Abstract
Methoxychlor (MXC) and its metabolites bind to estrogen receptors (ESRs) and increase ovarian atresia. To test whether ESR alpha (ESR1) overexpressing (ESR1 OE) antral follicles are more sensitive to atresia compared to controls, we cultured antral follicles with vehicle, MXC (1–100 mg/ml) or metabolites (0.1–10 mg/ml). Results indicate that MXC and its metabolites significantly increase atresia in ESR1 OE antral follicles at lower doses compared to controls. Activity of pro-apoptotic factor, caspase-3/7 was significantly higher in ESR1 OE treated antral follicles compared to controls. ESR1 OE mice dosed with MXC 64 mg/kg/day had an increased percentage of atretic antral follicles compared to controls. Furthermore, pro-caspase-3 levels were found to be significantly lower in ESR1 OE ovaries than controls dosed with MXC64. These data suggest that ESR1 OE ovaries are more sensitive to atresia induced by MXC and its metabolites in vitro and in vivo compared to controls.
Keywords: Methoxychlor, metabolites, atresia, antral follicles, reproduction, apoptosis, estrogenic
2. Introduction
The ovaries of a mammalian female are composed of structural and functional units called follicles at different stages of maturity. The ovary is responsible for nurturing and facilitating the oocyte-containing follicle through various stages of its development until it can release the oocyte for fertilization [1–3]. However, by a mechanism that is largely unknown, a relatively small number of follicles are recruited from the initial pool to ovulate and release an oocyte, while the rest of the follicles undergo atresia [1,4]. Therefore, atresia is a process of programmed cell death (apoptosis) that occurs naturally in the granulosa cells of the ovary at all stages of follicular development throughout the reproductive lifespan. During follicular growth and development, approximately 99% of follicles undergo atresia due to apoptosis of granulosa cells and hence, atresia remains a prominent feature of ovarian function until all the follicles are exhausted, resulting in reproductive senescence [1,5,6]. Because the numbers of follicles endowed to the female at birth is finite, it is critical that sufficient numbers of healthy follicles be available for ovulation for normal reproduction [6,7]. However, environmental toxicants such as the organochlorine pesticide, methoxychlor (MXC), have been shown to increase antral follicle atresia in mouse ovaries [8–10]. MXC specifically targets antral follicles, thus, raising the concern that exposure to MXC could cause depletion of antral follicles in the ovary, leading to infertility and reproductive senescence [9,11,12]. Early reproductive senescence is a concern because it has been associated with an increased risk of chronic diseases such as osteoporosis and cardiovascular disease [13,14].
Previous studies have shown that MXC may induce ovarian toxicity via the estrogenic pathway[15,16]. The metabolites of MXC, mono-hydroxy MXC (MOH) and bis-hydroxy MXC (HPTE) are more potent estrogenic compounds than MXC and can inhibit antral follicle growth to a greater extent than MXC [17,18]. There is limited information available that explains whether ovaries with increased expression of estrogen receptors will be more sensitive to estrogenic chemicals such as MXC and its metabolites. Several studies have shown that estrogen receptor expression can be induced in tissues by exposure to estrogenic chemicals or by genetic polymorphisms [19–21]. In this study, we have analyzed whether ovaries with increased expression of estrogen receptors are more sensitive to MXC and its metabolites. Specifically, because MXC and its metabolites can bind to estrogen receptor alpha (ESR1) [22,23], we tested the effects of MXC and its metabolites on a transgenic mouse model in which estrogen receptor alpha (ESR1) was overexpressed (ESR1 OE) in the ovaries. We hypothesized that ovaries overexpressing ESR1 are more sensitive to antral follicle toxicity induced by MXC and its metabolites because of an increased number of binding sites for the estrogenic chemicals.
In a previous study, we reported that antral follicles of ESR1 OE were more sensitive to inhibition of growth induced by MXC and its metabolites in vitro compared to controls [24]. Inhibition of growth was observed with lower doses of MXC and its metabolites in ESR1 OE, but not in control antral follicles. Disruption in the normal ratio of ESR1: ESR2 in ESR1 OE mouse ovaries may be critical in driving the sensitivity of the antral follicle to MXC and its metabolites. Hence, in this study, we analyzed atresia in antral follicles of control and ESR1 OE mice treated with MXC and its metabolites to determine whether antral follicles of ESR1 OE mice are more sensitive to atresia induced by lower doses of the chemicals compared to controls. To compare follicular atresia in both genotypes at the molecular level, we also evaluated the levels of key players in the apoptotic pathway, including the anti-apoptotic factor, B-cell lymphoma/leukemia protein-2 (Bcl-2) and the pro-apoptotic factors, Bcl-2 associated X protein (Bax), Bcl-2 interacting domain (Bid) and cysteine aspartate-specific protease – 3 (caspase-3).
3. Materials and Methods
3.1 Chemicals
MXC (99%) was purchased from Chemservice (West Chester, PA). For in vivo experiments, mice were dosed with 8, 16, 32 and 64 mg/kg/day. Four stock solutions of MXC were prepared by using sesame oil (SES; Thermo Fisher Scientific Inc., Rockford, IL) as the vehicle. The stock concentrations were 5mg/ml for 8 mg/kg/day dose, 10mg/ml for 16 mg/kg/day dose, 20 mg/ml for 32 mg/kg/day and 40 mg/ml for 64 mg/kg/day dose mg/kg/day. To keep the doses constant, mice were administered 1.6 ml/kg of the stock solutions.
For in vitro experiments, stock solutions of MXC, MOH and HPTE were prepared by dissolving them in dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO). MOH and HPTE (99%) were synthesized in Dr. Vincent Njar’s laboratory (University of Maryland, Baltimore now at Thomas Jefferson University, Philadelphia, PA). MXC stock solutions were prepared by dissolving 1.33, 13.3 and 133 mg/ml, while MOH and HPTE stock solutions were prepared by dissolving 0.133, 1.3 and 13.3 mg/ml, resulting in a final concentration of 0.1, 1 and 10 μg/ml per well. The doses selected for the in vitro and in vivo studies were based on previously published studies showing that these concentrations cause increased antral follicle atresia and inhibition of antral follicle growth in CD-1 mice [8–11].
