γ secretion, and activation of specific IgG isotypes for neutralization and opsonization as determinants of protective immunity [40, 56]. Our best information comes from studies on the immune response in cattle immunized with purified outer membranes, which induces protection against virulent challenge. In four trials, vaccinees were significantly protected against bacteremia upon challenge with homologous or heterologous A. marginale strains [44, 56–58]. This protection was shown for up to 75% of the vaccinees that were completely protected against infection; no organisms were detected microscopically during the 2.5 month period post-homologous strain challenge and, in the most recent trials, lack of infection was confirmed using the msp5 PCR [56, 58]. Importantly, protection correlated with antibody titer against the outer membrane immunogen with the highest antibody titers in cattle that were completely protected against infection [56]. In addition, complete protection was associated with induction of IgG2 antibody, the optimal opsonizing antibody subclass in cattle [59]. However, subsequent studies showed that immunization with gel-eluted native MSP2 containing multiple antigenic variants did not afford protection against challenge with homologous bacteria from which the MSP2 was derived, which expressed the same major MSP2 variants [21]. Furthermore, when comparing the MSP2-specific response in protected OM-vaccinees and unvaccinated, infected controls, there was no significant difference in antibody repertoires to MSP2 peptides in terms of breadth of response and titer [58]. Among the immunized animals, there was no association between either breadth or magnitude of the anti-MSP2 IgG response and either complete protection from infection or control of bacteremia [58]. We hypothesize that the protection afforded against heterologous strain challenge is due to responses directed against strain-conserved, sub-dominant antigenic epitopes in the outer membrane proteins used for immunization [45, 60–63]. A concerted effort is underway to identify such proteins and test them for conferring protective immunity, reviewed elsewhere [64].
3. Subversion and dysregulation of the immune response
It has recently become evident that Anaplasma pathogens have evolved mechanisms to thwart the innate and adaptive immune response. These include the manipulation of the host neutrophil by A. phagocytophilum to prevent cellular apoptosis and neutrophil killing mechanisms, antigenic variation of immunodominant surface proteins MSP2/P44 in A. phagocytophilum and of MSP2 and MSP3 in A. marginale to evade specific immune responses, and subversion of adaptive immune responses.
3.1 Evasion of innate defense mechanisms
Both A. phagocytophilum and A. marginale lack genes encoding biosynthetic pathways for LPS and peptidoglycan [65, 66] two important pathogen associated molecular patterns expressed by most gram negative bacteria that activate TLR and NOD receptors [67, 68]. Thus the absence of these molecules allows these pathogens to fall under the radar of important innate defense sensors. A. marginale inhabits a non-phagocytic cell, the erythrocyte, which lacks innate defense mechanisms. A. phagocytophilum, which inhabits the hostile environment of the neutrophil, uses several strategies to evade or block host cell antimicrobial function, which are extensively reviewed elsewhere [1, 69, 70]. Briefly, through binding to sialyated P-selectin glycoprotein ligand-1 and other glycans on the neutrophil, it bypasses complement and FcR-mediated phagocytic pathways. Caveolae-mediated endocytosis directs the pathogen to an intracellular membrane-bound inclusion that does not acquire components of NADPH oxidase or of late endosomes or lysosomes. A. phagocytophilum is further able to prevent superoxide anion production and neutrophil oxidative killing by scavenging oxygen, inhibiting NADPH oxidase assembly on its vacuolar membrane, and modifying promoter activity for a key NADPH oxidase component, gp91phox. It induces neutrophil release of IL-8 in humans and the murine homologs KC and MIP-2 and upregulates the expression of receptors for these chemokines, which results in recruitment of additional neutrophils that can be targeted for invasion and bacterial propagation. Uptake of this pathogen delays spontaneous neutrophil apoptosis. Furthermore, A. phagocytophilum dysregulates neutrophil gene expression leading to extension of the normally short-lived neutrophil lifespan to maximize its survival and dissemination within its mammalian host so until it can be acquired by tick feeding and transmitted to another host.
A. phagocytophilum and A. marginale are both small genome pathogens that have undergone reductive evolution, but retained a type IV secretion system (T4SS) [reviewed in ref. 1, 71–73]. In A. phagocytophilum the T4SS is a virulence factor, and it has been shown that the T4SS secretes effector proteins that include an ankyrin repeat domain-containing protein A (AnkA) and Anaplasma translocated substrate 1 (Ats-1) into the neutrophil cytoplasm. These effectors have distinct signaling mechanisms that may play a role in host cell attachment and invasion [73–75].
While the function of the T4SS in A. marginale has not been determined, retention of these genes by this small genome pathogen indicates their requirement for invasion and survival within erythrocytes and/or tick cells [66, 71]. T4SS structural proteins are definitely expressed in bacteria within host erythrocytes [45, 62, 63, 76], and recent studies have identified several T4SS effector molecules including AnkA [77]. Because of their immunogenicity, conservation among strains, and surface localization, T4SS proteins are being evaluated for vaccine development [45, 60–63].
3.2 Evasion of adaptive immunity
3.2.1 Antigenic variation
Persistent infection is established and maintained in large part through antigenic variation of immunodominant MSP2 and related MSP3 of A. marginale and MSP2/p44 of A. phagocytophilum. [19]. The msp2 locus was first described in A. marginale and is comprised of a variable number of functional pseudogenes (five to ten) and a single expression site. Full-length msp2 genes contain a central hypervariable region flanked by two conserved regions, and pseudogenes contain unique central hypervariable regions flanked by small conserved region sequences identical to those in the expression site msp2. Antigenic variation in MSP2 and MSP3 occurs by gene conversion of whole pseudogenes and small segments of pseudogenes (resulting in segmental gene conversion) into the msp2 expression site, providing an efficient mechanism to generate the large number of variants seen during sequential cycles of persistent infection [66, 78–82]. A. phagocytophilum has a similar gene locus designated msp2/p44 consisting of one msp2/p44 expression site gene and 112 msp2/p44 pseudogenes with a similar structure of hypervariable and conserved regions, and a similar gene conversion mechanism that gives rise to multiple variants [1, 83].
