Abstract
The effect of heavy metals at environmentally relevant concentrations on couple fecundity has received limited study despite ubiquitous exposure. In 2005–2009, couples (n=501) desiring pregnancy and discontinuing contraception were recruited and asked to complete interviews and to provide blood specimens for the quantification of cadmium (μg/L), lead (μg/dL) and mercury (μg/L) using inductively coupled plasma-mass spectrometry. Couples completed daily journals on lifestyle and intercourse along with menstruation and pregnancy testing for women. Couples were followed for 12 months or until pregnant. Fecundability odds ratios (FORs) and 95% confidence intervals (CIs) were estimated adjusting for age, body mass index, cotinine, and serum lipids in relation to female then male exposures. FORs <1 denote a longer time to pregnancy. In adjusted models, reduced FORs were observed for both female cadmium (0.78; 95% CI 0.63–0.97) and male lead (0.85; 95% CI 0.73–0.98) concentrations. When jointly modeling couples’ exposures, only male lead concentration significantly reduced the FOR (0.82; 95% CI 0.68, 0.97), though the FOR remained <1 for female cadmium (0.80; 95% CI 0.64, 1.00). This prospective couple based cohort with longitudinal capture of time to pregnancy is suggestive of cadmium and lead’s reproductive toxicity at environmentally relevant concentrations.
Keywords: cadmium, fecundity, lead, mercury, reproduction, time-to-pregnancy
1. Introduction
As a class of environmental exposures, heavy metals such as cadmium, lead and mercury are recognized reproductive toxicants, particularly for occupational workers with high levels of exposure (ATSDR 2004; Järup 2003). While there is some literature focusing on occupational lead exposure and human fecundity, most research has relied upon retrospective assessment of reproductive outcomes in relation to blood monitoring data including a recent study that reported a dose-dependent relation between male partners’ blood lead and couple fecundity as measured by a longer TTP (Shiau et al., 2004). Other occupational studies with retrospective TTP ascertainment, however, failed to observe dose dependency for lead exposure among monitored workers or their partners (Joffe et al., 2003; Sallmén et al., 2000). One possible explanation for the discrepant results is the limited validity of retrospectively ascertained TTP (Cooney et al., 2009).
To our knowledge, there have been no prospective cohort studies focusing on other heavy metals such as cadmium and mercury in relation to sensitive fecundity outcomes such as time-to-pregnancy. Thus, the potential reproductive toxicity of heavy metals at environmentally relevant concentrations remains unknown (Wirth and Mijal, 2010) despite the persistency of such exposures and possible sex-related differences in exposure response (Vahter et al., 2002). In addition, past research has largely focused on a single metal despite humans being exposed to mixtures with possible additive or synergistic effects (Iavicoli et al., 2009). These data gaps served as our impetus for study.
2 Materials and methods
2.1 Design and study population
The Longitudinal Investigation of Fertility and the Environment (LIFE) Study was specifically designed to assess persistent environmental pollutants and reproductive outcomes. Using a prospective cohort design, we enrolled 501 couples discontinuing contraception or for the purposes of becoming pregnant from two geographic areas in 2005–2009 to ensure a range of environmental exposures. Given the absence of sampling frameworks for delineating couples’ pregnancy planning intentions, we utilized a marketing database (e.g., filtered for persons with fishing interests) for recruitment in the four Michigan counties, and the state wildlife and fishing registry for the 12 counties in Texas to identify couples with presumed exposure to persistent environmental pollutants. Personalized letters were sent to our target study population (N=424,423) with the a priori expectation that 1% of couples would be planning a pregnancy in the next few months (Buck et al., 2004; Slama et al., 2006). Despite updating all addresses before mailings, 84% of individuals could not be contacted or screened for eligibility. Only 1,188 (2%) of the 51,715 individuals screened met the minimal eligibility criteria: female ages 18–44 years and male ages 18+ years; in a committed relationship; ability to communicate in English or Spanish; menstrual cycles between 21–42 days; no hormonal contraception injections during past year; and no sterilization procedures or physician diagnosed infertility. The study cohort comprised 501 (42%) of screened eligible couples; a complete description is presented elsewhere (Buck Louis et al., 2011). Full human subjects’ approval was granted prior to obtaining informed consent from all couples.
2.2 Data collection
Upon enrollment, in-person interviews were conducted separately with each partner to ascertain health and reproductive histories followed by standardized anthropometric assessment (Lohman et al., 1988). Both partners completed daily journals to capture lifestyle behaviors relevant for fecundity and sexual intercourse; women’s journals also recorded menstruation and pregnancy test results. Women were instructed in the use of the Clearblue® Easy fertility monitors consistent with the manufacturer’s guidance commencing on day six for tracking daily levels of estrone-3-glucuronide (E3G) and luteinizing hormone (LH). These varying ratios correspond to low/high/peak fertility with peak indicative of impending ovulation. Women also used the digital Clearblue® Easy home pregnancy test upon enrollment to ensure the absence of pregnancy at study start and on the day menses was expected for each cycle under observation in the study. The fertility monitor is accurate in detecting the LH surge (99%) and in predicting peak fertility (91%) as compared to the ultrasonography gold standard (Behre et al., 2000). During the baseline interview, the nurse obtained non-fasting blood for the quantification of heavy metals into a 3-ml EDTA purple top tube determined to be free of contaminants as provided by the participating toxicology laboratory. All blood collection equipment was prescreened by the participating laboratory before use. Samples were frozen at −20° or colder until shipment on ice to the participating laboratory. Each partner of the couple was remunerated $75 for complete participation in the study.