3.2 Animals
ESR1 OE and control mice (32–39 day old cycling females) that were used in this study were generated using C57BL6 and FVB mice as described previously [24,25]. ESR1 OE mice were validated for the overexpression of ESR1 gene and protein levels in the ovaries by quantitative real-time polymerase chain reaction (qPCR) and immunohistochemical staining techniques [24].
The mice were housed in the University of Illinois core animal facility under a 12:12 dark:light cycle and provided food and water ad libitum. The University of Illinois Institutional Animal Care and Use Committee approved all animal procedures used in this study.
3.3 In vivo dosing regimen
ESR1 OE and control mice were administered either sesame oil or MXC intraperitoneally using a 1 ml syringe at 1.6 ml/kg body weight. The doses were adjusted based on the animal’s most recent body weight. The mice were dosed continuously for a period of 20 days, as previous studies have shown that this length of exposure does not cause overt toxicity, but does induce antral follicle atresia [9]. In addition, to be consistent with previous studies and to minimize variability in results, intraperitoneal route of administration was used. After dosing, all samples were collected when the mice were in estrus to decrease variability at various days of the estrous cycle. Moreover, previous studies have shown that in vivo dosing with MXC causes persistent estrus [9,11].
3.4 Antral follicle culture
Antral follicles (determined by appearance and relative size) were isolated from ESR1 OE and control ovaries using fine watchmaker forceps. About 75–80 antral follicles (300–400 μm) were obtained from at least two mice of each genotype per experiment. The follicles were then randomly divided into five groups – non-treatment (NT), vehicle-control (DMSO) and three chemical treatments of MXC, MOH or HPTE. Increasing concentrations (1.33, 13.3 and 133 mg/ml) of MXC and (0.133, 1.33 and 13.3 mg/ml) of MOH and HPTE were made to allow an equal volume to be added to each of the treatment groups in the 96-well culture plate to control the solvent concentration. The final concentrations of MXC in each well of the culture were 1, 10 and 100 μg/ml. Similarly, final concentrations of MOH and HPTE in culture were 0.1, 1, and 10 μg/ml. MOH and HPTE were used at 10-fold lower concentrations than MXC because the metabolites of MXC are thought to be more toxic than the parent compound [10]. Moreover, from a physiological stance, a considerable quantity of MXC is likely broken down to its metabolites by the liver before it reaches the ovaries. In the vehicle treatment group, DMSO was used at 0.075%, which is not toxic to cultured follicles [8,10]. The doses selected for in vitro studies were based on previously published studies showing that these concentrations of MXC, MOH and HPTE induce toxicity in antral follicles and granulosa cell culture models [8,10]. A non-treatment group was included to control for culture conditions and follicles in this group were placed only in supplemented α-MEM devoid of either DMSO or chemicals.
The follicles were cultured in supplemented α-MEM as described previously [8,10]. Briefly, supplemented α-MEM was prepared using 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (FSH; Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). Follicles were incubated for 96 hrs at 37°C in 95% air and 5% CO2.
3.5 Histological evaluation of atresia
For the in vivo study, the ovaries of the mice in estrus were collected and fixed in Dietrick’s fixative. After fixation, the ovaries were dehydrated and embedded in Paraplast (VWR scientific, West Chester, PA), serially sectioned at 8 μm and stained with Weigert’s hematoxylin-picric acid methyl blue. Follicles were classified as antral if they contained five or more layers of granulosa cells and a clearly defined antral space. Antral follicles were classified as atretic if 10% of the granulosa cells were apoptotic (defined by the presence of pyknoticor apoptotic bodies in the granulosa cell layer), the granulosa cells were disorganized or the oocyte was fragmented or degenerating.
For evaluation of atresia in antral follicle cultures, the antral follicles exposed to DMSO or MXC/MOH/HPTE were fixed for at least 24 h in Dietrick’s fixative at the end of the culture. After a series of washes, the follicles were embedded in plastic (Technovit 7100), sectioned at 2μm, and stained using Lee’s methylene blue - basic fuchsin. The amount of follicular atresia was examined in each antral follicle by the presence of pyknotic bodies. Follicles were scored as healthy or atretic using a scale of 1–4 (1=healthy follicle; 2=10% apoptotic bodies/per follicle meaning an early atretic follicle; 3=11–30% of apoptotic bodies/per follicle meaning a mid atretic follicle; 4=greater than 30% apoptotic bodies/per follicle meaning a late atretic follicle) as previously described [8,18]. All scoring was done without knowledge of a genotype or treatment group. At least 2–3 antral follicles from three separate cultures were analyzed for atresia from each treatment group.
3.6 Caspase activity
Caspase activity levels were analyzed in antral follicles that were exposed to DMSO or MXC in vitro using the Caspase-Glo 3/7 Assay (Promega Corporation, Madison, WI). The Caspase-Glo 3/7 Assay is a homogeneous, luminescent assay that measures caspase-3 and -7 activities. Antral follicles were cultured with DMSO or MXC as described earlier. At the end of 96 hours, the antral follicle from each well and 100 μl of the supplemented media were placed into a white 96-well plate. As per manufacturer’s protocol, the reagents in the assay kit were mixed and added into each well containing the antral follicle in the supplemented media. The addition of the reagents in the assay kit results in cell lysis of the antral follicle, followed by the generation of a luminescent signal produced by luciferase. Luminescence is proportional to the amount of caspase activity present and was measured using a microplate reader with chemiluminescent filters.
