Abstract
Over the past fifty years a significant body of evidence has been compiled suggesting an interaction between the endocannabinoid (EC) system and alcohol dependence. However, much of this work has been conducted only in the past two decades following the elucidation of the molecular constituents of the EC system that began with the serendipitous discovery of the cannabinoid 1 receptor (CB1). Since then, novel pharmacological and genetic tools have enabled researchers to manipulate select components of the EC system, to determine their contribution to the motivation to consume ethanol. From these preclinical studies, it is evident that CB1 contributes the motivational and reinforcing properties of ethanol, and chronic consumption of ethanol alters EC transmitter levels and CB1 expression in brain nuclei associated with addiction pathways. These results are augmented by in vitro and ex vivo studies showing that acute and chronic treatment with ethanol produces physiologically relevant alterations in the function of the EC system. This report provides a current and comprehensive review of the literature regarding the interactions between ethanol and the EC system. We begin be reviewing the studies published prior to the discovery of the EC system that compared the behavioral and physiological effects of cannabinoids with ethanol in addition to cross-tolerance between these drugs. Next, a brief overview of the molecular constituents of the EC system is provided as context for the subsequent review of more recent studies examining the interaction of ethanol with the EC system. These results are compiled into a summary providing a scheme for the known changes to the components of the EC system in different stages of alcohol dependence. Finally, future directions for research are discussed.
Keywords: ethanol, endocannabinoid, alcohol dependence, CB1, anandamide, 2-arachidonyl glycerol
Introduction
Alcohol use disorders (AUDs) affect an estimated 8.5% of the US population over the age of 18, and problems associated with AUDs cost the United States economy up to $185 billion per year (NIAAA, 2009). This enormous economic and public health problem is due to the addictive behaviors of alcohol abusing and dependent individuals. These behaviors are driven by two hallmark features of addiction: an overriding compulsion to seek and use alcohol and an inability to control or inhibit these actions even though they result in a negative outcome (e.g. loss of job, imprisonment, family problems, etc). These two features of addiction are thought to emerge from aberrant learning processes and plasticity in the striatum and frontal cortices (For review see Kalivas, 2008; Koob and Volkow, 2010) a subset of which are general mechanisms that participate in driving the addictive phenotype for all drugs of abuse. Historically, dopamine (DA) has been seen as a key neuromodulator driving reward processes, but an increasing amount of attention has been focused on other neuromodulatory and neurendocrine systems that may serve as more tenable sites for pharmacotherapy. Along these lines, it is often instructive to examine disorders that are frequently comorbid in the hopes of understanding a common underlying etiology, and in this regard, AUDs and cannabis use disorders (CUDs) share some similarity.
Since the early 1970’s, a growing body of evidence has suggested a link between the neuropsychological effects of cannabis and ethanol consumption. Specifically, studies of the affective, cognitive, and psychomotor effects of these two substances in humans found evidence of a potential cross-tolerance to ethanol amongst cannabis users (Jones and Stone, 1970; MacAvoy and Marks, 1975). More recently, epidemiological studies of US populations indicate that for individuals with a CUD there is 45 – 81% lifetime prevalence of developing a comorbid AUD (Agosti et al., 2002; Regier et al., 1990; Stinson et al., 2006). In a clinical study of adolescents with an AUD, over 70% reported use of cannabis within the past year, and the mean frequency of cannabis use for this group was between 16 and 20 days per month (Martin et al., 1996). In addition, data from a national computerized telephone survey show that individuals who responded as using marijuana and ethanol simultaneously engaged in significantly more binge drinking and were more likely to have alcohol dependence (Midanik et al., 2007). Together, these findings demonstrate a high prevalence of comorbidity between AUDs and CUDs.
One possible explanation for these findings is that these drugs may serve as a substitute for one another. Ethanol and cannabis both have depressant effects on central nervous system function. In laboratory rodents, administration of either ethanol or Δ9-tetrahydrocannabinol (THC; the main psychoactive compound in cannabis) produces hypolocomotion, hypothermia, and ataxia (Crabbe et al., 2005; Martin et al., 1991). In human subjects acute administration of either compound produces euphoria and feelings of intoxication (Heishman et al., 1997; Jones and Stone, 1970), and both substances decrease response time and accuracy on neuropsychological tests measuring memory, attention, and psychomotor performance (Chait and Perry, 1994; Heishman et al., 1997; Heishman et al., 1988). In addition, daily cannabis users significantly increased self-reported ethanol craving and consumption during a two-week abstinence from marijuana (Peters and Hughes, 2010) and individuals in treatment for CUDs increased the frequency of ethanol use during 12 months following treatment (Stephens et al., 1994). However, there are conflicting data with respect to combined ethanol and cannabis use. Findings from other studies of post-treatment outcomes for CUDs show a decrease (Stephens et al., 2000) or no change (Kadden et al., 2009) in ethanol consumption associated with decreased use of cannabis.
It is possible that shared physiological and biochemical mechanisms contribute to the comorbid abuse and substitution of ethanol and cannabis, but at first glance, these two substances seem remarkably different. On one hand, ethanol is a remarkably low affinity ligand for the many enzymes and receptors with which it interacts. Ethanol’s low affinity is evidenced by the fact that it must be consumed in quantities sufficient to produce blood concentrations in the millimolar range before substantial behavioral effects can be observed. In contrast, THC produces the majority of its effects through high-affinity interactions with a small number of molecular targets. Almost all of the centrally mediated, behavioral effects of THC intoxication result from activation of the cannabinoid 1 (CB1) receptor. CB1 is a G-protein coupled receptor (GPCR) and is the main receptor for the endogenous cannabinoid (endocannabinoid; EC) system in the brain. ECs are a class of lipid-derived neuromodulators that serve as retrograde transmitters in central synapses. Research within the past decade has made it clear that ethanol interacts with the EC system, and a growing body of evidence suggests that the function of the EC system may be altered in alcohol dependence. In this review, we will begin by briefly discussing the early studies that demonstrated a link between the physiological and behavioral effects of cannabis and ethanol. Next we will cover the findings indicating that the EC system is a molecular target for ethanol and how the function of this neuromodulatory system is altered following chronic ethanol. Along these lines, we will attempt to place the behavioral ramifications of altered EC signaling in the context of circuitry associated with addictive processes. Finally, emerging topics in the fields of EC signaling and alcohol abuse will be discussed as future directions for research.
Historical Perspectives
Comparative Studies on the Effects of Cannabis and Ethanol
The first study to directly compare the effects of ethanol and marijuana was conducted by Jones and Stone (1970). Participants who claimed a history of heavy marijuana use were administered cannabis orally or in a cigarette, and in separate sessions, they were given ethanol. The authors reported cannabis significantly increased pulse, time estimation, and low-voltage, high-frequency EEG activity. Ethanol, on the other hand, was observed to only decrease subjective time estimation. The opposing effects of ethanol and cannabis on the subjective experience of time have been demonstrated elsewhere (Tinklenberg et al., 1976), but other studies have reported that acute ethanol intoxication has either no effect on time estimation (Heishman et al., 1997) or produces a lower magnitude overestimation similar to that of cannabis (Bech et al., 1973). Importantly, Jones and Stone (1970) reported that participants who claimed a history of heavy marijuana use were less intoxicated by ethanol and showed fewer ethanol-induced neuropsychological impairments than were found in previous studies. Although, this study did not include non-cannabis using control subjects, these results led the authors to suggest that prior heavy marijuana use may convey cross-tolerance to some of the acute effects of ethanol.
As reviewed by Zarcone (1973), ethanol and cannabis are also known to produce similar effects on sleep. Acute ethanol and THC administration reduces REM sleep early in the evening with a compensatory rebound occurring later in the night. These effects are probably due to acute tolerance to drug effects and/or elimination in the case of ethanol over the course of the evening. However, the depressive effects on REM sleep are attenuated with repeated administration of either marijuana or ethanol. The synergistic effects of acute ethanol and cannabinoids on sleep have also been observed in rodents where co-administration of ethanol with THC increases sleep time beyond that observed with either drug alone (Friedman and Gershon, 1974; Phillips et al., 1971). The effects of chronic ethanol and cannabis use differ, however. Chronic alcoholic patients experience a significant reduction or even an absence of deep-stage slow-wave sleep and an increase in REM following cessation of ethanol use, while chronic cannabis users show an increase in time spent in REM sleep. In the case of alcoholic patients, the alterations in sleep architecture seem to be more severe than in chronic cannabis users, and they can persist for many months into abstinence. In either case, resumption of drug use acts to normalize sleep patterns.
In addition to their subjective and physiological effects, cannabis and ethanol are also known to affect cognitive function. In a double-blinded placebo controlled study, Manno et al (1971) found that ethanol and marijuana interact synergistically to impair cognitive and psychomotor functions when co-administered. Studies of divided attention in human subjects have demonstrated that acute administration of marijuana and ethanol alone or in combination is sufficient to increase false-positive and false-negative errors in a task that requires simultaneous monitoring of two distinct stimuli (MacAvoy and Marks, 1975; Marks and MacAvoy, 1989). Interestingly, both of these studies reported a cross-tolerance to the effects of acute ethanol on signal sensitivity in a group of cannabis-users when compared to non-users. In addition to attentive processes, acute ethanol and marijuana impaired measures of accuracy and speed on the digit-symbol substitution task as well as word recall, but only acute ethanol impaired performance on a number recognition task (Heishman et al., 1997; Heishman et al., 1988). With regard to the cognitive effects of chronic ethanol and cannabis exposure, one study demonstrated significantly impaired learning in a Hebb-Williams maze and a punished moving belt test for rats that were chronically treated with either 6 g/kg ethanol or 10mg/kg THC for six months and allowed to recover for one month before training (Fehr et al., 1976). These studies suggest that although ethanol and cannabis have distinct subjective effects, these two drugs produce similar deficits in cognitive function after both acute and chronic administration.
Cross-Tolerance between Cannabinoids and Ethanol
Although Jones and Stone (1970) and later MacAvoy and Marks (1975) posited the existence of cross-tolerance between cannabinoid drugs and ethanol based upon their data from humans, preclinical studies in the rat were the first to definitively show a symmetrical cross-tolerance (Newman et al., 1974; Newman et al., 1972). In these studies, rats were trained in a one-way avoidance paradigm where they could escape to an elevated platform to avoid a foot shock. Acute administration of either THC or ethanol impaired avoidance behavior, but after repeated treatment with either THC or ethanol, subjects tested with the other compound (i.e. the one not given during treatment) displayed a marked tolerance where performance was not significantly impaired. Subsequent studies conducted in the 1970’s demonstrate that cross-tolerance also arises between the ataxic effects of cannabinoids and ethanol (Siemens and Doyle, 1979; Sprague and Craigmill, 1976). This cross-tolerance was not due to pharmacokinetic processes because ethanol-tolerant subjects did not metabolize THC more readily, and THC-tolerant rats did not show increased turnover of ethanol (Siemens and Doyle, 1979).
A more recent study demonstrates that a single dose of ethanol confers tolerance to a subsequent dose of THC, but pretreatment with a single dose of THC facilitates the ataxic effect of acute ethanol (da Silva et al., 2001). In a follow up to this study, rats that were given the CB1 antagonist SR 141716A (SR) with ethanol on the first day failed to develop tolerance to the ataxic effects of ethanol the following day, but SR treatment did not affect acute tolerance that develops within a single administration of ethanol (Lemos et al., 2007). Recent findings from our lab suggest a similar tolerance phenomenon is present for other behavioral measures as well. Mice treated chronically with ethanol for ten consecutive days displayed significantly reduced sensitivity to cannabinoid-induced hypomotility, hypothermia, and antinociception that was correlated with changes in CB1 receptor expression in the periaqueductal grey, hypothalamus, and ventral tegmental area (Pava et al., 2012). Importantly, cannabinoid-induced catalepsy was not altered by ethanol pre-treatment suggesting the modulation of CB1 function by ethanol is region or at least circuit specific. Together the studies on cross-tolerance between ethanol and cannabinoids indicate that pre-treatment with ethanol or THC can significantly alter the response to subsequent doses of the other compound, and the more recent studies implicate alterations in the EC system as central to this effect.
The Effect of Cannabinoids on Withdrawal from Ethanol
In the earliest report indicating a potential therapeutic potential of cannabinoid drugs for the treatment of AUDs, the authors describe the use of a synthetic analog of THC, pyrahexyl, given to ameliorate withdrawal symptoms (Thompson and Proctor, 1953). Although far from a rigorous, controlled clinical trial, this study provides anecdotal evidence indicating that acute withdrawal symptoms including irritability, loss of appetite, and insomnia are at least temporarily ameliorated by administration of this compound. Preclinical studies, however, dispute the beneficial effects of cannabinoid administration on ethanol withdrawal symptoms. Acute administration of THC to mice given three days of continuous ethanol vapor treatment produced higher handling-induced convulsion (HIC) scores and a longer duration withdrawal than subjects given vehicle upon removal from the chambers (Kralik et al., 1976; Sprague and Craigmill, 1978). One potential caveat to these studies is that acute administration of CB1 agonists can generate hyper-reflexive responses that manifest as eccentric jumping when the animal is slightly perturbed (Dewey, 1986; Patel and Hillard, 2001). It is possible that early studies examining the effect of acutely administered cannabinoids on HIC scores during withdrawal were confounded by these hyper-reflexive responses. Another measure of withdrawal severity in mice is the startle threshold to an electric foot shock. When compared with controls, mice undergoing ethanol withdrawal startle when a lower voltage is applied the cage floor. Using this measure of withdrawal severity, Sprague and Craigmill (1978) found that acute administration of THC, nabilone, or ethanol similarly ameliorated the decreased startle threshold during withdrawal from chronic vapor treatment.
Surprisingly, little follow up work has been done to address the potential utility of cannabinoids in the treatment of withdrawal symptoms. Considering the hyperphagic and sleep-inducing effects of cannabinoids (Dewey, 1986), it is possible that partial or indirect CB1 agonists may have utility for treatment of withdrawal symptoms, but the efficacy of these compounds must of course be weighed against their abuse liability. Furthermore, many alcohol dependent patients are at least regular users of cannabis if they do not qualify for comorbid CUDs, and the potential therapeutic benefit of cannabinoids on ethanol withdrawal may suggest a role for self-medication in the perpetuation of their CUD.