2.3 Laboratory analysis
Blood lead, cadmium and mercury were analyzed at the Centers for Disease Control and Prevention’s National Center for Environmental Health using inductively coupled plasma mass spectrometry (Centers for Disease Control and Prevention, 2009). The reported results met the Division of Laboratory Sciences’ accuracy and precision standards (Caudill et al., 2008). Cadmium and mercury are reported as μg/L and lead as μg/dl. Serum cotinine was analyzed using an enzyme-based immunoassay (Bernert et al., 1997), and categorized as active (≥100 ng/mL) or passive (<100 ng/mL) exposure (Wall et al., 1988). Serum lipids (ng/g serum) were quantified using commercially available enzymatic methods (Akins et al., 1989) and reported as total serum lipids (Phillips et al., 1989).
2.4 Statistical analysis and operational definitions
All data were entered into a web-based data management system capable of handling the study’s hierarchical data structure. Descriptive analyses included the inspection of missing data and extreme values, distributions of metals and menstrual cycles and assessment of potential confounders. A menstrual cycle was defined as the interval between initial bleeding as reported in diaries followed by at least two days of bleeding with increasing intensity to the onset of the next similar bleeding episode. After careful cycle specific assessment, this definition successfully ignored episodic spotting not indicative of menses. Fertility monitor data also were utilized in identifying the onset of menstruation and in defining the menstrual cycles, given that monitors record the onset of menstruation as indicated by the women. Further, a hybrid approach was used to identify the menstrual cycles by supplementing missing monitor information with the diary information. Pregnancy was defined as a positive test on the day of expected menstruation. The distribution of metals, cotinine and serum lipids are presented as tertiles along with the geometric mean and 95% confidence intervals (CIs).
Cox models for discrete survival time, which is a proportional odds model in SAS software (SAS version 9.2, SAS Institute, Inc., Cary, North Carolina) (Cox, 1972), were used to estimate the fecundability odds ratio (FOR) and 95% CIs. FORs estimate the odds of becoming pregnant each cycle given exposure conditional on not being pregnant in the previous cycle. FORs <1 denote a reduction in fecundity or a longer TTP, and FORs >1 a shorter TTP. Separate models were run for female and male exposures with final models including both partners’ metals exposures. A priori potential confounders include age (in years), body mass index (kg/m2 and categorized as underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0–34.9), serum cotinine (active/passive), serum lipids (continuous), research site, and parity (Augood et al., 1998; Dunson et al., 2002; Hassan and Killick, 2004; Ramlau-Hansen et al., 2007). Inclusion of serum lipids was intended to serve as a proxy for lipophilic chemicals reported to affect fecundity (Harley et al., 2010; Meeker et al., 2011). For the model that included both partners’ exposures, we simultaneously entered all metals and covariates, which assumes multiplicity as supported by the low to moderate correlations for partners’ cotinine (r=0.3) and metals concentrations (cadmium (r=0.5), lead (r=0.4), and mercury (r=0.5)). Given the colinearity (r=0.7) in partners’ ages, we entered female age and the difference of the couples’ ages into the final couple based exposure model. Only covariates meeting the criteria for confounding (Rothman and Greenland, 1998) were retained in final models, along with those believed to enhance statistical precision. Couples who withdrew from the study before pregnancy or who were not pregnant after 12 months of follow up were censored in all analyses.
The linearity and proportional hazards assumptions were verified for each of the metals (Grambsch and Therneau, 1994), and the nonparametric Kolmogorov-type supremum test for discrete time (Therneau and Grambsch, 2000). None of the tests were rejected denoting that that in no case was the null hypothesis rejected. No significant two-way interaction terms for age, site, BMI, lipids and metals were found. Under the missing random assumption, we implemented Markov Chain Monte Carlo (MCMC) methods to impute missing metals (≤2%) and lipids (≤4%) exposures, largely a result of insufficient blood for all analytes (Schafer, 1997). Model results are based on fecundability for all 501 couples accounting for both withdrawals and pregnancies. To facilitate interpretation within biologically plausible effect sizes, we rescaled metals by their standard deviations. The means and standard deviations for the chemicals for men and women, respectively, are: cadmium 0.27±0.34 and 0.29±0.31; lead 1.25±0.78 and 0.76±0.43 and mercury 1.81±1.96 and 1.40±1.38.
3 Results
Couples’ age ranges were 19–51 years for men and 19–40 years for women. Data were obtained from all 501 couples, though 100 (20%) couples withdrew from the study largely due to a change in interest or pregnancy intentions. To our knowledge, no couples were actively under infertility treatment while participating. Fifty-four (13%) couples were followed for 12 cycles without an hCG pregnancy. Among couples who became pregnant, 313 (90%) did so within first six cycles and 34 (10%) in cycles 7–12. A description of the cohort is provided in Table 1 by whether or not the couple had a pregnancy while enrolled. No significant differences were observed between couples not achieving pregnancy (n=54) and those withdrawing (n=100) from the study with the exception of higher mean parity for the former than latter group, i.e., 0.7 and 0.2, respectively.