3.7 Western Blotting
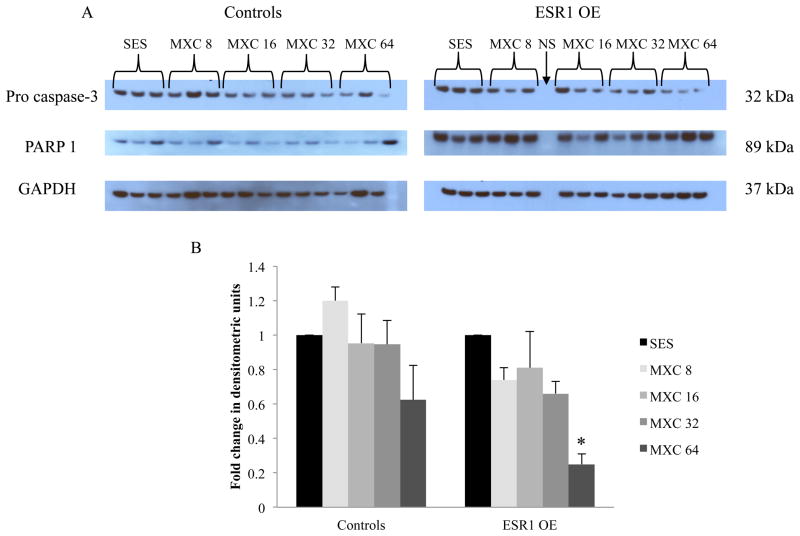
Protein analyses of pro-caspase-3 and PARP1 were carried out using ovaries from control and ESR1 OE mice that were treated with vehicle or MXC in vivo. Antral follicles from an ovary were isolated and homogenized using 250 μl T-Per (Thermo Fisher Scientific, Rockford, IL) containing protease inhibitors (Roche Diagnostics, Mannheim, Germany). After homogenization, the protein concentration in the lysate was determined by using a bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific, Rockford, IL). Electrophoresis and immunoblotting were performed using XCellSureLock Mini-Cell Blot Module Kit and recommended reagents as per manufacturer’s protocol (Invitrogen, Carlsbad, CA). The protein lysate (3–5μg) was loaded on precast 4–12% bis-tris sodium dodecyl sulphate-polyacrylamide gels (SDS-PAGE; Invitrogen, Carlsbad, CA), followed by wet transfer of the proteins to a blot at 4°C. As each precast gel has 17 wells/gel, we loaded ESR1 OE and control samples on separate gels. However, all treatment groups (5 treatments; n=3) of each genotype were loaded and run on the same gel (Fig. 5A right panel). The blots were incubated overnight at 4°C with primary antibodies, anti-pro-caspase-3 and anti-PARP1 (1:1000; Cell Signaling Technologies, Danvers, MA), followed by incubation with secondary HRP-linked antibodies (1:2000) for 1 hour at room temperature. To ensure uniform loading of the proteins, the blots were stripped and incubated with anti-GAPDH (1:2000; EnCor Biotechnology Inc., Gainesville, FL), followed by incubation with an HRP-linked secondary antibody. The immune complexes on the blots were visualized using an enhanced chemiluminescence detection kit (Cell Signaling Technologies, Danvers, MA) and captured on X-ray film. Scanning densitometry using the Image J software downloaded from the NIH website (http://rsb.info.nih.gov/ij/) was used to compare the protein levels. Densitometric units of pro-caspase-3 were normalized to GAPDH for quantification.
Figure 5.
Protein expression of pro-caspase-3 and PARP1 in control and ESR1 ovaries after 20 days of in vivo dosing with SES or MXC (8 – 64 mg/kg/day). (A) Ovaries of mice were collected and subjected to protein analysis by western blotting using specific antibodies for pro-caspase-3 (top panel), PARP1 (middle panel) and loading control, GAPDH (bottom panel). (B) Densitometric units of pro-caspase-3 bands normalized to GAPDH were quantified as fold change over SES in controls and ESR1 OE ovaries. Each bar represents means ± SEM; NS = no sample loaded; Asterisk (*) indicates significant difference from SES controls within genotype (n = 3; p≤0.05).
3.8 Gene expression analysis
Gene expression analysis was carried out on antral follicles that were exposed to vehicle or MXC in vitro. Total RNA was extracted from follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) by homogenizing the follicles in buffer according to the manufacturer’s protocol. Total RNA (100–500 ng) from the follicles was then reverse transcribed to obtain complementary DNA (cDNA) using an iScriptcDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules CA) according to the manufacturer’s protocol. Quantitative real-time (q-PCR) was conducted using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules CA) and accompanying software according to the manufacturer’s instructions. The machine quantifies the amount of q-PCR product generated by measuring the dye (SsoFastEvaGreen from Bio-Rad Laboratories, Inc., Hercules CA) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of purified q-PCR product. To detect genomic DNA contamination, a well containing total RNA was included each time. Gene-specific primers (synthesized by Integrated DNA Technologies, Inc., Coralville, Iowa) were used for the q-PCR (Table I) and mouse β-actin was used as the reference gene. An initial incubation of 95°C for 10 min was followed by 40–50 cycles of 94°C for 10 s (denaturation step), 55°– 60°C (annealing step) for 10 s, and 72 °C for 10 s (extension step), along with a final extension at 72 °C for 10 min. Starting quantity (SQ), an arbitrary term generated by the CFX96 software, denotes the relative gene expression level in the experimental samples. These values are calculated based on the standard curve and are read at the cycle at which the gene begins amplifying exponentially. For each gene, a melting curve was performed at 55–90 °C to monitor the generation of a single product. SQs from ESR1 OE and control samples were normalized by obtaining the ratio of gene:β-actin. The specificity of the primers was verified by the lack of amplification from genomic DNA and by a single melting curve. All experiments were performed in triplicate.
Table I.
Sequences of primer sets used for analysis of gene expression.
| Accession number | Gene name | Gene nomenclature | Forward | Reverse |
|---|---|---|---|---|
| NM_009741 | B-cell leukemia/lymphoma 2 | Bcl-2 | 5′-ATGCCTTTGTGGAA CTATATGGC-3′ | 5′-GGTATGCACCCAGA GTGATGC-3′ |
| NM_007527 | BCL2-associated X protein | Bax | 5′-TGAAGACAGGGGC CTTTTTG-3′ | 5′-AATTCGCCGGAGAC ACTCG-3′ |
| NM_007544 | BH3 interacting domain death agonist | Bid | 5′-GCCGAGCACATCA CAGACC-3′ | 5′-TGGCAATGTTGTGG ATGATTTCT-3′ |
| NM_007393 | Mouse Beta-actin | β-Actn | 5′-GGGCACAGTGTGG GTGAC-3′ | 5′-CTGGCACCACACCT TCTAC-3′ |
3.9 Statistical analysis
All data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± SEM from at least three separate experiments. Multiple comparisons between experimental groups were done using analysis of variance (ANOVA) followed by Tukey’s post hoc comparison. Comparison within treatments between the two genotypes was done using Student’s t-test. Statistical significance was assigned at p≤0.05.