As noted in section 2.2, MSP2 is the dominant protein recognized by sera from cattle infected with A. marginale or immunized with outer membranes, and Msp2/p44 is the dominant protein recognized during A. phagocytophilum infection [23, 43, 84, 85]. In cattle, the MSP2-specific antibody response is predominantly directed toward the hypervariable region rather than the flanking conserved regions [86, 87]. This is not surprising, as the hypervariable region is predicted to be surface exposed [86] and antigenic variation is detected under conditions of immune pressure and selection, which would not apply to surface exposed conserved sequences. Evasion of the anti-MSP2 antibody response by newly emerging variants during persistent infection was documented for A. marginale [49] and for A. phagocytophilum in sheep [7, 88]. However, the ovine antibody response to a particular variant may be short-lived and was not consistently associated with clearance of that variant [7]. In mice, immunization with one MSP2/p44 variant failed to prevent A. phagocytophilum infection, which can be explained by the emergence of other MSP2/p44 variants [89].
In addition to escaping antibody recognition, variation in A. marginale MSP2 generated by segmental gene conversion resulted in lack of T cell recognition of naturally occurring variants when CD4+ T cell clones from MSP2-vaccinated cattle were tested with synthetic peptides representing these variant sequences [90]. Oligoclonal CD4+ T cell lines from the MSP2-immunized cattle did respond to some of the variant peptides, indicating their presence in the MSP2 immunogen. Antigenic variation involving just one amino acid substitution has been shown to result in immune evasion by a loss of T cell recognition or induction of T cell antagonism or anergy [91–93]. The biological significance of T cell epitope variation in MSP2 during infection with regard to potential effects on antigen-specific T cell recognition have not been determined. Nevertheless, these data suggest that the generation of MSP2 and MSP3 or MSP2/p44 variants allows for escape of antibody, and potentially CD4+ T cell responses, which contribute to the ability of Anaplasma pathogens to establish long-term persistent infection.
3.2.2 CD4+ T cell suppression, exhaustion and deletion in Anaplasma infection
A. phagocytophilum infection results in a generalized immune suppression, which has been most thoroughly described in sheep [reviewed in ref. 10]. The immune suppression is characterized by severe leukopenia due to early lymphocytopenia, prolonged neutropenia and thrombocytopenia. Neutrophil phagocytosis and bacterial killing are impaired, and as discussed in section 2.1, this involves signaling pathways related to respiratory burst [94–96]. This results in increased susceptibility to concurrent bacterial infections that would otherwise be controlled by unimpaired neutrophil and lymphocyte functions.
In addition to impaired innate defenses, lymphocyte function is also dysregulated during A. phagocytophilum infection. Antibody responses to other bacterial and viral pathogens and T cell responses to mitogens were affected, which could result from severe lymphopenia [10]. Specifically, there was a reduction in the number of CD4+ T cells and changes in the ratio of CD4+ T cells to CD8+ T cells. Downregulated expression of the IL-2 receptor CD25 in ovine CD4+ T cells was also observed during infection and up to five weeks post-infection [9]. As CD25 is expressed on antigen activated CD4+ T cells as well as T regulatory cells (Treg), both of these cell types could be impaired during infection.
Antibody responses specific for A. phagocytophilum to variant MSP2/P44 and conserved MSP5 also diminished over time during infection [7]. Together, these results support suppression of both A. phagocytophilum-specific and non-specific adaptive immune responses during infection. Mechanistically, this could be due to lymphopenia or failure to generate long-lived memory T and B cells and long-lived plasma cells [87]. The isotype of antibody was not determined in this study so it is unknown whether the antibody response to MSP2/P44 and MSP5 is a T-dependent IgG response or T-independent IgM response.
In contrast to the nonspecific immune suppression observed during A. phagocytophilum infection of sheep, infection of cattle with A. marginale does not result in a generalized immunosuppression, and altered numbers of circulating leukocytes, including lymphocytes, have not been reported. However suppression of antigen-specific T cell responses does occur upon A. marginale infection [21, 97]. Immunization using A. marginale MSP2 or immunogenic portions of MSP1a has shown that infection induced a rapid downregulation of a strong pre-challenge CD4+ T cell response. In the first experiment, cattle were hyperimmunized with gel-purified native MSP2 in Th1 adjuvants and challenged six months later with organisms expressing the same major MSP2 variants that were present in the immunogen [21]. As described above, protection was not observed and anemia and rickettsemia were comparable in vaccinated and control (adjuvant only) animals. What was surprising was a dramatic loss in MSP2-specific CD4+ T cell responses occurring just prior to the peak bacteremia. This included T cell responses specific for both conserved and variable epitopes in MSP2, and was determined as a loss of both proliferation and IFN-γ secreting cells, detected by ELISPOT assay. The loss of response to epitopes conserved in all MSP2 variant proteins indicated that the loss of the MSP2-specific response was not only due to emergence of new antigenic variants. Importantly, antigen-specific responses were never detected in vaccinated animals monitored periodically for a year after infection. The long-term dysregulated response was antigen-specific, as responses to the unrelated Clostridium sp. antigen against which animals were also vaccinated remained normal. Antigen presenting cells (APC) obtained from peripheral blood of cattle at non-responding time points were fully functional when used to present antigen to autologous CD4+ T cells obtained at responding time points, ruling out dysfunctional APC as a cause for loss of responsiveness to antigen. Functional suppressor cells in the peripheral blood of non-responding animals were also not detected when mixing different ratios of non-responding and responding cells in proliferation assays. Furthermore, the frequencies of circulating CD25+ CD4+ lymphocytes, measured before and during the course of infection, did not vary significantly, and the production of Treg cytokines TGF-β and IL-10 by peripheral blood mononuclear cells in response to antigen did not increase following infection. These results suggested the sudden loss of MSP2-specific T cell responses in the peripheral blood was not simply due to an increase in Treg cells in response to infection [21]. However, spleen lymphocytes were not evaluated for functional suppressor T cells, which if present, could explain these results, as this lymphoid organ is important for removing infected erythrocytes during hemoparasite infections and spleen lymphocytes would have ample opportunity to encounter antigen [98].