Table 1.
Description of Study Cohort by Observed Pregnancy, LIFE Study, 2005–2009.
| Characteristic | Pregnancy (n=347) | No Pregnancy (n=54) | Withdrew (n=100) | |||
|---|---|---|---|---|---|---|
| # | % | # | % | # | % | |
| Female race/ethnicity: | ||||||
| Non-Hispanic white | 285 | 82 | 39 | 72 | 66 | 66 |
| Non-Hispanic black | 5 | 1 | 4 | 7 | 13 | 13 |
| Hispanic | 29 | 8 | 8 | 15 | 13 | 13 |
| Othera | 15 | 4 | 1 | 2 | 4 | 4 |
| Multiracial | 13 | 4 | 2 | 4 | 3 | 3 |
| Male race/ethnicity: | ||||||
| Non-Hispanic white | 278 | 80 | 42 | 78 | 64 | 64 |
| Non-Hispanic black | 7 | 2 | 4 | 7 | 12 | 12 |
| Hispanic | 30 | 9 | 6 | 11 | 12 | 12 |
| Othera | 10 | 3 | -- | -- | 3 | 3 |
| Multiracial | 21 | 6 | 2 | 4 | 8 | 8 |
| Female education: | ||||||
| <High school | -- | -- | 1 | 2 | 2 | 2 |
| High school/equivalent | 15 | 4 | 1 | 2 | 8 | 8 |
| College | 331 | 95 | 52 | 96 | 90 | 90 |
| Male education: | ||||||
| <High school | 2 | 1 | -- | -- | 1 | 1 |
| High school/equivalent | 16 | 5 | 5 | 9 | 20 | 20 |
| College | 328 | 95 | 49 | 91 | 77 | 77 |
| Household income: | ||||||
| ≤29,999 | 10 | 3 | 3 | 6 | 13 | 13 |
| 30,000–49,999 | 35 | 10 | 14 | 26 | 15 | 15 |
| 50,000–69,999 | 46 | 13 | 7 | 13 | 16 | 16 |
| ≥70,000 | 250 | 72 | 30 | 56 | 55 | 55 |
| Female health insurance: | ||||||
| No | 15 | 4 | 5 | 9 | 20 | 20 |
| Yes | 332 | 96 | 49 | 91 | 80 | 80 |
| Male health insurance: | ||||||
| No | 21 | 6 | 7 | 13 | 15 | 15 |
| Yes | 326 | 94 | 47 | 87 | 85 | 85 |
| Mean | SD | Mean | SD | Mean | SD | |
| Female age (years) | 29.8 | 3.9 | 30.6 | 4.3 | 30.3 | 4.8 |
| Age at menarche (years) | 12.5 | 1.4 | 12.7 | 1.7 | 12.7 | 1.9 |
| Male age (years) | 31.6 | 4.6 | 32.4 | 5.3 | 32.1 | 5.8 |
| Gravidity (# pregnancies) | 1.1 | 1.2 | 0.8 | 1.3 | 1.3 | 1.5 |
| Parity (# live births)b | 0.7 | 0.8 | 0.2 | 0.5 | 0.7 | 1.0 |
| Female BMI (kg/m2) | 27.0 | 6.7 | 28.7 | 9.1 | 29.4 | 7.9 |
| Male BMI (kg/m2) | 29.7 | 5.5 | 30.3 | 5.5 | 29.9 | 5.8 |
NOTE: All estimates rounded to two decimal places.
BMI, body mass index; CI, confidence interval; SD, standard deviation.
Other includes American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander.
Difference between couples not achieving pregnancy and who withdrew achieved significance (p<0.0001).
Table 2 presents the distribution of metals, cotinine and serum lipids in tertiles by partner and observed pregnancy status during the 12 months of follow up. The upper ranges of tertiles for all compounds were higher for males than females. Females in the highest tertile of cadmium and lead were significantly more likely not to have achieved pregnancy within 12 observed months relative to women in the lower tertiles, as were women with higher cotinine concentrations. Identical patterns were observed for males for these same compounds. The association between tertiles of male serum lipids and pregnancy status within 12 observed months achieved borderline (P=0.06) significance. However, blood mercury was not associated with pregnancy status within 12 observed months irrespective of partners’ concentration.
Table 2.
Distribution of Blood Metals, Cotinine and Lipids for Partner’s Exposures by Pregnancy Status, LIFE Study, 2005–2009.