4. Results
4.1 Atresia caused by MXC in vivo
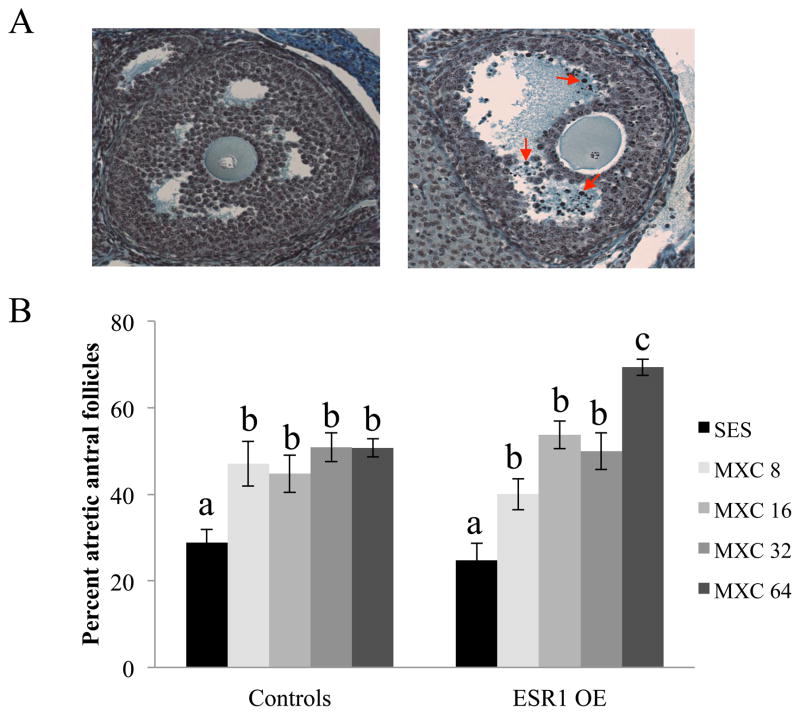
Control and ESR1 OE mice were dosed with vehicle or MXC (8, 16, 32 and 64 mg/kg/day) for a period of 20 days. The percentage of atretic antral follicles was compared between sesame oil treated mice and MXC treated mice of both genotypes based on the presence of pyknotic bodies in histologically prepared ovarian sections as shown in (Fig. 1A). Within genotype, all chemically treated control and ESR1 OE mice had a significantly higher percentage of atretic antral follicles compared to sesame oil treated control and ESR1 OE mice (Fig. 1B). However, in ESR1 OE mice, the highest MXC treatment group (64 mg/kg/day) had a significantly higher percent of atretic antral follicles compared to any other treatment groups in both genotypes (Fig. 1B).
Figure 1.
Evaluation of atresia in control and ESR1 OE ovaries treated in vivo with sesame oil (SES) or MXC (8 – 64 mg/kg/day). Ovaries of mice were collected and fixed for histological evaluation of atresia. (A) Histological sections of ovaries were analyzed for atresia by comparing the presence of apoptotic bodies. Representative sections of a healthy antral follicle (left panel) and an atretic antral follicle (right panel) with red arrows pointing to a few apoptotic bodies are shown. (B) The percentages of atretic antral follicles were quantified and plotted on a graph. Each bar represents means ± SEM. Bars with different letters are significantly different from each other (n = 6; p≤0.05).
4.2 Atresia caused by MXC and its metabolites in vitro
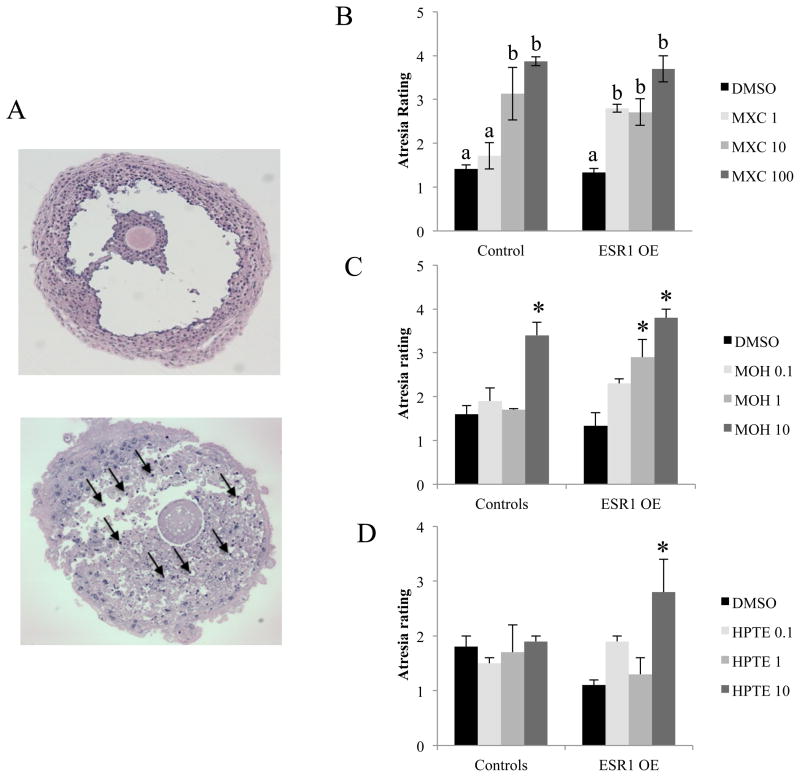
Antral follicles from control and ESR1 OE mice were isolated, treated with MXC, MOH, HPTE or DMSO for 96 hours in vitro and subjected to histological analysis of atresia. The antral follicles were rated by the presence of pyknotic bodies(Fig. 2A). The results indicate that antral follicles from control mice had a significantly higher rating of atresia in MXC 10 and 100 μg/ml treatment groups compared to DMSO treatment. However, in ESR1 OE antral follicles, the atresia rating was higher in MXC 1, 10 and 100 μg/ml treatments compared DMSO treated follicles. Furthermore, MXC 1 treated ESR1 OE antral follicles had a significantly higher atresia rating compared to MXC 1 treated control follicles(Fig. 2B). With MOH treatment, antral follicles from control mice that were treated with MOH 10 μg/ml had a significantly higher atresia rating compared to DMSO treated mice. However, in ESR1 OE antral follicles, MOH 1 and 10 μg/ml had significantly higher ratings of atresia compared to DMSO treatment (Fig. 2C). With HPTE treatment, the atresia rating in control antral follicles was not different between treatment groups and DMSO treated follicles. However, in ESR1 OE antral follicles, HPTE 10 μg/ml caused a significantly higher atresia rating compared to DMSO treatment (Fig. 2D).
