Abstract
Luteinizing hormone receptor and follicle stimulating hormone receptor play a crucial role in female and male reproduction. Significant new information has emerged about the structure, mechanism of activation, and regulation of expression of these receptors. Here we provide an overview of the current information on those aspects with an in-depth discussion of the recent developments in the post-transcriptional mechanism of LH receptor expression mediated by a specific LH receptor mRNA binding protein, designated as LRBP. LRBP was identified by electrophoretic gel mobility shift assay using cytosolic fractions from ovaries in the down regulated state. LRBP was purified, its binding site on LH receptor mRNA was identified and characterized. During ligand-induced down regulation, LRBP expression is increased through the cAMP/PKA and ERK signaling pathway, is translocated to translating ribosomes, binds LH receptor mRNA and forms an untranslatable ribonucleoprotein complex. This complex is then routed to the mRNA degradation machinery resulting in diminished levels of both LHR mRNA and cell surface expression of LH receptor. The studies leading to these conclusions are presented.
1. Introduction
The LHReceptor (LHR) play a crucial role in the regulation of reproductive functions including ovarian steroidogenesis, ovulation in the female, and testosterone production by the Leydig cells of testis (Ascoli et al., 2002; Dufau, 1998; Menon et al., 2005; Menon et al., 2004; Segaloff and Ascoli, 1992). Owing to its ability to bind both LH and hCG with high affinity, LHR is designated as LH/hCG receptor. The FSH receptor (FSHR), by contrast, binds only FSH. FSHR is also a crucial molecule in the regulation of ovarian function through its action in follicle development and stimulation of estrogen production (Dias et al., 2002; Richards and Hedin, 1988). In the male, FSHR regulates sertoli cell functionsandspermatogenesis. Additionally, LHR is also expressed in a number of extra gonadal sites (Rao and Lei, 2007) with several potential physiological and pathophysiological manifestations (Abdallah et al., 2004; Apaja et al., 2004; Casadesus et al., 2005; Fields and Shemesh, 2004; Horiuchi et al., 2000; Saner-Amigh et al., 2006). FSHR and LHR belong to rhodopsin/2 adrenergic receptor-like family A of G protein coupled receptors (GPCR) consisting of a large extracellular domain, seven membrane spanning α helices with intracellular and extracellular loops connecting them and a short intracellular domain. Aberrations in reproductive functions in the female and male have been reported due to naturally occurring mutations in LHR and FSHR (Ascoli and Puett, 2009; Costagliola et al., 2005; Latronico and Segaloff, 1999; Piersma et al., 2007; Segaloff, 2009; Tao and Segaloff, 2009; Themmen, 2005; Themmen and Huhtaniemi, 2000). This review will provide a brief overview of the structure/function relationship of the two receptors and will focus on recent advances in the molecular regulation of LHR expression in the ovary.
2. Genomic organization and tissue expression
Human LHR and FSHR are encoded by a single copy gene on the short arm of chromosome 2 and separated by 200kb. These are the only two functional genes present in this region (LHCGR and FSHR; www.ensembl.org). The rat LHR is encoded by a 70kb genomic DNA and consists of 11 exons. All but the terminal 47 amino acid residues in the carboxyl terminus are encoded by exons 1-10 and the remaining portion of the receptor is encoded by exon 11. The transcript sizes vary among different species. For example, the rat LHR expresses four transcripts ranging from 6.7 to 1.8 Kb while for human LHR, only two transcripts are seen in human granulosa cells (Jia et al., 1991; McFarland et al., 1989; Minegishi et al., 1990). The FSHR is encoded by a 190 kb genomic DNA and contains 10 exons. The majority of the amino terminal domain of the FSHR is encoded by exons 1-9 and the remaining portion of the receptor that include stransmembrane and the carboxyl terminus region is encoded by exon 10. Northern blot analyses of ovaries and testis in rat revealed two predominant FSHR transcripts of 7.0 and 2.5 Kb in size (LaPolt et al., 1992; Rannikki et al., 1995). Additionally, two minor transcripts of 4.2 and 1.8 kb in size were also identified in rat ovaries (LaPolt et al., 1992). Multiple FSHR transcript sizes were also detected in human testis (Gromoll et al., 1992) and bovine ovary (Houde et al., 1994).