| Compound (tertiles) | Pregnant (n=347) | Not Pregnant (n=154) | ||
|---|---|---|---|---|
| # | % | # | % | |
| Female Exposurea | ||||
| Cadmium (μg/L): | ||||
| 1st (0.02, 0.16) | 113 | 33 | 36 | 23 |
| 2nd (0.17, 0.27) | 125 | 36 | 46 | 30 |
| 3rd (0.28, 2.87) | 102 | 29 | 71 | 46 |
| Geometric mean (95% CI) | 0.21 (0.19, 0.22) | 0.28 (0.24, 0.31) | ||
| Mercury (μg/L): | ||||
| 1st (−0.10, 0.61) | 108 | 31 | 48 | 31 |
| 2nd (0.62, 1.37) | 118 | 34 | 55 | 36 |
| 3rd (1.38, 9.88) | 114 | 33 | 50 | 33 |
| Geometric mean (95% CI)a | 0.98 (0.89, 1.08) | 0.93 (0.81, 1.08) | ||
| Lead (μg/dL): | ||||
| 1st (0.23, 0.57) | 125 | 36 | 35 | 23 |
| 2nd (0.58, 0.78) | 120 | 35 | 49 | 32 |
| 3rd (0.79, 5.84) | 95 | 27 | 69 | 45 |
| Geometric mean (95% CI) | 0.66 (0.63, 0.69) | 0.76 (0.71, 0.82) | ||
| Cotinine (ng/mL): | ||||
| No exposure (0, 9.99) | 310 | 89 | 121 | 79 |
| Passive smoking (10,99.99) | 13 | 4 | 11 | 7 |
| Active smoking (100,299.99) | 13 | 4 | 14 | 9 |
| Heavy smoking (300, 595.31) | 1 | 0.3 | 6 | 4 |
| Serum lipids (ng/g serum): | ||||
| 1st (301.01, 560.37) | 115 | 33 | 46 | 30 |
| 2nd (560.38, 653.05) | 115 | 33 | 50 | 33 |
| 3rd (653.06, 1288.57) | 105 | 30 | 57 | 37 |
| Male Exposure | ||||
| Cadmium (μg/L): | ||||
| 1st (0.01, 0.13) | 111 | 32 | 43 | 28 |
| 2nd(0.14, 0.22) | 136 | 39 | 39 | 25 |
| 3rd (0.23, 3.64) | 96 | 28 | 69 | 45 |
| Geometric mean (95% CI) | 0.17 (0.16, 0.19) | 0.23 (0.20, 0.27) | ||
| Mercury (μg/L): | ||||
| 1st (−0.05, 0.77) | 110 | 32 | 52 | 34 |
| 2nd (0.78, 1.72) | 112 | 32 | 56 | 36 |
| 3rd (1.73, 16.06) | 121 | 35 | 43 | 28 |
| Geometric mean (95% CI) | 1.19 (1.08, 1.31) | 1.18 (1.03, 1.35) | ||
| Lead (μg/dL): | ||||
| 1st (0.34, 0.88) | 129 | 37 | 33 | 21 |
| 2nd (0.89, 1.27) | 114 | 33 | 50 | 33 |
| 3rd (1.28, 6.91) | 100 | 29 | 68 | 44 |
| Geometric mean (95% CI) | 1.03 (0.98, 1.08) | 1.27 (1.17, 1.38) | ||
| Cotinine (ng/mL): | ||||
| No exposure (0, 9.99) | 283 | 82 | 104 | 68 |
| Passive smoking (10,99.99) | 13 | 4 | 9 | 6 |
| Active smoking (100,299.99) | 23 | 7 | 21 | 14 |
| Heavy smoking (300, 926.06) | 23 | 7 | 17 | 11 |
| Serum lipids (ng/g serum): | ||||
| 1st (342.42, 617.83) | 123 | 35 | 39 | 25 |
| 2nd (617.84, 774.84) | 113 | 33 | 53 | 34 |
| 3rd (774.85, 2148.98) | 104 | 30 | 59 | 38 |
NOTE: Observed pregnancy within 12 months of follow up. Not pregnant includes 100 couples who withdrew before pregnancy.
Excludes 5 women.
Figure 1 illustrates the Kaplan-Meier curves for metals (stratified by tertiles) and TTP. The cycle 1 probability accounts for couples found pregnant at enrollment; the probability at cycle 12 is censored consistent with the clinical definition of infertility. The longest TTPs were observed for the highest tertile of cadmium and lead irrespective of partner’s exposure. No association was observed between mercury and TTP.
Figure 1. Kaplan-Meier survival curves for time-to-pregnancy by partners’ metal exposures (in tertiles), LIFE Study, 2005–2009.
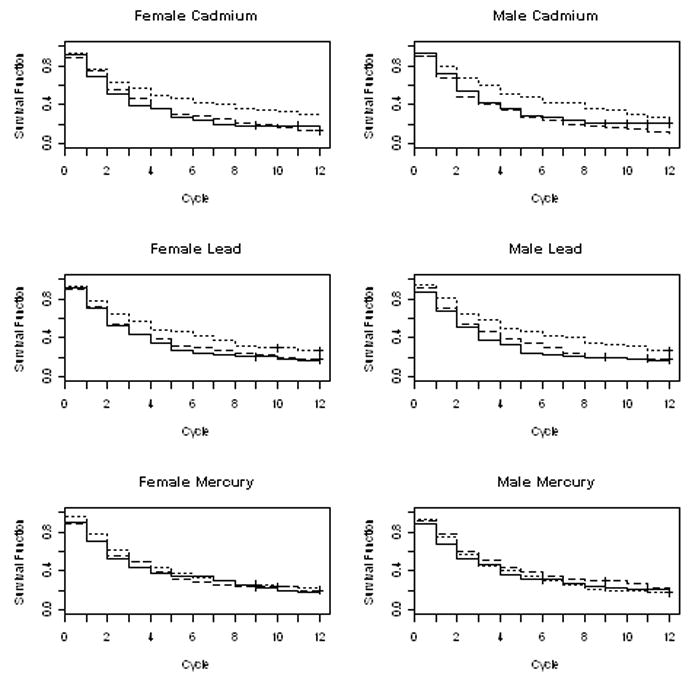
Black solid line corresponds to the lowest tertile, black dashed line corresponds to the middle tertile, and the black dotted line corresponds to the highest tertile.