Figure 2.
Evaluation of atresia in control and ESR1 OE antral follicles treated in vitro with vehicle, DMSO MXC, MOH or HPTE. (A) Histological sections of antral follicles were rated for atresia by comparing the presence of apoptotic bodies. Representative sections of a healthy antral follicle (top panel) and an atretic antral follicle (bottom panel) with black arrows pointing to apoptotic bodies are shown. (B) MXC (1–100 μg/ml) treated follicles; (C) MOH (0.1–10 μg/ml) treated follicles; (D) HPTE (0.1–10 μg/ml) treated follicles. Each bar represents means ± SEM. Asterisks (*) indicate significant differences from DMSO controls within genotype Bars with different letters are significantly different from each other. (n = 2–3 follicles per treatment from three separate experiments; p≤0.05).
4.3 Gene expression levels of pro- and anti-apoptotic factors in antral follicles treated with MXC in vitro
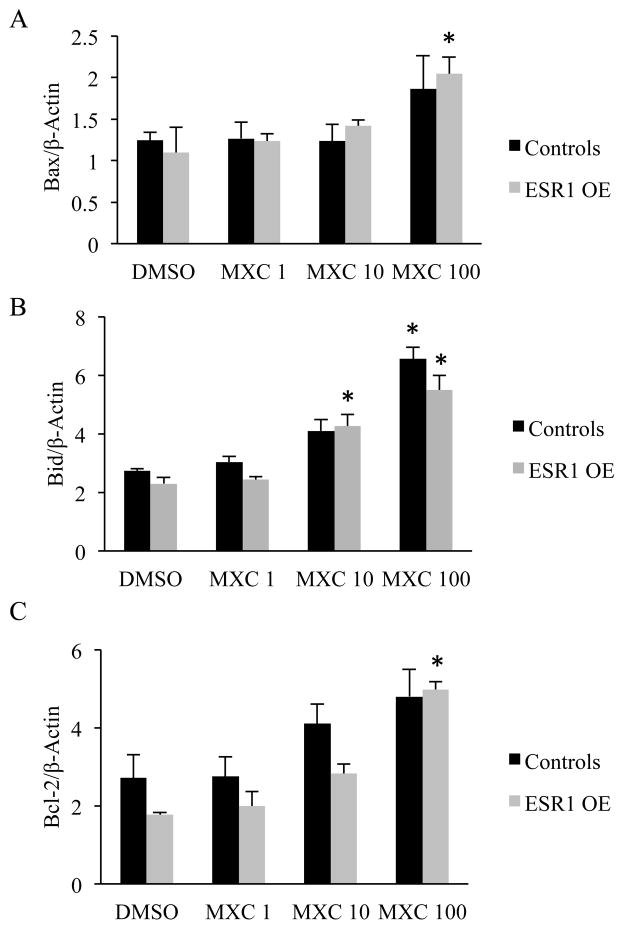
To analyze the levels of pro-apoptotic factors, Bax and Bid, and the anti-apoptotic factor, Bcl-2, cultured antral follicles from control and ESR1 OE mice were subjected to qPCR. The results indicated that, in control antral follicles, mRNA levels of Bax were not different between treatment groups. However, in ESR1 OE antral follicles, Bax was significantly upregulated in MXC (100 μg/ml) compared to DMSO treatment (Fig. 3A). Gene expression analysis of Bid revealed that mRNA levels of Bid were significantly upregulated in controls treated with MXC 100 μg/ml compared to DMSO treatment. Interestingly, in ESR1 OE antral follicles, mRNA levels of Bid were significantly higher in MXC 10 and 100 μg/ml compared to DMSO treatments (Fig. 3B). Gene expression levels of the anti-apoptotic factor Bcl-2 were not altered between treatment groups of control antral follicles, but were up-regulated significantly in ESR1 OE antral follicles treated with MXC 100 μg/ml (Fig. 3C).
Figure 3.
Effect of MXC (1–100 μg/ml) on gene expression levels of pro and anti-apoptotic factors in antral follicles of control and ESR1 OE mice in vitro. The relative gene expression quantities of were normalized to β-Actin. (A) Gene expression levels of pro-apoptotic factor, Bax; (B) Gene expression levels of pro-apoptotic factor, Bid; (C) Gene expression levels of anti-apoptotic factor, Bcl-2. Each bar represents means ± SEM. Asterisks (*) indicate significant differences from DMSO controls within genotype (n = 13 –15 follicles per treatment from three separate experiments; p≤0.05).
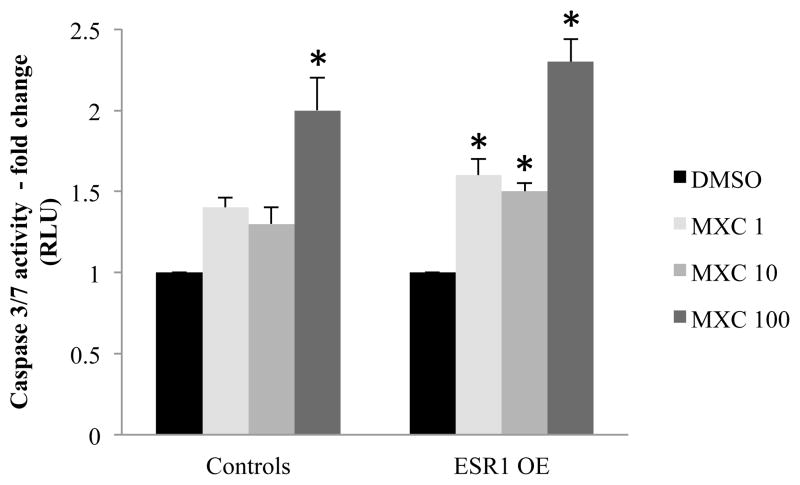
4.4 Caspase-3/7 activity in antral follicles treated with MXC in vitro
Caspase-3 and 7 are the main effector molecules in the caspase cascade leading to apoptosis [26]. Hence, the enzyme activity of caspase-3/7 was analyzed in antral follicles treated with vehicle or MXC in vitro. The results indicate that in control mouse follicles, significantly high levels of caspase-3/7 activity were found in MXC 100 μg/ml treatment compared to DMSO treatment groups. However, in ESR1 OE antral follicles, significantly higher caspase-3/7 activity levels were found in MXC 1, 10 and 100 μg/ml compared to DMSO treatment (Fig 4).