Molecular Targets of Ethanol and Cannabis
For many years, both ethanol and cannabinoid drugs were thought to exert the majority of their psychotropic effects through non-specific disruption of membrane lipids or alterations in the content of membrane lipids (Bruggemann and Melchior, 1983; Chin and Goldstein, 1981; Hillard et al., 1985; Hillard et al., 1990; Sun and Sun, 1985). Although there is currently not a specific receptor associated with the mechanism of action for ethanol, a robust data set has emerged in the past 20 years that strongly indicates the majority of ethanol’s actions at intoxicating concentrations are mediated by relatively weak interactions with a wide variety of molecular targets that include membrane receptors and enzymes. It is beyond the scope of this review to exhaustively describe the molecular mechanisms by which ethanol alters central nervous system (CNS) physiology (for review see Harris et al., 2008).
Cannabinoids on the other hand are now known to produce their psychotropic effects via high affinity, specific interactions with relatively few trans-membrane receptors, the cannabinoid receptors. The first cannabinoid receptor to be cloned (CB1) was serendipitously discovered from cDNA isolated from rat cerebral cortex and found to be a GPCR (Matsuda et al., 1990). CB1 is expressed throughout the brain, and it is particularly enriched in cortical structures, the striatum, and the cerebellum (Tsou et al., 1998). In contrast to ethanol, the low lethality associated with cannabinoid drugs is likely due to the relative absence of the CB1 receptor in brainstem nuclei responsible for respiration and cardiac regulation (Adams and Martin, 1996). Another cannabinoid-activated receptor, CB2, has also been cloned (Munro et al., 1993), but this receptor is primarily expressed in the periphery, suggesting that most of the psychotropic effects of cannabinoids are mediated by the CB1 receptor. Both cannabinoid receptors are Gi/o-coupled and inhibit adenylate cyclase and the production of cAMP (Howlett, 2005).
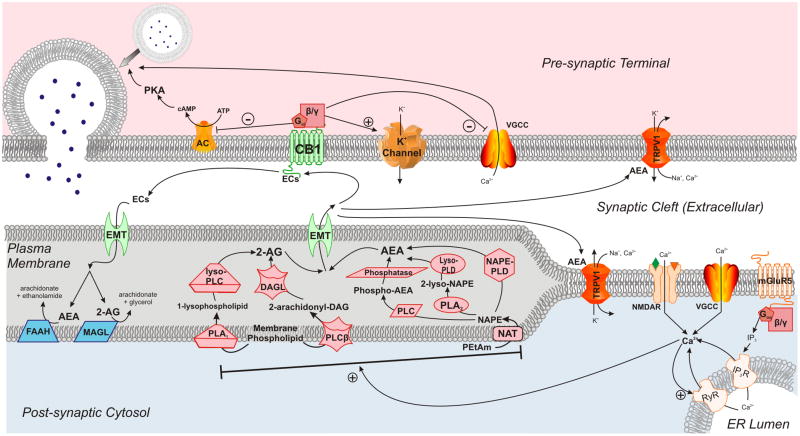
The discovery of receptors with high-affinity for cannabinoids propelled a series of findings shortly thereafter that elucidated the basic components of the EC system (figure 1; for a comprehensive review see Kano et al., 2009). The membrane-lipid derived EC transmitters fall into two categories: ethanolamides (Devane et al., 1992; Hanus et al., 1993) and monoacylglycerols (Mechoulam et al., 1995; Sugiura et al., 1995). The most well studied of the ethanolamide transmitters, arachidonyl ethanolamide (anandamide; AEA), is derived from phosphatidyl ethanolamine precursors via N-acyl transferase and an N-acyl phosphatidyl ethanolamine (NAPE) specific isoform of phospholipase D (NAPE-PLD; Leung et al., 2006; Okamoto et al., 2004; Schmid, 2000). However, other pathways of AEA synthesis exist including sequential deacylation of NAPE by α, β hydrolase 4 (ABHD4; Liu et al., 2008) and a two-step hydrolysis by phospholipase A2 (PLA2) and lyso-phospholipase D (Cadas et al., 1996; Sun et al., 2004).
Figure 1.
Biochemistry of the EC System in the CNS. ECs are synthesized in the post-synaptic membrane and produce most of their actions via activation of the CB1 receptor which decreases neurotransmitter release from the pre-synaptic terminal (for details, see text). Synthetic enzymes in the post-synaptic membrane are in red, and degradative enzymes are shown in blue. Abbreviations: 2-AG = 2-arachidonylglycerol, AC = adenylate cyclase, AEA = anandamide, ATP = adenosine triphosphate, Ca2+ = calcium ion, cAMP = cyclic adenosine monophosphate, CB1 = cannabinoid receptor 1, DAG = diacylglycerol, DAGL = diacylglycerol lipase, ECs = endocannabinoids, EMT = endocannabinoid membrane transporter, ER = endoplasmic reticulum, FAAH = fatty acid amide hydrolase, IP3 = inositol triphosphate, IP3R = inositol triphosphate receptor, K+ = potassium ion, lyso-PLC = lyso-specific isoform of phospholipase C, lyso-PLD = lyso-specific isoform of phospholipase D, MAGL = monoacylglycerol lipase, mGluR5 = metabotropic glutamate receptor 5, Na+ = sodium ion, NAPE = N-arachidonyl phosphotidylethanolamine, NAPE-PLD = NAPE-specific isoform of phospholipase D, NAT = N-acyl transferase, NMDAR = N-methyl-D-aspartate receptor, PEtAM= phosphotidylethanolamine, PKA = protein kinase A, PLA1 = phospholipase A1, PLA2 = phospholipase A2, PLC = phospholipase C, PLCβ = β-isoform of phospholipase C, RyR = ryanodine receptor, TRPV1 = transient receptor vanilloid 1, VGCC = voltage-gated calcium channel.
To date, the only two monoacylglycerol derivatives that have been identified as endogenous ligands for cannabinoid receptors in the CNS are 2-arachidonyl glycerol (2-AG) and 2-arachidonyl glyceryl ether (nolandin ether; 2-AGE; Fezza et al., 2002; Hanus et al., 2001). 2-AG is synthesized from phosphatidyl inositol (PI) precursors via a PI-specific phosoholipase C (PLC) and diacylglycerol lipase (DAGL; Stella et al., 1997), but the synthetic pathway for 2-AGE has yet to be determined. In addition to the lipid-derived transmitters of the EC system, other putative and novel ligands of cannabinoid receptors have been discovered. Hemopressin, a peptide derived from cleavage of α-hemoglobin, acts an inverse agonist at the CB1 receptor (Heimann et al., 2007), and recently discovered N-terminally extended hemopressin-like peptides derived from both α- and β-hemoglobin have been found to act as CB1 agonists (Gomes et al., 2009). Interestingly, these peptide agonists produce activation of distinct signal transduction cascades from those of the lipid-derived transmitters suggesting that the functional consequences of CB1 activation are determined by the ligand. However, the relevance of these peptides for physiological processes is unclear, at present, and more work is needed to determine if the molecules are significant components of the EC system. Because these peptide ligands are derived from hemoglobin, it may be difficult to demonstrate that they are present in brain tissue and not an artifact from the homogenization of tissue along with the neurovasculature. Nevertheless, these findings indicate that our understanding of EC transmitters will continue to evolve as novel ligands and their associated synthetic pathways will likely be discovered.
In the CNS, CB1 is localized to presynaptic terminals of both GABAergic (Katona et al., 1999) and glutamatergic (Rodriguez et al., 2001) neurons, and activation of CB1 results in decreased neurotransmitter release from these terminals (Gerdeman and Lovinger, 2001; Lévénés et al., 1998; Shen et al., 1996; Szabo et al., 1998). Unlike classical neurotransmitters like those derived from amino acids or peptides, ECs are not stored in vesicles. Their synthesis and release occurs on-demand during periods of intense synaptic activity and membrane depolarization via Ca2+-dependent processes. Depolarization-induced suppression of inhibition and excitation are two physiological protocols that depend on EC synthesis in the post-synaptic neuron to activate pre-synaptic CB1 receptors on GABAergic (Kreitzer and Regehr, 2001a; Wilson et al., 2001; Wilson and Nicoll, 2001; Yoshida et al., 2002) and glutamatergic (Kreitzer and Regehr, 2001b) terminals, respectively. These physiological protocols demonstrate how prolonged cell depolarization can lead to EC release; however, there are other mechanisms that can give rise to EC release and CB1 activation. These include activation of other GPCRs, such as the metabotropic glutamate receptor 5 (mGluR5; Maejima et al., 2001; Varma et al., 2001), and activation of ligand-gated ion channels like the NMDA receptor (Ohno-Shosaku et al., 2007; Stella and Piomelli, 2001). These studies demonstrate that ECs act as retrograde neurotransmitters that serve to decrease synaptic transmission during periods of intense cellular activity.
Inactivation of ECs occurs predominantly through enzymatic hydrolysis of the transmitters. Fatty Acid Amide Hydrolase (FAAH) is the enzyme primarily responsible for the inactivation of AEA (Cravatt et al., 1996), and monoacylglycerol lipase (MAGL) is chiefly responsible for the breakdown of 2-AG (Dinh et al., 2002a; Dinh et al., 2002b; Karlsson et al., 1997; Karlsson et al., 2000). The existence of an EC membrane transport (EMT) mechanism is another possible means of inactivation that has been hotly debated (Lovinger, 2007). Proponents of the EMT’s existence cite data from pharmacological studies that show an increase in AEA without inhibiting FAAH activity (López-Rodríguez et al., 2003; Mahadevan and Razdan, 2005), but critics argue that to date no gene or specific molecular mechanism has been implicated in this process and passive diffusion mechanisms are sufficient for EC uptake (Deutsch et al., 2001; Glaser et al., 2003; Kaczocha et al., 2006). EC inactivation can also proceed through oxidation mediated by cyclooxygenase-2 (Fowler, 2007; Kozak et al., 2004), and recently, 2-AG was found to be degraded by α, β hydrolase domain 6 (ABHD6) in the cerebral cortex (Marrs et al., 2010).
The neurophysiological consequences of intoxication with ethanol and cannabinoids are now understood to arise from the interaction of these substances with specific molecular substrates, particularly with membrane bound receptors and enzymes. Cannabinoid drugs interact specifically with the components of the EC system to modulate neurotransmission primarily at glutamatergic and GABAergic synapses. In contrast, the molecular substrates with which ethanol interacts are numerous and vary greatly with regard the neurochemical processes they participate in. However, mounting evidence from biochemical, genetic, electrophysiological, and behavioral studies conducted over the past decade indicates that the EC system plays an important role in mediating the acute effects of ethanol. Furthermore, the function of the EC system is perturbed following chronic exposure to ethanol, and these data suggest that altered EC signaling may contribute to the underlying neuropathology that drives alcohol abuse and dependence disorders. The remainder of this review will focus specifically on the interactions between ethanol and the EC system.
Ethanol and the EC System
Despite the early flurry of research concerning the interactions between marijuana and ethanol in the 1970s, there was a marked paucity of studies examining the intersection of ethanol and cannabinoid substances through the 1980’s and most of the 90’s. Although it was not realized at the time, the first reports to implicate ethanol as a modulator of EC biosynthetic enzymes demonstrated that chronic exposure to ethanol in mice up-regulated the activity of PLA2 and that this effect persisted into withdrawal (Hungund et al., 1994; John et al., 1985). Hungund’s lab followed up on these studies demonstrating the specific up-regulation of a Ca2+-dependent, arachidonic acid-specific isoform of PLA2 following chronic ethanol (Basavarajappa et al., 1997). Shortly thereafter, it was found that CB1 receptor expression and function were decreased in the brains of mice chronically exposed to ethanol vapor (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999b), and levels of AEA and 2-AG were enhanced in cultured cells treated chronically with ethanol presumably due to activation of PLA2 (Basavarajappa and Hungund, 1999a; Basavarajappa et al., 2000). Around this time, studies from other labs regarding the effects of CB1 agonists (Gallate et al., 1999) and antagonists (Arnone et al., 1997; Colombo et al., 1998; Gallate and McGregor, 1999; Rodríguez de Fonseca et al., 1999) on ethanol consumption were concluding that modulation of this receptor could significantly alter ethanol consumption. Following on these investigations and armed with a greater understanding of the biochemistry and physiology of the EC system, a plethora of studies conducted within the past decade has firmly established a role for the EC system in mediating the reinforcing properties of ethanol and the pathology of alcohol dependence. The remainder of this review will focus on recent literature examining the effects of ethanol on the EC system and the role the EC system plays in reward, ethanol consumption, and relapse.
Physiological Effects of Acute Ethanol on the EC System
Given the CNS depressant effects of ethanol, the ability of acute ethanol administration to activate release of ECs seems, at first, to be contradictory as synthesis and release of ECs are highly dependent on excitability-induced elevations intracellular Ca2+ concentrations. However, brief incubation with ethanol is known to increase intracellular Ca2+ in forebrain synaptosomes (Davidson et al., 1988) and in hippocampal neurons via release from intracellur stores (Mironov and Hermann, 1996). Along these lines, several pieces of evidence suggest that the effects of acute ethanol are mediated in part by the liberation of ECs from neural tissue and their subsequent actions on neurotransmission (table 1). Basavarajappa et al (2008) found that a 30 min application of 50 mM ethanol (approximately 200 mg %) was sufficient to increase both AEA and 2-AG in hippocampal cultures and that this increase in EC content was Ca2+-dependent. Furthermore, these authors reported that the ethanol-mediated increase in EC levels resulted in reduced pre-synaptic glutamate release. Additional evidence comes from in vivo studies where SR or chronic pre-treatment with the CB1 agonist, WIN 55,212-2 (WIN), reduced the effect of acute ethanol administration to alter the spontaneous firing rate of basolateral amygdala (BLA; Perra et al., 2008) and ventral tegmetal area (VTA; Perra et al., 2005)projection neurons. Similar findings were also obtained from evoked activity in nucleus accumbens (NAc) neurons (Perra et al., 2005). The effect of chronic WIN treatment on the response of VTA neurons to acute ethanol is particularly interesting because it implies that the rewarding properties of ethanol may be reduced following chronic exposure to cannabinoids, and this may be of significance to the high prevalence of comorbidity between AUDs and CUDs discussed previously. Data regarding the role of the EC system in mediating the rewarding properties of ethanol will be discussed in the next section.
Table 1.