Several alternatively spliced variants have been reported for both LHR and FSHR mRNAs, some of which are presented here. Transcripts encoding alternatively spliced variants of the LHR have been detected in the sheep (Bacich et al., 1994), horse (Saint-Dizier et al., 2003), cow (Kawate and Okuda, 1998), and human (Madhra et al., 2004; Nishimori et al., 1995) corpus luteum (CL). A splice variant of LHR that lacks exon 9 has also been reported in human (Minegishi et al., 2007; Nakamura et al., 2004). In the case of FSHR, up to four alternatively spliced transcripts have been described in the ovary (reviewed in (O’Shaughnessy et al., 1996; Sairam and Babu, 2007; Simoni et al., 1997), the expression of which varied at different stages of artificially induced ovulatory cycles (Yaron et al., 1998). More recently,four abnormal FSHR splice variants with reduced responsiveness to FSH have been reported in the cumulus cells surrounding the oocytes isolated from human follicular aspirates (Gerasimova et al., 2010).
3. Receptor structure and mechanism of activation
Both LH and FSH receptors contain an N-terminal extracellular domain that binds the respective ligands with high affinity and specificity, and seven transmembrane domains responsible for signal transduction (Ascoli et al., 2002; Dias et al., 2002). The extracellular domains of LHR and FSHR contain a number of irregular leucine-rich repeats (LRR) (Ascoli and Segaloff, 1989). The LRR region is thought to contain successive -strands and -helices that organize into a cusp-shaped structure (Bhowmick et al., 1996; Jiang et al., 1995; Kajava et al., 1995). The -strands form a parallel -sheet along the concave surface of this structure, which is a common feature of all the three glycoprotein hormone receptors (LHR, FSHR and thyroid stimulating hormone receptor). Angelova et al (Angelova et al., 2010) have recently proposed that some of the highly identical or conserved amino acid residues present in different -strands of the LRRs of these receptors may have different functions.Based on the sequence similarity, it would appear that the structure of the hormone binding domain is conserved across the glycoprotein hormone receptors. Co-crystallization studies of deglycosylated human FSH with the ectodomain of FSHR revealed that all glycoprotein hormones bind to their receptors in a hand clasp fashion to the concave surface of the receptor and that the binding specificity is mediated by key interaction sites involving the hormone specific as well as the common subunits (Fan and Hendrickson, 2005; Fan and Hendrickson, 2008). The same group also found evidence for dimerization of FSH-FSHR ectodomain complex in solution by chemical crosslinking, analytical centrifugation and light scattering (Fan and Hendrickson, 2005). The hinge region between the LRR and transmembrane domain of both LH and FSH receptors reflects shared characteristics, including a signaling-sensitive serine residue (LHR, S277; FSHR, S273) and the sulfation motif, emphasizing the significance of the structurally undefined hinge region for receptor function (Mueller et al., 2010; Nakabayashi et al., 2000). Although there are no structural data available for LHR, comparative modeling with other LRR proteins has allowed generation of models of the ectodomain of LHR (Puett et al., 2007), which resembles the crystal structure of truncated FSHR ectodomain with bound FSH.