The second study was designed to track antigen specific CD4+ T cells following infection using bovine MHC class II tetramers [99] in both blood and spleen [97]. Cattle were inoculated intradermally with a DNA construct encoding a T cell epitope (F2-5) and B cell epitopes (the N-terminal repeats) from the conserved MSP1a [100], boosted once with recombinant protein [101] and subsequently challenged months later with A. marginale. As observed for MSP2, the strong CD4+ T cell responses from blood measured after immunization and before challenge disappeared just prior to peak rickettsemia [97]. As before, the loss of response was specific for A. marginale, as Clostridium spp.-specific responses remained intact. Unlike the situation with A. phagocytophilum, percentages of CD4+ T cells did not change significantly over the course of infection. However, the dramatic loss of MSP1a-specific T cell proliferation and IFN-γ secreting cells was mirrored by a rapid drop in MHC class II tetramer positive T cells, using a tetramer constructed with the MSP1a F2-5 epitope and DRA/DRB3 MHC class II proteins known to present this peptide [99] and expressed by each of the experimental animals. Antigen-specific T cells were apparently not sequestered in the spleen or lymph node, as samples taken at necropsy had baseline levels of tetramer positive T cells in lymph nodes of all cattle and in spleens of three of the five animals. In the other two animals, tetramer-positive cells were two- to seven-fold higher than baseline levels. Ten-fold higher levels of tetramer positive cells were detected in liver biopsies obtained at necropsy from all cattle. However, none of the liver or spleen lymphocytes could be expanded in vitro with antigen, with or without IL-2, suggesting they were destined for apoptosis.
For both studies described above, A. marginale-specific T cell responses were also not detected in peripheral blood lymphocytes from sham immunized control animals following challenge [21, 97], in spite of high levels of bacteremia and high A. marginale specific IgG antibody titers. This suggested that dysfunctional T cell priming to A. marginale antigens, in addition to the MSP2 and MSP1a immunogens, occurs during infection, as was subsequently shown [20] and is described above in section 2.2. When naïve cattle were infected with A. marginale, either by intravenous injection of infected blood or by tick transmission, detection of T cell responses were delayed, and then appeared sporadically and lasted only one-two weeks. It is possible that these transient T cell responses result from a periodic escape from a regulatory environment, described for other persistent infections [102]. The dysfunctional CD4+ T cell response to infection is also consistent with continual down regulation or deletion of newly primed effector T cells, similar to what was observed for MSP-specific T cells induced by immunization after animals were challenged with A. marginale [21, 97]. As new antigenic variants of MSP2 or MSP3 arise during persistent infection, T cells may be continually primed to these new variants, which are then suppressed or deleted as antigen load increases. We have shown that both hypervariable as well as conserved regions of MSP2 contain CD4+ T cell epitopes [86, 90]. During persistent infection, there may also be continuous priming and expansion of CD4+ T cells to epitopes on subdominant outer membrane proteins [61].
We have hypothesized that the loss of immunization-induced CD4+ T cell responses after infection, and the failure to establish a strong memory CD4+ T cell response during A. marginale infection are the result of high antigen load, leading to T cell apoptosis [20, 21, 97]. T cell apoptosis was supported by the loss of antigen-specific T cells detected by tetramer staining, but the possibility remained that a small population of antigen-specific T cells were present but unable to proliferate or secrete IFN-γ due to anergy or downregulation via Treg cells that arise in response to infection [102]. It was, therefore, of interest to determine whether memory T cells became dysfunctional in cattle, protected from high levels of bacteremia by immunization with the outer membrane immunogen, upon infection. Cattle were immunized with outer membranes and T cell responses monitored for several months. As observed before [56, 60, 62, 63], all five cattle had strong T cell responses to the outer membrane immunogen. Following tick-transmitted challenge with homologous strain A. marginale three months after the last outer membrane immunization, all cattle lost A. marginale-specific T cell responses, but not Clostridium spp. vaccine-induced T cell responses. However, all animals did become infected, albeit with significantly lower bacteremias than controls. We then asked whether completely clearing the bacterial infection with antibiotics would enable T cell responses to once again be detected. This would indicate whether antigen-specific T cells were present and anergic/suppressed or deleted. (It should be noted that it was technically impossible to detect antigen-specific CD4+ T cells using MHC class II tetramers as the outer membrane vaccine would elicit immune responses to numerous proteins and epitopes [61] with unknown MHC class II restriction elements). Four months after challenge, when antigen-specific T cell responses were still negative, cattle were treated with an antibiotic regimen that completely cured infection [103]. The infection was shown to be completely cleared by PCR and Southern blot analysis of msp5 gene expression and confirmed by subinoculation of blood into a splenectomized calf, which failed to develop infection by 45 days. Importantly, in all animals significant T cell responses to A. marginale returned. While this is not definitive proof that the presence of infection resulted in a suppressed response, the results are consistent with this interpretation. The manuscript describing these results is in preparation [Turse, JE, Morse K, Scoles, GA, Sutten EL, and Brown WC]. Experiments are also in progress to evaluate the potential role of FoxP3+ T reg cells during acute infection of cattle on immunization-primed MSP1a and MSP2 responses.
3.2.3 T cell exhaustion and depletion during other chronic infections
During a primary immune response, naïve T cells expand many fold and differentiate into highly activated effector cells which may become polarized to different subsets (Th1, Th2, Th17, Treg, etc.) or may express multiple cytokines. Contraction of this antigen-specific T cell response occurs after infection is controlled to limit potential tissue damage from T cells that secrete inflammatory cytokines [104]. The ensuing memory cells have the potential to respond rapidly to a subsequent antigen exposure, develop into effector cells, and again contract to a relatively stable homeostatic level of memory cells. However, during chronic infection with pathogens that reach high and persistent levels, this contraction does not always result in strong memory cell development, and in fact the T cells can become rapidly exhausted or deleted [105]. The fate of antigen-specific effector T cells is determined by many factors, including the cytokines they produce [104, 106].
For example, in a murine systemic intracellular bacterial infection with Bacille Calmette-Guérin (BCG), antigen-specific CD4+ T cells rapidly expanded after infection, and then underwent homeostatic contraction to normal levels [107]. The contraction phase was due to IFN-γ-dependent T cell apoptosis, and was also dependent on NO production by macrophages. However, in this study not all effector cells were deleted, and considerable numbers remained in the spleens of wild-type mice following effector cell contraction [107]. It was subsequently shown in this model that IFN-γ acted directly on CD4+ T cells to upregulate expression of genes encoding intrinsic apoptosis machinery involved in promoting damage to the cell mitochondria [108]. It was further shown that IFN-γ induced expression of cell-extrinsic pro-apoptotic signals TRAIL and TNF-α from CD4+ T cells and NO and TNF-α from macrophages [108]. These studies point to mechanisms by which IFN-γ produced by antigen-specific CD4+ T cells in response to antigen or infection promotes T cell apoptosis to restore a normal homeostatic response.
