Abstract
An elevated plasma concentration of serotonin ([5-HT]) is a common feature of cardiovascular disease often associated with enhanced platelet activation and thrombosis. Whether elevated in vivo plasma 5-HT per se represents an independent risk factor for platelet hyperreactivity or only is an epiphenomenon of cardiovascular disease is poorly understood. We examined in vitro and in vivo platelet function following a 24 hr elevation of plasma [5-HT] in mice. In vivo administration of 5-HT using osmotic minipumps increased plasma [5-HT] in treated mice compared to control mice instrumented with saline loaded pumps. 5-HT infusion did not increase systolic blood pressure, but markers of platelet activation including P-selectin and PEJon/A staining were increased and these findings coincided with the enhanced aggregation of isolated platelets in response to type I fibrillar collagen. Tail bleeding times and the time to occlusion following chemical damage to the carotid artery were shortened in 5-HT-infused mice. 5-HT-infused mice were treated with paroxetine (Prx) to block 5-HT uptake via the serotonin transporter (SERT). Prx lowered platelet [5-HT] and attenuated platelet activation and aggregation. These results and our biochemical indices of enhanced 5-HT intracellular signaling in the platelets of 5-HT-infused mice reveal a mechanistic link between elevated plasma [5-HT], abnormal intracellular 5-HT signaling and accentuated platelet aggregation. Although a down-regulation of the serotonin transporter (SERT) on the platelet surface may counteract the pro-thrombotic influence of elevated plasma [5HT], this compensatory mechanism may fail to prevent the increased thrombotic risk caused by elevated plasma [5-HT].
Keywords: serotonin transporter, platelet, serotonin
1. Introduction
5-Hydroxytryptamine (5-HT, serotonin) is a well characterized neurotransmitter in the central nervous system [1]. However, it also plays diverse roles in the circulation by regulating vascular tone and platelet function [2–3]. 5-HT is synthesized and secreted into blood by enterochromaffin cells of the intestine, but its plasma concentration is primarily regulated by circulating platelets [4]. Specifically, platelets express serotonin transporters (SERT) on their membrane surface providing a saturable reuptake mechanism that can regulate the concentration of 5-HT ([5-HT]) in the plasma. Once in the platelet cytoplasm, 5-HT is sequestered by the vesicular monoamine transporter type 2 (VMAT2) into intracellular dense granules [5], but prior to sequestration, the 5-HT molecules are capable of activating intracellular signaling pathways linked to platelet activation and aggregation. Notably, plasma [5-HT] is in the low nanomolar range, but the dense granules of resting platelets store millimolar concentrations of 5-HT [6–7]. Thus, platelets apparently are designed to tightly control the intracellular concentration of 5-HT.
The biological role of 5-HT during cardiovascular disease is incompletely understood. However, it seems apparent that plasma [5-HT] is elevated in a subset of patients with cardiovascular disorders including hypertension [8–10], coronary artery disease (CAD) [11–14], atherothrombosis [12–15] and myocardial infarction [16–17]. For example, Brenner et al. [10] reported a 33% increase of [5-HT] in the plasma of patients diagnosed with hypertension after emergency room admission. Vikens et al. [13] noted a 10–fold elevation of plasma [5-HT] in patients undergoing angiography after admission for myocardial infarction. In these patients, the elevated plasma [5-HT] was associated with CAD and cardiac events [13]. A 16-fold rise in [5-HT] was reported in the coronary sinus of patients following angioplasty by Leosco and colleagues [17]. In the same patients, the [5-HT] assayed in systemic plasma samples was normal, inferring that locally elevated [5-HT] may not be routinely detected as a risk factor. Thus, a physiological in vivo interplay between circulating 5-HT and platelet function may be a predictor of coronary artery disease. Additionally, atherothrombosis, cerebrovascular ischemia and myocardial infarction have been linked to elevated [5-HT] [8–21]. Indeed, tryptophan hydroxylase 1 (TPH1) knockout mice lacking 5-HT have a mild bleeding abnormality [22]. Notably, patients diagnosed with cardiovascular disorders also may show a blunted release of endothelium-derived prostacyclin and nitric oxide. It has been proposed that the deficit of these anti-coagulant molecules may permit amplification of the opposing, pro-coagulant actions of 5-HT [18]. However, it is not fully understood if elevated in vivo plasma 5-HT per se represents an independent risk factor for platelet hyperreactivity or simply is an epiphenomenon of cardiovascular disease.
To address this important issue, we established an experimental model of 5-HT-infused mice. Adult C57BL/6J mice were implanted with osmotic mini-pumps infusing 5-HT for 24 hours to elevate plasma [5-HT] to levels observed in humans with cardiovascular disease without increasing systolic blood pressure (SBP). In-vivo hemostasis assays combined with biochemical and aggregation studies in isolated platelets provided evidence that elevated plasma 5-HT directly affects platelet function, both in vitro and in vivo, establishing prohemostatic and prothrombotic consequences. Indeed, elevated extracellular 5-HT has been shown to promote platelet aggregation by receptor independent-[22, 23] and –dependent [24, 25] signaling pathways. In a receptor-dependent pathway, 5-HTex can bind to 5-HT surface membrane receptors [24–25] initiating a G-protein signaling pathway that mobilizes calcium from intracellular stores to trigger the vesicular release of pro-coagulant molecules from α-granules [7]. In a receptor independent pathway, 5-HT in the platelet cytosol is transamidated on small GTPases converting them to their active, GTP-bound form [22–23]. One of the downstream events is an association between Rab4-GTP and SERT which tethers the transporter to an intracellular compartment [26] resulting in the loss of SERT on the platelet surface and reduced uptake of 5-HT. Using our 5-HT-infused mice model we have examined the physiological relevance of both pathways, elevated 5-HTex and then the consequences of SERT down-regulation in the cytoplasmic milieu of the hyperreactive platelet.
In this manuscript we did biochemical analysis of platelets from 5-HT-infused mice and showed an elevation in calcium mobilization and activation of platelet transglutaminase (TGase). While the association between Rab4-GTP and SERT could only be found in the platelets of 5-HT-, this association was abolished if the mice were injected with Paroxetine (Prx) prior to the 5-HT-infusion. Prx lowers 5-HT levels in platelet cytoplasm via disabling SERT [22–26]. Interestingly, Prx also reduced markers of platelet activation in 5-HT-infused mice. These findings emphasize the role of platelet 5-HT levels in initiating platelet activation. Additional studies with the transamidation of Rab4 with 3H-5HT and its GTP-binding ability with 35S-γ-GTP in 5-HT-treated platelets revealed a mechanistic link between SERT and the accentuated platelet response to 5-HTex. Based on these findings we propose that the down-regulation of 5-HT uptake rates of SERT counteract the pro-thrombotic influences of elevated 5-HT levels in platelet cytoplasm that predominates in the platelet activation and aggregation processes. However, this important compensatory mechanism may fail to prevent the increased thrombotic risk caused by elevated plasma [5-HT].
2. Materials and methods
2.1. Animal protocols
Adult C57BL/6J male mice (10–12 weeks old) were anesthetized with isoflurane (2.5% at 1.5 L/min oxygen) for implantation of subcutaneous osmotic mini-pumps (Alzet model 1003, Alza Corp). The mini-pumps were filled with saline or 5-HT (0.05 mg/ml dissolved in saline) and set for an infusion rate of 1 μl/hr. A subset of mice was injected immediately after surgery with Prx (3 mg/kg i.p.), a SERT inhibitor [27–29]. For a typical experiment, 5 mice from a single experimental group contributed blood to a pooled sample. Procedures involving animals were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Blood sampling and platelet preparation
After 24 hours of continuous infusion with either saline or 5-HT, blood was withdrawn into a syringe containing 3.8% sodium citrate solution by cardiac puncture from each animal. Samples of platelet and plasma were prepared from the whole blood [10]. In biochemical studies, each assay was performed using the same number of platelets (300,000 platelets/μL blood). The [5-HT] in platelets and plasma was quantified by competitive enzyme-linked immunosorbent assay (ELISA). The platelet 5-HT uptake assay was performed as described [10].
2.3. Blood pressure measurement
SBP was measured in mice using tail cuff plethysmography. Baseline SBP was calculated by averaging a minimum of 6 trials on 2 consecutive days prior to insertion of mini-pumps containing saline or 5-HT. The SBP was recorded again by taking a minimum of 6 trials in each mouse after 24 hours of saline or 5-HT infusion and averaging the values.