Effects of Acute EtOH Exposure on Components of the EC System
| Measure: | EtOH Exposure: | System: | Species: | Genetic Background: | Method of Analysis: | Brain Region: | Effect: | Reference: |
|---|---|---|---|---|---|---|---|---|
| 2-AG | 30 min application 50 mM EtOH | In vitro | Mus | C57BL6/J | Cell and medium extract | HC | ↑ | Basavarajappa et al., 2008 |
| 24 hr access 8% v/v EtOH in liquid diet | Ex vivo | Rattus | Sprague | Tissue Content | AMG | none | Rubio et al., 2007 | |
| CPu | none | |||||||
| HyTh | none | |||||||
| PFC | ↓ | |||||||
| 30 min Self-administration of 10% (w/v) EtOH | In vivo | Rattus | Wistar | microdialysis | NAc | ↑ | Caillé et al., 2007 | |
| AEA | 30 min application 50 mM EtOH | In vitro | Mus | C57BL6/J | Cell and medium extract | HC | ↑ | Basavarajappa et al., 2008 |
| 24 hr access 8% v/v EtOH in liquid diet | Ex vivo | Rattus | Sprague | Tissue Content | AMG | ↓ | Rubio et al., 2007 | |
| CPu | ↓ | |||||||
| HyTh | ↓ | |||||||
| PFC | none | |||||||
| 4g/kg i.p. injection | Ex vivo | Rattus | Wistar | Tissue Content | Cereb | ↓ | Ferrer et al., 2007 | |
| HC | ↓ | |||||||
| NAc | ↓ | |||||||
| microdialysis | NAc | ↓ | ||||||
| 30 min Self- administration of 10% (w/v) EtOH | In vivo | Rattus | Wistar | microdialysis | NAc | none | Caillé et al., 2007 | |
| CB1 | 30 – 60 min application 50 mM EtOH | In vitro | Mus | C57BL6/J | protein | HC | none | Basavarajappa et al., 2008 |
| 24 hr access 8% v/v EtOH in liquid diet | Ex vivo | Rattus | Sprague | protein | AMG | ↓ | Rubio et al., 2009 | |
| CPu | none | |||||||
| HyTh | none | |||||||
| PFC | ↓ | |||||||
| 4g/kg i.p. injection | Ex vivo | Rattus | Wistar | mRNA | Cereb | none | Ferrer et al., 2007 | |
| HC | none | |||||||
| FAAH | 24 hr access 8% v/v EtOH in liquid diet | Ex vivo | Rattus | Sprague | Activity/ Protein | AMG | none | Rubio et al., 2009 |
| CPu | none | |||||||
| HyTh | ↓ (act)/↑ (protein) | |||||||
| PFC | ↓ (act)/none (protein) | |||||||
| 4g/kg i.p. injection | Ex vivo | Rattus | Wistar | Activity/ mRNA | Cereb | none | Ferrer et al., 2007 | |
| HC | ↓ (act)/none (mRNA) | |||||||
| NAc | none |
2-AG – 2-arachidonyl glycerol, AEA – anandamide, AMG – amygdala, CB1 – cannabinoid receptor 1, CPu – caudate-putamen, Cereb – cerebellum, EtOH – ethanol, FAAH – fatty acid amide hydrolase, HC – hippocampus, HyTh – hypothalamus, NAc – nucleus accumbens, PFC – prefrontal cortex
In contrast to the findings mentioned above, there is also evidence that acute ethanol inhibits EC signaling in a number of brain regions. Direct measures of EC tissue content have found that acute or short-term exposure to ethanol reduces EC levels in the hippocampus, striatum, prefrontal cortex, amygdala, and cerebellum (Ferrer et al., 2007; Rubio et al., 2007). Futhermore, these reductions in EC levels are not associated with enhanced FAAH activity (Ferrer et al., 2007; Rubio et al., 2009) suggesting that the effects of acute and short-term ethanol are not mediated by increased metabolism.
On the other hand, several lines of evidence suggest that the physiological effects of acute ethanol oppose or are antagonized by the EC system. For instance, EC release from medium-spiny neurons in the dorsomedial striatum is associated with a long-lasting disinhibition of these cells, and this effect is blocked by pre-treatment with ethanol (50 mM) via a pre-synaptic mechanism that is independent of EC synthesis and CB1 activation (Clarke and Adermark, 2010). Additionally, activation of CB1 by WIN is sufficient to block the pre-synaptic facilitation of GABAergic signaling on pyramidal neurons (PNs) in the central amygdala that is produced by acutely applied ethanol (Roberto et al., 2010). This effect of ethanol is likely due to presynaptic signal transduction mechanisms that converge with and oppose those of CB1 activation. Studies of cerebellar purkinje neurons have found that ethanol facilitates GABA release from pre-synaptic terminals via a PKA-dependent mechanism that liberates Ca2+ from internal stores and does not require EC synthesis (Kelm et al., 2007). However, activation of CB1 blocks the enhancement IPSC frequency by ethanol in these neurons leading the authors conclude that this is likely due to the Gi-mediated inhibition of PKA produced by CB1 activation (Kelm et al., 2008). In vivo studies in FAAH KO mice also suggest that AEA opposes some of the acute effects of ethanol including loss of righting reflex and hypothermia while exacerbating others (i.e. hypothermia; Basavarajappa et al., 2006; Vinod et al., 2008a). These results suggest that the EC system interacts with the effects of ethanol in region dependent manner.
Given the discrepancies between studies examining the interaction of acute ethanol administration and EC signaling, there is clearly much debate as to the role of the EC system in mediating the intoxicating effects of ethanol. However, several variables that differ in the aforementioned studies may explain the contrasting datasets and may provide future avenues of research. It seems probable that ethanol’s effect on the EC system varies greatly based upon brain the region in question. In support of this hypothesis, the results from Rubio et al (2007) demonstrate that short-term ethanol consumption produced a complex pattern of alterations in the levels of six different ECs that varied substantially by brain region. Additionally, because the EC system mediates retrograde signaling at both glutamatergic and GABAergic synapses there is more biochemical complexity than in most classical neurotransmitter systems. The contrast between the effects of ethanol observed at hippocampal glutamate synapses (Basavarajappa et al., 2008) and at GABAergic synapses (Clarke and Adermark, 2010; Kelm et al., 2008; Roberto et al., 2010) are examples of this complexity. Lastly, the state of the system under study may have a large impact on EC signaling. The in vivo studies in the BLA, VTA, and NAc were all conducted in urethane anesthetized animals raising the possibility that the anesthetic used in these studies alters EC signaling itself. Along these lines, the EC system has been implicated in mediating some of the effects of the general anesthetic propofol (Patel et al., 2003), but there are currently no data available as to the effects of urethane on the EC system, making data from the in vivo studies difficult to interpret in light of conflicting results. Additionally, a nascent but growing body of evidence has linked the EC system to the regulation of the sleep/wake cycle (for review see Murillo-Rodriguez et al., 2011). As a consequence, future studies should be sensitive to the possible interactions between the EC system and the state of the organism under study.
Endocannabinoids and the Reinforcing Properties of Ethanol
A large body of data collected over the past 15 years has demonstrated that the EC system is intricately involved in the function of reward neurocircuitry for both natural reinforcers like food (as reviewed by Fattore et al., 2010) and drugs of abuse (for review see Serrano and Parsons, 2011; Solinas et al., 2007). It is beyond the scope of the present review to exhaustively discuss the data implicating the EC system in all reward processes, so the following sections will be focused on an in depth review of the literature concerning EC’s and the reinforcing properties of ethanol (table 2). Along these lines, evidence spanning multiple domains including behavioral, biochemical, genetic, and physiological data will be discussed.
Table 2.
Effect of Modulating EC System Function on Alcohol Consumption and Seeking Behaviors
| Manipulation: | Method: | Behavioral Paradigm: | Drug Delivery: | Genus: | Strain: | Effect: | Reference: |
|---|---|---|---|---|---|---|---|
| CB1 Activation | Pharmacological | Operant EtOH Self-administration | Systemic | Rattus | Wistar | ↓ | Cippitelli et al., 2007 |
| Progressive Ratio Operant Responding for Beer | Systemic | Rattus | Wistar | none | Gallate et al., 1999 | ||
| Drinking in the Dark | Systemic | Mus | C57B6 | ↓ | Linsenbardt and | ||
| VTA microinjection | Mus | C57BL6 | ↑ | Boehm, 2009 | |||
| Two-bottle Choice | Systemic | Mus | C57BL6/J & DBA/2 | ↑ | Vinod et al., 2008b | ||
| Deprivation-induced Escalation of Operant Response for EtOH | Systemic during forced abstinence | Rattus | Wistar | ↑ |
López-Moreno et al., 2004 Alén et al., 2009 |
||
| CB1 Inactivation | Genetic deletion of CB1 | EtOH Conditioned Place Preference | - | Mus | CD1 | ↓ | Houchi et al., 2005 |
| Two-bottle Choice | - | Mus | C57BL6/J & DBA/2 | ↓ | Vinod et al., 2008b | ||
| - | Mus | C57BL6/J | ↑ | Racz et al., 2003 | |||
| ↓ | Wang et al., 2003 | ||||||
| - | Mus | CD1 | ↓ | Naassila et al., 2004 | |||
| - | Mus | 129/Ola x C57BL6/J | ↓ | Lallemand and De Witte, 2005 | |||
| Two-bottle Choice in Dependent Mice | - | Mus | 129/Ola x C57BL6/J | ↓ | Lallemand and De Witte, 2005 | ||
| Pharmacological | Operant EtOH Self-administration | PFC microinjection | Rattus | AA | ↓ | Hansson et al., 2007 | |
| NAc microinjection | Rattus | Wistar | ↓ | Caillé et al., 2007 | |||
| Systemic | Rattus | Long-Evans | ↓ | Freedland et al., 2001 | |||
| msP | ↓ | Cippitelli et al., 2005 | |||||
| Wistar | ↓ |
Economidou et al., 2006 Cippitelli et al., 2005 |
|||||
| none | Cippitelli et al., 2008 | ||||||
| Operant EtOH Self-administration in Dependent rats Compared to Controls | Systemic | Rattus | Wistar | ↓ | Rodríguez de Fonseca et al., 1999 | ||
| Progressive Ratio Operant Responding for Beer | Systemic | Rattus | Wistar | ↓ | Gallate and McGregor, 1999 | ||
| Two-bottle Choice | Systemic | Mus | C57BL6/J & DBA/2 | ↓ | Vinod et al., 2008b | ||
| C57BL6/J | ↓ |
Arnone et al., 1997 Wang et al., 2003 |
|||||
| Rattus | sP | ↓ | Colombo et al., 1998 | ||||
| Cue-induced Reinstatement to Operant EtOH Self-administration | Systemic | Rattus | Wistar | ↓ |
Cippitelli et al., 2005 Economidou et al., 2006 Economidou et al., 2007 |
||
| Stess-induced Reinstatement to Operant EtOH Self-administration | Systemic | Rattus | Wistar | none |
Economidou et al., 2006 Economidou et al., 2007 |
||
| FAAH Inactivation | Genetic deletion of FAAH | Two-bottle Choice | - | Mus | 129/SvJ x C57BL6/J | ↑ | Basavarajappa et al., 2006 |
| - | ↑ | Blednov et al., 2007 | |||||
| - | Mus | C57BL6/J | ↑ | Vinod et al., 2008a | |||
| Pharmacological | Operant EtOH Self-administration | PFC microinjection | Rattus | Wistar | ↑ | Hansson et al., 2007 | |
| Systemic | none | Cippitelli et al., 2008 | |||||
| Two-bottle Choice | Systemic | Mus | 129/SvJ x C57BL6/J | ↑ | Blednov et al., 2007 | ||
| Mus | C57BL6/J | ↑ | Vinod et al., 2008a | ||||
| Rattus | msP | none | Cippitelli et al., 2008 | ||||
| Cue-induced Reinstatement to Operant EtOH Self-administration | Systemic | Rattus | Wistar | none | Cippitelli et al., 2008 | ||
| Yohimbine-induced Reinstatement to Operant EtOH Self- administration | Systemic | Rattus | Wistar | none | Cippitelli et al., 2008 |
CB1 – cannabinoid receptor 1, EtOH – ethanol, FAAH – fatty acid amide hydrolase, NAc – nucleus accumbens, PFC – prefrontal cortex, VTA – ventral tegmental area
The activity of mesolimbic DA neurons in the VTA is closely associated with the reward and learning processes thought to drive the transition to compulsive drug use (Morikawa and Morrisett, 2010), and one of the major targets for these neurons is the NAc. Along these lines, Cheer et al (2004) reported that acute administration of WIN enhanced the frequency of fast, spontaneous DA transients in the NAc of freely moving rats. In a subsequent study, they found that acute ethanol administration also increased DA release in the NAc, and this effect could be blocked by pre-treating the rats with SR, suggesting that CB1 function was required for the ethanol-mediated activation of VTA DA neurons (Cheer et al., 2007). As mentioned in the previous section, similar results have also been obtained from single-unit recordings of VTA DA neurons where pre-treatment with SR blocks the increased firing rate observed after acute ethanol administration (Perra et al., 2005). Consistent with these findings, CB1 KO mice lack the increase in NAc DA concentrations observed in wt mice following an acute injection of ethanol (Hungund et al., 2003), and CB1 KO mice also display blunted conditioned place preference for ethanol (Houchi et al., 2005). Data from humans also implicates a polymorphism in the CNR1 gene in modulating the reinforcing properties of ethanol (Hutchison et al., 2008). Together, these studies support the hypothesis that the rewarding properties of ethanol are produced in part by the EC-mediated facilitation of VTA DA neuron activation, and they suggest that genetic differences in CB1 signaling and expression may predispose some individuals to abuse and dependence.
ECs and Ethanol Consumption/Self-Administration
In keeping with the concept that a genetic predisposition to ethanol abuse and dependence can arise from altered function of the EC system, C57BL/6 mice have a higher preference for ethanol and lower CB1 expression than DBA/2 mice (Hungund and Basavarajappa, 2000). In addition, rats bred selectively for high ethanol preference (AA rats) have decreased activity of FAAH and reduced CB1 expression in the prefrontal cortex compared to rats with low ethanol preference (Hansson et al., 2007). Furthermore, the AA phenotype can be reconstituted in non-selected rats by injecting the FAAH inhibitor, URB597, into the PFC. Similar results have been obtained in mice where genetic knockout of FAAH confers a higher preference for ethanol, and systemic treatment of wt mice with URB597 enhanced ethanol consumption (Basavarajappa et al., 2006; Blednov et al., 2007; Vinod et al., 2008a). FAAH KO mice also have reduced expression and function of CB1 in several brain regions including the dorsal striatum, NAc, and hippocampus (Vinod et al., 2008a). A consistent finding from these studies is that reduced FAAH activity is correlated with decreased expression of the CB1 receptor and increased ethanol preference. These results are in line with those in the preceding section indicating that acute ethanol facilitates EC synthesis and release (Basavarajappa et al., 2008; Perra et al., 2008; Perra et al., 2005), and the reduction of CB1 expression observed in these studies is likely a compensatory mechanism for enhanced AEA tone.