High antigen load observed during both acute and persistent viral infections causes dysregulation in the form of T cell exhaustion leading to apoptosis of antigen-specific T cells. This has been described extensively for virus specific CD8+ T cells, and to a lesser extent for CD4+ T cells [reviewed in ref. 105, 106, 109]. During chronic infection, such as those caused by HIV and hepatitis B (HBV) and hepatitis C (HCV) viruses in humans, and lymphocytic choriomeningitis virus (LCMV) in mice, under conditions of high viral load during persistent infection, both CD4+ and CD8+ T cells become dysfunctional and lose the ability to produce IL-2 and TNF-α. They may, however, continue to produce IFN-γ. T cells that produce high levels of IFN-γ in the absence of TNF-α and IL-2 are programmed to undergo apoptosis, rather than differentiate into memory cells [106]. Similarly, influenza virus-specific CD4+ memory T cells that produced IL-2 underwent normal contraction in comparison with IL-2-deficient cells that underwent a rapidly enhanced contraction during infection, suggesting IL-2 may increase resistance to various mechanisms of cell death in responding CD4+ memory T cell populations [104]. However, it has also been shown that dysfunctional T cells from HIV infected individuals could be rescued with IL-2 [110]. In our study with A. marginale infected cattle, unresponsive T cells did not proliferate to antigen (i.e. made insufficient IL-2) and could not be stimulated to proliferate with exogenous IL-2 [97]. However, in general there were decreasing frequencies of IFN-γ secreting cells responding to A. marginale antigen ex vivo after infection of immunized cattle, which is consistent with a switch from IL-2+ IFN-γ+ cells to IL-2− IFN-γ+ cells that stop proliferating and are targeted towards apoptosis [21, 97].
Finally, it is of interest to compare the dysfunctional CD4+ T cell response observed during A. marginale infection with the CD4+ T cell response during infection with another intraerythrocytic pathogen, Plasmodium. Development of protective immunity against malaria infection in humans is difficult to achieve and it is believed that memory T cell responses are impaired [111]. Studies performed with mouse malarial parasites have addressed the failure to establish long-term CD4+ T cell memory to the intra-erythrocyte stage of the parasite. In studies with Plasmodium berghei, adoptively transferred, CD4+ T cells specific for malarial antigens were deleted upon infection, and the deletion was antigen-specific, as T cells specific for ovalbumin were not deleted. Studies using gene knockout mice showed that deletion was not dependent on either TNF or Fas, indicating upregulation of these death receptors was not involved in T cell death. However, IFN-γ was shown to be important, as mice depleted of IFN-γ with specific antibody did not undergo deletion of adoptively transferred T cells following infection [112].
In a different infection model with P. chabaudi chabaudi, CD4+ and CD8+ T cells were shown to undergo apoptosis in the spleen during high levels of parasitema, which was associated with increased expression of death receptors Fas and FasL [113, 114]. More recent studies tracked mice for 200 days following untreated (high parasite load) or drug-treated (low parasite load) infection with P. chabaudi AS strain [115]. It was concluded that although T cell apoptosis was observed during acute infection in association with high antigen load, this did not impair the generation of P. chabaudi-specific memory T cells, which were detected by 20 days. Rather, there was a progressive decline in the memory T cell response over time, leading to impaired immunity.
T cell exhaustion has been attributed to upregulated expression of inhibitory molecules on the T cell surface that results in inhibition of cytokine production. The potential roles of TNF and Fas receptors were mentioned above in malaria infection models. Other death receptors have been implicated in viral infections. One is programmed death-1 (PD-1), is a member of the B7 family, which binds to its ligands, PD-L1 and PD-L2, and results in inhibition of T cells and IL-2 expression [116]. Upregulated T cell PD-1 plays a role in T cell exhaustion in HIV and HCV infection [117–119]. Another recently described inhibitory molecule is the Tim-3 receptor, a T cell Ig mucin family of proteins with galectin-9 as its ligand [120]. Tim-3 was significantly upregulated on CD4+ and CD8+ T cells from HIV-infected individuals and Tim-3 positive CD8+ T cells were shown to be functionally exhausted [121]. Tim-3 was also upregulated during HCV and HBV infections [122, 123]. The mechanisms leading to dysfunction and deletion of CD4+ T cells during A. marginale infection have not been determined, but experiments should be performed to investigate a potential role of lymphocyte surface inhibitory molecules such as Fas, PD-1, and TIM-3.
3.3 Conclusions
Intracellular A. marginale and A. phagocytophilum pose unique problems for vaccine development. In spite of very different intracellular niches, both bacteria achieve persistent infection, which is critical for tick transmission to susceptible individuals. These bacteria have evolved unique mechanisms to persist, including loss of LPS and peptidoglycan that activate innate immune defense mechanisms, manipulation of the host target neutrophil designed to destroy the pathogen or establishment of infection in a rather benign erythrocyte, suppression of innate and adaptive immune responses to favor pathogen survival, extensive antigenic variation in immunodominant surface proteins MSP2 (and MSP3 for A. marginale) to permit evasion the adaptive immune response, and secretion of virulence factors through the type IV secretion system. In humans and mice, A. phagocytophilum infection induces a mild immune suppression, is generally not persistent, and control is mediated in a CD4+ T cell-dependent mechanism. IFN-γ both aids in pathogen clearance and induces host pathology. In sheep, A. phagocytophilum infection induces a more severe generalized immune suppression attributed to lymphocytopenia, neutropenia and thrombocytopenia, leading to secondary infections. On the other hand, A. marginale infection causes deletion and/or anergy of a previously elicited A. marginale MSP-specific T cell response. In naïve animals infection induces a delayed and transient effector CD4+ T cell response leading to a poor memory response in spite of high titers of A. marginale-specific IgG (directed mainly at MSP2), which fails to clear infection. If a goal is to develop vaccines that prevent infection, understanding the mechanisms of immune regulation by these pathogens is critical to develop ways to effectively prime T cells to that will be stimulated upon infection to expand rapidly into an effector population capable of controlling infection.