LHR is thought to remain in an inactive state via extensive intra helical hydrogen bonding network. Upon binding the ligand on the extracellular domain, the receptor is activated by disrupting some of these hydrogen bonding interactions. The identification of a constitutively activating mutation of rhodopsin, a member of GPCR, provided the first vital clue to the mechanism of receptor activation. This mutation was seen in a patient with autosomal dominant retinitis pigmentosa (Farrar et al., 1992). Since then a constitutively activating mutation of human LHR was identified in a boy with precocious puberty (Shenker et al., 1993). In the proband, the conserved Asp residue 578 in the sixth transmembrane domain of LHR is found to be replaced by Gly. The mutation caused LH-independent production of adult levels of testosterone during the prepubertal period leading to male precocious puberty (Shenker, 2002; Shenker et al., 1993). The simplest explanation for the constitutive activation of LHR was that the mutation caused disruption of the interaction between Asp578 and Asn615 in the transmembrane helix 7 to mimic ligand-occupied state of the receptor resulting in the activation of Gs protein. (Angelova et al., 2002; Bradbury and Menon, 1999; Kawate et al., 1995; Kremer et al., 1993; Shenker et al., 1993). Using chimeric receptors with or without a point mutation, Kudo et al demonstrated that the trans membrane 5 to 6 region of human LH receptor is important for its constitutive activation, whereas the corresponding domains of human FSH receptor might be more constrained and keep it in an inactive state (Kudo et al., 1996).Since then, approaches including molecular simulation and single amino acid substitutions of conserved residues creating activating and inactive mutants coupled with limited crystallographic data have provided several models to explain the mechanism of LHR and FSHR activation (Fan and Hendrickson, 2005; Lapthorn et al., 1994). These studies led to the conclusion that transmembrane helices 3, 6 and 7 play an important role in receptor activation (Angelova et al., 2002). The available data also show that the extracellular domains of both LHR and FSHR are involved in ligand binding. Based upon available evidence on structure/function studies of LHR and FSHR, Ascoli and Puett (Ascoli and Puett, 2009; Costagliola et al., 2005; Vassart et al., 2004) have summarized three potential models for receptor activation. The first model envisions that a portion of the ligand bound to the extracellular domain interacts with the hinge region or the transmembrane helices leading to receptor activation. A second possible scenario is that the interaction of ligand on the extracellular domain would allow the extracellular domain to interact with the transmembrane helices to cause receptor activation. The third model proposes that the extracellular domain confers an inhibitory effect on the transmembrane region keeping it in an inactive state, and the binding of the ligand to the extracellular domain relieves this inhibitory effect. Further insight into the mechanism of receptor activation will come from advances in elucidation of the crystal structure of the receptor in the presence and absence of ligand.
4. Post-translational modification of the gonadotropin receptors
Both LHR and FSHRare glycosylated at asparagine residues within the consensus sequence Asn-X-Ser/Thr, where X is any amino acid except proline (Bause and Legler, 1981; Gavel and von Heijne, 1990) and palmitoylatedat the conserved cysteine residues in the carboxyl terminus (Kawate et al., 1995; Kawate and Menon, 1994; Munshi et al., 2005; Zhu et al., 1995). Glycosylation is believed to be required for the proper folding of the protein by interaction with chaperones. Both of these post-translational modifications occur during biosynthesis and trafficking of the mature form of the receptor to the cell surface. These post -translational modifications have been more extensively studied for LHR. Six potential glycosylation sites have been identified in the rat, human and porcine LHR (Davis et al., 1997; Vu-Hai et al., 2000; Zhang et al., 1995). The extent of glycosylation appears to vary among different species. The rat FSHR is glycosylated at two of the three glycosylation consensus sequences (N174, N182 and N276) on the extracellular domain. Mutagenesis of potential glycosylation sites suggested that glycosylation at one of the three sites is sufficient for proper folding of the receptor since mutation of the other two sites produced minimal effect on cell surface expression and ligand binding (Davis et al., 1995). The second post-translational modification that occurs during biosynthesis is palmitoylation of the two conserved cysteine residues (621 and 622) of the rat LHR and cysteine residues 643 and 644 of the human LHR (Kawate and Menon, 1994; Munshi et al., 2001; Zhu et al., 1995). While palmitoylation might provide an anchoring site for the carboxyl terminus, abrogation of the palmitoylation site by mutating to Gly or Ser does not appear to affect neither the efficiency of cell surface expression nor the responsiveness to ligand (Kawate and Menon, 1994; Moench et al., 1994). Further studies on Human LHR showed that palmitoylation affects the post-endocytic trafficking of the internalized LHR since palmitoylation deficient mutants undergo rapid internalization and impaired recycling of the receptor back to the cell surface (Munshi et al., 2005). One explanation for this finding is that the palmitoylation deficient mutants might undergo hyperphosphorylation and might redirect the internalized receptor from endosomes to the degradative pathway (Charest and Bouvier, 2003). The palmitoylation of FSHR has not been examined to the same extent as the LHR.