2.4. Quantitative measurement of 5HT levels by ELISA
The 5-HT concentration ([5-HT]) in platelets and plasma prepared from the blood samples of each animal model was measured by competitive ELISA following the manufacturer’s instructions (IBL Immuno-Biological Laboratories, Hamburg, Germany) [10]. Briefly, samples were acylated with acetic anhydride in acetone and samples or standards were applied to 96-well microtitre plates coated with goat anti-rabbit IgG. Biotinylated 5-HT and rabbit antiserum to 5-HT were added to each well and incubated overnight at 4°C. Para-nitrophenylphosphate in a diethanolamine solution was used as a substrate following the application of alkaline phosphatase conjugated goat anti-biotin antibody (Ab). Samples were read at 405 nm on an ELISA plate reader (Molecular Devices Union City, CA, USA). The concentrations of 5-HT were quantified using standards supplied by the manufacturer and analyzed using Origin software (Microcal Software, Northampton, MA).
2.5 Platelet 5-HT uptake assay
Platelet pellets were quickly washed with phosphate-buffered saline (PBS) containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM) then resuspended in PBS/CM with 14.6 nM 3H-5-HT at room temperature (RT) for 10 min, to include only the initial linear phase of 5-HT transport [10]. Platelets were collected by rapid filtration through Whatman GF/B filters and were washed twice with 5 ml of ice-cold PBS. Filters were placed in scintillation vials containing 5 ml scintillation cocktail and immediately counted.
Background accumulation of 3H-5-HT that occurred independently of SERT was measured in the same experiment using platelets incubated with the high-affinity cocaine analog, 0.1 μM 2β-carbomethoxy-3-tropane (β-CIT) (Chemical Synthesis Service, NIMH, Bethesda, MD). The value was subtracted from each experimental value to estimate 5-HT uptake mediated by SERT [10]. In parallel, the protein concentration of 0.2 × 106 (0.015 mg cellular protein) platelets was determined using the Micro BCA protein Assay Kit. 5-HT uptake rates were calculated as means of standard deviation values from three independent experiments.
2.6. Immunoprecipitation (IP)
Binding of Rab4 to SERT was assayed in platelets (300,000/μL of platelets) as described previously [10, 26]. The precleared lysate was combined with an equal volume of a 1:1 slurry of protein A Sepharose beads and mixed overnight at 4°C with a polyclonal Rab4-Ab (BD Transduction Laboratories). Samples were separated on an SDS-PAGE and analyzed by Western blot (WB) with monoclonal anti-SERT Ab (1:1,000 dilution). The signals were developed using an ECL detection system.
For radio-immunoprecipitation (RIP), platelets (300,000/μL of platelets) were treated with 10 mM cold and [3H]-5HT (100 μCi/ml) or 35S-γ-GTP (125 μCi/ml) in PBS/CM at RT. After 1 hr, the cells were washed quickly with ice-cold PBS and harvested, and Rab4 was immunoprecipitated using monoclonal Rab4-Ab [26]. Immune precipitates were eluted from the beads by incubation in SDS sample buffer, analyzed by 12.5% SDS-PAGE, and visualized by fluorography.
2.7. Western blot analysis
Platelets (300,000/μL of platelets) were washed, lysed and solubilized in PBS containing 0.44% SDS, 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor mixture (PIM). The PIM contained 5 mg/ml pepstatin and 50 mg/ml leupeptin, and 5 mg/ml aprotinin was included in the lysis buffer, which also contained the alkylating agent N-ethylmaleimide (NEM, 5 mM) to prevent oxidation and formation of nonspecific disulfide bonds during lysis and to retain the native monomeric structures in the gel. Samples were analyzed by 8% SDS-PAGE and transferred to the nitrocellulose membrane. WB analysis was performed, first with monoclonal hSERT-Ab (MAb Technologies, Inc. Stone Mountain, GA) (diluted 1:1,000), and then with HRP-conjugated anti-mouse secondary antibodies at a dilution of 1:10,000. The signals were visualized using the ECL WB detection system. Blots were visualized under a VersaDoc 1000 gel visualization and analysis system (BioRad Lab) [10].
2.8. Cell surface biotinylation
Platelet plasma membrane (PM) expression levels of SERT were compared using the membrane-impermeant biotinylation reagent, NHS-SS-biotin (Pierce, Inc., Rockford, IL) [10, 26]. Platelets from blood samples (300,000/μL of platelets) were prepared and washed twice using ice-cold PBS/CM. Next, the platelets were incubated with NHS-SS-biotin (1.5 mg/mL) on ice during gentle shaking. After biotinylation, platelets were first briefly rinsed and then incubated with PBS/CM containing 100 mM glycine on ice. Platelets were lysed with 1% SDS-1% TX100. Then the biotinylated proteins were recovered from the platelet lysate using streptavidin-agarose beads (Pierce, Inc., Rockford, IL). The beads were washed with high salt, low salt and 50 mM Tris-HCl (pH 7.5). The biotinylated proteins were eluted from the beads in SDS-PAGE sample buffer. The mercaptoethanol cleaves the disulfide bond of NHS-SS-biotin, releasing the recovered proteins from the biotin moiety and consequently from the streptavidin- agarose beads. The cell surface proteins were separated using SDS-PAGE. SERT was detected by WB using the monoclonal hSERT-Ab (MAb Technologies). In platelets a single SERT band migrating at 90 kDa is recognized.
2.9. Fura 2-AM loading platelets and Ca2+ measurements
Isolated platelets (300,000/μL) were suspended in Tyrode-HEPES buffer. Platelets were loaded with fura 2-AM (2 μM) for 45 min at RT protected from light. Thereafter, the platelet suspension was centrifuged at 700 × g for 10 min and the platelet pellet was washed and resuspended in Tyrode-HEPES and stored in the dark until the measurement was performed. A standard curve was prepared by using calcium calibration buffers (Invitrogen) containing 10-fold concentrates of the K2EGTA and CaEGTA reagents, which were used for Kd determination. Calcium-dependent fluorescence signals were obtained using excitation at 340 nm and 380 nm and fluorescence intensity ratios were detected at 510 nm [30, 41]. The intracellular Ca2+ levels were calculated according to the formula: [Ca2+]i = kd × (F − Fmin)/(Fmax − F), where kd is the fura dissociation constant. The Fmax value was obtained with triton X-100 (0.1%) in the presence of saturating calcium (CaCl2 1 mM), whereas Fmin value was obtained with the calcium chelator EGTA (10 mM) plus Tris (20 mM; pH = 8).
2.10. TGase Assay
Tissue TGase enzymatic activity was assayed by measuring the incorporation of [1.4(n)-3H]-putrescine dihydrocloride (14.4 Ci/mM; Perkin-Elmer) into N,N′-dimethylcasein (Sigma) as described [31, 32]. Briefly, platelet (300,000/μL of platelets) homogenates were incubated for 90 min at 37°C with [1,4-3H]putrescine (2.5 mM, 3.97 mCi/mmol), dithiothreitol (3.8 mM), CaCl2 (2.5 mM), and dimethylcasein (5 mg/ml). Purified tissue TGase (1μg) from guinea pig liver (Sigma, MO) was used as a positive control The reaction was stopped by the addition of 50% trichloroacetic acid and precipitated with 10% BSA overnight at 4°C. After several wash steps, the amount of putrescine incorporated into the precipitated proteins was determined by scintillation counting.
2.11. Ferric chloride (FeCl3)-induced thrombus formation
Ferric chloride (FeCl3)-induced carotid artery injury was performed as described [31]. Briefly, the carotid artery was exposed in anesthetized mice (2.5% isoflurane) followed by placement for 3 min of a 1 × 2 mm strip of Whatman No. 1 filter paper soaked with 10% FeCl3 (0.62 mol/L) on the exposed artery. After removal of the filter paper, the exposed area was thoroughly rinsed with isotonic saline. Blood flow was monitored using a laser Doppler system (Vasamedics Inc., Eden Prairie, MN) connected to a BPM2 Blood Perfusion Monitor (Vasamedics) interfaced to digital output (PowerLab System, AD Instruments, Castle Hill, Australia). The readout was a determination of the time to occlusion.
2.12. Mouse tail bleeding time assay
Tail bleeding time was performed by 2 independent researchers blind to group assignment. Mice were immobilized using a restrainer (Braintree Scientific Inc.). The distal 3-mm portion of the tail was removed. Immediately the tail was inserted into 37°C isotonic saline and the time until bleeding stopped recorded [34].