Figure 4.
Caspase-3/7 activity measured in antral follicles treated in vitro with MXC (1–100 μg/ml) for 96h. Antral follicles were treated with either DMSO or MXC for 96 h and treated with reagents in the Caspase-Glo 3/7 assay kit. Luminescence is proportional to the amount of caspase activity present, which was measured and plotted as fold change over DMSO treatment. Each bar represents means ± SEM. Asterisks (*) indicate significant differences from DMSO controls within genotype (n = 4–6 follicles per treatment from three separate experiments; p≤0.05).
4.5 Caspase-3 and PARP1 expression in MXC treated ovaries in vivo
Pro-caspase-3 is cleaved to form active caspase-3, which targets and cleaves PARP1, driving the cells further into becoming apoptotic [26,27]. Hence, pro-caspase-3 and PARP1 levels were analyzed in control and ESR1 OE ovaries treated with MXC in vivo by western blotting. The results indicate that in control mice, levels of pro-caspase-3 were not different in MXC treated ovaries compared to sesame oil treated mice (Fig. 5A top left panel). However, in ESR1 OE mice, pro-caspase-3 levels were significantly decreased in ovaries from MXC 64 mg/kg/day treatment compared to sesame oil treatment groups (Fig. 5A top right panel). Densitometric analysis of pro-caspase-3 normalized to GAPDH was quantified as fold change over vehicle treatment (Fig. 5B). The results indicate that in control mice, there was no difference in the levels of pro-caspase-3 protein, but in ESR1 OE ovaries, levels of pro-caspase-3 were significantly reduced in the MXC 64 mg/kg/day treatment group compared to sesame oil treated controls. Cleaved PARP1 expression was found in all samples of controls and ESR1 OE mice treated with MXC and sesame oil (Fig. 5A middle panels). GAPDH levels were not different in ovaries of control or ESR1 OE mice across treatment groups (Fig. 5A bottom panels).
5. Discussion
The ovary is a dynamic tissue, which is comprised of follicles that are continuously going through phases of growth and atresia. Hence, inhibition of follicle growth or increased amount of follicular atresia could result in the depletion of the finite number of antral follicles in the ovary available for ovulation [28]. Excessive atresia may be induced as a result of toxic injury by estrogenic chemicals such as MXC and its metabolites. Previous studies have shown that MXC and its metabolites increase atresia of mouse antral follicles in vivo and in vitro [9,10]. Since it is known that MXC and its metabolites act via estrogen receptors [17,18], ovarian cells that have disrupted levels of estrogen receptors may respond differently to MXC and its metabolites compared to the normal ovary. Several studies have shown that in tissues that co-express ESR1 and ESR2, the ratio of ESR1:ESR2 is critical for the response of the tissue to environmental estrogens [29,30]. Besides, in our previous in vitro study, we have shown that antral follicles of ESR1 OE mice are more sensitive to inhibition of growth compared to antral follicles of control mice treated with MXC and its metabolites[25]. Hence, in this study we evaluated whether the ovaries of ESR1 OE mice are more sensitive to atresia induced by MXC and its metabolites compared to ovaries of control mice. To evaluate atresia, in this study we carried out in vivo and in vitro experiments on control and ESR1 OE antral follicles. In the in vivo experiments, we dosed controls and ESR1 OE mice with increasing doses of MXC (8, 16, 32 and 64 mg/kg/day). In contrast to a previous in vivo study [31], we included lower doses (8 & 16 mg/kg/day) of MXC to analyze whether ovaries of ESR1 OE mice are more sensitive to these doses compared to ovaries of controls. Our results show that at 8, 16 and 32 mg/kg/day MXC doses, control and ESR1 OE mice have an increased percentage of atretic antral follicles compared to sesame oil treated mice. However, consistent with our previous results, ESR1 OE mice have a significantly higher percentage of atretic antral follicles at the 64 mg/kg/day dose compared to other treatment groups of both genotypes [31], suggesting that ESR1 OE ovaries are only sensitive to the highest dose of MXC in vivo compared to control ovaries.
To test our hypothesis using a direct approach, we also conducted in vitro studies to evaluate the levels of atresia in ESR1 OE and control antral follicles using MXC and its metabolites. In contrast to in vivo experiments, where the concentration of MXC or its metabolites reaching the ovaries cannot be controlled, in the in vitro experiments, individual antral follicles were isolated and treated with various concentrations of MXC, MOH and HPTE directly. The results indicate that control mice treated with 10 and 100 μg/ml MXC have a significantly increased atresia rating compared to 1 μg/ml and DMSO treated follicles. However, ESR1 OE antral follicles have significantly higher ratings of atresia at 1, 10 and 100 μg/ml compared to DMSO treatment, indicating an increased sensitivity of ESR1 OE antral follicles to the lowest dose of MXC compared to control follicles. Furthermore, ESR1 OE antral follicles treated with MXC 1 μg/ml had significantly higher atresia rating compared to control mice treated with the same concentration of MXC. This is in concurrence with our previous study in which we reported increased sensitivity of ESR1 OE antral follicles to growth inhibition caused by lower doses of MXC compared to control mice [24]. With MOH treatment, control antral follicles were significantly atretic at the highest dose of 10 μg/ml MOH compared to DMSO treatment, whereas ESR1 OE antral follicles had significantly higher atresia rating at 1 and 10 μg/ml compared to DMSO treatment. Moreover, there was a trend towards increased atresia in MOH 1 μg/ml treated ESR1 OE antral follicles when compared to control follicles at the same dose. With HPTE treatment, atresia was not induced in controls by any of the treatments, whereas in ESR1 OE antral follicles, 10 μg/ml HPTE significantly increased the atresia rating compared to DMSO treatment. The effect of MOH and HPTE in controls is in concurrence with previous data, which show that MOH 10 μg/ml caused atresia in antral follicles from CD-1 mice, but not with HPTE 10 μg/ml [18]. However, unlike MXC, we did not find significant differences between genotypes treated with MOH or HPTE suggesting that ESR1 OE antral follicles are more sensitive to atresia induced by MXC compared to the metabolites. Collectively, these data suggest that ESR1 OE antral follicles are sensitive to MXC induced toxicity but may be sensitive to a lesser extent to MOH and HPTE induced toxicity (MOH being slightly more toxic than HPTE) compared to controls.