While glycosylation serves an essential role in LHR and FSHR folding to facilitate intracellular trafficking for cell surface expression, phosphorylation of these receptors is believed to occur after the interaction of the receptor with its ligand. Both LHR and FSHR are phosphorylated in transfected cells. The major sites of phosphorylation are serine residues located in the carboxyl terminus (Hipkin et al., 1995; Lazari et al., 1998; Min and Ascoli, 2000; Wang et al., 1996; Wang et al., 1997) and catalyzed by GPCR kinase family of enzymes. In the case of rat FSHR, phosphorylation has been reported to occur on residues on the first, third and the carboxyl terminus portion (Hipkin et al., 1995; Kara et al., 2006; Nakamura et al., 1998). While the exact role of phosphorylation has not been determined, it is thought to facilitate internalization of the ligand-bound receptor to the intracellular sites through the interaction of phosphorylated receptor with arrestin. However, phosphorylation independent association of LHR with arrestin has also been reported by Lamm and Hunzicker-Dunn (Lamm and Hunzicker-Dunn, 1994). Further studies are needed to clarify the precise role of phosphorylation in LHR and FSHR internalization and/or recycling.
5. Mutations of LHR and FSHR
Several naturally occurring mutations with reproductive phenotypes have been reported for human LHR and FSHR (reviewed in (Ascoli and Puett, 2009; Segaloff, 2009; Tao and Segaloff, 2009; Themmen, 2005)). As mentioned earlier, the first mutation to be identified for the gonadotropin receptors was the constitutively active mutation of LH receptor in a patient with precocious puberty (Shenker et al., 1993). Since then, similar mutations of LHR involving the substitution of Asp578 by Gly,Glu, Tyr or His leading to male familial precocious pubertyhave been reported by others (Gromoll et al., 1998; Kawate et al., 1995; Kremer et al., 1993; Yano et al., 1994; Yano et al., 1995). It has also been shown that Asp578Gly is the most common activating mutation of LHR (Laue et al., 1995). Asp578His, which is the only somatic mutation identified so far (reviewed in (Segaloff, 2009)), has been shown to be responsible for hyperplasia and leydig cell adenoma (Boot et al., 2011). Activating mutations of LHR occur mainly on exon 11 which codes for the transmembrane domain and the carboxyl terminus region (Ascoli et al., 2002; Ascoli and Puett, 2009; Costagliola et al., 2005; Latronico and Segaloff, 1999; Themmen and Huhtaniemi, 2000). These mutations are localized on the sixth transmembrane domain and the third intracellular loop (Ascoli et al., 2002; Ascoli and Puett, 2009; Costagliola et al., 2005; Latronico and Segaloff, 1999; Themmen and Huhtaniemi, 2000). On the other hand, several of the LHR mutations causing loss of function occur on exon 10 leading to developmental or other disorders of male reproductive function (Gromoll et al., 2000). Single amino acid substitution leading to inactivating mutations of LHR have been reported to cause loss of leydig cell function (Laue et al., 1995) and pseudohermaphroditism (Qiao et al., 2009), hypospadias and micropenis in homozygous or compound heterozygous 46XY individuals (Latronico and Segaloff, 1999; Qiao et al., 2009; Themmen and Huhtaniemi, 2000). Inactivating mutations of the LH receptor have been reported in women with oligo-amenorrhea and infertility (Arnhold et al., 2009; Latronico and Segaloff, 1999; Themmen and Huhtaniemi, 2000). Recently, a missense mutation of LHR with impaired hCG responsiveness has been implicated for the empty follicle syndrome (Yariz et al., 2011). Loss of function and gain of function mutations,associated with altered reproductive functions, have also been reported in human FSHR (Tao and Segaloff, 2009; Themmen, 2005). Specifically, mutations of FSHR have been linked to primary amenorrhea (Achrekar et al., 2010; Nakamura et al., 2008), ovarian hyperstimulation syndrome (De Leener et al., 2008; Dieterich et al., 2010; Montanelli et al., 2004; Rodien et al., 2010; Smits et al., 2003), primary ovarian failure (Doherty et al., 2002) and infertility (Peltoketo et al., 2010; Themmen and Huhtaniemi, 2000).