However, several studies dispute the role of increased levels of AEA in ethanol consumption. The earliest study to suggest that enhanced AEA levels are not associated with increased ethanol intake utilized the putative EMT inhibitor, AM404. Treatment with AM404 reduced the number of active lever responses in rats trained to self-administer ethanol (Cippitelli et al., 2007). However, this effect was not blocked by CB1, CB2, or TRPV1 antagonists raising the possibility that AM404 produced these effects via a mechanism independent of AEA inactivation. In marked contrast to the study by Hannson et al (2007), systemic administration of URB597 failed to enhance ethanol self-administration or the reinforcing properties of ethanol in Wistar rats, and treatment with URB597 did not affect ethanol consumption in Marchigian Sardinian alcohol preferring rats (msP rats; Cippitelli et al., 2008). It is possible that the lack of effect of URB597 on ethanol drinking in msP rats was due to a ceiling effect where these rats consumed enough ethanol at baseline that further enhancement by FAAH inhibition was not possible. However, the discrepancy between the ethanol self-administration data in these two studies is more difficult to reconcile because of methodological differences. In Hansson et al (2007), URB597 was microinjected into the PFC where Cippitelli et al (2008) delivered the drug systemically altering AEA concentrations throughout the organism. These widespread changes in AEA levels could produce effects that mute those elicited by localized injection into the PFC. As a result, the sum of available data support the hypothesis that brain AEA concentrations are enhanced following ethanol consumption, and the increased AEA levels contribute to the reinforcing and motivational actions of the drug.
The relative paucity of data implicating 2-AG in mediating the reinforcing effects of ethanol is due to the lack of selective pharmacological and genetic tools available when the previous studies were conducted. However, some of the data presently available do implicate this EC transmitter in mediating the reinforcing properties of ethanol. Notably, self-administration of ethanol increases interstitial levels of 2-AG in the NAc that are correlated with the amount of ethanol consumed, but does not alter levels of AEA in this brain region (Caillé et al., 2007). Additionally, a methamphetamine-induced neurotoxic lesion of nigrostriatal dopaminergic projections (Granado et al., 2010; Sanchez et al., 2003) is associated with increased levels of ethanol consumption and preference, and this mouse model of enhanced ethanol intake displays increased tissue concentrations of 2-AG in limbic forebrain sections containing anterior cingulate and NAc (Gutierrez-Lopez et al., 2010). Furthermore, these investigators found that enhanced ethanol consumption and preference were also observed in intact mice treated with the MAGL inhibitor N-arachidonyl maleimide. However, future studies using more specific MAGL inhibitors and MAGL KO mice should be employed to discriminate the roles of AEA and 2-AG in mediating the effects of ethanol.
The effects of increased AEA on ethanol consumption are likely mediated by activation of the CB1 receptor as numerous studies have demonstrated that direct modulation of CB1 alters ethanol intake. Studies utilizing CB1 agonists to modulate ethanol consumption are confronted with the potential confound that CB1-mediated reductions in motor activity may act synergistically with those of ethanol thereby occluding any enhancement in the motivation to consume ethanol. In keeping with this effect, two studies have reported that decreased ethanol consumption and self-administration following systemic injection of WIN is correlated with reduced locomotor activity (Cippitelli et al., 2007; Linsenbardt and Boehm, 2009). In addition, CB1 agonists are known to induce hyperphagia that may confound studies examining ethanol preference where sweetened or otherwise flavored solutions are used as controls (Dewey, 1986). However, Gallate et al (1999) concluded that pre-treatment with a CB1 agonist increased the motivation of rats to self-administer beer despite increased responding for both control substances (near beer and sucrose) used in this study. More convincing results for the role of CB1 activation in the reinforcing properties of ethanol have been obtained by microinjecting WIN into the posterior VTA (Linsenbardt and Boehm, 2009). In this study, WIN microinjection into the posterior VTA increased binge-like ethanol consumption during the second half of a drinking in the dark paradigm supporting the hypothesis that CB1-modulation of VTA neurons contributes the motivation to consume ethanol. Finally, treatment of mice with a CB1 agonist was found to increase ethanol preference in both high-consuming C57BL/6 and low-consuming DBA/2 strains (Vinod et al., 2008b).
Given the confounds associated with CB1 agonists, it is not surprising that most data supporting the role of CB1 in mediating the rewarding effects of ethanol have been obtained by studies using genetic and pharmacological inactivation of CB1 signaling. Arnone et al (1997) published the earliest account of CB1 modulation of ethanol consumption, where they reported that SR treatment blocked both ethanol and sucrose solution intake in C57BL/6 mice. This report was followed by others indicating that systemic SR treatment reduced ethanol intake in several preclinical models of ethanol consumption. SR administration reduced the break-point in progressive ratio self-administration experiments where rats were trained to lever press for beer (Gallate and McGregor, 1999) or 10% ethanol solution (Economidou et al., 2006) indicating that blockade of the CB1 receptor decreased the reward value of ethanol. A study of operant responding for sipper tube access found that SR treatment reduced responding for both ethanol and sucrose (Freedland et al., 2001). Economidou et al (2006) reported a similar dose-dependent reduction in sucrose- and ethanol-associated lever presses following SR treatment, and this effect was not due to motoric impairment by SR. In Sardinian alcohol-preferring rats, SR injection reduced both ethanol and food consumption while only affecting water intake at the highest dose used (10mg/kg; Colombo et al., 1998), and similar results were obtained from AA rats trained to self-administer ethanol in an operant chamber (Hansson et al., 2007). Interestingly a subsequent self-administration study in Wistar and msP rats replicated the findings that that SR reduced ethanol, saccharin, and sucrose intake, but it failed to reduce food intake (Cippitelli et al., 2005). These authors also reported reduced levels of CB1 mRNA in several brain regions including frontoparietal cortex, dorsal striatum, and hippocampus in msP rats that was normalized following ethanol consumption. Using another in-bred alcohol preferring rat strain, Adams et al (2010) reported that low doses of SR that did not alter ethanol self-administration on their own could be combined with sub-threshold doses of either an adenosine A2A or mGluR5 antagonist to reduce ethanol responding. Lastly, one study examining the interaction between alcohol dependence and the effect of SR treatment on operant responding for ethanol found that SR reduced ethanol self-administration in dependent rats but did not affect the response rate of control treated rats (Rodríguez de Fonseca et al., 1999).
The neuroanatomical loci of the SR mediated reduction in ethanol self-administration are likely to involve many of the brain regions typically associated with addiction, but at present there is a paucity of datasets with this level of resolution. Hansson et al (2007) report that microinjection of SR into medial PFC reduces ethanol self-administration, but injections into the dorsal striatum did not affect the number responses for ethanol. In a complementary study, microinjection of SR in the NAc was found to reduce ethanol self-administration (Caillé et al., 2007). Future studies will need to replicate these findings as well as examine the role of other brain regions like the VTA and amygdala in mediating the effects of SR on ethanol consumption.
Studies in mice have been able to make use of transgenic strains that lack functional copies of the CB1 gene. Vinod et al (2008b) found that treatment of either C57BL/6 or DBA/2 mice with SR reduced ethanol preference to levels comparable to mice with these genetic backgrounds but lacking a functional copy of the CNR1 gene. Reduced ethanol consumption and preference in CB1 KO mice has been replicated in other labs (Naassila et al., 2004) including in ethanol dependent mice (Lallemand and De Witte, 2005), and Racz et al (2003) found that CB1 KO mice do not exhibit the increase in ethanol consumption observed in wt controls following acute stress. Interestingly, this study failed to find the reduced ethanol preference in CB1 KO mice reported elsewhere, and in fact, it reported that these mice had a higher preference for ethanol during the first few days of access (Racz et al., 2003). However, an important methodological difference separating this study from the others is that the ethanol was available ad libitum for several weeks, and while the initial pattern of drinking for CB1 KOs was different from wt mice, the consumption of the two groups was no different beyond the first few days of drinking. Lastly, the reduction in drinking associated with CB1 inhibition and deletion is decreased in aged mice suggesting that the interaction between the EC system and ethanol may be particularly important for the onset of AUDs in adolescence and early adulthood (Wang et al., 2003).
One explanation for the lower ethanol preference of CB1 KO mice found in most studies may be due to increased sensitivity to its effects. Several labs have reported that CB1 KO mice display increased hypolocomotion, ataxia, hypothermia, and sleep time following acute injection with ethanol (Naassila et al., 2004; Racz et al., 2003; Vinod et al., 2008b; Warnault et al., 2007), and one study reported increased plasma ethanol concentration in CB1 mice following a high dose i.p. injection (5 g/kg) and forced pulmonary alcoholization (Lallemand and De Witte, 2005). It should be noted, however, that in this study and in others (Naassila et al., 2004; Warnault et al., 2007) differences in blood ethanol concentration (BEC) between genotypes were not observed with more moderate doses of ethanol suggesting that a null mutation of the CB1 gene produces only modest effects on ethanol metabolism. As a consequence of the numerous preclinical studies investigating the role of CB1 function in regulating ethanol consumption a clear picture emerges that activation of this receptor facilitates ethanol consumption while antagonism of CB1 reduces the motivational properties of ethanol perhaps by enhancing sensitivity to its other effects.
Given the highly consistent data from preclinical studies that SR reduces the reinforcing value of appetitive stimuli, clinical trials for CB1 antagonists were undertaken primarily as a way to quell appetite in the treatment of obesity and reduce smoking. Two studies were conducted regarding the use of SR treatment in alcohol abuse and dependence prior to the discontinuation of all clinical trials due to negative psychiatric effects (Maccioni et al., 2010). In marked contrast to the results obtained in mice and rats, both of these studies concluded that SR failed to alter any parameters associated with ethanol consumption or abstinence in treatment seeking (Soyka et al., 2008) and non-seeking (George et al., 2010) subjects. Because all human trials with CB1 antagonists have been discontinued for safety reasons, it is unlikely that the reason for the discrepancy in clinical and preclinical data can be resolved. However, as future work examines the involvement of other components of the EC system in alcohol dependence and abuse it may be possible to leverage this neurotransmitter system for therapeutic potential.
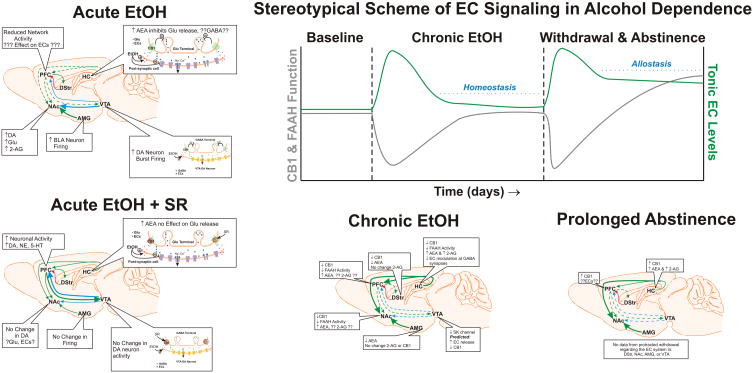
ECs in the Development of Ethanol Tolerance and Dependence
Two of the major hallmarks of alcohol dependence are the establishment of tolerance to the acute effects of ethanol and the susceptibility to a withdrawal syndrome in the absence of the drug. Reports from various labs have indicated that the EC system is associated with both of these traits of dependence. In fact, most of the early work suggesting an interaction between ethanol and cannabinoid drugs (see historical perspectives section of this review) supports the view that the EC system is involved in mediating these two traits of addiction, but at the time these studies were conducted, the existence of the EC system and the molecular mechanisms by which ethanol and cannabinoids produce their effects was unknown. More recent work has demonstrated the symmetrical cross-tolerance that develops to the ataxic effects of cannabinoids and ethanol is CB1-dependent (da Silva et al., 2001; Lemos et al., 2007). In addition, studies in our lab have found that the cross-tolerance between ethanol and cannabinoids is a wide-spread phenomenon in the brain, and that this effect is correlated with alterations in CB1 expression (Pava et al., 2012).
Hungund’s lab was the first to publish that chronic ethanol treatment reduced CB1 expression and function (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999b). In these studies, mice were exposed chronically to ethanol vapor for three days and then tissue from whole-brain homogenates was assayed for CB1 binding and G-protein coupling. Since these seminal experiments, altered CB1 expression and function have been reported by a number of labs implementing various chronic and sub-chronic ethanol regimens (table 3). Replicating their previous findings, Hungund’s lab reported that 3-day ethanol vapor inhalation produces a down-regulation of CB1 expression and function in mouse cortex, hippocampus, striatum, and cerebellum (Vinod et al., 2006). In another study, sub-chronic administration of ethanol (2 g/kg; twice daily) for 7 days decreased sensitivity to WIN-induced changes in monoamine synthesis in the striatum, hippocampus, and locus coeruleus (Moranta et al., 2006). In addition, rats made ethanol dependent using 52 days of forced access to a 10% ethanol solution had reduced CB1 gene expression in the striatum, hippocampus, and hypothalamus, but expression was not altered in the cingulate cortex suggesting region specific reductions in expression (Ortiz et al., 2004). Another dependence study in rats, found that hippocampal expression of CB1 mRNA and protein was reduced following chronic intermittent treatment with ethanol, and these changes in expression corresponded with reduced physiological measures of CB1-mediated inhibition of GABAergic synaptic transmission indicating a loss of function as well (Mitrirattanakul et al., 2007). Importantly, this study also reported that CB1 expression rebounded above control levels following 40 days of withdrawal, and similar results have been reported for CB1 expression in the cingulate cortex following a 3 week withdrawal from chronic ethanol treatment (Rimondini et al., 2002). Despite these reports, Gonzalez et al (2002) found no change in CB1 binding or gene expression in any of fifteen brain regions examined, including cingulate cortex, hippocampus, dorsal striatum, NAc, BLA, and cerebellum. In this study, rats were treated with ethanol (7.2%) via a liquid diet for 15 days prior to tissue collection, but the BECs obtained by these animals were not reported. Therefore, the discrepancy between these results and others may be due to methodological differences resulting in lower or more variable BECs than those obtained in other labs. Additionally, a subsequent study using the same treatment regimen for 10 days found that CB1 was reduced in the striatum and substantia nigra after 3 hrs of abstinence from ethanol when withdrawal symptoms were present (Rubio et al., 2008). These results indicate that this treatment paradigm produces alterations in CB1 expression that are highly dependent on the duration of abstinence. Regardless, the large majority of data suggest that ethanol treatments associated with the development of tolerance and dependence produce an initial down-regulation of CB1 expression that is followed by an up-regulation as acute withdrawal symptoms subside.