Since apoptosis is the molecular basis of atresia, it is important to evaluate the levels of apoptotic factors that may cause the increased sensitivity of ESR1 OE to MXC compared to controls [4]. Previous studies have shown that MXC increases oxidative stress in the ovaries and that it causes mitochondrial dysfunction [8,32]. This is a likely trigger for apoptosis, as increased production of hydrogen peroxide in the cells is known to cause apoptosis. Apart from this, previous studies have shown that MXC causes an increase in Bax in the ovaries, whereas mice that overexpress Bcl-2 are protected from MXC induced atresia [10,11]. Increased oxidative stress and increased Bax production followed by mitochondrial dysfunctions are key factors that activate the apoptotic pathway by causing an alteration in the mitochondrial inner transmembrane potential. This results in the formation of a conductance channel known as the mitochondrial permeability transition pore, through which cytochrome c is released. Cytochrome c complexes with Apaf-1 and caspase-9 to form an active apoptosome that can cleave pro caspase-3 and 7. Once the mitochondrial pore is formed resulting in activation of caspases, an infinite loop is created, in which an increase in caspase activity results in increased mitochondrial permeability and vice-versa. Caspase-3 and 7 are known as effector caspases since they cleave critical proteins needed for maintaining homeostasis and disable vital processes essential for cell survival [26]. One of the key proteins cleaved by capsase-3 is PARP-1, a key signaling enzyme and a prompt DNA damage sensor, involved in triggering single-strand DNA repair [26]. Cleavage of PARP-1 by caspase-3 disables it and drives the cell towards apoptosis [27,33].
Our data indicate significantly high levels of caspase-3/7 activity in ESR1 OE antral follicles that were treated in vitro with MXC compared to DMSO treated follicles. Moreover, gene expression levels of the pro-apoptotic factor, Bax were not different between treatment groups in controls, while they were significantly higher in ESR1 OE antral follicles treated with MXC 100 μg/ml compared to DMSO treatment. Further, in controls, gene expression levels of pro-apoptotic factor Bid were significantly higher in 100 μg/ml MXC compared to DMSO treatment, whereas in ESR1 OE antral follicles, 10 and 100 μg/ml MXC had higher levels of Bid compared to DMSO treatment. Gene expression levels of the anti-apoptotic factor Bcl-2 were higher in antral follicles treated with MXC in both genotypes, possibly as an attempt to rescue the follicles. Collectively, the results from our in vitro studies indicate that the higher levels of caspase-3/7 activity in ESR1 OE antral follicles treated with MXC compared to controls is possibly due to an increase in up-stream pro-apoptotic factors such as Bax and Bid. Since caspase-3/7 is known as the final executioner of apoptosis, an up-regulation of its activity by MXC in ESR1 OE antral follicles will result in increased atresia compared to controls. Although we did not find significant differences in caspase-3/7 enzyme activity levels or gene expression levels of Bax, Bid and Bcl-2 within treatments between genotypes, our results suggest that overexpression of ESR1 plays a role in increasing atresia in antral follicles by altering gene expression levels and activity of pro-and anti-apoptotic factors.
We analyzed the levels of pro-caspase-3 protein in control and ESR1 OE antral follicles after in vivo treatment with MXC. Since controls and ESR1 OE samples were run on separate gels and transferred to separate blots, we represented the data as fold-change over vehicle treatment (sesame oil) in each genotype. The results showed that the protein levels of pro-caspase-3 were significantly decreased in ESR1 OE mice treated with 100 mg/kg/day MXC compared to vehicle treatment. We could not detect cleaved caspase-3 in the western blot, likely because of the small amount of protein loaded (5 μg) and the small size of the cleaved protein (17 KDa). However, since GAPDH levels were uniform in all treatment groups of controls and ESR1 OE, we are confident that the significant decrease in pro-caspase-3 in ESR1 OE ovaries treated with MXC 64 mg/kg/day was indeed due to cleavage of the protein. Moreover, cleaved PARP1 was found in all treatment groups of controls and ESR1 OE, further confirming that active caspase-3 was present in these cells. In concurrence with our results, several studies have shown that a decrease of pro-caspase-3 expression and the presence of cleaved PARP-1 are indicative of active caspase-3 resulting in apoptosis of the cells [34, 35]. However, fold-change of PARP1 was not different between treatment groups and between genotypes (data not shown), indicating that other targets of downstream caspase-3 signaling may be involved in causing an increase in atresia in ESR1 OE antral follicles compared to controls.
6. Conclusion
In conclusion, this study has shown that ESR1 OE antral follicles are more sensitive to atresia induced by MXC compared to controls, as seen by histological analysis of antral follicles in vivo and in vitro. Moreover, ESR1 OE antral follicles exhibit increased atresia when treated with MOH and HPTE in vitro compared to vehicle treatment. Since MXC, and not its metabolites, is the primary chemical that is sprayed as pesticides on crops, fruits and vegetables, we thought it relevant to analyze the molecular signaling of apoptosis in antral follicles exposed to MXC. Hence, we have shown that, anti-apoptotic factors, Bax, Bid and caspase-3/7 play crucial roles in mediating increased atresia in ESR1 OE antral follicles compared to controls. Future studies will include investigating other signaling molecules downstream of caspase-3/7, which are cleaved and activated, causing increased sensitivity of ESR1 OE antral follicles to atresia induced by MXC and its metabolites compared to controls.
HIGHLIGHTS.
Estrogen receptor alpha overexpressing (ESR1 OE) antral follicles are more susceptible to atresia (cell-death) caused by methoxychlor (MXC) and its metabolites in vitro and in vivo compared to controls.
MXC increased caspase-3 activity in ESR1 OE antral follicles compared to controls.