In the model based upon female exposures, unadjusted FORs were below one suggesting a significantly longer TTP for female cadmium, lead, cotinine, serum lipids, and age, while parity was associated with an elevated FOR (Table 3). In the adjusted models, FORs remained below one only for cadmium (0.78; 95% CI 0.63, 0.97) and age (0.79; 95% CI 0.69, 0.91) while parity conferred an elevated FOR (1.75; 95% CI 1.36, 2.25). Similar findings were observed for males in unadjusted models with FORs <1 for cadmium, lead, and age. After adjustment, only lead (0.85; 95% CI 0.73, 0.98) and male age (0.85; 95% CI 0.75, 0.97) remained significant while parity increased the FOR (1.68; 95% CI 1.32, 2.13). When both partners’ exposures were jointly modeled, male lead concentration was the only metal that significantly reduced couple fecundability (FOR=0.82; 95% CI: 0.68, 0.97). Female age also conferred a reduction in fecundability (0.81; 95% CI 0.69, 0.94) while parity increased the FOR (1.80; 1.39, 2.31).
Table 3.
Blood Metals by Partner and Fecundability Odds Ratios, LIFE Study, 2005–2009.
| Model Metals & Covariates | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| FOR | 95% CI | FOR | 95% CI | |
| Female Exposure | ||||
| Cadmium (μg/L) | 0.70 | 0.58, 0.84 | 0.78 | 0.63, 0.97 |
| Lead (μg/dL) | 0.83 | 0.71, 0.96 | 0.97 | 0.85, 1.11 |
| Mercury (μg/L) | 0.95 | 0.85, 1.07 | 0.99 | 0.88, 1.13 |
| Cotinine (ng/ml) | ||||
| None/passive | 1.00 | referent | 1.00 | referent |
| Active/heavy | 0.51 | 0.29, 0.90 | 0.85 | 0.44, 1.64 |
| Serum lipids (ng/g) | 0.85 | 0.75, 0.96 | 0.94 | 0.82, 1.07 |
| Age | 0.81 | 0.72, 0.91 | 0.79 | 0.69, 0.91 |
| BMI | ||||
| < 18.5 | 1.00 | referent | 1.00 | referent |
| 18.5 – 24.9 | 0.85 | 0.32, 2.30 | 1.12 | 0.40, 3.13 |
| 25.0 – 29.9 | 0.68 | 0.25, 1.86 | 0.91 | 0.32, 2.59 |
| ≥30 | 0.61 | 0.22, 1.67 | 0.90 | 0.31, 2.56 |
| Site | 1.29 | 0.97, 1.73 | 1.23 | 0.91, 1.67 |
| Parity | 1.57 | 1.25, 1.98 | 1.75 | 1.36, 2.25 |
| Male Exposure | ||||
| Cadmium (μg/L) | 0.76 | 0.65, 0.90 | 0.85 | 0.71, 1.02 |
| Lead (μg/dL) | 0.78 | 0.68, 0.90 | 0.85 | 0.73, 0.98 |
| Mercury (μg/L) | 0.96 | 0.86, 1.08 | 0.98 | 0.87, 1.12 |
| Cotinine (ng/ml) | ||||
| None/passive | 1.00 | referent | 1.00 | referent |
| Active/heavy | 0.64 | 0.46, 0.90 | 0.89 | 0.60, 1.32 |
| Serum lipids (ng/g) | 0.94 | 0.84, 1.05 | 0.98 | 0.87, 1.10 |
| Age (years) | 0.83 | 0.74, 0.93 | 0.85 | 0.75, 0.97 |
| BMI | ||||
| < 18.5 | 1.00 | referent | 1.00 | referent |
| 18.5 – 24.9 | 0.15 | 0.02, 1.17 | 0.08 | 0.01, 0.63 |
| 25.0 – 29.9 | 0.16 | 0.02, 1.20 | 0.08 | 0.01, 0.63 |
| ≥30 | 0.17 | 0.02, 1.29 | 0.08 | 0.01, 0.65 |
| Site | 1.29 | 0.97, 1.73 | 1.34 | 0.99, 1.81 |
| Parity | 1.57 | 1.25, 1.98 | 1.68 | 1.32, 2.13 |
| Couple Exposure | ||||
| Female cadmium (μg/L) | -- | -- | 0.80 | 0.64, 1.00 |
| Female lead (μg/dL) | -- | -- | 1.06 | 0.91, 1.24 |
| Female mercury (μg/L) | -- | -- | 1.01 | 0.86, 1.17 |
| Female Cotinine (ng/ml) | -- | -- | ||
| None/passive | -- | -- | 1.00 | referent |
| Active/heavy | -- | -- | 0.95 | 0.48, 1.87 |
| Female lipids (ng/g) | -- | -- | 0.91 | 0.79, 1.04 |
| Female age (years) | -- | -- | 0.81 | 0.69, 0.94 |
| Female BMI | -- | -- | ||
| < 18.5 | -- | -- | 1.00 | referent |
| 18.5 – 24.9 | -- | -- | 1.02 | 0.36, 2.90 |
| 25.0 – 29.9 | -- | -- | 0.84 | 0.29, 2.41 |
| ≥30 | -- | -- | 0.83 | 0.28, 2.41 |
| -- | -- | |||
| Male cadmium (μg/L) | -- | -- | 0.94 | 0.77, 1.13 |
| Male lead (μg/dL) | -- | -- | 0.82 | 0.68, 0.97 |
| Male mercury ((μg/L) | -- | -- | 0.98 | 0.85, 1.15 |
| Male Cotinine (ng/ml) | -- | -- | ||
| None/passive | -- | -- | 1.00 | referent |
| Active/heavy | -- | -- | 0.92 | 0.61, 1.37 |
| Male lipids (ng/g) | -- | -- | 1.00 | 0.87, 1.13 |
| Delta parental agesb | -- | -- | 1.00 | 0.84, 1.19 |
| Male BMI | -- | -- | ||
| < 18.5 | -- | -- | 1.00 | referent |
| 18.5 – 24.9 | -- | -- | 0.07 | 0.01, 0.58 |
| 25.0 – 29.9 | -- | -- | 0.07 | 0.01, 0.58 |
| ≥30 | -- | -- | 0.08 | 0.01, 0.67 |
| Site | -- | -- | 1.30 | 0.95, 1.77 |
| Parity | -- | -- | 1.80 | 1.39, 2.31 |
NOTE: All covariates (excluding parity and site) were scaled by their standard deviation. CI, confidence interval; FOR, fecundability odds ratio