2.13. Stirred platelet aggregation
For aggregation assays, platelets in plasma were prepared and platelet counts were normalized (300,000/μL) using a Hemavet 950 (Drew Scientific, Waterbury, CT). The responses to different platelet agonists including thrombin (0.5, 1 and 3 U/ml), ADP (5, 10 and 20 μM) and collagen (2, 4 and 5 μg/ml) were monitored by light transmittance (Chrono-log Corp., Havertown, PA) [35].
2.14. Flow cytometry
The impact of elevated plasma [5-HT] on platelet activation was assessed using phycoerythrin (PE) labeled anti-αIIbβ3 (Jon/A) Ab developed to follow integrin activation on mouse platelets [36]. Platelets (300,000/μL) were incubated in the dark with PEJon/A (Emfret Analytics, Cat M023-2) and at the end of the incubation, 300 μL of 2% formaldehyde in PBS was added to stop the reaction. The samples were gated for single platelets based on forward and side scatter profiles and 20,000 events were recorded and read at the UAMS Flow Cytometry Core Facility. Additional Abs used for flow cytometry included anti-P-selectin (Emfret Analytics, Cat M130-1), anti-granulophysin (Santa Cruz Technology, Cat SC-15363), and anti-glycoprotein Ib (Emfret Analytics, Cat M040-0).
2.15. Data analysis
Nonlinear regression fits of experimental and calculated data were performed with Origin, which uses the Marquardt-Levenberg non-linear least squares curve fitting algorithm. Each figure shows a representative experiment that was performed at least three times. The statistical analyses given in the Results section is from multiple experiments. Data with error bars are represented as mean ± SEM for triplicate samples. Data were analyzed by ANOVA (analysis of variance) to compare data sets and two-sided t-tests based on the ANOVA mean squared error.
3. Results
3.1. Murine models of 5-HT infusion
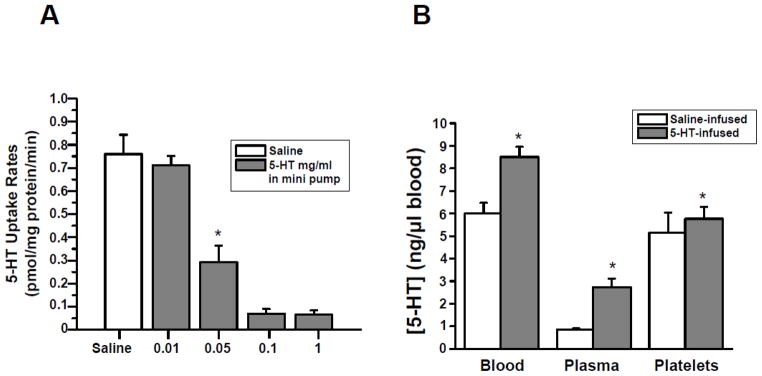
Adult C57BL/6J male mice were randomized to receive osmotic mini-pumps filled with isotonic saline or filled with isotonic saline in which 5-HT (0.01 – 1.0 mg/ml) was dissolved. The platelets were isolated from each group of mice and the 5-HT uptake rates were measured (Fig. 1, Panel A). The 5-HT uptake rate was reduced by 61% in platelets of mice (n = 15) instrumented with mini-pumps loaded with 0.05 mg/ml 5-HT compared to control mice infused with saline. Higher concentrations of 5-HT (0.1 and 1 mg/ml) in the osmotic mini-pumps reduced the 5HT uptake rate of platelets by 90%. In order to only mildly elevate circulating 5-HT levels in our studies (Fig. 1, Panel B) less than increases reported in patients with cardiovascular disease, mice were infused with 0.05 mg/ml 5-HT at an infusion rate of 1.66 μg/kg/hr.
Fig. 1.
(A) Effect of 5-HT concentration in mini-pump on 5-HT uptake rates of platelets. Osmotic mini-pumps were filled with 90 ± 10 μl of saline or 5-HT (dissolved in saline to provide a dose of 0.01. 0.05, 0.1 and 1 mg/ml) (n=15 group). At the end of a 24-hr infusion, platelets were isolated and the 5-HT uptake rates of platelets measured [10]. Elevated plasma 5-HT in 5-HT-infused mice was associated with a 61% decrease in the platelet 5-HT uptake rate compared to saline-infused mice. 5-HT uptake rates of platelets were assayed in triplicate. (B) Blood 5-HT concentration. The [5-HT] in blood samples of the saline- and 5-HT-infused mice was measured by a competitive ELISA technique (6.00±0.47 ng/μl blood in saline-infused, and 8.50±0.44 ng/μl blood in 5-HT-infused) [10]. 5-HT-infusion increased plasma [5-HT] by 3.2 –fold. All assays were performed in triplicate (n =15 group). * = statistical difference between saline- and 5-HT-infused mice (n = 15 for each group).
Twenty-four hours after mini-pump insertion, platelets were counted in blood samples from saline and 5-HT –infused mice. The circulating platelet count was not altered by 5-HT infusion. The [5-HT] in blood and its plasma and platelet components were determined by ELISA. Platelet [5-HT] was 5.15 ± 0.89 ng/μl blood for saline-infused animals (n= 15) and 5.77 ± 0.54 ng/μl blood for 5-HT-infused animals (n= 15) (Fig. 1, Panel B). Although platelet [5-HT] was not significantly elevated in 5-HT compared to saline–infused mice at 24 hours, the plasma to platelet distribution of 5-HT differed markedly between the two animal groups. As anticipated, saline-infused mice showed a low blood [5-HT] (0.85 ± 0.04 ng/μl blood) with 86% of total blood 5-HT concentrated in platelets (5.15 ng/μl blood). In contrast, 5-HT-infused mice showed a 3.2–fold increase in plasma [5-HT], but only 68% of total 5-HT was attributed to platelets. Interestingly, the 5-HT concentration (5.77 ng/μl blood) in these platelets was not significantly different from the platelets of saline –infused mice (5.15 ng/μl blood) (Fig. 1, Panel B and Table 1).
TABLE 1.
The summary of our findings in 4 group of mice model system.
| Saline-infused | Prx-injected saline-infused | 5-HT-infused | Prx-injecte 5-HT-infused | |
|---|---|---|---|---|
| [5-HT] in plasma (ng/μl blood) | 0.85 ± 0.04 | 2.95 ± 0.05 | 2.74 ± 0.37 | 5.54 ± 0.16 |
| [5-HT] in platelet (ng/μl blood) | 5.15 ± 0.89 | 3.07 ± 0.09 | 5.77 ± 0.54 | 2.98 ± 0.01 |
| SERT on the plasma membrane (% of total) | 100 ± 0.8 | 95 ± 0.5 | 58 ± 5.9 | 94 ± 6 |
| 5-HT uptake rates (pmol/min/mg protein) | 0.76 ± 0.08 | 0.18 ± 0.04 | 0.29 ± 0.07 | 0.15 ± 0.06 |
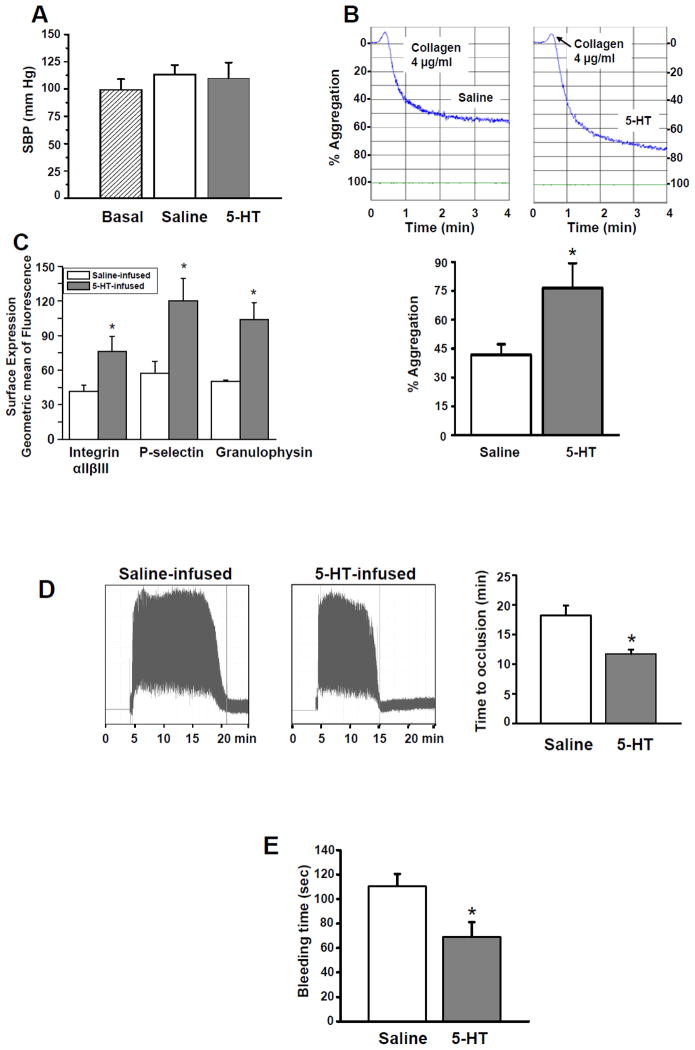
Reports from patients with cardiovascular disease indicate that plasma [5-HT] may rise as much as 4–fold in chronic disorders including hypertension, diabetes and CAD [10, 13, 14, 20, 21]. More profound plasma 5-HT elevations averaging 10 to 16-fold higher than normal subjects have been detected during surgeries for acute coronary conditions [13, 14]. In our study, 5-HT infusion did not significantly elevate SBP and thus a rise in blood pressure was not a confounding factor underlying observed changes in platelet function (Fig. 2A).