Table 3.
Effects of Repeated EtOH Exposure and Withdrawal on Components of the EC System
| Measure: | EtOH Treatment Regimen: |
Duration of EtOH Exposure: |
System: | Genus: | Genetic Background: |
Method of Analysis: |
Brain Region: |
Effect: | Duration of Abstinence: |
Effect of Abstinence/ Withdrawal: |
Reference: |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-AG | 100–150 mM chronic EtOH treatment | 48–72 hr | In vitro | Rattus | Sprague | Cell and medium extract | Cereb | ↑ | - | - | Basavarajappa et al., 2000 |
| 7.2% (v/v) EtOH Liquid Diet | 10–15 days | Ex vivo | Rattus | Wistar | Tissue Content | AMG | - | 3 hr | none |
Rubio et al., 2008 González et al., 2002 |
|
| Cereb | none | - | - | ||||||||
| Cortex | none | - | - | ||||||||
| CPu | - | 3 hr | none | ||||||||
| HC | none | - | - | ||||||||
| HyTh | - | 3 hr | none | ||||||||
| Limbic Forebrain | none | - | - | ||||||||
| Midbrain | ↓ | - | - | ||||||||
| Striatum | none | - | - | ||||||||
| Oral chronic intermittent 5–6 g/kg every other day | 120 days + 2 day withdrawal | Ex vivo | Rattus | Sprague | Tissue Content | HC | ↑ | 40 days | ↑ | Mitrirattanakul et al., 2007 | |
| AEA | 50–150 mM chronic EtOH treatment | 24–72 hr | In vitro | Homo | neuroblastoma SK-N-SH cells | Cell and medium extract | - | ↑ | - | - | Basavarajappa and Hungund, 1999a |
| 100–150 mM chronic EtOH Treatment | 72 hr | In vitro | Rattus | Sprague | Tissue Content | Cereb | ↑ | - | - | Basavarajappa et al., 2003 | |
| 7.2% (v/v) EtOH Liquid Diet | 10–15 days | Ex vivo | Rattus | Wistar | Tissue Content | AMG | - | 3 hr | ↓ |
Rubio et al., 2008 González et al., 2002 |
|
| Cereb | none | - | - | ||||||||
| CPu | - | 3 hr | ↓ | ||||||||
| HC | none | - | - | ||||||||
| HyTh | - | 3 hr | none | ||||||||
| Limbic Forebrain | ↓ | - | - | ||||||||
| Midbrain | ↓ | - | - | ||||||||
| Striatum | none | - | - | ||||||||
| Forced Vapor Inhalation | 72 hr | Ex vivo | Mus | Swiss-Webster | Tissue Content | Cortex | ↑ | 24 hr | none | Vinod et al., 2006 | |
| Oral chronic intermittent 5–6 g/kg every other day | 120 days + 2 day withdrawal | Ex vivo | Rattus | Sprague | Tissue Content | HC | none | 40 days | ↑ | Mitrirattanakul et al., 2007 | |
| CB1 | 7.2% (v/v) EtOH Liquid Diet | 10–15 days | Ex vivo | Rattus | Wistar | mRNA & Binding | AMG | - | 3 hr | none |
Rubio et al., 2008 Gonzalez et al., 2002 |
| none | - | - | |||||||||
| Cereb | none | - | - | ||||||||
| CPu | - | 3 hr | none | ||||||||
| none | - | - | |||||||||
| Cortex | - | 3 hr | none | ||||||||
| none | - | - | |||||||||
| HC | none | - | - | ||||||||
| HyTh | none | - | - | ||||||||
| NAc | none | - | - | ||||||||
| Forced Consumption of 10% (v/v) EtOH | 52 days | Ex vivo | Rattus | Wistar | mRNA | CPu | ↓ | - | - | Ortiz et al., 2004 | |
| Cortex | none | ||||||||||
| HC | Region Dependent | ||||||||||
| HyTh | ↓ | ||||||||||
| Operant EtOH Self-administration | 10 days | Ex vivo | Rattus | mSP | mRNA | AMG | none | - | - | Cippitelli et al., 2005 | |
| CPu | ↓ | ||||||||||
| Cortex | none | ||||||||||
| HC | none | ||||||||||
| Twice daily 4 g/kg i.p. | 10 days + 24 hr abstinence | Ex vivo | Mus | C57BL6/J | Protein | Cereb | none | 10 day | none | Pava et al., 2012 | |
| Cortex | none | ||||||||||
| HC | none | ||||||||||
| HyTh | ↓ | ||||||||||
| NAc | none | ||||||||||
| Striatum | none | ||||||||||
| VTA | ↑ | ||||||||||
| Continuous Forced Vapor Inhalation | 72–96 hr | Ex vivo | Mus | Swiss-Webster | Binding & GTPγS | Cereb | ↓ | 24 hr | none |
Vinod et al., 2006 Basavarajappa et al., 1998 Basavarajappa and Hungund, 1999b |
|
| Cortex | ↓ | none | |||||||||
| HC | ↓ | ↑ | |||||||||
| Striatum | ↓ | none | |||||||||
| Whole Brain | ↓ | - | - | ||||||||
| Chronic Intermittent Forced Vapor Inhalation | 49 days | Ex vivo | Rattus | Wistar | mRNA | Cortex | - | 3 weeks | ↑ | Rimondini et al., 2002 | |
| Oral chronic intermittent 5–6 g/kg every other day | 120 days + 2 day withdrawal | Ex vivo | Rattus | Sprague | Protein & mRNA | HC | ↓ | 40 days | ↑ | Mitrirattanakul et al., 2007 | |
| Alcohol dependent patients vs. controls | Lifetime | Post-mortem | Homo | - | Protein & GTPγS | NAc | ↓ | - | - | Vinod et al., 2010 | |
| FAAH | 100–150 mM chronic EtOH Treatment | 72 hr | In vitro | Rattus | Sprague | Activity | Cereb | ↓ | - | - | Basavarajappa et al., 2003 |
| Forced Vapor Inhalation | 72 hr | Ex vivo | Mus | Swiss-Webster | Activity | Cortex | ↓ | - | - | Vinod et al., 2006 | |
| Alcohol dependent patients vs. controls | Lifetime | Post-mortem | Homo | - | Protein & Activity | NAc | ↓ | - | - | Vinod et al., 2010 |
2-AG – 2-arachidonyl glycerol, AEA – anandamide, AMG – amygdala, CB1 – cannabinoid receptor 1, CPu – caudate-putamen, Cereb – cerebellum, EtOH – ethanol, FAAH – fatty acid amide hydrolase, HC – hippocampus, HyTh – hypothalamus, NAc – nucleus accumbens, VTA – ventral tegmental area
The results demonstrating altered CB1 expression following withdrawal from chronic ethanol beg the question of what effect reduced CB1 signaling has on the neuroadaptions occurring through withdrawal. One effect of the down-regulation in CB1 may be to counteract neuroadaptations at GABAergic and glutamatergic synapses following chronic ethanol consumption. Warnault et al (2007) observed that CB1 KO mice did not exhibit the chronic ethanol-induced changes in cortical, hippocampal, and cerebellar NMDA and GABAA receptor expression found in wt mice. However, future studies utilizing conditional knockouts, CB1 antagonists, or genetic knockdown experiments would be useful to determine if this observation is due to developmental mechanisms specific to constitutive knockouts.
A likely cause of the reduced CB1 expression found by these studies is an enhancement of EC concentrations induced by chronic ethanol. The first data to support this hypothesis came from experiments performed in cultured cells where it was observed that chronic incubation with intoxicating concentrations of ethanol enhanced both AEA and 2-AG content (Basavarajappa and Hungund, 1999a; Basavarajappa et al., 2000). These authors posited that the enhanced EC levels were due to activation of PLA2 by chronic ethanol thereby increasing EC synthesis (Basavarajappa et al., 1997; Hungund et al., 2002). Other labs have made similar observations following chronic treatment. In rats forced to consume ethanol (7.2%) in a liquid diet, tissue content of AEA was increased in the limbic forebrain, and interestingly, AEA levels were reduced in the midbrain (González et al., 2002), amygdala, and striatum (Rubio et al., 2008). Mitrirattanakul et al (2007) reported that rats made ethanol dependent using a chronic intermittent ethanol regimen displayed increased levels of AEA and 2-AG in the hippocampus that persisted 40 days into withdrawal. Additionally, mice treated with ethanol vapor for 72 hrs displayed increased concentrations of AEA in cortex that were correlated with decreased activity of FAAH (Vinod et al., 2006), and similar results were obtained in cultured cerebellar neurons where enhanced media levels of AEA were associated with reduced activity of FAAH and EC transport mechanisms following chronic ethanol treatment (Basavarajappa et al., 2003). Results from a post-mortem study of human tissue also found reduced expression and activity of FAAH in the ventral striatum of alcoholic patients, and these changes in FAAH were correlated with enhanced tissue content of AEA and reduced expression of CB1 (Vinod et al., 2010). In addition to studies with ethanol, FAAH KO mice display reduced CB1 expression and G-protein coupling in several brain regions including the striatum, hippocampus, NAc, and amygdala (Vinod et al., 2008a). Together these results suggest that the increased levels of ECs following chronic ethanol may arise from enhanced synthesis and reduced inactivation mechanisms. However, to fully establish the causative role of the ethanol-induced increases in EC content as the mechanism responsible for down-regulation of CB1, studies utilizing genetic and pharmacological tools to block EC metabolism will need to be conducted. Unfortunately, the multiple pathways of AEA biosynthesis make the selective reduction of AEA a difficult task, so new methodologies will be required to advance our understanding of the molecular mechanisms associated with the EC systems role in ethanol tolerance.
The relevance of a reduction in CB1 expression for tolerance mechanisms is somewhat obvious given the preponderance of evidence suggesting that ethanol acts, in part, to facilitate EC release. However, it is much less clear what effect reduced CB1 expression has on withdrawal symptoms. Ethanol withdrawal is characterized by enhanced CNS excitability that can result in hallucinations and seizures (Saitz, 1998). Because CB1 is located at both GABAergic and glutamatergic terminals, activation of this receptor could produce either a facilitation or inhibition of withdrawal symptoms. Consequently, it is not surprising that there is some discrepancy in the literature as to the effect of CB1 on withdrawal. Two studies report decreased HIC scores in CB1 KO mice following chronic ethanol vapor inhalation (Vinod et al., 2008b) and 4 week forced consumption (Racz et al., 2003) while a third reports enhanced HICs following a 12-day forced consumption paradigm (Naassila et al., 2004). Importantly, Vinod et al (2008b) also observed that the reduction in withdrawal severity could be reconstituted in wt mice by treating them with SR. Taken in the context of earlier studies reporting increased withdrawal severity following administration of CB1 agonists (Kralik et al., 1976; Sprague and Craigmill, 1978), these results suggest a positive correlation between CB1 activation and HIC severity. However, these early studies used THC, a full CB1 agonist, to enhance withdrawal severity, and in addition to the potential confounds associated with using CB1 agonists to modulate HIC measures (see historical perspectives section), these studies reported differential findings based on the measure of withdrawal. To more fully delineate the relationship between CB1 activation and withdrawal severity studies implementing partial agonists are useful, but at present these data are nearly absent from the literature. One study using FAAH KO mice found that these mice displayed reduced severity of HICs following withdrawal from chronic ethanol (Vinod et al., 2008a). These data indicate that the EC system is involved in modulating ethanol withdrawal symptoms in a complex way, and future work should focus on the utility of partial CB1 agonists as well as FAAH and MAGL inhibitors for the control of withdrawal symptoms.
ECs and Relapse
The goal of all addiction therapies is to prevent a relapse to binge use, and given the EC system’s association with ethanol reward, consumption, and withdrawal processes, it is logical to assume that this neuromodulatory system is involved in the mechanisms underlying relapse. Data supporting of the involvement of CB1 in relapse have been obtained primarily from reinstatement models of ethanol self-administration in rodents where CB1 agonists facilitate reinstatement and CB1 antagonists block it. Several studies have reported that non-contingent administration of WIN during abstinence facilitates the subsequent reinstatement to ethanol responding (Alén et al., 2009; López-Moreno et al., 2004), and this increase in ethanol-seeking behavior is correlated with reduced hippocampal neurogenesis (Alén et al., 2010). Furthermore, sub-chronic administration of WIN reduces the DA response in the NAc shell to a subsequent dose of ethanol administered 24 hrs later, and these effects may be compounded by similar reductions observed after chronic treatment with ethanol (Lopez-Moreno et al., 2008). These data suggest that the enhancement of ethanol-seeking following the administration of CB1 agonists during periods of abstinence may be due to a blunted DA release in the NAc above and beyond that observed in subjects that only receive ethanol. However, all of these studies have employed non-contingent treatment with WIN, and it would be useful to determine if rotating schedules of WIN and ethanol self-administration can produce similar effects. Pending the outcome of these studies, these results may have implications for the understanding and treatment of co-morbid CUDs and AUDs.
Along with work involving CB1 agonists, a several studies have been performed to examine the effects of CB1 blockade on reinstatement to ethanol self-administration. Results from this work suggest that treatment with the CB1 antagonist SR prior to the reinstatement session reduces cue-induced relapse (Cippitelli et al., 2005; Economidou et al., 2006; Economidou et al., 2007), and combining sub-threshold doses of SR with either an adenosine A2A or mGluR5 antagonist also reduces ethanol responding in cued relapse sessions (Adams et al., 2010). This latter approach may prove especially useful for clinical applications seeking to circumvent the negative psychiatric side-effects of higher doses of SR. Importantly, SR treatment has no effect on foot-shock-induced relapse suggesting that CB1 receptors are not involved in mediating stress-induced relapse (Economidou et al., 2006; Economidou et al., 2007). Lastly, Cippitelli et al (2008) found that enhancing brain AEA levels with FAAH inhibitors failed to alter either cue- or stress-induced relapse to ethanol-seeking behavior. These results are not surprising given the number of studies reporting increased AEA levels that persist following chronic ethanol. Together the data from reinstatement experiments support the involvement of CB1 in cue-induced relapse behavior, but more work needs to be conducted to determine the neuroanatomical loci of the effects of SR to reduce reinstatement. In addition, future studies will need to consider the role of ECs in mediating relapse to ethanol self-administration.