Adjusted for age (years), body mass index (kg/m2, categorized), cotinine (ng/mL, categorized), parity (nulliparous/parous), serum lipids (ng/g serum), and site (Texas/Michigan).
Age denoted the difference between male and female ages (in years).
4. Discussion
The LIFE Study is the first prospective cohort study with preconception enrollment of couples designed specifically to assess persistent environmental chemicals and human fecundity. Approximately 13% of couples did not achieve pregnancy within 12 months of trying, which is below the range (18%–38%) previously reported for cohort studies following women/couples for 12 months/cycles (DeMouzon et al., 1988; Zinaman et al, 1996); however, neither study measured chemical exposures.
Heavy metal exposure (i.e., cadmium and lead) was observed to be significantly associated with a reduction in couple fecundity requiring higher exposed couples a longer time to conceive an hCG confirmed pregnancy than couples with lower blood concentrations. When assessing partners’ exposures separately, female cadmium exposure was associated with approximately a 22% reduction and male lead exposure with approximately a 15% reduction in the odds of conception per standard deviation increase of blood concentrations. We did not observe any effect for mercury, which may reflect our inability to distinguish the more persistent organic versus inorganic forms of the compound. Of particular note is the observation that the magnitude of the metals’ effects was comparable to that observed for female and male age. Parity was the only factor conferring a significantly shorter TTP as measured by a FOR >1, with parous women requiring less time to conceive than nulliparous women consistent with previous findings (Axmon et al., 2006; Zinaman et al., 1996). Our findings were upheld even after conducting extensive sensitivity analyses such as removing parity, which may induce over-adjustment bias (Schisterman et al., 2009), removing serum lipids as a proxy for lipophilic chemical exposures and correcting for any time (≤2 months) couples may have been off contraception before enrollment (see Supplemental Table 1 for the latter results). The consistency of the findings for metals underscores their negative association with couple fecundity, as measured by TTP in the context of biology and lifestyle factors.
The loss of a significant female cadmium effect when modeling couples’ exposures is intriguing and has various possible explanation including: higher male exposures for all metals, our inability to separate pathway specific effects when assessing couple fecundability, a loss in power given an increased number of covariates in the joint model, left censoring, gender-specific differences in toxicity, or residual correlated partners’ exposures. With regard to the latter, Kappa measures of agreement for partners’ exposures in tertiles suggest otherwise: Cd (0.20; 95% CI 0.13–0.27); Pb (0.23; 955 CI 0.17, 0.30); and Hg (0.39; 95% CI 0.32, 0.45). Gender-specific differences may support higher lead concentrations in males than females as a function of higher blood hematocrit levels, thereby, allowing for the binding of lead to erythrocytes (Pirkle et al., 1998). Our findings do corroborate the well-documented reductions in fecundity for couples’ ages (Dunson et al., 2002) and higher fecundity for parous women with regard to successive pregnancies (Axmon et al., 2006; Buck Louis et al., 2009; Zinaman et al., 1996).
To our knowledge, only one previous prospective cohort study with preconception enrollment of women has assessed heavy metals and time-to-pregnancy (Bloom et al., 2011). Blood lead concentrations but not cadmium were associated with a reduction even when controlling for polychlorinated biphenyls and other relevant covariates, but the CI included one, possibly due to limited statistical power given the cohort (n=80) size. In a cross-sectional study comprising 41 couples with a pregnant partner, female’s mercury concentration >1.2 μg/L was associated with a significant reduction in adjusted FOR (0.22; 95% CI 0.07, 0.72), whereas no significant effects were observed for male or couple-based exposures (Cole et al., 2006).