6. Regulation of FSHR and LHR during ovarian cycle
Expression of LHR undergoes dynamic changes during the normal ovarian cycle. Granulosa cells from early antral follicles express FSHR and the expression increases with follicle growth.Antral follicles also express small amounts of LHR which is mostly confined to theca-interstitial cells. The LHR expression levels increase with follicle growth in response to FSH, estradiol and other paracrine factors reaching maximum levels prior to ovulation (Menon et al., 2005; Zeleznik et al., 1974; Zeleznik et al., 1981) LHR expression is transiently down regulated in response to preovulatory LH surge during the differentiation of estrogen producing granulosa cells to luteal cells (Hoffman et al., 1991; LaPolt et al., 1990; Lu et al., 1993; Peegel et al., 1994; Segaloff et al., 1990). During this period, the differentiating granulosa cells remain refractory to LH due to desensitization of the G protein coupled responsive system (Azhar et al., 1980; Ghosh et al., 1988; Hunzicker-Dunn et al., 1979; Sen et al., 1979). There is no evidence for similar down regulation of FSHR during this period. This is followed byfull recovery of LHR as well as the responsiveness of the differentiated granulosa cells to LH. LHR expression reaches maximum levels during mid-luteal phasewith increased progesterone production. The receptor levels then declinewith the regression of the corpus luteum (Hoffman et al., 1991; LaPolt et al., 1990; Lu et al., 1993; Peegel et al., 1994). LHR expression appears to be regulated both through transcriptional and more prominently via post-transcriptional mechanisms. The latter mechanism has been described in detail below. Although FSH receptor expression appears to be regulated mainly atthe transcriptional level (Goetz et al., 1996; Heckert et al., 1998; Heckert et al., 2000; Hermann et al., 2008; Xing and Sairam, 2001), there are also reports showing that regulation can also occur through post-transcriptional processes (Tano et al., 1997; Themmen et al., 1991). The factors that are shown to control the expression of FSHR include FSH, members of the transforming growth factor (TGF)- family, TGF- and epidermal growth factor (reviewed in (Findlay and Drummond, 1999)).
7. Post-transcriptional regulation of LHR expression
The downregulation of LH receptor in response to preovulatory LH surge canbe mimicked by the administration of a pharmacological dose of hCGto PMSG-hCG primed rats (Hoffman et al., 1991; Lu et al., 1993; Peegel et al., 1994). The ligand-induced down regulation ofLHR is not confined to rodent ovaries, since this phenomenon has also been observed in human.For example, granulosa cells isolated from the follicular aspirates derived from women undergoing ovulation induction for in vitro fertilization show a sharp decline in the levels of LH receptor mRNA transcriptsdue to treatment with high doses of hCG prior to oocyte retrieval. When the cells are cultured for 48 hours, the receptor levels return to normallevels, a phenomenon similar to that seen in rodent ovaries (Nair et al., 2006).