The EC System and the Susceptibility to Alcohol Abuse Disorders
In addition to data that suggest the function of EC system is altered following chronic ethanol use and withdrawal, it is possible that allelic variation in the CNR1 gene may contribute to innate susceptibility to alcohol dependence. Unfortunately, the genetic association between CNR1 alleles and the susceptibility for alcohol dependence is clouded by conflicting datasets in most cases. Homozygosity for a CNR1 allele having five or more repeats of a microsatellite polymorphism is associated with a reduction of the P300 potential in the frontal lobes (Johnson et al., 1997), and reduced amplitude of the P300 wave is known to be a physiological marker that runs in families with a history of alcohol dependence and is associated with attentional processes (Begleiter et al., 1984). However, a companion study found no correlation between geneotype and self-reported alcohol-associated problems (Comings et al., 1997). In contrast, subsequent studies have found correlations between this allele and comorbidity between alcohol dependence and ADHD (Ponce et al., 2003) or psychopathy (Hoenicka et al., 2007), but at present the data still do not support a direct correlation between this allele and a susceptibility for alcohol dependence.
Some single nucleotide polymorphisms (SNPs) have been identified that confer a susceptibility to specific aspects of alcohol dependence. The 1359G/A polymorphism (rs1049353) has been implicated in mediating the withdrawal severity experienced by chronic alcoholic patients where individuals homozygous for the A allele display more severe symptoms than other genotypes (Schmidt et al., 2002). However, the effect of this SNP on withdrawal severity is contested as another report failed to find evidence of 1359G/A’s association with the incidence of ethanol withdrawal seizures (Preuss et al., 2003). Furthermore, another study failed to find a correlation between rs1049353 and alcohol dependence, so it seems unlikely that this SNP is important for mediating a susceptibility to alcohol dependence (Zuo et al., 2007). However, the study by Zou et al (2007) did identify two SNPs (rs806371 and rs645467) conferring susceptibility and one SNP (rs806377) conferring resistance to alcohol dependence, but to date, no subsequent work has been published on these polymorphisms as regards alcohol dependence. In addition to these, another CNR1 SNP (rs202323) has been implicated in mediating cue-reactivity to alcohol in heavy drinkers, where individuals with at least one copy of the C allele display increased craving and salivary response to an ethanol associated cue (van den Wildenberg et al., 2007). A follow up to this study found that the C allele was correlated with increased CB1 expression in post-mortem samples of human PFC, and alcohol dependent patients with the C allele had increased activation in the PFC, orbitofrontal cortex, and NAc in response to ethanol-associated cues (Hutchison et al., 2008). These individuals also reported increased sensation of reward and positive affect following consumption of several alcoholic beverages, suggesting the C allele may confer increased likelihood of binge ethanol use. These results suggest that genetic variation in the CNR1 may contribute susceptibility to the development of AUDs, but future studies are needed to confirm and extend the data regarding the role these SNPs play in alcohol dependence.
In addition to the genetic studies, several post-mortem studies of brain tissue from chronic alcohol dependent patients have indicated CB1 expression is altered in the prefrontal cortex and ventral striatum (Vinod et al., 2005; Vinod et al., 2010). Notably, alcohol dependent patients who commit suicide display increased CB1 expression in the PFC and NAc compared to alcoholic patients who died of other causes. In addition, one study also reported that recently abstinent alcohol dependent patients do not display the increase in circulating AEA correlated with ethanol craving in control subjects (Mangieri et al., 2009). These studies raise the possibility that altered function of the EC system could be useful as a biomarker of alcohol dependence or a risk factor for suicide in alcoholic patients.
Summary of Ethanol and the EC System
The results outlined in the previous sections strongly argue for the involvement of the EC system in regulating aspects of the acute response to ethanol, the establishment of tolerance/dependence, and the propensity to relapse. In this section we will briefly summarize the salient points mentioned earlier and provide a tentative model for the change in function of the EC system across these stages of addiction (figure 2). To begin, a consistent finding has been that ethanol interacts with the EC system to produce its acute, reinforcing effects which may drive further drug seeking. Studies of VTA DA neuron activity and of DA concentrations in the NAc provide support for the hypothesis that ethanol-induced EC release in the VTA serves to activate DA neurons (Cheer et al., 2004; Cheer et al., 2007; Hungund et al., 2003; Perra et al., 2005). One mechanism that likely contributes to this effect is a disinhibition of DA neurons produced by a CB1-mediated decrease of GABA release onto these neurons (Riegel and Lupica, 2004). In addition, acute ethanol inhibits hippocampal neurons via an EC-mediated reduction in glutamate release (Basavarajappa et al., 2008). If this can be extended to cortical neurons then ethanol-mediated EC release would be expected to reduce cortical output thus producing a synergistic mechanism with the increased activity of the mesolimbic DA pathway. Alternatively, the effect of SR to reduce the activation of mesolimbic DA pathways could be due to an enhancement of mesocortical DA function in the presence of this compound (Tzavara et al., 2003), but numerous studies have reported that ethanol increases tissue content of ECs. For instance, Caillé et al (2007) reported increased 2-AG levels in the NAc of rats engaged in ethanol self-administration, and infusion of CB1 agonists into the posterior VTA facilitates ethanol consumption suggesting a common mechanism (Linsenbardt and Boehm, 2009). To clarify this point, future studies should seek to directly measure interstitial EC concentrations in the VTA following acute ethanol administration. However, the present data suggest that EC release from mesolimbic DA neurons in response to ethanol is a mechanism by which these cells could become disinhibited and drive the reinforcing properties of this drug.
Figure 2.
Summary of ethanol-induced alterations in the EC system and a putative model of the generalized changes in EC signaling observed in different stages of chronic alcoholization. Acute ethanol consumption is associated with a CB1-dependent increase in dopamine neuron activity and dopamine release in the nucleus accumbens as well as reduced output from frontal cortical structures and the hippocampus. These effects are reversed by pre-treatment with rimonabant. In most brain structures, chronic ethanol treatment is associated with reductions in CB1 expression and FAAH activity that are paralleled by increased concentrations of anandamide. These changes normalize as the duration of chronic treatment is extended. The hyper-excitability associated with the initial phase of withdrawal results in a large increase in EC release and concomitant reductions in CB1 expression. Over time EC levels remain elevated and CB1 expression is up-regulated to compensate. For details see text. Green arrows denote glutamate or excitatory connections. Red arrows denote GABA or inhibitory connections. Blue arrows indicate dopamine projections. Thick lines represent enhanced output while hashed lines represent diminished connection strength. Text symbols and abbreviations: ↑= increase in concentration/expression/activity, ↓ = decrease in concentration/expression/activity, ? = unknown effect/no data, 2-AG = 2-arachidonyl glycerol, 5-HT = 5-hydroxytryptamine, AEA = anandamide, AMG = amygdala, BLA = basolateral amygdala, CB1 = cannabinoid receptor 1, DA = dopamine, DStr = dorsal striatum, ECs = endocannabinoids, EtOH = ethanol, FAAH = fatty acid amide hydrolase, GABA = γ-amino-butyric acid, Glu = glutamate, HC = hippocampus, NAc = nucleus accumbens, NE = norepinephrine, PFC = prefrontal cortex, SK = Small conductance calcium-activated potassium channel, SR = rimonabant, VTA = ventral tegmental area.
In studies where ethanol is applied chronically, there is a high degree of variability concerning the pattern and duration of ethanol treatment, and this variability is both a blessing and a curse when deciphering the effect of chronic alcohol on the EC system. On the positive side, the broad variety of treatment paradigms employed by these studies provides a wider perspective on the timescale of changes to the EC system, particularly the CB1 receptor. However, the discrepancy between methodologies is also a confound when trying to disentangle contrasting datasets. Nevertheless, some trends regarding the effects of chronic ethanol on the EC system do emerge. Hungund’s lab has consistently relied on 72 hr continuous treatment regimens for their work in both mice and cell cultures. While this paradigm does induce withdrawal and tolerance in mice, it is distinct from long-term regimens used in other studies where animals are subject to repeated periods of abstinence or discontinuous exposure to ethanol due to metabolism or voluntary drinking patterns. As a consequence, data from 72 hr exposure regimens provide a better understanding of what might be occurring to the EC system following a prolonged binge. The results from this work consistently show an increase in EC concentrations that is associated with reduced function of FAAH and CB1, but these effects reverse to basal levels after only 24 hr of withdrawal. In studies where the exposure is somewhat longer and the BEC oscillates because subjects are not under chronic pulmonary treatment, CB1 expression appears much more variable, and alterations to CB1 are brain region dependent (Gonzalez et al., 2002; Moranta et al., 2006; Pava et al., 2012). However, in long-term studies (over one month of ethanol exposure) where experiments are performed soon after the discontinuation of ethanol CB1 expression seems to be consistently reduced, and at least one study has reported elevated EC content (Mitrirattanakul et al., 2007; Ortiz et al., 2004). Interestingly, two studies that allowed subjects to go through a several week-long withdrawal prior to testing EC content or CB1 expression found elevated levels of both (Mitrirattanakul et al., 2007; Rimondini et al., 2002). These data suggest that EC tone may be persistently increased well into abstinence, and CB1 expression is up-regulated over time to allow the EC system to respond to phasic changes. An important caveat in interpreting these findings is that most of this data pertains to forebrain structures including the hippocampus, cortex, amygdala, striatum and NAc. Data from our lab suggests that sub-chronic treatment with ethanol produces an up-regulation in the expression of CB1 in midbrain (Pava et al., 2012), but to date, no published studies of long-term treatment have examined midbrain CB1 or EC levels. However, EC release at GABAergic synapses onto VTA DA neurons is under control of SK channels, and treatment of these cells with the SK channel blocker, apamin, is known to reduce GABA release via a CB1-dependent mechanism (Riegel and Lupica, 2004). SK channel function is reduced in the VTA following chronic ethanol (Hopf et al., 2007) suggesting that EC release may be facilitated. If this results in enhanced EC tone, then CB1 expression would be predicted to decrease as this receptor is rapidly internalized and targeted for lysosomal degradation in response to agonist binding (Martini et al., 2007; Tappe-Theodor et al., 2007). As a consequence, we hypothesize that CB1 expression is likely to be reduced in the midbrain during the initial phase of withdrawal.
Reinstatement studies provide clear evidence that CB1 activation is involved in cue-induced but not stress-induced relapse. However, no studies to date have employed strategies to understand which neuroanatomical structures are responsible for the effects of SR to block reinstatement of ethanol-seeking. Considering the role the EC system plays in regulating VTA burst firing, it is possible that CB1 activation may contribute to the DA release observed in response to drug-paired cues (Schultz, 1998). However, the complete lack of any data regarding the function of the EC system in discrete brain regions during cued-reinstatement renders this hypothesis as utter speculation. As a consequence, much work needs to be done to elucidate the mechanisms by which this important neuromodulatory system contributes to relapse.
Future Directions for Research
As is evident from the preceding sections, a fundamental basis of knowledge exists for the changes to EC system function over the development of ethanol dependence, but much work needs to be done to establish a mechanism for how these changes contribute to addiction pathology. In particular, studies employing region specific modulation of EC signaling are needed to establish the brain nuclei important for EC-mediated contributions to the various phases of alcohol dependence, including: the escalation of use, the negative affect and stress associated with withdrawal, and the reinstatement to drug-seeking following abstinence. Beyond establishing the mechanistic contributions of the EC in the development of alcohol dependence, there are additional areas of EC research that may prove fruitful in our understanding of addiction. Some of these topics have recently emerged as our knowledge of the basic molecular components of the EC system continues to grow, and others involve complex experimental designs that are appropriate only now that a fundamental understanding of the alterations to EC signaling in alcohol dependence is in hand. This section of the review will seek to provide a list of these burgeoning issues along with research relevant to their potential role in AUDs.
CB1 Binding Proteins
A consistent finding from studies using sub-chronic or chronic ethanol treatments is that CB1 expression is reduced either immediately after treatment or during acute withdrawal. Why does this occur, and what is the molecular machinery responsible for this process? The CB1 receptor is known to be rapidly internalized and transported to lysosomal degradation pathways in periods of saturable activation, and the tolerance that develops to the analgesic effects of CB1 agonists is attributed to this process. One mechanism by which this can occur is through binding proteins like G-protein-associated sorting protein 1 (GASP1) that target CB1 for degradation (Martini et al., 2007; Tappe-Theodor et al., 2007), and a recent study has found that mice lacking the GASP1 gene do not develop the analgesic tolerance to WIN observed in wt mice (Martini et al., 2010). Our lab has recently reported that sub-chronic treatment with ethanol induces a cross-tolerance to several WIN-induced behaviors including its antinociceptive effects (Pava et al., 2012), and it is possible that this reduction in CB1 function may be mediated by GASP1. Alternatively, cross-tolerance between the analgesic effects of μ-opioid and CB1 receptor activation is mediated by receptor desensitization that involves signaling through Gαz, and mice with a knockdown of Gαz do not develop this cross-tolerance (Garzón et al., 2009). As a consequence, the ethanol-induced knockdown of CB1 function may be facilitated through Gαz signaling.
In addition to these CB1-associated proteins, the cannabinoid receptor associated protein 1a (CRIP1a) has been implicated in modulating the function of CB1 in response to antagonists. Notably, over-expression of CRIP1a with CB1 reduces the increased Ca2+ conductance observed when SR is applied to cells where only CB1 is expressed (Niehaus et al., 2007). Furthermore, over-expression of CRIP1a in cultured cortical neurons reveals a neuroprotective effect of SR treatment in response to glutamate excitotoxicity (Brower, 2001). As a consequence, an understanding of CRIP1a modulation of CB1 may help to clarify the conflicting results regarding the effect of cannabinoid pharmacotherapies on withdrawal severity. However, few studies have followed up on the original report, and much work still needs to be done to fully characterize the involvement of CRIP1a-regulation of CB1 signaling.