Acknowledgements
I would like to thank Kelly Brayton for providing the phylogenetic tree.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Micro. 2010;8:328–339. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- 2.Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 3.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1824–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein MB, Miller JS, Nelson CM, Goodman JL. Primary bone marrow progenitors of both granulocytic and monocytic lineases are susceptible to infection with the agent of human granulocytic ehrlichiosis. J Infect Dis. 1997;176:1405–1409. doi: 10.1086/517332. [DOI] [PubMed] [Google Scholar]
- 6.Herron MJ, Ericson ME, Kurtti TJ, Munderloh UG. The interactions of Anaplasma phagocytophilum, endothelial cells, and human neutrophils. Ann NY Acad Sci. 2005;1063:374–382. doi: 10.1196/annals.1355.090. [DOI] [PubMed] [Google Scholar]
- 7.Granquist EG, Stuen S, Crosy L, Lundgren AM, Alleman AR, Barbet AF. Variant-specific and diminishing immune responses towards the highly variable MSP2(P44) outer membrane protein of Anaplasma phagocytophilum during persistent infection in lambs. Vet Immunol Immunopathol. 2010;133:117–124. doi: 10.1016/j.vetimm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scorpio DG, Leutenegger C, Berger J, Barat N, Madigan JE, Dumler JS. Sequential analysis of Anaplasma phagocytophilum msp2 transcription in murine and equine models of human granulocytic anaplasmosis. Clin Vaccine Immunol. 2008;15:418–424. doi: 10.1128/CVI.00417-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whist SK, Storset AK, Johansen GM, Larsen HJS. Modulation of leukocyte populations and immune responses in sheep experimentally infected with Anaplasma (formerly Ehrlichia) phagocytophilum. Vet Immunol Immunopathol. 2003;94:163–175. doi: 10.1016/s0165-2427(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 10.Woldehiwet Z. Immune evasion and immunosuppression by Anaplasma phagocytophilum, the causative agent of tick-borne fever of ruminants and human granulocytic anaplasmosis. The Vet Journal. 2008;175:37–44. doi: 10.1016/j.tvjl.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Stuen S, Longbottom D. Treatment and control of chlamydial and rickettsial infections in sheep and goats. Vet Clin North Am Food Anim Pract. 2011;27:213–233. doi: 10.1016/j.cvfa.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Theiler A. Report of the Government Veterinary Bacteriologist, 1908–1909. South Africa: Transvaal; 1910. Anaplasma marginale (gen. spec. nov.) The marginal points in the blood of cattle suffering from a specific disease; pp. 7–64. [Google Scholar]
- 13.Aubry P, Geale DW. A review of bovine anaplosmsis. Transboundary and Emerging Diseases. 2010;58:1–30. doi: 10.1111/j.1865-1682.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 14.Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing SA. The natural history of Anaplasma marginale. Vet Parasitol. 2010;167:95–107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Munderloh UG, Lynch MJ, Herron MJ, Palmer AT, Kurtti TJ, Nelson RD, Goodman JL. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet Microbiol. 2004;101:53–64. doi: 10.1016/j.vetmic.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Carreño AD, Alleman AR, Barbet AF, Palmer GH, Noh SM, Johnson CM. In vivo endothelial cell infection by Anaplasma marginale. Vet Pathol. 2007;44:116–118. doi: 10.1354/vp.44-1-116. [DOI] [PubMed] [Google Scholar]
- 17.Wamsley JL, Alleman AR, Johnson CM, Barbet AF, Abbott JR. Investigation of endothelial cells as an in vivo nidus of Anaplasma marginale infection in cattle. Vet Microbiol. 2011;153:264–273. doi: 10.1016/j.vetmic.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Futse JE, Ueti MW, Knowles DP, Palmer GH. Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction, J Clin Microbiol. 2003;41:3829–3834. doi: 10.1128/JCM.41.8.3829-3834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer GH, Bankhead T, Lukehart SA. 'Nothing is permanent but change'-antigenic variation in persistent bacterial pathogens. Cell Microbiol. 2009;11:1697–1705. doi: 10.1111/j.1462-5822.2009.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Norimine J, Brayton KA, Palmer GH, Scoles GA, Brown WC. Anaplasma marginale infection with persistent high-load bacteremia induces a dysfunctional memory CD4+ T lymphocyte response but sustained high IgG titers. Clin Vaccine Immunol. 2010;17:1881–1890. doi: 10.1128/CVI.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott JR, Palmer GH, Kegerreis KA, Hetrick P, Howard CJ, Hope JC, Brown WC. Rapid and long-term disappearance of CD4+ T-lymphocyte responses specific for Anaplasma marginale major surface protein-2 (MSP2) in MSP2 vaccinates following challenge with live A. marginale. J Immunol. 2005;174:6702–6715. doi: 10.4049/jimmunol.174.11.6702. [DOI] [PubMed] [Google Scholar]
- 22.Dumler JS, Bakken JS. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 23.IJdo JW, Zhang Y, Hodzic E, Magnarelli LA, Wilson ML, Telford SR, 3rd, Barthold SW, Fikrig E. The early humoral response in human granulocytic ehrlichiosis. J Infect Dis. 1997;176:687–692. doi: 10.1086/514091. [DOI] [PubMed] [Google Scholar]
- 24.Artursson K, Gunnarsson A, Wikström UB, Engvall EO. A serological and clinical follow-up in horses with confirmed equine granulocytic ehrlichiosis. Equine Vet J. 1999;31:473–477. doi: 10.1111/j.2042-3306.1999.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 25.Dumler JS, Trigiani ER, Bakken JS, Aguero-Rosenfeld ME, Wormser GP. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin Diag Lab Immunol. 2000;7:6–8. doi: 10.1128/cdli.7.1.6-8.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HY, Mott J, Zhi N, Tajima T, Rikihisa Y. Cytokine gene expression by peripheral blood leukocytes in horses experimentally infected with Anaplasma phagocytophila. Clin Diagn Lab Immunol. 2002;9:1079–1084. doi: 10.1128/CDLI.9.5.1079-1084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster KA, Mitchell GB. Use of counter immunoelectrohphoresis in detection of antibodies to tickborne fever. Res Vet Sci. 1988;45:28–30. [PubMed] [Google Scholar]
- 28.Paxton EA, Scott GR. Detection of antibodies to the agent of tick-borne fever by indirect immunofluorescence. Vet Microbiol. 1989;21:133–138. doi: 10.1016/0378-1135(89)90025-4. [DOI] [PubMed] [Google Scholar]