Fig. 2.
(A) Blood pressure. SBP was evaluated using tail-cuff plethysmography. Saline or 5-HT infusions did not change SBP (basal 99±9.7, Saline 113±8.6, 5-HT 110±14X mm Hg; n = 15 group). (B) Platelet aggregation. Representative tracings of stirred platelet aggregation reactions are shown. In response to stimulation with 4 μg/ml collagen, platelets from 5-HT-infused mice showed a 45% higher aggregation rate than the platelets from saline-infused mice. * = statistical difference between saline- and 5-HT-infused mice. All assays were performed in triplicate (n = 15 group). (C) Integrin αIIbβ3, P-selectin and granulophysin flow cytometry. The impact of plasma 5-HT on the platelet activity was evaluated by measuring the binding of integrin αIIbβ3 to the activation-dependent antibody PEJon/A. The flow cytometry data revealed an 83% elevation in the expression level of PEJon/A binding in platelets of 5-HT-infused mice. Increases also were observed for other markers of platelet activation, such as P-selectin and granulophysin. This assay was performed in triplicate. (D) In vivo thrombosis. Tracings of the laser Doppler signal corresponding to red blood cell velocity in carotid arteries exposed to FeCl3 injury 24 hr after mice were infused saline- or 5-HT. After damage by FeCl3 of an exposed carotid artery, the Doppler signal in an anesthetized animal was followed for approximately 16 min. A representative tracing from each group is shown but is indicative of results obtained from 15 different animals. Occlusion time. Average time to occlusion was plotted for saline-and 5-HT-infused mice (mean ± SEM; n=15 for both groups). (E) Bleeding time. We used a tail bleeding model to assess hemostasis. The tail bleeding time for 5-HT-infused mice was shortened by 38% compared to saline-infused mice (n = 15 for each group). * = statistical difference between saline- and 5-HT-infused mice.
3.2. Platelets of 5-HT-infused mice
After validating in our model that elevated plasma [5-HT] did not cause hypertension, platelets of 5-HT -infused mice were evaluated for aggregation responses and markers of activation. Isolated platelets from saline and 5-HT-infused mice were stimulated with thrombin (0.5, 1 and 3 U/ml), ADP (5, 10 and 20 μM) and collagen (2, 4 and 5 μg/ml) to test the effect of elevated plasma [5-HT] on stirred platelet aggregation assays. Platelets from both groups of mice showed the maximal aggregation response to 4 μg/ml collagen. This finding concurs with reports that 5-HT -induced platelet activation depends on collagen metabolites [37–38]. Subsequently, the responses of isolated platelets from 5-HT- and saline-infused mice to 4 μg/ml collagen were monitored. Representative tracings (Fig. 2, Panel B) reveal that platelets from 5-HT –infused mice showed an increased aggregation response to collagen. Overall, aggregation was increased by 83% using platelets from 5-HT-infused animals (76 ± 12% versus 42 ± 5 in saline-infused mice; n=15). Platelets from both mouse models also examined for markers of platelet activation. Fig. 2, Panel C shows an increase in PEJon/A binding indicating activated platelet integrin αIIbβ3 detected by flow cytometry. Other markers of platelet activation including P-selectin and granulophysin, also were increased in 5-HT infused mice. Thus, exposure to elevated plasma [5-HT] coincides with heightened stirred platelet aggregation and increased markers of platelet activation.
3.3. In vivo correlate of platelet hyperreactivity
To evaluate the potential in vivo significance of increased platelet reactivity, assays of chemical-induced thrombus formation and normal hemostasis were examined. First, carotid arteries of saline and 5-HT-infused animals were examined for their ability to form occlusive thrombi following exposure to FeCl3 (0.62 mol/L, 3 min) [39]. Fig. 2, Panel D shows representative Doppler signals corresponding to red blood cell velocity following FeCl3 injury. In animals achieving a stable occlusion due to the formation of a platelet-rich thrombus, a significant reduction in time to occlusion was found in mice with elevated plasma [5-HT]. Time to occlusion averaged 18±2 min for saline-infused mice compared to 11±1 min for 5HT-infused animals (n=15). Tail bleeding times were also shortened in animals with increased plasma [5-HT] (Fig. 2, Panel E). 5-HT-infused mice had a bleeding time of 67±10 sec, which was 38% shorter than the bleeding time of 110±3 sec recorded in saline-infused mice (n=15). Tail bleeding time is a crude measurement of mouse hemostasis, which depends on platelet status, coagulation factors, and additional blood and vascular wall components. However, these findings collectively suggest that the presence of elevated plasma [5-HT] may predispose mice to a prothrombotic condition.
3.4. Intraplatelet changes following 5-HT infusion
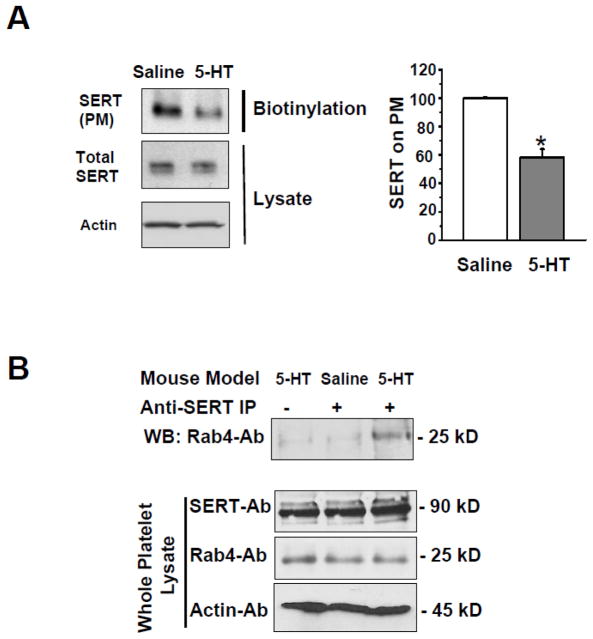
Previously we showed that 5-HT pretreatment in vitro alters the distribution of SERT in the plasma membrane (PM) versus cytoplasm of SERT -expressing CHO cells. In particular, SERT was lost from the PM and retained intracellularly causing a loss of 5-HT uptake [26]. Here, in both mouse models the prevalence of SERT in the platelet PM and cytoplasm was assessed by biotinylation of surface proteins, which were retrieved on streptavidin beads. The SERT protein in bound and unbound fractions was assessed using WBs probed with SERT-Ab (Fig. 3, Panel A). Whole platelet lysates from saline- and 5-HT-infused mice revealed no difference in total SERT, but SERT expression was reduced 42% on the platelet PM of the 5-HT-infused animals. These findings were confirmed by a 61% lower 5-HT uptake rate in platelets from 5-HT versus saline-infused mice (Table 1).
Fig. 3.