ESR1 OE ovaries had significantly low levels of pro-caspase 3 with MXC treatment in vivo, suggesting greater cleavage of pro-caspase-3 in ESR1 OE ovaries compared to controls.
Acknowledgments
The authors thank National Institute of Environmental Health Sciences for funding the study (ES019178 to J.A.F.), UIUC Billie A. Field Fellowship in Reproductive Biology (Z.R.C) and all members of Dr. Jodi A. Flaws’ laboratory for technical help.
Footnotes
7. Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tessie Paulose, Email: tessiepaulose@gmail.com.
Patrick R. Hannon, Email: phannon2@illinois.edu.
Jackye Peretz, Email: peretz@illinois.edu.
Zelieann R. Craig, Email: zelieann@illinois.edu.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- 1.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 2.Richards JS, Jahnsen T, Hedin L, Lifka J, Ratoosh S, Durica JM, et al. Ovarian follicular development: from physiology to molecular biology. Recent ProgHorm Res. 1987;43:231–74. doi: 10.1016/b978-0-12-571143-2.50012-5. [DOI] [PubMed] [Google Scholar]
- 3.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–14. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 4.Tilly JL. The molecular basis of ovarian cell death during germ cell attrition, follicular atresia, and luteolysis. Front Biosci. 1996;1:d1–d11. doi: 10.2741/a111. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman S. The number of oocytes in the mature ovary. Recent ProgHorm. 1951;6:63–109. [Google Scholar]
- 6.Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Andersen CY, et al. On regenerating the ovary and generating controversy. Cell. 2005;122:821–22. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Byskov AG, Faddy MJ, Lemmen JG, Andersen CY. Eggs forever? Differentiation. 2005;73:438–46. doi: 10.1111/j.1432-0436.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–9. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 9.Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–8. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- 10.Miller KP, Gupta RK, Greenfeld C, Babus J, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–21. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- 11.Borgeest C, Miller KP, Gupta R, Greenfeld C, Hruska KS, Hoyer P, et al. Methoxychlor-induced atresia in the mouse involves Bcl-2 family members, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–35. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- 12.Swartz WJ, Eroschenko VP. Neonatal exposure to technical methoxychlor alters pregnancy outcome in female mice. Reprod Toxicol. 1998;6:565–73. doi: 10.1016/s0890-6238(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 13.Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;6:1492–8. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 14.Bagur AC, Mautzlen CA. Risk for developing osteoporosis in untreated premature menopause. Calcif TissInt. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- 15.Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–79. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- 16.Gray LE, Jr, Ostby J, Cooper RL, Kelce WR. The estrogenic pesticide and antiandrogenic pesticide methoxychlor alters the reproductive tract and behavior without affecting pituitary size or LH and prolactin secretion on male rats. Toxicol Ind Health. 1999;15:37–47. doi: 10.1177/074823379901500105. [DOI] [PubMed] [Google Scholar]
- 17.Bulger WH, Muccitelli RM, Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27:2417–23. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- 18.Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–8. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- 19.Newbold RR, Jefferson WN, Padilla-Banks E, Haseman J. Developmental exposure to diethylstilbesterol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol. 2004;18:399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Mol Brain Res. 1999;67:165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Sasaki M, Kaneuchi M, Fujimoto S, Dahiya R. Estrogen receptor alpha polymorphisms and renal cell carcinoma - a possible risk. Mol Cell Endocrinol. 2003;202:109–116. doi: 10.1016/s0303-7207(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 22.Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor a and b, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–8. [PubMed] [Google Scholar]
- 23.Waters KM, Safe S, Gaido KW. Differential gene expression in response to methoxychlor and estradiol through ERalpha, ERbeta, and AR in reproductive tissues of female mice. Toxicol Sci. 2001;63:47–56. doi: 10.1093/toxsci/63.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Paulose T, Hernandez-Ochoa I, Basavarajappa MS, Peretz J, Flaws JA. Increased sensitivity of estrogen receptor alpha overexpressing antral follicles to methoxychlor and its metabolites. Toxicol Sci. 2011;120:447–59. doi: 10.1093/toxsci/kfr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomic D, Frech MS, Babus JK, Symonds D, Furth PA, Koos RD, et al. Effects of ERalpha overexpression on female reproduction in mice. Reprod Toxicol. 2007;23:317–25. doi: 10.1016/j.reprotox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Boone DL, Tsang BK. Capase-3 in the rat ovary: Localization and possible role in follicular atresia and luteal regression. Biol Reprod. 1998;58:1533–9. doi: 10.1095/biolreprod58.6.1533. [DOI] [PubMed] [Google Scholar]
- 27.Fenwick MA, Hurst PR. Immunohistochemical localization of active caspase-3 in the mouse ovary: growth and atresia of small follicles. Reproduction. 2002;124:659–65. doi: 10.1530/rep.0.1240659. [DOI] [PubMed] [Google Scholar]
- 28.Hirshfield AN. Overview of ovarian follicular development: considerations of the toxicologist. Environ Mol Mutagen. 1997;29:10–15. [PubMed] [Google Scholar]
- 29.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, et al. Estrogen receptors alpha and beta as determinants of gene expression: Influence of ligand, dose, and chromatin binding. Mol Endocrinol. 2008;22:1032–3. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall JM, McDonnell DP. The estrogen receptor b-isoform (ERb) of the human estrogen receptor modulates ERa transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–78. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 31.Tomic D, Frech MS, Babus JK, Gupta RK, Furth PA, Flaws JA. Methoxychlor induces atresia of antral follicles in ERalpha-overexpressing mice. Toxicol Sci. 2006;93:196–204. doi: 10.1093/toxsci/kfl040. [DOI] [PubMed] [Google Scholar]
- 32.Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006;216:436–45. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AL, Bridgham JT. Caspase-mediated apoptosis in the vertebrate ovary. Reproduction. 2002;124:19–27. doi: 10.1530/rep.0.1240019. [DOI] [PubMed] [Google Scholar]
- 34.Sleiman RJ, Stewart BW. Early caspase activation in leukemic cells subject to etoposide-induced G2-M arrest: Evidence of commitment to apoptosis rather than mitotic cell death. Clin Can Res. 2000;6:3756–65. [PubMed] [Google Scholar]
- 35.Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. 2002;63:225–36. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]