- 29.Martin ME, Bunnell JE, Dumler JS. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J Infect Dis. 2000;181:374–378. doi: 10.1086/315206. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, Ijdo JW, Telford SR, 3rd, Hodzic E, Zhang Y, Barthold SW, Fikrig E. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J Clin Invest. 1997;100:3014–3018. doi: 10.1172/JCI119855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akkoyunlu M, Fikrig E. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect Immun. 2000;68:1827–1833. doi: 10.1128/iai.68.4.1827-1833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerjee R, Anguita J, Fikrig E. Granulocytic ehrlichiosis in mice deficient in phagocyte oxidase or inducible nitric oxide synthase. Infect Immun. 2000;68:4361–4362. doi: 10.1128/iai.68.7.4361-4362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin M, Caspersen K, Dumler JS. Immunopathology and ehrlichial propagation are regulated by interferon-γ and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am J Pathol. 2001;158:1881–1888. doi: 10.1016/s0002-9440(10)64145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telford SR, III, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunnell JE, Trigani ER, Srinivas SR, Dumler JS. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J Infect Dis. 1999;180:546–550. doi: 10.1086/314902. [DOI] [PubMed] [Google Scholar]
- 36.von Loewenich FD, Scorpio DG, Reischl U, Dumler JS, Bogdan C. Control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR)2 and TLR4, or the TLR adaptor molecule MyD88. Eur J Immunol. 2004;34:1789–1797. doi: 10.1002/eji.200425029. [DOI] [PubMed] [Google Scholar]
- 37.Choi K-S, Webb T, Oelke M, Scorpio DG, Dumler JS. Differential innate immune cell activation and proinflammatory response in Anaplasma phagocytophilum infection. Infect Immun. 2007;75:3124–3130. doi: 10.1128/IAI.00098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkner K, Steiner B, Rinkler C, Kern Y, Aichele P, Bogdan C, von Loewenich FD. The elimination of Anaplasma phagocytophilum requires CD4+ T cells, but is independent of Th1 cytokines and a wide spectrum of effector mechanisms. Eur J Immunol. 2008;38:3395–3410. doi: 10.1002/eji.200838615. [DOI] [PubMed] [Google Scholar]
- 39.Scorpio DG, von Loewenich FD, Göbel H, Bogdan C, Dumler JS. Innate immune responses to Anaplasma phagocytophilum contributes to hepatic injury. Clin Vaccine Immunol. 2006;13:806–809. doi: 10.1128/CVI.00092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer GH, Rurangirwa FR, Kocan KM, Brown WC. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 41.Estes DM, Closser NM, Allen GK. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-µ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 42.Brown WC, McElwain TF, Palmer GH, Chantler SE, Estes DM. Bovine CD4+ T-lymphocyte clones specific for rhoptry associated protein-1 (RAP-1) of Babesia bigemina stimulate enhanced immunoglobulin G1 (IgG1) and IgG2 synthesis. Infect Immun. 1999;67:155–164. doi: 10.1128/iai.67.1.155-164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer GH, Eid G, Barbet AF, McGuire TC, McElwain TF. The immunoprotective Anaplasma marginale major surface protein-2 (MSP-2) is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tebele N, McGuire TC, Palmer GH. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez JE, Siems WF, Brayton KA, Palmer GH, McGuire TC, Brown WC. Identification of novel antigenic proteins in a complex Anaplasma marginale outer membrane immunogen by mass spectrometry and genomic mapping. Infect Immun. 2005;73:8109–8118. doi: 10.1128/IAI.73.12.8109-8118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer GH, McGuire TC. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984;133:1010–1015. [PubMed] [Google Scholar]
- 47.Palmer GH, McElwain TF. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–253. doi: 10.1016/0304-4017(94)03123-e. [DOI] [PubMed] [Google Scholar]
- 48.Eid G, French DM, Lundgren AM, Barbet AF, McElwain TF, Palmer GH. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.French D, Brown WC, Palmer GH. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gale KR, Leatch G, Gartside M, Dimmock CM. Anaplasma marginale: failure of sera from immune cattle to confer protection in passive-transfer experiments. Parasitol Res. 1992;78:410–415. doi: 10.1007/BF00931697. [DOI] [PubMed] [Google Scholar]
- 51.Buening GM. Cell-mediated immune response in anaplasmosis as measured by a micro cell-mediated cytotoxicity assay and leukocyte migration inhibition test. Am J Vet Res. 1976;34:1215–1218. [PubMed] [Google Scholar]
- 52.Carson CA, Sells DM, Ristic M. Cell mediated immunity in bovine anaplasmosis and correlation with protection induced by vaccination. Vet Parasitol. 1976;2:75–81. [Google Scholar]
- 53.Wyatt CR, Davis WC, Knowles DP, Goff WL, Palmer GH, McGuire TC. Effect of intraerythrocyte Anaplasma marginale of soluble factors from infected calf blood mononuclear cells. Infect Immun. 1996;64:4846–4849. doi: 10.1128/iai.64.11.4846-4849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gale KR, Leatch G, Dimmock CM, Wood PR. Anaplasma marginale: effect of the treatment of cattle with an interferon γ-neutralizing monoclonal antibody or the nitric oxide synthetase inhibitor aminoguanidine on the course of infection. Parasite Immunol. 1997;19:411–417. doi: 10.1046/j.1365-3024.1997.d01-237.x. [DOI] [PubMed] [Google Scholar]
- 55.Gale KR, Gartside MG, Dimmock CM, Zakrzewski H, Leatch G. Peripheral blood lymphocyte proliferative responses in cattle infected or vaccinated with Anaplasma marginale. Parasitol Res. 1996;82:551–562. doi: 10.1007/s004360050161. [DOI] [PubMed] [Google Scholar]