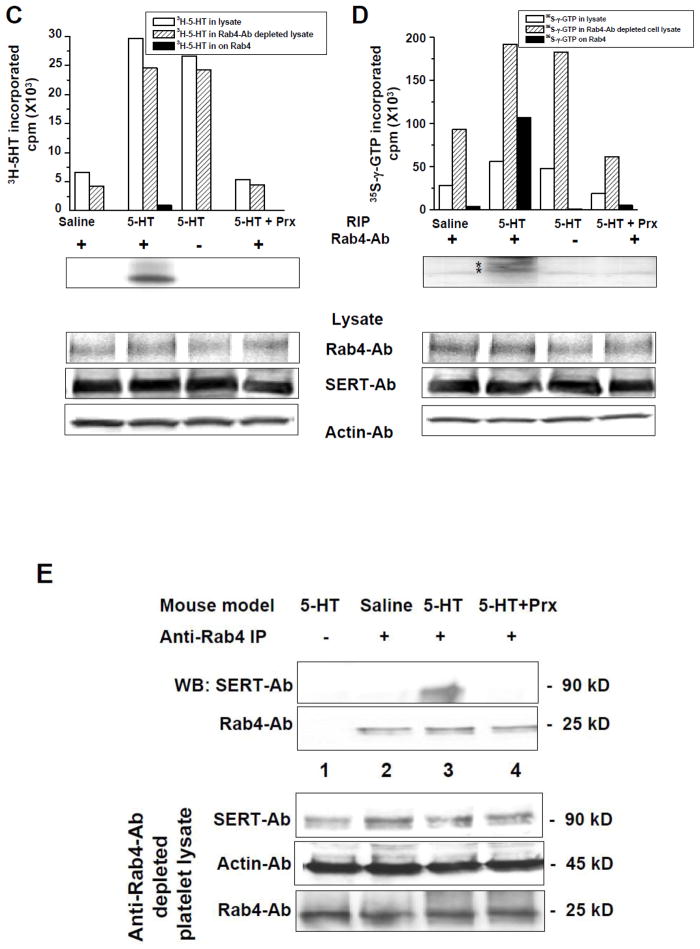
(A) Western blot analysis of SERT expression in platelets from saline- and 5-HT-infused mice. Representative images are from one of four independent experiments. Total SERT in platelet lysates was similar between platelets from the two groups of animals, but SERT was depleted in the platelet plasma membrane (PM) of 5-HT-infused mice. Actin was used as a loading control for each lysate. Average immunodensity values reveal that the density of SERT on the PM of platelets from 5-HT-infused mice was 42% less compared to control mice. * = statistical difference between saline- and 5-HT- infused mice. All assays were performed in triplicate (n = 15 group). (B) SERT-Rab4 association is enhanced in platelets from 5-HT-infused mice. Platelets were isolated from saline- and 5-HT-infused mice. Platelet lysates were incubated with an anti-SERT Ab (lanes 2 and 3) or pre-immune serum (lane 1). Proteins were eluted from PA beads and resolved on SDS-PAGE followed by WB using an anti-Rab4 Ab to determine Rab4-SERT association; or to determine the expression level of Rab4 in whole lysates. Expression of SERT in total platelet lysates was determined by WB. A distinct 25 kD band was only observed in platelets from 5-HT-infused mice. Rab-4 was not detected in anti-SERT immunoprecipitate using platelets from saline-infused mice. Actin levels were used as a sample loading controls.
5-HT acts as a signaling molecule through several pathways to alter platelet function. For example, in vitro studies suggest that elevations of intracellular [5-HT] caused by increased SERT uptake promote the association between SERT and the small GTPase, Rab4, which sequesters SERT intracellularly [25, 40]. This compensatory mechanism limits excessive uptake of 5-HT by reducing the number of SERT molecules on the platelet PM. We examined Rab4-SERT association by co-IP in platelet lysates to determine if this event occurred in vivo. Platelet pellets from saline- and 5-HT-infused mice were lysed and subjected to co-IP [25, 40]. Briefly, platelet lysates were incubated with anti-Rab4-Ab coated protein Sepharose A beads. The Rab4-Ab bound proteins were eluted and analyzed by WB. Probing the proteins with a SERT-Ab revealed that despite similar platelet [5-HT] after 24 hr infusion, only the platelets from 5-HT-infused mice showed Rab4-SERT association (Fig. 3, Panel B, top panel). This finding raised the possibility that an initial elevation of intracellular [5-HT] in the platelets from 5HT-infused mice may have initiated 5-HT intracellular signaling that persisted at 24 hours. Total SERT and Rab4 expression were similar in the presence or absence of elevated 5-HT.
3.5. Calcium level and the TGase activity of the platelets of 5-HT-infused mice
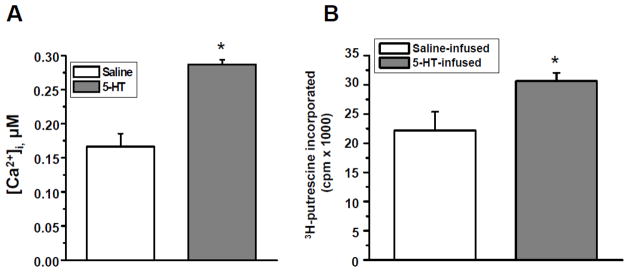
High intracellular [5-HT] also can modify (transamidate) Rab4, a process defined as serotonylation [22, 26, 37, 40]. This modification reportedly involves calcium mobilization and the activation of TGase in platelets. We explored if platelets from 5-HT-infused showed evidence of Rab4 serotonylation as evidence of enhanced intracellular 5-HT signaling. Platelets of 5-HT and saline-infused mice were loaded with 2 μM of fura-2/AM, a calcium ion–binding fluorochrome. As reported previously, Fura-2 does not lead to activation or interfere with platelet activation [30, 41]. Our data showed a 72% elevation of intracellular calcium concentration in platelets from 5-HT-infused mice compared to saline-infused mice (Fig. 4, Panel A). TGase activity was determined using earlier methods [31, 32] by evaluating 3H-putrescine incorporation into precipitated platelet proteins. These studies revealed a 38% elevation in platelet TGase activity in the presence of elevated 5-HT (Fig. 4, Panel B).
Fig. 4.
(A) Calcium mobilization in isolated platelets. Fura2-AM was loaded in platelets isolated from saline- and 5HT-infused mice and allowed to hydrolyze prior to the experiment. Platelets were excited at 340 and 380 nm and emission was read at 510 nm. [Ca2+]i was determined to be 0.184 ± 0.036 μM and 0.283 ± 0.054 μM in saline- and 5HT-infused mice, respectively. This reflects a 72% elevation of [Ca2+]i in 5HT-treated platelets (Student t-test p<0.05). (B) TGase activity in platelets. The incorporation of 3H-putrescine into N,N-dimethylcasein was measured using a purified bovine TGase (Sigma) which served as a positive control. TGase activity was measured using platelet lysates and revealed a 38% increased activity in platelets from 5-HT-infused mice compared to the saline group. Asterisk indicates statistically significant compared to saline- and positive control (Student t-test p<0.05). (C) RIP of 3H-5HT and (D) of 35S-γ-GTP labeled Rab4. Platelets were isolated from 5-HT- and saline-infused mice and Prx-injected 5-HT-infused mice and incubated with either [3H]-5HT (C) or 35S-γ-GTP in PBS/CM at RT. After 1 hr, platelets were washed with ice cold PBS and harvested. Samples from: (i) platelet lysate; (ii) the proteins pulled down by Rab4-Ab; and (iii) depleted platelet lysates after Rab4-Ab pull were normalized based on their protein concentration. Then, an equal amount of samples from each group was mixed with 5 ml scintillation cocktail and measured in the scintillation counter (bar graphs in Panels C and D). Immune precipitates were eluted from the beads by incubation in SDS sample buffer, analyzed by 12.5% SDS-PAGE, and Rab4 was visualized by fluorography. A 25 kD major band representing Rab4 was precipitated with anti-Rab4 Ab only from the platelets of 5-HT-infused mice. In platelets from Prx-injected 5-HT-infused mice in which 5-HT uptake by SERT was blocked before the 5-HT infusion, the 25 kD band was not detectable. Rab4 and SERT were detected by WB in total platelet lysates, showing that their total expression was the same between preparations. Data are representative of at least three independent experiments. (E) Effect of Prx on Rab4-SERT association. Proteins bound to anti-Rab4 Ab coated PA beads (lanes 2–4) were analyzed to determine the Rab4-SERT association in platelets isolated from different mouse models including saline- (lane 2), 5-HT- (lanes 1 and 3) or Prx-injected and 5-HT-infused mice (lane 4). The co-IP data did not show an association between SERT and Rab4 in the platelets isolated from saline-infused mice (lane 2) or Prx-injected and 5-HT-infused mice (lane 4) . Nonspecific adsorption of SERT to Sepharose beads was determined in the absence of Rab4 Ab (lane 1). Expressions of SERT and Rab4 in total platelet lysates were determined by WB. Actin was used as a loading control. A distinct 90 kD band was only observed in platelets isolated from 5-HT-infused mice. SERT could not be co-IP from saline-infused mice.