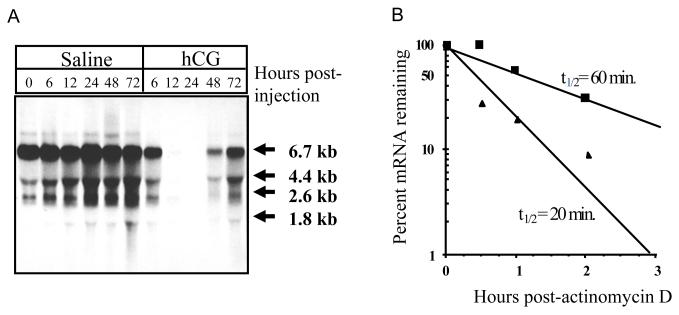
The mechanism of regulation of LHR has been extensively studied in our laboratory by employing the ligand-induced downregulation as a model system. Administration of a single dose of hCG to PMSG-hCG primed rats resulted in a loss of mRNA transcripts starting at 6 hours, persisting upto 48 hours (Fig 1A) (Hoffman et al., 1991). Although there was a dramatic decline in the steady state levels of LHR mRNA expression by 24 hours, surprisingly, the transcription rates remained unchanged (Lu et al., 1993). A comparison of the half-life of the LHR mRNA between the control and down regulated ovaries showed a threefold decline in the down regulated group suggesting that the decrease in steady state levels of LHR mRNA occurs through its increased degradation (Fig 1B) (Lu et al., 1993). It is now recognized thatmany highly regulated mRNAs are controlled by regulating their stability by post-transcriptional mechanisms (Ross, 1995). In a majority of instances these changes in stability are caused by RNA binding proteinsthat bind to specific sequences or structures in the target mRNA. These trans-factors can bind to the coding region (c-fos, c-myc, thymidylate synthase and dihydrofolate reductase), 3′ untranslated region (beta adrenergic receptor mRNA and Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) mRNA) or 5′ untranslated region(ferritin mRNA) (Bernstein et al., 1992; Iwai et al., 1991; Leibold and Munro, 1988; Lin et al., 2000; Shyu et al., 1991; Tai et al., 2004; Tholanikunnel and Malbon, 1997) of the corresponding mRNAs,thereby increasing or decreasing their stability. Further studies were therefore focused onexamining if the post-transcriptional regulation of LHR mRNA involves the participation of an RNA binding protein.
Fig 1.
Hormonal control of LHR mRNA expression in the ovary.
A. Northern blot hybridization analysis of steady state LHR mRNA levels during hCG-induced down-regulation. Autoradiogram of Northern blot hybridization analysis of total RNA isolated at the indicated times from the ovaries of saline-injected (control) (lanes 1-6) or hCG-injected (down-regulated) (lanes 7-11) rats. Blots were probed using a labeled cDNA encoding the LHR carboxyl terminus and a portion of the 3′-UTR (nucleotides 1936-2682). B. LHR mRNA half-life determination in control and 12-h downregulated rat ovaries. Cell suspensions were incubated with 10 g/ml actinomycin D for 2h. Duplicate aliquots of 20 × 106 cells were removed at the indicated times. Total RNA was isolated and assayed for LHR mRNA by solution hybridization. Each data point represents the average of duplicate determinations. ■ Contol, ▲ downregulated. A-“This research was originally published in J Biol Chem. Kash JC, Menon KM. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mRNA binding during receptor down-regulation. J Biol Chem. 1998. 273:10658-64. © the American Society for Biochemistry and Molecular Biology”. B-Copyright 1998, Endocrine Society 1993.
Cytosolic fractions from control and downregulated ovaries were subjected to RNA electrophoretic mobility shift assay using [32P] labeled LHR mRNA that was prepared by transcribing the full length cDNA encoding LHR in the presence of [32P] UTP (Kash and Menon, 1998). Two LHR mRNA bound protein complexes of approximately 50 and 45 kDawere identified. The 50 kDa bandwas induced in response to hCG treatment while the shorter form showed no increase. This 50kDa form was designated as LHR mRNA binding protein (LRBP). Further studies revealed that the expression of LRBP showed a reciprocal relationship with LHR mRNA expression during different physiological states in the ovary as well as during the life span of corpus luteum (Nair et al., 2002). These results suggested that LRBP is a negative regulator of LHR mRNA expression. Hydroxyl radical foot printing analysis identified an 18 nucleotide polypyrimidine-rich bipartite sequence between nucleotides 203 and 220 in the coding region of LHR mRNA as the sequence that interacted with LRBP with high affinity and specificity (Kash and Menon, 1999). This region of the rat LHR mRNA was designated as the LHR mRNA binding site (LBS).