The Role of Non-CB1 Cannabinoid Receptors in AUDs
To date, most studies involving the role of the EC system in AUDs have focused on EC transmitters, their related synthetic and inactivating enzymes, and the CB1 receptor. This is almost certainly due to the well-ingrained dogma in the cannabinoid field that CB1 represents the central cannabinoid receptor (Matsuda et al., 1990) and CB2 is the peripheral cannabinoid receptor (Bayewitch et al., 1995). However, the existence and role of central CB2 receptors is beginning to gain some traction (Atwood and Mackie, 2010; Onaivi et al., 2011), and a recent behavioral study indicates that CB2 is involved anxiogenic, pneumonic, and motoric processes (Ortega-Alvaro et al., 2011). Furthermore, ethanol treatment and consumption is known to alter CB2 gene expression in the brain (Onaivi et al., 2008). As a consequence, future research should be mindful of the lack of specificity of most cannabinoid agonists and should attempt to discriminate CB1- versus CB2-mediated drug actions before ascribing a role to CB1. In addition to CB2, evidence from the past decade has indicated that the TRPV1 receptor is also associated with the EC system namely through activation by AEA, and several studies have demonstrated that ethanol potentiates TRPV1 mediated responses (Nicoletti et al., 2008; Trevisani et al., 2002; Vetter et al., 2008). More importantly, TRPV1 null mutant mice display higher ethanol preference and reduced sensitivity to ethanol induced sleep and ataxia, and the reduced behavioral sensitivity to ethanol are also observed in wt mice administered a TRPV1 antagonist (Hinson and Siegel, 1986). These results suggest that AEA signaling through TRPV1 may prove important to mediating some effects of EC signaling in response to ethanol, and future studies should examine alterations in TRPV1 expression and function following chronic ethanol treatment.
Models of Co-morbid Cannabinoid and Alcohol Dependence
As mentioned at the beginning of this review, the rate of co-abuse of ethanol and cannabis is strikingly high, but surprisingly few studies have been conducted to examine the effect of co-abuse on the EC system or behaviors associated with ethanol/cannabis consumption. A few studies in rodents have reported that administration of CB1 agonists facilitates ethanol consumption (Gallate et al., 1999; Linsenbardt and Boehm, 2009; Vinod et al., 2008b), and a series of reports have implicated the activation of CB1 by exogenous cannabinoids as a factor in enhancing reinstatement to ethanol seeking (Alén et al., 2009; López-Moreno et al., 2004). In addition, one study found that administration of THC to ethanol-consuming rats produced a synergistic effect to impair memory processes (Ciccocioppo et al., 2002). Beyond the effect of these drugs on the reward circuitry of the brain, several reports suggest that co-administration of ethanol and CB1 agonists may facilitate the neurotoxic effects of ethanol on neural tissue (Alén et al., 2010; Hansen et al., 2008). Interestingly, mice with a null mutation of DAGLα have reduced neurogenesis in the hippocampus (Gao et al., 2010). Co-administration of ethanol with a CB1 agonist is predicted to down-regulate cannabinoid signaling beyond that of ethanol administration alone, so the effects observed in DAGLα KO mice are in line with this effect. Additionally, these results suggest that the reduced neurogenesis observed in the ethanol-cannabinoid co-administration models may be mediated by reduced 2-AG signaling. It is important to note that all of these studies have employed non-contingent administration of the CB1 agonist, and this reduces the face validity of these studies for co-abuse models. Consequently, future studies should be performed using non-contingent ethanol administration paired with self-administration procedures for CB1 agonists in addition to studies employing contingent methods of drug delivery for both substances.
EC System and Stress in Ethanol Consumption
A large and growing body of evidence indicates that the EC system is involved in mediating the response to stress. For instance, activation of a novel, non-genomic glucocorticoid receptor in the hypothalamus produces an EC-mediated reduction in glutamate EPSCs on parvocellular neurons of the paraventricular nucleus, and this provides feedback inhibition along the hypothalamic-pituitary-adrenal axis (Di et al., 2003; Di et al., 2005; Di et al., 2009). Additionally, chronic treatment with glucocorticoid receptor agonists is associated with decreased levels of 2-AG and CB1 in the hippocampus (Hill et al., 2008), and similar findings are also observed in a rodent model of chronic unpredictable stress (CUS) that results in a persistent increase in circulating levels of corticosterone (CORT; Hill et al., 2005). Furthermore, stress is one of the predominant causes associated with relapse to alcohol abuse, and chronic ethanol treatment and withdrawal produce persistent changes in glucocorticoid signaling. CORT levels are known to remain elevated in brain tissue up to several months following chronic ethanol treatment even after circulating CORT has returned to basal levels (Little et al., 2008), and this persistent elevation in CORT may interact with CB1 expression and 2-AG levels along the lines observed following CUS. Other studies have implicated the EC system in dampening the stress response to an acute injection of ethanol (Cippitelli et al., 2008) and in mediating stress-induced drinking in mice (Racz et al., 2003). Additionally, the increased stress response during early phase of withdrawal is attenuated by acute injection of SR (Rubio et al., 2008). Given the data from studies of alcohol dependence in humans and in rodents that demonstrate altered functioning of the EC system, it seems likely that concomitant changes in glucocorticoid signaling may be involved. These data suggest that the interaction between stress, ethanol, and the EC system may be fruitful ground for new research.
Conclusions
A retrospective examination of the early literature on the interactions between ethanol and cannabinoids finds clear evidence for the involvement of the EC system in the acute reinforcing properties of ethanol and the neuroadaptive changes that occur with its chronic abuse. However, it was not until the 1990’s that the molecular constituents of the EC system were discovered, and only in the past decade has the interaction between ethanol and the EC system been directly investigated. From these studies it is apparent that EC release in response to ethanol mediates the reinforcing properties of this drug and that chronic ethanol use resulting in tolerance and dependence dramatically alters the function of EC signaling. However, our understanding of the mechanisms responsible for the changes in EC signaling in response to chronic ethanol is lacking, and the role of EC system in regulating specific circuits associated with addiction processes is limited. Future work with more standardized methodologies will be needed to better grasp the complexity underlying the interaction between this neuromodulatory system and alcohol dependence. In addition, novel areas of research continue to emerge with basic discoveries surrounding the molecular constituents of the EC system.
Acknowledgments
This work was supported by National Institutes of Health Grants P50AA10761 (Charleston Alcohol Research Center; JJW) and F31AA018908 (MJP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CL, Short JL, Lawrence AJ. Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors. Br J Pharmacol. 2010;159:534–542. doi: 10.1111/j.1476-5381.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614. [PubMed] [Google Scholar]
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Alén F, Mouret A, Viveros M-P, Llorente R, Lepousez G, Lledo P-M, López-Moreno JA. Converging action of alcohol consumption and cannabinoid receptor activation on adult hippocampal neurogenesis. Int J Neuropsychopharmacol. 2010;13:191–205. doi: 10.1017/S1461145709991118. [DOI] [PubMed] [Google Scholar]
- Alén F, Santos A, Moreno-Sanz G, González-Cuevas G, Giné E, Franco-Ruiz L, Navarro M, López-Moreno JA. Cannabinoid-induced increase in relapse-like drinking is prevented by the blockade of the glycine-binding site of N-methyl-D-aspartate receptors. Neuroscience. 2009;158:465–473. doi: 10.1016/j.neuroscience.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999a;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTP gamma S binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999b;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem. 2008;107:1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Activation of arachidonic acid-specific phospholipase A2 in human neuroblastoma cells after chronic alcohol exposure: prevention by GM1 ganglioside. Alcohol Clin Exp Res. 1997;21:1199–1203. [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bayewitch M, Avidor-Reiss T, Levy R, Barg J, Mechoulam R, Vogel Z. The peripheral cannabinoid receptor: adenylate cyclase inhibition and G protein coupling. FEBS Lett. 1995;375:143–147. doi: 10.1016/0014-5793(95)01207-u. [DOI] [PubMed] [Google Scholar]
- Bech P, Rafaelsen L, Rafaelsen OJ. Cannabis and alcohol: effects on estimation of time and distance. Psychopharmacology (Berl) 1973;32:373–381. doi: 10.1007/BF00429474. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Bruggemann EP, Melchior DL. Alterations in the organization of phosphatidylcholine/cholesterol bilayers by tetrahydrocannabinol. J Biol Chem. 1983;258:8298–8303. [PubMed] [Google Scholar]
- Cadas H, Schinelli S, Piomelli D. Membrane localization of N-acylphosphatidylethanolamine in central neurons: studies with exogenous phospholipases. J Lipid Mediat Cell Signal. 1996;14:63–70. doi: 10.1016/0929-7855(96)00510-x. [DOI] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 1994;115:340–349. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien MLAV, Phillips PEM, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, Aragona BJ, Phillips PEM, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JH, Goldstein DB. Membrane-disordering action of ethanol: variation with membrane cholesterol content and depth of the spin label probe. Mol Pharmacol. 1981;19:425–431. [PubMed] [Google Scholar]
- Ciccocioppo R, Antonelli L, Biondini M, Perfumi M, Pompei P, Massi M. Memory impairment following combined exposure to delta(9)-tetrahydrocannabinol and ethanol in rats. Eur J Pharmacol. 2002;449:245–252. doi: 10.1016/s0014-2999(02)01999-4. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, Ciccocioppo R, Rodríguez de Fonseca F. The anandamide transport inhibitor AM404 reduces ethanol self-administration. Eur J Neurosci. 2007;26:476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, Massi M, Bermudez-Silva FJ, Navarro M, Ciccocioppo R, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Clarke RBC, Adermark L. Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology. 2010;58:799–805. doi: 10.1016/j.neuropharm.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fà M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray J. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2:161–168. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- da Silva GE, Morato GS, Takahashi RN. Rapid tolerance to Delta(9)-tetrahydrocannabinol and cross-tolerance between ethanol and Delta(9)-tetrahydrocannabinol in mice. Eur J Pharmacol. 2001;431:201–207. doi: 10.1016/s0014-2999(01)01449-2. [DOI] [PubMed] [Google Scholar]
- Davidson M, Wilce P, Shanley B. Ethanol increases synaptosomal free calcium concentration. Neurosci Lett. 1988;89:165–169. doi: 10.1016/0304-3940(88)90375-8. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, Abumrad N. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002a;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002b;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Ubaldi M, Lourdusamy A, Soverchia L, Hardiman G, Campolongo P, Cuomo V, Ciccocioppo R. Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Delta9-tetrahydrocannabinol. ToxicolAppl Pharmacol. 2007;223:73–85. doi: 10.1016/j.taap.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Pistis M, Fratta W. The endocannabinoid system and nondrug rewarding behaviours. Exp Neurol. 2010;224:23–36. doi: 10.1016/j.expneurol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Fehr KA, Kalant H, LeBlanc AE. Residual learning deficit after heavy exposure to cannabis or alcohol in rats. Science. 1976;192:1249–1251. doi: 10.1126/science.1273591. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Bermúdez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Di Marzo V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol. 2007;152:594–601. doi: 10.1038/sj.bjp.0707379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- Friedman E, Gershon S. Effect of delta8-THC on alcohol-induced sleeping time in the rat. Psychopharmacology (Berl) 1974;39:193–198. doi: 10.1007/BF00421026. [DOI] [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl) 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;70:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang M-Y, Strassle BW, Lu P, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón J, de la Torre-Madrid E, Rodríguez-Muñoz M, Vicente-Sánchez A, Sánchez-Blázquez P. Gz mediates the long-lasting desensitization of brain CB1 receptors and is essential for cross-tolerance with morphine. Mol Pain. 2009;5:11. doi: 10.1186/1744-8069-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Heilig M, Ramchandani VA, Geyer C, Spero DE, et al. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology. 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Grushko J, Golebiewska U, Hoogendoorn S, Gupta A, Heimann A, Ferro E, Scarlata S, Fricker L, Devi L. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009 doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend. 2002;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, O’Shea E, Vicario-Abejon C, Colado MI, Moratalla R. Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration : early loss of TH in striosomes after methamphetamine. Neurotox Res. 2010;18:48–58. doi: 10.1007/s12640-009-9106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Lopez MD, Llopis N, Feng S, Barrett DA, O’Shea E, Colado MI. Involvement of 2-arachidonoyl glycerol in the increased consumption of and preference for ethanol of mice treated with neurotoxic doses of methamphetamine. Br J Pharmacol. 2010;160:772–783. doi: 10.1111/j.1476-5381.2010.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Krutz B, Sifringer M, Stefovska V, Bittigau P, Pragst F, Marsicano G, Lutz B, Ikonomidou C. Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Ann Neurol. 2008;64:42–52. doi: 10.1002/ana.21287. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermúdez-Silva FJ, Malinen H, Hyytiä P, Sanchez-Vera I, Rimondini R, Rodríguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Gopher A, Almog S, Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem. 1993;36:3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Science signaling. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci USA. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE. Alcohol and marijuana: comparative dose effect profiles in humans. Pharmacol Biochem Behav. 1988;31:649–655. doi: 10.1016/0091-3057(88)90244-4. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WSV, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Harris RA, Bloom AS. Effects of the cannabinoids on physical properties of brain membranes and phospholipid vesicles: fluorescence studies. J Pharmacol Exp Ther. 1985;232:579–588. [PubMed] [Google Scholar]
- Hillard CJ, Pounds JJ, Boyer DR, Bloom AS. Studies of the role of membrane lipid order in the effects of delta 9-tetrahydrocannabinol on adenylate cyclase activation in heart. J Pharmacol Exp Ther. 1990;252:1075–1082. [PubMed] [Google Scholar]
- Hinson RE, Siegel S. Pavlovian inhibitory conditioning and tolerance to pentobarbital-induced hypothermia in rats. J Exp Psychol Anim Behav Process. 1986;12:363–370. [PubMed] [Google Scholar]