3.6. Rab4-GTP formation in platelets of 5-HT-infused mice
TGase transamidates 5-HT on the GTP-GDP hydrolysis domain of Rab4 which keeps this small GTPase in its active form, Rab4-GTP [22]. We determined if this reaction that requires intracellular 5-HT occurred in platelets of 5-HT-infused mice by incubating platelets from these animals and saline-infused mice with either 3H-5-HT or 35S-γ-GTP for 1 hr, followed by a RIP on Rab4-Ab coated protein A beads [23]. Samples included: (i) whole platelet lysates to measure the total radioactive material in platelets; (ii) the eluent of protein A beads coated with Rab4-Ab to measure the level of radioactive material associated with the Rab4-Ab; and (iii) depleted platelet lysate to measure the amount of radioactive material left after Rab4-Ab pull down from whole platelet lysate. All samples were normalized for protein concentration and then radioactivity detected by a scintillation counter (Fig. 4, Panels C and D). The Rab4-Ab bound proteins were resolved by SDS-PAGE and subjected to fluorography. Only one major band corresponding to 3H-5-HT-Rab4 serotonylation was observed at 25 kD in platelet lysates from 5-HT-infused mice (Fig. 4C, top blot). Similarly, the platelet lysates from 5-HT-infused mice showed a preponderance of the GTP-bound form of Rab4. A single band corresponding to 35S-GTP-Rab4 was evident in platelet proteins from these animals (Fig. 4D). Collectively, these findings are consistent with TGase –mediated 5-HT transamidation of Rab4 to maintain its active state and they provide further evidence of enhanced serotonin signaling in the platelets of 5-HT-infused mice. The total levels of Rab4, SERT and actin were similarly between platelet lysates from 5-HT and saline-infused mice.
3.7. Abrogation of SERT in hyperreactive platelets
Next, we hypothesized that the initial uptake of plasma 5-HT by platelet SERT prior to the down-regulation of SERT as an end-effect of serotonylation, is the critical event that promotes 5-HT –signaling and also contributes to the abnormal biochemical and functional profile of platelets from 5-HT-infused mice. To test this hypothesis, we injected mice with a well characterized inhibitor of SERT, Prx (3 mg/kg i.p.) [27–29] prior to the insertion of the 5-HT-containing minipumps. The injection of Prx at 3 mg/kg i.p. reportedly inhibits 90% of SERT activity in rats for 24 hrs [27–29]. The serotonylation and GTP-binding abilities of Rab4 in the platelets of Prx-injected saline- or 5HT-infused mice were analyzed in RIP as described above. Neither 3H-5HT binding nor 35S-γ-GTP –binding to Rab4 was detected in the platelets of Prx-injected 5-HT-infused mice (Fig. 4, Panels C and D). These findings infer that the serotonylation and subsequently enhanced GTP-binding of Rab4 in platelets of 5-HT-infused mice depend on SERT-mediated 5-HT uptake. Additionally, the SERT-Rab4 interaction dependent on elevation of intracellular [5-HT] and detected in the platelets of 5-HT-infused mice (Fig. 3, Panel B) was abolished after Prx treatment (Fig. 4, Panel E). Additionally, an anti-SERT WB of anti-Rab4 precipitate detected SERT protein in platelet lysates of untreated but not Prx-treated 5-HT-infused mice. The GTP-binding of Rab4 appears to be a key event in platelet exocytosis [22–23] in addition to underlying the loss of SERT on the PM during 5-HT elevation [26].
3.8. 5-HT level in the platelet cytoplasm may contribute to platelet aggregation
Next, we examined platelets from Prx-injected mice to evaluate if a SERT-mediated uptake of 5-HT underlies the heightened hemostatic and thrombotic in vivo phenotype of 5-HT-infused mice. Pre-injection of Prx in saline-infused mice resulted in a 76% reduction in platelet 5-HT uptake after 24 hr but did not reduce SERT expression (Table 1). Prx administration also did not change total 5-HT blood levels, but it increased the 5-HT plasma/platelet ratio from 6% (−Prx) to 94% (+Prx), respectively. These findings suggested an effective block by Prx of SERT-mediated 5-HT uptake in platelets (Table 1).
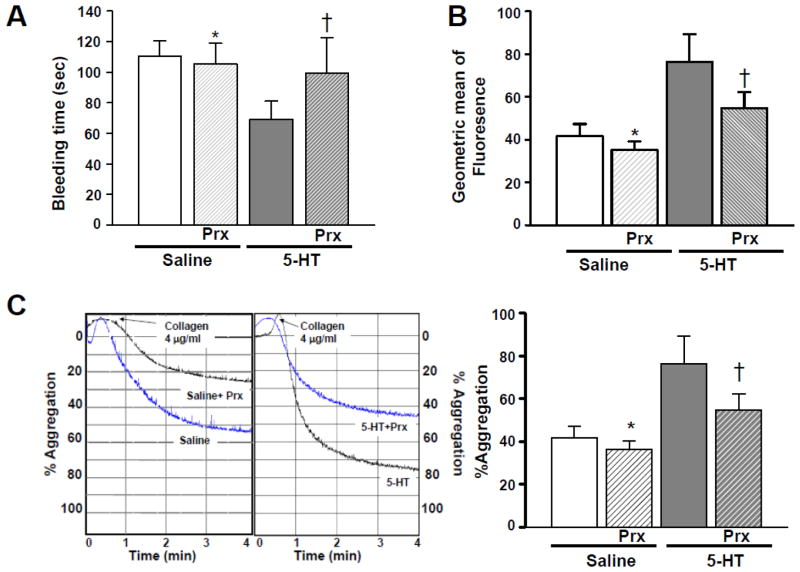
Subsequently, we performed platelet aggregation and bleeding time assays in untreated and Prx-treated saline- and 5-HT-infused mice. The Prx-treated mice infused with 5-HT showed a 44% increase in tail bleeding time compared to untreated 5-HT-infused animals (Fig. 5, Panel A); their bleeding time was not significantly different from mice infused with saline. Accordingly, isolated platelets from 5-HT-infused mice treated with Prx showed a 28% reduction in the expression of the PEJon/A platelet activation marker (Fig. 5, Panel B). Prx partially restored this marker of platelet activation to the values obtained in saline-infused mice. In addition, stirred platelet aggregation assays revealed that pretreatment with Prx attenuated collagen-induced aggregation in platelets from 5-HT-infused mice (Fig. 5, Panels C and D). Collectively, these findings suggest that the Prx effect in 5-HT infused animals may not be entirely platelet-dependent. Additionally, in response to 5-HT infusion, an enhanced uptake of 5-HT by SERT rather than elevated plasma [5-HT] per se appears to initiate the intracellular 5-HT signaling associated with an abnormal platelet phenotype. Presumably the increased 5HT uptake by platelets occurs prior to the down-regulation of SERT on the platelet PM, since the latter event is triggered by 5-HT-dependent Rab4-SERT association.
Fig. 5. Impact of Prx-injection on 5-HT-infused mice.
(A) Tail bleeding time. Prx injection before 5-HT infusion restored the short bleeding time of 5-HT-infused mice toward the values seen in saline-infused mice. (B) Effect of Prx on platelet activation. The effect of Prx-injection on the activation of integrin αIIbβ3 was measured by FACS analysis using PEJon/A. The elevated expression of the PEJon/A epitope in platelets of 5-HT-infused mice was restored to lower values in mice treated with Prx. (C) Effect of Prx on platelet aggregation. Platelets were isolated from untreated or Prx-injected saline or 5-HT-infused mice and stimulated with collagen. Their aggregation profile was monitored using an aggregometer. Approximately 25% of the platelets from Prx-injected saline-infused mice and 45% of platelets from Prx-injected 5-HT-infused mice were aggregated at the end of 4 min, whereas in the absence of Prx, platelets of saline- and 5-HT –infused mice showed ~53% and ~75% aggregation. The average of 5 measurements is presented in the bar graph. * = statistical difference between saline- and 5-HT-infused mice. Asterisks indicate statistical difference between saline- and Prx-injected saline-infused (*) and 5-HT- and Prx-injected 5-HT-infused (†) mice. All assays were performed in triplicate (n =15 group).