8. Identification and characterization of LRBP
LRBP was purified and subjected to both N-terminal analysis and determination of partial sequences using MALDI-TOF (Nair and Menon, 2004). The LHR mRNA binding protein was identified as mevalonate kinase, a metabolic enzyme involved in cholesterol biosynthesis. The identity of LRBP as being mevalonate kinase is not surprising since many mRNA binding proteins identified so far are multifunctional proteins (Nagy and Rigby, 1995; Pioli et al., 2002; Rouault et al., 1991). We have chosen to refer the LHR mRNA binding protein as LRBP rather than mevalonate kinase in the context of its function in binding LHR mRNA. The identity of LRBP was further confirmed by immunoblotting the partially purified and the gel purified protein with anti-mevalonate kinase antibody (Nair and Menon, 2004), by the ability of the purified protein to bind LHR mRNAusing electrophoretic mobility shift assay and by molecular cloning and overexpression in 293 cells (Nair and Menon 2004). The LHRmRNA binding characteristics of the overexpressed protein were consistent with the results obtained using cytosolic fractions from downregulated ovary as well as the gel purified protein (Nair and Menon, 2004). Endogenous association of LHR mRNA with LRBP was assessed by immunoprecipitation of the ribonucleoprotein complex from the cytosolic fraction with antibody against mevalonate kinase followed by reverse transcription-PCR of the RNA associated from the immune complexand amplification using LHR specific primers (Nair and Menon, 2005). The role of LRBP in the regulation of LHR mRNA expression was independently confirmed by Ikeda et al (Ikeda et al., 2008) by demonstrating that overexpression of LRBP in granulosa cells inhibited the FSH-induced increase in LHR mRNA expression in the cultured rat granulosa cells. (Ikeda et al., 2008).
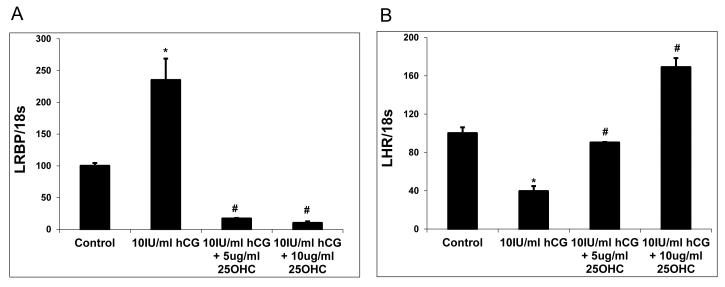
The functional role of LRBP was established by depleting LRBP levels by treating human granulosa cells with 25-hydroxycholesterol (Wang et al., 2007). It has been established that treatment with25 hydroxycholesterol will deplete mevalonate kinase (LRBP) (Adams et al., 2004). Accordingly, cultured human granulosa cells were treated with 25-hydroxy cholesterol. Thistreatment resulted in the suppression of both mRNA and protein expression of LRBP (Wang et al., 2007). The ability of LRBP-depleted granulosa cells to undergo down regulation was then examined by incubating the cultured cells with hCG in the presence and absence of 25-hydroxycholesterol. In the absence of 25 hydroxycholesterol, hCG produced down regulation of LHR mRNA expression, as expected. Most interestingly, the hCG-induced down-regulation of LHR mRNA was abrogated by treatment with 25-hydroxy cholesterol (Wang et al., 2007) (fig 2). These results show that the hCG-induced down-regulation of LHR mRNA was abolished when LRBP was depleted from the cells. These results together with the findings of Ikeda et al provide convincing evidence for the role of LRBP as a negative regulator of LHR mRNA expression in the ovary (Ikeda et al., 2008).
Fig 2.
Suppression of LRBP Expression abrogates LHR mRNA downregulation.
After culture in serum free medium for 48 h, human granulosa cells were treated with serum free medium alone, 10 IU/ml hCG, 10 IU/ml hCG plus 5 g/ml 25-OHC or 10 IU/ml hCG plus 10 g/ml 25-OHC, and harvested at 12 h. Steady-state levels of LRBP (A) and LHR mRNA (B) were measured by real-time PCR. Mean values ± SE (n = 3) were normalized to 18S rRNA and graphed as percent of control (time 0 h). *P< 0.05 vs. Control, # P< 0.05 vs. 10 IU/ml hCG.