- 56.Brown WC, Shkap V, Zhu D, McGuire TC, Tuo W, McElwain TF, Palmer GH. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect Immun. 1998;66:5406–5413. doi: 10.1128/iai.66.11.5406-5413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer GH, Munodzana D, Tebele N, Ushe T, McElwain TF. Heterologous strain challenge of cattle immunized with Anaplasma marginale outer membranes. Vet Immunol Immunopathol. 1994;42:265–273. doi: 10.1016/0165-2427(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 58.Noh SM, Zhuang Y, Futse JE, Brown WC, Brayton KA, Palmer GH. The immunization-induced response to the Anaplasma marginale major surface protein 2 and its association with protective immunity. Vaccine. 2010;28:3471–3477. doi: 10.1016/j.vaccine.2010.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGuire TC, Musoke AJ, Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils, and skin. Immunology. 1979;38:249–256. [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez JE, Palmer GH, Brayton KA, Dark MJ, Leach SE, Brown WC. Immunogenicity of Anaplasma marginale type IV secretion system proteins in a protective outer membrane vaccine. Infect Immun. 2007;75:2333–2342. doi: 10.1128/IAI.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez JE, Beare PA, Heinzen RA, Norimine J, Lahmers KK, Palmer GH, Brown WC. High-throughput identification of T-lymphocyte antigens from Anaplasma marginale expressed using in vitro transcription and translation. J Immunol Methods. 2008;332:129–141. doi: 10.1016/j.jim.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Sutten EL, Norimine J, Beare PA, Heinzen RA, Lopez JE, Morse K, Brayton KA, Gillespie JJ, Brown WC. Anaplasma marginale type IV secretion system proteins VirB2, VirB7, VirB11, and VirD4 are immunogenic components of a protective bacterial membrane vaccine. Infect Immun. 2010;78:1314–1325. doi: 10.1128/IAI.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morse K, Norimine J, Palmer GH, Sutten EL, Baszler TV, Brown WC. Association and evidence for linked recognition of type IV secretion system proteins VirB9-1, VirB9-2, and VirB10 in Anaplasma marginale. Infect Immun. 80(1) doi: 10.1128/IAI.05798-11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noh SM, Brown WC. Adaptive immune responses to infection and opportunities for vaccine development (Anaplasmataceae) In: Palmer GH, Azad AF, editors. Intracellular Pathogens: Rickettsiales. Washington D.C.: ASM Press; in press. [Google Scholar]
- 65.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brayton KA, Kappmeyer LS, Herndon DR, Dark MJ, Tibbals DL, Palmer GH, McGuire TC, Knowles DP., Jr Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc Natl Acad Sci USA. 2005;102:844–849. doi: 10.1073/pnas.0406656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ulevitch RJ, Mathison JC, da Silva Correia J. Innate immune responses during infection. Vaccine. 2004;22(Suppl 1):S25–S30. doi: 10.1016/j.vaccine.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 68.Sorbara MT, Philpott DJ. Peptidoglycan: a critical activator of the mammalian immune system during infection and homeostasis. Immunol Rev. 2011;243:40–60. doi: 10.1111/j.1600-065X.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 69.Carlyon JA, Fikrig E. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr Opin Hematol. 2006;13:28–33. doi: 10.1097/01.moh.0000190109.00532.56. [DOI] [PubMed] [Google Scholar]
- 70.Rikihisa Y. Molecular events in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet Parasitol. 2010;167:155–166. doi: 10.1016/j.vetpar.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillespie JJ, Brayton KA, Williams KP, Quevo Diaz MA, Brown WC, Azad AF, Sobral BW. Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infect Immun. 2010;78:1809–1823. doi: 10.1128/IAI.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rikihisa Y. Type IV secretion in the obligatory intracellular bacterium Anaplasma phagocytophilum. Cell Microbiol. 2010;12:1213–1221. doi: 10.1111/j.1462-5822.2010.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 75.Niu H, Kozjak-Pavlovic V, Rudel T, Rikihisa Y. Anaplasma phagocytophilum Ats-1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000774. e1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noh S, Brayton KA, Brown WC, Norimine J, Munske GR, Davitt CM, Palmer GH. Composition of the surface proteome of Anaplasma marginale and its role in protective immunity induced by outer membrane immunization. Infect Immun. 2008;76:2219–2226. doi: 10.1128/IAI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lockwood S, Voth DE, Brayton KA, Beare PA, Brown WC, Heinzen RA, Broschat SL. Identification of Anaplasma marginale Type IV secretion system effector proteins. PLoS ONE. doi: 10.1371/journal.pone.0027724. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbet AF, Lundgren A, Yi J, Rurangirwa FR, Palmer GH. Antigenic variation of the ehrlichia Anaplasma marginale by expression of MSP2 sequence mosaics. Infect Immun. 2000;68:6133–6138. doi: 10.1128/iai.68.11.6133-6138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbet AF, Yi J, Lundgren A, McEwen BR, Blouin EF, Kocan KM. Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle. Infect Immun. 2001;69:3057–3066. doi: 10.1128/IAI.69.5.3057-3066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brayton KA, Palmer GH, Lundgren A, Yi J, Barbet AF. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol Microbiol. 2002;43:1151–1159. doi: 10.1046/j.1365-2958.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- 81.Brayton KA, Meeus PF, Barbet AF, Palmer GH. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect Immun. 2003;71:6627–6632. doi: 10.1128/IAI.71.11.6627-6632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meeus PF, Brayton KA, Palmer GH, Barbet AF. Conservation of a gene conversion mechanism in two distantly related paralogues of Anaplasma marginale. Mol Microbiol. 2003;47:633–643. doi: 10.1046/j.1365-2958.2003.03331.x. [DOI] [PubMed] [Google Scholar]
- 83.Barbet AF, Meeus PF, Belanger M, Bowie MV, Yi J, Lundgren AM, Alleman AR, Wong SJ, Chu FK, Munderloh UG, Jauron SD. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect Immun. 2003;71:1706–1718. doi: 10.1128/IAI.71.4.1706-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhi N, Ohashi N, Rikihisa Y, Horowitz HW, Wormser GP, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy CI, Storey JR, Recchia J, Doros-Richert LA, Gingrich-Baker C, Munroe K, Bakken JS, Coughlin RT, Beltz GA. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect Immun. 1998;66:3711–3718. doi: 10.1128/iai.66.8.3711-3718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott JR, Palmer GH, Howard CJ, Hope JC, Brown WC. Anaplasma marginale major surface protein 2 CD4+ T cell epitopes are evenly distributed in conserved and hypervariable regions (HVR), whereas linear B cell epitopes are predominantly located in the HVR. Infect Immun. 2004;72:1096–1106. doi: 10.1128/IAI.72.12.7360-7366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhuang Y, Futse JE, Brown WC, Brayton KA, Palmer GH. Maintenance of antibody to pathogen epitopes generated by segmental gene conversion is highly dynamic during long-term persistent infection. Infect Immun. 2007;75:5185–5190. doi: 10.1128/IAI.00913-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Granquist EG, Stuen S, Lundgren AM, Braten M, Barbet AF. Outer membrane protein sequence variation in lambs experimentally infected with Anaplasma phagocytophilum. Infect Immun. 2008;76:120–126. doi: 10.1128/IAI.01206-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.IJdo JW, Wu C, Telford SR, III, Fikrig E. Differential expression of the p44 gene family in the agent of human granulocytic ehrlichiosis. Infect Immun. 2002;70:5295–5298. doi: 10.1128/IAI.70.9.5295-5298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown WC, Brayton KA, Styer CM, Palmer GH. The hypervariable region of Anaplasma marginale major surface protein 2 (MSP2) contains multiple immunodominant CD4+ T lymphocyte epitopes that elicit variant-specific proliferative and IFN-γ responses in MSP2 vaccinates. J Immunol. 2003;170:3790–3798. doi: 10.4049/jimmunol.170.7.3790. [DOI] [PubMed] [Google Scholar]