4. DISCUSSION
Cardiovascular diseases including CAD, atherothrombosis, cerebrovascular ischemia, and myocardial infarction have been linked to elevated plasma [5-HT] [10, 13, 14, 20, 21]. Clinical studies have reported an increased [5-HT] in the plasma of patients following myocardial infarction or angioplasty. Of 121 patients undergoing angiography, plasma [5-HT] was 10-fold higher in patients diagnosed with CAD compared to those with a normal angiogram [13]. Excessive transcardiac accumulation of 5-HT has been demonstrated in patients as chronic stable angina converts to unstable coronary syndromes [14]. Importantly, clinical studies also suggest that platelet activation is enhanced in the presence of elevated blood [5-HT] [8, 13–14].
Studies in platelets of mice lacking the gene for TPH, the rate-limiting enzyme in the synthesis of 5-HT in peripheral cells, show blunted α-granule secretion and a reduced risk of thrombosis [22–23]. Other reports indicate that blocking the 5-HT2A receptor in animal models of hypertension reduces ex vivo platelet aggregation by inhibiting the 5-HT-induced platelet release reaction [42]. Some of the most definitive studies implicating 5-HT in hemostasis have demonstrated that selective 5-HT reuptake inhibitors (SSRI) can predispose to bleeding [42–46]. However, for the most part, our current knowledge related to the impact of elevated extracellular [5-HT] on platelet physiology is based on studies using isolated platelets exposed to 5-HT in an in vitro setting. It still is unclear if increased plasma [5-HT] is prothrombotic, and if 5-HT can alter the intravascular micro-environment to affect coagulation and platelet pathways. Thus, we sought to determine if elevated plasma [5-HT] in otherwise normal experimental animals results in abnormalities of in vivo platelet function. It remains unclear if an elevation of plasma [5-HT] in patients with cardiovascular disease is a risk factor for thrombosis or simply is an epiphenomenon of the disease process with little or no functional impact.
In order to assess if elevated blood [5-HT] in the absence of other risk factors alters platelet function, we established a mouse model of elevated blood [5-HT] by implanting 5-HT –containing osmotic mini-pumps in C57BL/6J mice. Then, platelets isolated from saline and 5-HT -infused mice were compared for physiological and biochemical properties. When mice were infused with 5-HT (1.66 μg/kg/hr), their total blood 5-HT concentration was elevated by 42% at 24 hours. At this time-point, the [5-HT] in platelets of 5-HT-infused mice was not significantly elevated compared to saline-infused mice. However, our biochemical results strongly suggested enhanced 5-HT signaling in the platelets of 5-HT –infused mice. In this regard, we reported earlier that 5-HT treatment of CHO cells expressing SERT “paralyzed” the translocation of intracellular SERT to the PM by enabling an association between SERT and co-expressed Rab4-GTP [26]. In the present study, we similarly observed a loss of surface SERT, SERT-Rab4 association, and a rise in Rab4-GTP in platelets of 5-HT-infused mice indicative of enhanced 5-HT signaling in vivo. Platelets of 5-HT –infused mice showed elevated intracellular calcium and activation of TGase, two events regarded as essential for 5-HT –mediated SERT/Rab4 interaction. Thus, multiple biochemical assays suggested enhanced 5-HT signaling in platelets from the 5-HT-infused mice.
Based on these findings, we theorized that the initial rise in plasma [5-HT] after implantation of 5-HT-containing minipumps in mice caused an early increase of SERT-mediated uptake of 5-HT to promote 5-HT signaling and protein modification. Subsequently, the compensatory down-regulation of SERT on the PM slowed 5-HT uptake so that platelet [5-HT] was no longer elevated at 24 hours although biochemical evidence of enhanced 5-HT signaling persisted. To test this hypothesis, we injected mice with Prx to block SERT-mediated 5-HT uptake prior to 5-HT infusion. Pre-injection of Prx lowered platelet 5-HT levels (Table 1) without reducing SERT expression in platelets, and abolished Rab4-SERT association in the platelets of 5-HT-infused mice. Interestingly, Prx not only blocked the SERT/Rab4 interaction mediated by intracellular 5-HT but also reduced the pro-aggretory and prohemostatic profile of platelets from 5-HT-infused mice. Prx-pretreatment did not fully eliminate activation of integrin IIb/IIa in platelets of 5-HT –infused mice, although tail bleeding time and aggregation responses appeared to be fully restored to the control levels observed in saline-infused mice. The reason for this difference between responses is not readily apparent. Collectively, these findings confirm the participation of intracellular 5-HT in establishing platelet activation. Additionally, our results raise the possibility that even short-lived rises in plasma [5-HT] may enhance SERT-mediated uptake of 5-HT into platelets to enact a pro-thrombotic phenotype before the compensatory down-regulation of surface SERT can occur. Indeed, in vitro studies by our laboratory in isolated human platelets showed that increases in extracellular [5-HT] initially and dose-dependently enhanced the 5-HT uptake rate in platelets, which was followed by a loss of SERT on the platelet PM and the expected attenuation of 5-HT uptake [10, 26, 47].
Importantly, other investigators have detected the release of α–granule proteins in 5-HT –exposed platelets concurrent with Rab4-GTP activation [22–23, 26]. The findings of these studies have linked enhanced intracellular 5-HT signaling in isolated platelets to the release of pro-thrombotic molecules. Our new findings support the concept that enhanced 5-HT signaling is associated with a platelet phenotype of hyperreactivity. Additionally, we provide initial evidence that elevated plasma [5-HT] in an vivo mouse model apparently free from cardiovascular risk factors is associated with decreased tail bleeding time and increased carotid thrombus formation in response to chemical injury. Thus, our collective data suggest that elevated plasma [5-HT] in the absence of overt cardiovascular risk factors may adversely impact platelet function in an experimental animal model. Our findings strongly suggest that elevated in vivo plasma [5-HT] represents an independent risk factor for platelet hyperreactivity and potentially an increased risk of thrombosis.
Highlights.
Cardiovascuar diseases have been linked to elevated plasma 5-HT levels.
platelets SERT regulate the concentration of 5-HT in blood plasma.
platelet SERT counteract the pro-thrombotic influence of elevated plasma 5HT levels.
Acknowledgments
We gratefully acknowledge the UAMS Animal Facility and Flow Cytometry Core. We thank Dr. Sung Rhee for assisting with the fura-2 measurements and Mr. Terry Fletcher for his assistance in animal handling. This work was supported by the American Heart Association [Grant 0660032Z] and by the National Heart Lung and Blood Institute of the National Institutes of Health [Grants R01HL091196 and R01HL091196-01A2W1 to FK].