Our findings are the first to be based upon a prospective cohort design including the preconception recruitment of couples with intensive longitudinal measurement of TTP and relevant covariates. The LIFE Study utilized population based sampling frameworks that generated a 2% recruitment yield underscoring the limited percentage of couples planning a pregnancy in the next few months at any point in time (Buck Louis et al., 2011). However, our yield is within the range (0.8% to 4.0%) reported for TTP studies with a defined denominator (Brown et al., 1997; Buck Louis et al., 2009). The large percentage of individuals who could not be contacted at their reported current address may introduce selection bias, though we know of no evidence to suggest metals exposure or TTP systematically varies by household moving. In addition despite sampling from two geographic locations and using two different frameworks, we observed few differences in study participants (Buck Louis et al., 2011). Other study strengths include a standardized anthropometric assessment of both partners of the couple by the research assistant and the use of cotinine measured at baseline as a marker of cigarette smoking exposure. Still, our findings are limited by the absence of attention to gene-environmental interactions as recently summarized (Wirth and Mijal, 2010), quantification of trace metals such as copper or manganese purportedly associated with adverse reproductive effects including those resulting from male exposures (Jurasovic et al., 2004; Meeker et al., 2008), and residual confounding. Of note are the relatively similar distributions for metals observed in our cohort with those reported for females and males participating in the National Health and Nutrition Evaluation Survey (NHANES), 2005–2006 (Centers for Disease Control and Prevention, 2010).
The exact mechanisms by which heavy metals may exert their toxicity remain to be established, though recent summaries support a role for both direct and indirect pathways for both male and females. Potential mechanisms for reproductive toxicity for male lead exposure may include hormonal disruption of spermatogenesis or direct toxicity to the seminiferous tubules (Assennato et al., 1986; Caserta et al., 2011; Iavicoli et al., 2009; Wirth and Mijal, 2010). Of note is our observed association of a longer TTP for male blood lead concentrations below the limit (>40 μg/dL) purported to be associated with decrements in semen quality (Apostoli et al., 1998). Despite the absence of an independent significant effect for female blood lead concentrations when jointly modeling partners’ exposures in our study, previous findings suggest that concentrations >2.5 μg/dL conferred a significant threefold increased risk of infertility in comparison to women with lower exposures (Chang et al., 2006). The mechanisms underlying heavy metals and adverse reproductive effects are yet to be delineated, though a recent prospective cohort of 252 premenopausal women with comparable blood metals concentrations reported a negative relation between blood cadmium and follicle stimulating hormone (Pollack et al., 2011).
5. Conclusions
Our findings suggest that environmentally relevant concentrations of blood lead and, possibly, cadmium are associated with a longer TTP. Our limited understanding regarding the impact of the male, female and couple based exposures in relation to reproductive endpoints remains a critical data gap from both mechanistic and public health intervention aspects.
Supplementary Material
Highlights.
Female cadmium and male lead blood concentrations associated with a longer time-to-pregnancy.
Male blood lead effect remained in the context of female exposures.
Environmentally-relevant concentrations of metals adversely affect couple fecundity.
Acknowledgments
Supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts #N01-HD-3-3355; N01-HD-3-3356; N01-HD-3-3358).
Abbreviations
- Cd
cadmium
- CI
confidence interval
- FOR
fecundability odds ratio
- Hg
mercury
- Pb
lead
- TTP
time-to-pregnancy
Footnotes
CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Germaine M. Buck Louis, Email: louisg@mail.nih.gov.
Rajeshwari Sundaram, Email: sundaramr2@mail.nih.gov.
Enrique F. Schisterman, Email: schistee@mail.nih.gov.
Zhen Chen, Email: chenzhe@mail.nih.gov.
Sungduk Kim, Email: kims2@mail.nih.gov.