- 91.Sloan-Lancanster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Ann Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 92.Plebanski M, Lee EA, Hill AV. Immune evasion in malaria: altered peptide ligands of the circumsporozoite protein. Parasitology. 1997;115:S55–S66. doi: 10.1017/s0031182097002035. [DOI] [PubMed] [Google Scholar]
- 93.McMichael A. T cell responses and viral escape. Cell. 1998;93:673–676. doi: 10.1016/s0092-8674(00)81428-2. [DOI] [PubMed] [Google Scholar]
- 94.Carlyon JA, Chan WT, Galán J, Roos D, Fikrig E. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J Immunol. 2002;169:7009–7018. doi: 10.4049/jimmunol.169.12.7009. [DOI] [PubMed] [Google Scholar]
- 95.Whist SK, Storset AK, Larsen HJS. Functions of neutrophils in sheep experimentally infected with Ehrlichia phagocytophila. Vet Immunol Immunopathol. 2002;86:183–193. doi: 10.1016/s0165-2427(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 96.Borjesson DL, Kobayashi SD, Whitney AR, Voich JM, Agrue CM, DeLeo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174:6364–6372. doi: 10.4049/jimmunol.174.10.6364. [DOI] [PubMed] [Google Scholar]
- 97.Han S, Norimine J, Palmer GH, Mwangi W, Lahmers KK, Brown WC. Rapid deletion of antigen-specific CD4+ T cells following infection represents a strategy of immune evasion and persistence for Anaplasma marginale. J Immunol. 2008;181:7759–7769. doi: 10.4049/jimmunol.181.11.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 99.Norimine J, Han S, Brown WC. Quantitation of Anaplasma marginale major surface protein (MSP)1a and MSP2 epitope-specific CD4+ T lymphocytes using bovine DRB3*1101 and DRB3*1201 tetramers. Immunogenetics. 2006;58:726–739. doi: 10.1007/s00251-006-0140-3. [DOI] [PubMed] [Google Scholar]
- 100.Brown WC, McGuire TC, Mwangi W, Kegerreis KA, Macmillan H, Lewin HA, Palmer GH. Major histocompatibility complex class II DR-restricted memory CD4+ T lymphocytes recognize conserved immunodominant epitopes of Anaplasma marginale major surface protein 1a. Infect Immun. 2002;70:5521–5532. doi: 10.1128/IAI.70.10.5521-5532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mwangi W, Brown WC, Splitter GA, Davies CJ, Howard CJ, Hope JC, Aida Y, Zhuang Y, Hunter B, Palmer GH. A DNA vaccine construct incorporating intercellular trafficking and intracellular targeting motifs effectively primes and induces memory B and T cell responses in outbred animals. Clin Vacc Immunol. 2007;14:304–311. doi: 10.1128/CVI.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 103.Reinbold JB, Coetzee JF, Hollis LC, Nickell JS, Reigel C, Olson KC, Ganta RR. The efficacy of three chlortetracycline regimens in the treatment of persistent Anaplasma marginale infection. Vet Microbiol. 2010;145:69–75. doi: 10.1016/j.vetmic.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T cell contraction during pathogen challenge. Immunol Rev. 2010;236:110–124. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 106.Seder RA, Darrah PA, Roederer M. T cell quality in memory and protection: implications for vaccine development. Nature Rev Microbiol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 107.Dalton DK, Haynes L, Chu C-Q, Swain SL, Wittmer S. Interferon γ eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, McKinstry KK, Swain SL, Dalton DK. IFN-γ acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptosis signals. J Immunol. 2007;179:939–949. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1902–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev. 2004;201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 112.Xu H, Wipsa J, Yan H, Zeng M, Makobongo MO, Finkelman FD, Kelso A, Good MF. The mechanism and significance of deletion of parasite-specific CD4+ T cells in malaria infection. J Exp Med. 2002;195:881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Helmby HG, Jonsson G, Troye-Blomberg M. Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect Immun. 2000;68:1485–1490. doi: 10.1128/iai.68.3.1485-1490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez-Torres L, Rodriguez-Ropon A, Aguilar-Medina M, Favila-Castillo L. Mouse splenic CD4+ and CD8+ T cells undergo extensive apoptosis during a Plasmodium chabaudi chabaudi AS infection. Parasite Immunol. 2001;23:617–626. doi: 10.1046/j.1365-3024.2001.00422.x. [DOI] [PubMed] [Google Scholar]
- 115.Freitas do Rosário AP, Muxel SM, Rodríguez-Málaga SM, Sardinha LR, Zago CA, Castillo-Méndez SI, Alvarez JM, D'Império Lima MR. 2008 Gradual decline in malaria-specific memory T cell responses leads to failure to maintain long-term protective immunity to Plasmodium chabaudi AS despite persistence of B cell memory and circulating antibody. J Immunol. 2008;181:8344–8355. doi: 10.4049/jimmunol.181.12.8344. [DOI] [PubMed] [Google Scholar]
- 116.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honio T, Freeman GJ, Careno BM. PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 117.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 118.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 120.Hafler DA, Kuchroo V. TIMs: central regulators of immune responses. J Exp Med. 2008;205:2699–2701. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones RB, Ndhlovu LC, Barbour J, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, Luan F, Shi W, Zhy FL, Sun WS, Zhang LN, Gao CJ, Gao LF, Liang XH, Ma CH. Blockade of Tim-3 pathway ameliorates interferon-γ production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell Mol Immunol. 2009;6:35–43. doi: 10.1038/cmi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]