Footnotes
DISCLOSURE STATEMENT: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubenstein JL. Development of serotonergic neurons and their projections. Biol Psychiatry. 1998;44:145–50. doi: 10.1016/s0006-3223(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 2.Rudnick G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. J Biol Chem. 1977;252:2170–74. [PubMed] [Google Scholar]
- 3.Vanhoutte PM. Platelet-derived serotonin, the endothelium, and cardiovascular disease. J Cardiovasc Pharmacol. 1991;17 (Suppl 5):S6–12. [PubMed] [Google Scholar]
- 4.Barter R, Pearse AG. Mammalian enterochromaffin cells as the source of serotonin (5-hydroxytryptamine) J Pathol Bacteriol. 1955;69:25–31. doi: 10.1002/path.1700690106. [DOI] [PubMed] [Google Scholar]
- 5.Brunk I, Blex C, Rachakonda S, et al. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 2006;281:33373–85. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- 6.Holmsen H, Weiss HJ. Secretable storage pools in platelets. Annu Rev Med. 1979;30:119–34. doi: 10.1146/annurev.me.30.020179.001003. [DOI] [PubMed] [Google Scholar]
- 7.McNicol A, Israels SJ. Platelets and anti-platelet therapy. J Pharmacol Sci. 2003;93:381–96. doi: 10.1254/jphs.93.381. [DOI] [PubMed] [Google Scholar]
- 8.Linder L, Kiowski W, Buhler FR, et al. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–67. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 9.Saxena PR, Villalon CM. Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol. 1990;15 (Suppl 7):S17–S34. [PubMed] [Google Scholar]
- 10.Brenner B, Harney JT, Ahmed BA, et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–15. doi: 10.1111/j.1471-4159.2007.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaumann AJ, Levy FO. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Golino P, Maseri A. Serotonin receptors in human coronary arteries. Circulation. 1994;90:1573–75. doi: 10.1161/01.cir.90.3.1573. [DOI] [PubMed] [Google Scholar]
- 13.Vikens K, Farstad M, Nordrehaug JE. Serotonin is associated with coronary artery disease and cardiac events. Circulation. 1999;100:483–89. doi: 10.1161/01.cir.100.5.483. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg EK, Schmitz JM, Benedict CR, et al. Transcardiac serotonin concentration is increased in selected patients with limiting angina and complex coronary lesion morphology. Circulation. 1989;79:116–24. doi: 10.1161/01.cir.79.1.116. [DOI] [PubMed] [Google Scholar]
- 15.Ban Y, Watanabe T, Miyazaki A, et al. Impact of increased plasma serotonin levels and carotid atherosclerosis on vascular dementia. Atherosclerosis. 2007;195:153–59. doi: 10.1016/j.atherosclerosis.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Yamada S, Akita H, Kanazawa K, et al. T102C polymorphism of the serotonin (5-HT) 2A receptor gene in patients with non-fatal acute myocardial infarction. Atherosclerosis. 2000;150:143–48. doi: 10.1016/s0021-9150(99)00356-1. [DOI] [PubMed] [Google Scholar]
- 17.Leosco D, Fineschi M, Pierli C, et al. Intracoronary serotonin release after high-pressure coronary stenting. Am J Cardiol. 1999;84:1317–22. doi: 10.1016/s0002-9149(99)00564-0. [DOI] [PubMed] [Google Scholar]
- 18.Nebigil CG, Choi DS, Dierich A, et al. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A. 2000;97:9508–13. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pino R, Cerbai E, Calamai G, et al. Effect of 5-HT4 receptor stimulation on the pacemaker current I(f) in human isolated atrial myocytes. Cardiovasc Res. 1998;40:516–22. doi: 10.1016/s0008-6363(98)00198-9. [DOI] [PubMed] [Google Scholar]
- 20.Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100(23):13525–30. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietraszek MH, Takada Y, Takada A, et al. Blood serotonergic mechanisms in type 2 (non-insulin-dependent) diabetes mellitus. Thromb Res. 1992;66:765–74. doi: 10.1016/0049-3848(92)90052-c. [DOI] [PubMed] [Google Scholar]
- 22.Walther DJ, Peter JU, Winter S, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–62. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 23.Shirakawa R, Yoshioka A, Horiuchi H, et al. Small GTPase Rab4 regulates Ca2+-induced alpha-granule secretion in platelets. J Biol Chem. 2000;275:33844–49. doi: 10.1074/jbc.M002834200. [DOI] [PubMed] [Google Scholar]
- 24.Przyklenk K, Frelinger AL, 3rd, Linden MD, Whittaker P, Li Y, Barnard MR, Adams J, Morgan M, Al-Shamma H, Michelson AD. Targeted inhibition of the serotonin 5HT2A receptor improves coronary patency in an in vivo model of recurrent thrombosis. J Thromb Haemost. 2010 Feb;8(2):331–40. doi: 10.1111/j.1538-7836.2009.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishihira K, Yamashita A, Tanaka N, Kawamoto R, Imamura T, Yamamoto R, Eto T, Asada Y. Inhibition of 5-hydroxytryptamine receptor prevents occlusive thrombus formation on neointima of the rabbit femoral artery. J Thromb Haemost. 2006 Jan;4(1):247–55. doi: 10.1111/j.1538-7836.2005.01702.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed BA, Jeffus BC, Bukhari SI, et al. Serotonin transamidates Rab4 and facilitates its binding to the C terminus of serotonin transporter. J Biol Chem. 2008;283:9388–98. doi: 10.1074/jbc.M706367200. [DOI] [PubMed] [Google Scholar]
- 27.Abdelmalik N, Ruhe HG, Barwari K, et al. Effect of the selective serotonin reuptake inhibitor paroxetine on platelet function is modified by a SLC6A4 serotonin transporter polymorphism. J Thromb Haemost. 2008;6:2168–74. doi: 10.1111/j.1538-7836.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 28.De Groote L, Olivier B, Westenberg HG. The effects of selective serotonin reuptake inhibitors on extracellular 5-HT levels in the hippocampus of 5-HT(1B) receptor knockout mice. Eur J Pharmacol. 2002;439(1–3):93–100. doi: 10.1016/s0014-2999(02)01417-6. [DOI] [PubMed] [Google Scholar]
- 29.Kreilgaard M, Smith DG, Brennum LT, Sánchez C. Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice. Br J Pharmacol. 2008;155(2):276–84. doi: 10.1038/bjp.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–50. [PubMed] [Google Scholar]
- 31.Robinson NA, Eckert RL. Identification of transglutaminase-reactive residues in S100A11. J Biol Chem. 1998;273(5):2721–28. doi: 10.1074/jbc.273.5.2721. [DOI] [PubMed] [Google Scholar]
- 32.Fabbi M, Marimpietri D, et al. Tissue transglutaminase is a caspase substrate during apoptosis. Cleavage causes loss of transamidating function and is a biochemical marker of caspase 3 activation. Cell Death Differ. 1999;6(10):992–1001. doi: 10.1038/sj.cdd.4400573. [DOI] [PubMed] [Google Scholar]
- 33.Guerrero JA, Shafirstein G, Russell S, et al. In vivo relevance for platelet glycoprotein Ibα residue Tyr276 in thrombus formation. J Thromb Haemost. 2008;6:684–91. doi: 10.1111/j.1538-7836.2008.02916.x. [DOI] [PubMed] [Google Scholar]
- 34.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci USA. 2000;97:2803–08. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philipp CS, Dilley A, Miller CH, et al. Platelet functional defects in women with unexplained menorrhagia. J Thromb Haemost. 2003;1:477–84. doi: 10.1046/j.1538-7836.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 36.Bergmeier W, Schulte V, Brockhoff G, et al. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry. 2002;48:80–86. doi: 10.1002/cyto.10114. [DOI] [PubMed] [Google Scholar]
- 37.Szasz R, Dale GL. COAT Platelets. Curr Opin Hematol. 2003;10(5):351–5. doi: 10.1097/00062752-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Tseng YL, Chiang ML, Huang TF, Su KP, Lane HY, Lai YC. A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb Res. 2010;126(6):517–23. doi: 10.1016/j.thromres.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Guerrero JA, Shafirstein G, Russell S, Varughese KI, Kanaji T, Liu J, Gartner TK, Bäumler W, Jarvis GE, Ware J. In vivo relevance for platelet glycoprotein Ibalpha residue Tyr276 in thrombus formation. J Thromb Haemost. 2008;6(4):684–91. doi: 10.1111/j.1538-7836.2008.02916.x. [DOI] [PubMed] [Google Scholar]
- 40.Mercado CP, Ziu E, Kilic F. Communication between 5-HT and small GTPases. Curr Opin Pharmacol. 2011;11(1):23–8. doi: 10.1016/j.coph.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117(4):953–60. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry CN, Lorrain J, Lochot S, Delahaye M, Lalé A, Savi P, Lechaire I, Ferrari P, Bernat A, Schaeffer P, Janiak P, Duval N, Grosset A, Herbert JM, O’Connor SE. Antiplatelet and antithrombotic activity of SL65. 0472, a mixed 5-HT1B/5-HT2A receptor antagonist. Thromb Haemost. 2001;85:521–28. [PubMed] [Google Scholar]
- 43.Ottervanger JP, Stricker BH, Huls J, Weeda JN. Bleeding attributed to the intake of paroxetine. Am J Psychiatry. 1994;151:781–82. doi: 10.1176/ajp.151.5.781. [DOI] [PubMed] [Google Scholar]
- 44.Layton D, Clark DW, Pearce GL, Shakir SA. Is there an association between selective serotonin reuptake inhibitors and risk of abnormal bleeding? Results from a cohort study based on prescription event monitoring in England. Eur J Clin Pharmacol. 2001;57:167–76. doi: 10.1007/s002280100263. [DOI] [PubMed] [Google Scholar]
- 45.Dalton SO, Johansen C, Mellemkjaer L, Nørgård B, Sørensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 46.de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319:1106–09. doi: 10.1136/bmj.319.7217.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv. 2010;10(4):231–41. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118(4):1544–52. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]