References
- 1.Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chem Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 2.Apostoli P, Kiss P, Porru S, Bonde JP, Vanhoorne M. Male reproductive toxicity of lead in animals and humans. ASCLEPIOS Study Group Occup Environ Med. 1998;55:364–374. doi: 10.1136/oem.55.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assennato G, Paci C, Baser ME, Molinini R, Candela RG, Altamura BM, Giorgino R. Sperm count suppression without endocrine dysfunction in lead exposed men. Arch Environ Health. 1986;41:387–390. doi: 10.1080/00039896.1986.9935784. [DOI] [PubMed] [Google Scholar]
- 4.ATSDR. Health and Human Services. US Department of Health and Human Services. Public Health Service; Atlanta, GA: 2004. Interaction profile for arsenic, cadmium, chromium and lead. [Google Scholar]
- 5.Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13:1532–1539. doi: 10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- 6.Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006;21:1279–1284. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- 7.Behre HM, Kuhlage J, Gahner C, Sonntag B, Schem C, Schneider HP, Nieschlage E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan® Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- 8.Bernert JT, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW, Miller BB, Patterson DG, Needham LL, Hannon WH, Sampson EJ. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chemistry. 1997;43:2281–2291. [PubMed] [Google Scholar]
- 9.Bloom MS, Buck Louis GM, Sundaram R, Kostyniak PJ, Jain J. Associations between blood metals and fecundity among women residing in New York State. Reprod Toxicol. 2011;31:158–163. doi: 10.1016/j.reprotox.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JE, Jacobs DRJ, Barosso GM, Potter JD, Hannan PJ, Kopher RA, Rourke MJ, Hartman TJ, Hose K. Recruitment, retention and characteristics of women in a prospective study of preconceptional risks to reproductive outcomes: experience of the Diana Project. Paediatr Perinat Epidemiol. 1997;11:345–358. doi: 10.1111/j.1365-3016.1997.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 11.Buck GM, Lynch CD, Stanford JB, Sweeney AM, Schieve LA, Rockett JC, Selevan SG, Schrader SM. Prospective Pregnancy Study Designs for Assessing Reproductive Developmental Toxicants. Environ Health Perspect. 2004;112:79–86. doi: 10.1289/ehp.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck Louis GM, Dmochowski J, Lynch CD, Kostyniak PJ, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod. 2009;24:451–458. doi: 10.1093/humrep/den373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton R, Lynch CD, Barr DD, Schrader SM, Kim S, Chen Z, Sundaram R. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction – the LIFE Study. Paediatr Perinatal Epidemiol. 2011;25:413–424. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C, Maranghi F, Moscarini M. Environment and women’s reproductive health. Hum Reprod Update. 2011;17:418–433. doi: 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- 15.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/PbCd_E_met_lead_cadmium.pdf.
- 17.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables. 2010 http://www.cdc.gov/exposurereport.
- 18.Chang S-H, Cheng B-H, Lee S-L, Chuang HY, Yang CY, Sung FC, Wu TN. Low blood lead concentration in association with infertility in women. Environ Res. 2006;101:380–386. doi: 10.1016/j.envres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Cole DC, Wainman B, Sanin LH, Weber JP, Muggah H, Ibahim S. Environmental contaminant levels and fecundability among non-smoking couples. Reprod Toxicol. 2006;22:13–19. doi: 10.1016/j.reprotox.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Cooney MA, Buck Louis GM, Sundaram R, McGuinness B, Lynch CD. Validty of self-reported time to pregnancy. Epidemiol. 2009;20:56–59. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox DR. Regression Models and Life Tables (with discussion) J Royal Stat Soc, Series. 1972;20:187–220. [Google Scholar]
- 22.De Mouzon J, Spira A, Schwartz D. A prospective study of the relation between smoking and fertility. Int J Epidemiol. 1988;17:378–384. doi: 10.1093/ije/17.2.378. [DOI] [PubMed] [Google Scholar]
- 23.Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17:1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- 24.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 25.Harley KG, Marks AR, Chevrier J, Bradman A, Sjödin A, Eskenazi E. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12:206–223. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- 28.Järup L. Hazards of heavy metal contamination. Br Med Bullet. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 29.Joffe M, Bisanti L, Apostoli P, Kiss P, Dale A, Roeleveld N, Lindbohm ML, Sallmén M, Vanhoorne M, Bonde JP. Time to pregnancy and occupational lead exposure. Occup Environ Med. 2003;60:752–758. doi: 10.1136/oem.60.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurasovic J, Cvitkovic P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals. 2004;17:735–743. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 31.Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 32.Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008;116:1473–1479. doi: 10.1289/ehp.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meeker JD, Maity A, Missmer SA, Williams PL, Mahalingaiah S, Ehrlich S, Berry KF, Altshul L, Perry MJ, Cramer DW, Hauser R. Serum concentrations of polychlorinated biphenyls in relation to in vitro fertilization outcomes. Environ Health Perspect. 2011;119:1010–1016. doi: 10.1289/ehp.1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 35.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991–1994. Environ Health Perspect. 1998;106:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, Wactawski-Wende J. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119:1156–1161. doi: 10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ, Greenland S, editors. Modern Epidemiology. 2. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 39.Sallmén M, Lindbohm ML, Anttila A, Taskinen H, Hemminki K. Time to pregnancy among the wives of men occupationally exposed to lead. Epidemiol. 2000;11:141–147. doi: 10.1097/00001648-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall; 1997. [Google Scholar]
- 41.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiau CY, Wang JD, Chen PC. Decreased fecundity among male lead workers. Occup Environ Med. 2004;61:915–923. doi: 10.1136/oem.2004.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slama R, Ducot B, Carstensen L, Lorente C, de La Rocherbrochard E, Leridon H, Keiding N, Bouyer J. Feasibility of the current-duration approach to studying human fecundity. Epidemiol. 2006;17:440–449. doi: 10.1097/01.ede.0000221781.15114.88. [DOI] [PubMed] [Google Scholar]
- 44.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer Series, Statistics for Biology and Health; 2000. [Google Scholar]
- 45.Vahter M, Berglund M, Åkesson A, Lindén C. Metals and women’s health. Environ Res. 2002;88:145–155. doi: 10.1006/enrs.2002.4338. [DOI] [PubMed] [Google Scholar]
- 46.Wall MA, Johnson J, Jacob P, Benowitz NL. Coitine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78:699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 2010;56:147–167. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- 48.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