- Hoenicka J, Ponce G, Jiménez-Arriero MA, Ampuero I, Rodríguez-Jiménez R, Rubio G, Aragüés M, Ramos JA, Palomo T. Association in alcoholic patients between psychopathic traits and the additive effect of allelic forms of the CNR1 and FAAH endocannabinoid genes, and the 3' region of the DRD2 gene. Neurotox Res. 2007;11:51–60. doi: 10.1007/BF03033482. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS. Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. J Neurosci Res. 2000;60:122–128. doi: 10.1002/(SICI)1097-4547(20000401)60:1<122::AID-JNR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS, Vadasz C, Kunos G, Rodriguez de Fonseca F, Colombo G, Serra S, Parsons L, Koob GF. Ethanol, endocannabinoids, and the cannabinoidergic signaling system. Alcohol Clin Exp Res. 2002;26:565–574. [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Zheng Z, Lin L, Barkai AI. Ganglioside GM1 reduces ethanol induced phospholipase A2 activity in synaptosomal preparations from mice. Neurochem Int. 1994;25:321–325. doi: 10.1016/0197-0186(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Horton WJ, Filbey F. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Littleton JM, Nhamburo PT. Increased activity of Ca2+-dependent enzymes of membrane lipid metabolism in synaptosomal preparations from ethanol-dependent rats. J Neurochem. 1985;44:1235–1241. doi: 10.1111/j.1471-4159.1985.tb08749.x. [DOI] [PubMed] [Google Scholar]
- Johnson JP, Muhleman D, MacMurray J, Gade R, Verde R, Ask M, Kelley J, Comings DE. Association between the cannabinoid receptor gene (CNR1) and the P300 event-related potential. Mol Psychiatry. 1997;2:169–171. doi: 10.1038/sj.mp.4000246. [DOI] [PubMed] [Google Scholar]
- Jones RT, Stone GC. Psychological studies of marijuana and alcohol in man. Psychopharmacology (Berl) 1970;18:108–117. doi: 10.1007/BF00402390. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Hermann A, Glaser ST, Bojesen IN, Deutsch DG. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem. 2006;281:9066–9075. doi: 10.1074/jbc.M509721200. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Increased drinking in a trial of treatments for marijuana dependence: substance substitution? Drug Alcohol Depend. 2009;105:168–171. doi: 10.1016/j.drugalcdep.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol Rev. 2009;89:309. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Tornqvist H, Holm C. Expression, purification, and characterization of histidine-tagged mouse monoglyceride lipase from baculovirus-infected insect cells. Protein Expr Purif. 2000;18:286–292. doi: 10.1006/prep.1999.1194. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M, Criswell H, Breese G. The Role of Protein Kinase A in the Ethanol-induced Increase in Spontaneous GABA Release onto Cerebellum Purkinje Neurons. J Neurophysiol. 2008 doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JJ, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des. 2004;10:659–667. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- Kralik PM, Ho BT, Matthews HR. Effect of delta-THC on ethanol withdrawal in mice. Experientia. 1976;32:723–725. doi: 10.1007/BF01919854. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001a;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001b;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lallemand F, De Witte P. Ethanol induces higher BEC in CB1 cannabinoid receptor knockout mice while decreasing ethanol preference. Alcohol Alcohol. 2005;40:54–62. doi: 10.1093/alcalc/agh115. [DOI] [PubMed] [Google Scholar]
- Lemos JI, Takahashi RN, Morato GS. Effects of SR141716 and WIN 55,212–2 on tolerance to ethanol in rats using the acute and rapid procedures. Psychopharmacology (Berl) 2007;194:139–149. doi: 10.1007/s00213-007-0804-1. [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévénés C, Daniel H, Soubrié P, Crépel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol (Lond) 1998;510(Pt 3):867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Moreno JA, González-Cuevas G, Rodríguez de Fonseca F, Navarro M. Long-lasting increase of alcohol relapse by the cannabinoid receptor agonist WIN 55,212-2 during alcohol deprivation. J Neurosci. 2004;24:8245–8252. doi: 10.1523/JNEUROSCI.2179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Scherma M, Rodriguez de Fonseca F, Gonzalez-Cuevas G, Fratta W, Navarro M. Changed accumbal responsiveness to alcohol in rats pre-treated with nicotine or the cannabinoid receptor agonist WIN 55,212-2. Neurosci Lett. 2008;433:1–5. doi: 10.1016/j.neulet.2007.11.074. [DOI] [PubMed] [Google Scholar]
- López-Rodríguez ML, Viso A, Ortega-Gutiérrez S, Fowler CJ, Tiger G, de Lago E, Fernández-Ruiz J, Ramos JA. Design, synthesis, and biological evaluation of new inhibitors of the endocannabinoid uptake: comparison with effects on fatty acid amidohydrolase. J Med Chem. 2003;46:1512–1522. doi: 10.1021/jm0210818. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Endocannabinoid liberation from neurons in transsynaptic signaling. J Mol Neurosci. 2007;33:87–93. doi: 10.1007/s12031-007-0043-2. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Marks DF. Divided attention performance of cannabis users and non-users following cannabis and alcohol. Psychopharmacology (Berl) 1975;44:147–152. doi: 10.1007/BF00445566. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Colombo G, Carai MAM. Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets. 2010;9:55–59. doi: 10.2174/187152710790966623. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Mahadevan A, Razdan RK. Further advances in the synthesis of endocannabinoid-related ligands. The AAPS journal. 2005;7:E496–502. doi: 10.1208/aapsj070250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Hong KIA, Piomelli D, Sinha R. An endocannabinoid signal associated with desire for alcohol is suppressed in recently abstinent alcoholics. Psychopharmacology (Berl) 2009;205:63–72. doi: 10.1007/s00213-009-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno JE, Kiplinger GF, Scholz N, Forney RB. The influence of alcohol and marihuana on motor and mental performance. Clin Pharmacol Ther. 1971;12:202–211. doi: 10.1002/cpt1971122part1202. [DOI] [PubMed] [Google Scholar]
- Marks DF, MacAvoy MG. Divided attention performance in cannabis users and non-users following alcohol and cannabis separately and in combination. Psychopharmacology (Berl) 1989;99:397–401. doi: 10.1007/BF00445566. [DOI] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS-J, Woodruff G, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Martin CS, Kaczynski NA, Maisto SA, Tarter RE. Polydrug use in adolescent drinkers with and without DSM-IV alcohol abuse and dependence. Alcohol Clin Exp Res. 1996;20:1099–1108. doi: 10.1111/j.1530-0277.1996.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Martini L, Thompson D, Kharazia V, Whistler JL. Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology. 2010;35:1363–1373. doi: 10.1038/npp.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, Weisner C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend. 2007;90:72–80. doi: 10.1016/j.drugalcdep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Hermann A. Ethanol actions on the mechanisms of Ca2+ mobilization in rat hippocampal cells are mediated by protein kinase C. Brain Res. 1996;714:27–37. doi: 10.1016/0006-8993(95)01456-x. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, García-Sevilla JA. Ethanol desensitizes cannabinoid CB1 receptors modulating monoamine synthesis in the rat brain in vivo. Neurosci Lett. 2006;392:58–61. doi: 10.1016/j.neulet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Poot-Ake A, Arias-Carrio NO, Pacheco-Pantoja E, de la Fuente-Ortego NA, Arankowsky-Sandoval G. The Emerging Role of The Endocannabinoid System in the Sleep-Wake Cycle Modulation. Central nervous system agents in medicinal chemistry. 2011 doi: 10.2174/187152411798047780. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Newman LM, Lutz MP, Domino EF. Delta9-tetrahydrocannabinol and some CNS depressants: evidence for cross-tolerance in the rat. Archives internationales de pharmacodynamie et de thérapie. 1974;207:254–259. [PubMed] [Google Scholar]
- Newman LM, Lutz MP, Gould MH, Domino EF. 9 -Tetrahydrocannabinol and ethyl alcohol: evidence for cross-tolerance in the rat. Science. 1972;175:1022–1023. doi: 10.1126/science.175.4025.1022. [DOI] [PubMed] [Google Scholar]
- NIAAA. Five year strategic plan, FY 09–14. 2009. p. 110. [Google Scholar]
- Nicoletti P, Trevisani M, Manconi M, Gatti R, De Siena G, Zagli G, Benemei S, Capone JA, Geppetti P, Pini LA. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia. 2008;28:9–17. doi: 10.1111/j.1468-2982.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Liu Y, Wallis KT, Egertová M, Bhartur SG, Mukhopadhyay S, Shi S, He H, Selley DE, Howlett AC, et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol (Lond) 2007;584:407–418. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. AnnNY Acad Sci. 2008;1139:426–433. doi: 10.1196/annals.1432.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gu S, Liu Q-R. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 2011 doi: 10.1177/0269881111400652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, ndez AAFarrez, Navarrete F, Manzanares J. Deletion of CB2 Cannabinoid Receptor Induces Schizophrenia-Related Behaviors in Mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Pérez-Rial S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid CB(1) receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther. 2001;297:629–637. [PubMed] [Google Scholar]
- Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, Falck JR, Nithipatikom K, Campbell WB, Hillard CJ. The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. 2003;139:1005–1013. doi: 10.1038/sj.bjp.0705334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Blake EM, Green ST, Mizroch BJ, Mulholland PJ, Woodward JJ. Tolerance to cannabinoid-induced behaviors in mice treated chronically with ethanol. Psychopharmacology. 2012;219:137–147. doi: 10.1007/s00213-011-2387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol Clin Exp Res. 2008;32:443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Peters EN, Hughes JR. Daily marijuana users with past alcohol problems increase alcohol consumption during marijuana abstinence. Drug Alcohol Depend. 2010;106:111–118. doi: 10.1016/j.drugalcdep.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Phillips RN, Brown DJ, Forney RB. Enhancement of depressant properties of alcohol or barbiturate in combination with aqueous suspended delta 9-tetrahydrocannabinol in rats. J Forensic Sci. 1971;16:152–161. [PubMed] [Google Scholar]
- Ponce G, Hoenicka J, Rubio G, Ampuero I, Jiménez-Arriero MA, Rodríguez-Jiménez R, Palomo T, Ramos JA. Association between cannabinoid receptor gene (CNR1) and childhood attention deficit/hyperactivity disorder in Spanish male alcoholic patients. Mol Psychiatry. 2003;8:466–467. doi: 10.1038/sj.mp.4001278. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Koller G, Zill P, Bondy B, Soyka M. Alcoholism-related phenotypes and genetic variants of the CB1 receptor. Eur Arch Pychiatry Clin Neurosci. 2003;253:275–280. doi: 10.1007/s00406-003-0440-7. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The Endocannabinoid System Tonically Regulates Inhibitory Transmission and Depresses the Effect of Ethanol in Central Amygdala. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Acta pharmacologica Sinica. 1999;20:1109–1114. [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M, de Miguel R, Fernández-Ruiz J, Gutiérrez-López D, Carai MAM, Ramos JA. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009;99:354–358. doi: 10.1016/j.drugalcdep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Rubio M, Fernández-Ruiz J, de Miguel R, Maestro B, Michael Walker J, Ramos JA. CB1 receptor blockade reduces the anxiogenic-like response and ameliorates the neurochemical imbalances associated with alcohol withdrawal in rats. Neuropharmacology. 2008;54:976–988. doi: 10.1016/j.neuropharm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Rubio M, McHugh D, Fernández-Ruiz J, Bradshaw H, Michael Walker J. Short-term exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci Lett. 2007;421:270–274. doi: 10.1016/j.neulet.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World. 1998;22:5–12. [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Zeini M, Camarero J, O’Shea E, Bosca L, Green AR, Colado MI. The nNOS inhibitor, AR-R17477AR, prevents the loss of NF68 immunoreactivity induced by methamphetamine in the mouse striatum. J Neurochem. 2003;85:515–524. doi: 10.1046/j.1471-4159.2003.01714.x. [DOI] [PubMed] [Google Scholar]
- Schmid HH. Pathways and mechanisms of N-acylethanolamine biosynthesis: can anandamide be generated selectively? Chem Phys Lipids. 2000;108:71–87. doi: 10.1016/s0009-3084(00)00188-2. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132:215–241. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens AJ, Doyle OL. Cross-tolerance between delta9-tetrahydrocannabinol and ethanol: the role of drug disposition. Pharmacol Biochem Behav. 1979;10:49–55. doi: 10.1016/0091-3057(79)90168-0. [DOI] [PubMed] [Google Scholar]
- Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF, Investigators AS. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol. 2008;28:317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- Sprague GL, Craigmill AL. Ethanol and delta-9-tetrahydrocannabinol: mechanism for cross-tolerance in mice. Pharmacol Biochem Behav. 1976;5:409–415. doi: 10.1016/0091-3057(76)90104-0. [DOI] [PubMed] [Google Scholar]
- Sprague GL, Craigmill AL. Effects of two cannabinoids upon abstinence signs in ethanol-dependent mice. Pharmacol Biochem Behav. 1978;9:11–15. doi: 10.1016/0091-3057(78)90005-9. [DOI] [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sun GY, Sun AY. Ethanol and membrane lipids. Alcohol Clin Exp Res. 1985;9:164–180. doi: 10.1111/j.1530-0277.1985.tb05543.x. [DOI] [PubMed] [Google Scholar]
- Sun Y-X, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LJ, Proctor RC. The use of pyrahexyl in the treatment of alcoholic and drug withdrawal conditions. N C Med J. 1953;14:520–523. [PubMed] [Google Scholar]
- Tinklenberg JR, Roth WT, Kopell BS. Marijuana and ethanol: differential effects on time perception, heart rate, and subjective response. Psychopharmacology (Berl) 1976;49:275–279. doi: 10.1007/BF00426830. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg E, Janssen RG, Hutchison KE, van Breukelen GJ, Wiers RW. Polymorphisms of the dopamine D4 receptor gene (DRD4 VNTR) and cannabinoid CB1 receptor gene (CNR1) are not strongly related to cue-reactivity after alcohol exposure. Addict Biol. 2007;12:210–220. doi: 10.1111/j.1369-1600.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter I, Wyse BD, Roberts-Thomson SJ, Monteith GR, Cabot PJ. Mechanisms involved in potentiation of transient receptor potential vanilloid 1 responses by ethanol. Eur J Pain. 2008;12:441–454. doi: 10.1016/j.ejpain.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Arango V, Xie S, Kassir SA, Mann JJ, Cooper TB, Hungund BL. Elevated levels of endocannabinoids and CB1 receptor-mediated G-protein signaling in the prefrontal cortex of alcoholic suicide victims. Biol Psychiatry. 2005;57:480–486. doi: 10.1016/j.biopsych.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–597. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008a;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, Hungund BL. Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse. 2008b;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Houchi H, Barbier E, Pierrefiche O, Vilpoux C, Ledent C, Daoust M, Naassila M. The lack of CB1 receptors prevents neuroadapatations of both NMDA and GABA(A) receptors after chronic ethanol exposure. J Neurochem. 2007;102:741–752. doi: 10.1111/j.1471-4159.2007.04577.x. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone VP. Marijuana and ethanol: effects on sleep. Int J Psychiatry Med. 1973;4:201–212. doi: 10.2190/ba5b-1lvx-0lq5-xv02. [DOI] [PubMed] [Google Scholar]
- Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 variation modulates risk for drug and alcohol dependence. Biol Psychiatry. 2007;62:616–626. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]