9. Translational suppression of LHR mRNA by LRBP
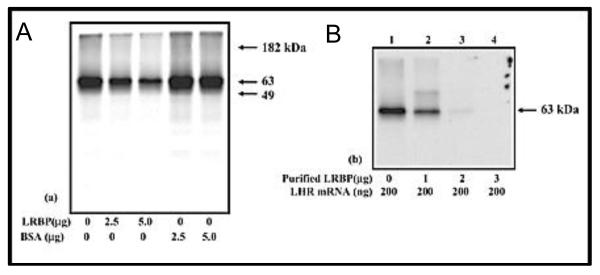
Since post-transcriptional regulation of mRNA mediated by RNA binding protein is believed to be coupled to translation, the possibility of LRBP acting as inhibitor of LHR mRNA translation was examined. A full-length FLAG-tagged rat LHR mRNA was synthesized and translated in vitro in a rabbit reticulocyte lysate system using [35S] methionine in the presence and absence of LRBP. The translation products were immunoprecipitated and analyzed on SDS-PAGE. The results showed that LHR mRNA translation was suppressed by LRBP (fig 3) (Nair and Menon, 2005). To confirm the specificity of translation inhibition by LRBP, in vitrotranslation reactions were performed in the presence of wild type and mutated LHR mRNA fragments (LBS) to test whether sequestration of LRBP might reverse the translational suppression by LRBP (Kash and Menon, 1999). As expected, excess LBS was able to reverse translation inhibition by LRBPwhile the mutant LBS was not able to reverse the inhibition. Addition of mevalonate, the endogenous substrate for LRBPwas also found toreduce the extent of translation inhibition by LRBP (Nair and Menon, 2004). These studies provide strong evidence that the LRBP-mediated translational suppression of LHR mRNA is indeed due to the binding of LRBP to LHR mRNA.
Fig 3.
Translation of LHR mRNA in vitro; effect of LRBP.
A. 200 ng of FLAG-tagged rat LHR mRNA was in vitro translated using 15 μCi of [35S]methionine in the presence or absence of partially purified rat LRBP from the ovary or fatty acid-free bovine serum albumin (BSA) at different concentrations (2.5 and 5.0 μg). The translated LHR protein was immunoprecipitated, separated on 10% SDS-PAGE, and developed for autoradiogram. B. 200 ng of FLAG-tagged rat LHR mRNA was in vitro translated using 15 μCi of [35S]methionine in the absence and presence of purified rat LRBP at different concentrations (1, 2, and 3 μg).The translated LHR protein was immunoprecipitated and processed for developing the autoradiogram.“This research was originally published in J Biol Chem. Regulation of luteinizing hormone receptor expression: evidence of translational suppression in vitro by a hormonally regulated mRNA-binding protein and its endogenous association with luteinizing hormone receptor mRNA in the ovary. Nair AK, Menon KM. J Biol Chem. 2005. 280. 42809-16. © the American Society for Biochemistry and Molecular Biology”.
The translocation of LRBP to translating ribosomes was then demonstrated by showingthat this translocation occurs during hCG-induced LHR mRNA downregulation (Menon et al., 2009). Immunoprecipitation of polysomes with antibody against LRBP followed by the analysis of LHR mRNA levels in the immunoprecipitates confirmed that direct interaction of LRBP with LHR mRNA occurs in the ribosomes during downregulation. The functional role of this association was established by demonstrating that translational suppression of LHR mRNA can be induced by LRBP-rich polysomes isolated from downregulated ovaries (Menon et al., 2009). These studies suggest that accelerated degradation of LHR mRNA occurs during down regulation by the binding of LRBP to LHR mRNA in the ribosomes resulting in the formation of an untranslatable complex which is then targeted for degradation.This is consistent with the notion that translational arrest/inhibition or aberrant termination can lead to the degradation of eukaryotic mRNA (Blume and Shapiro, 1989; Jacobson and Peltz, 1996; Pachter et al., 1987; Ross, 1995).
10. Signaling pathways in LH/hCG-induced LRBP expression
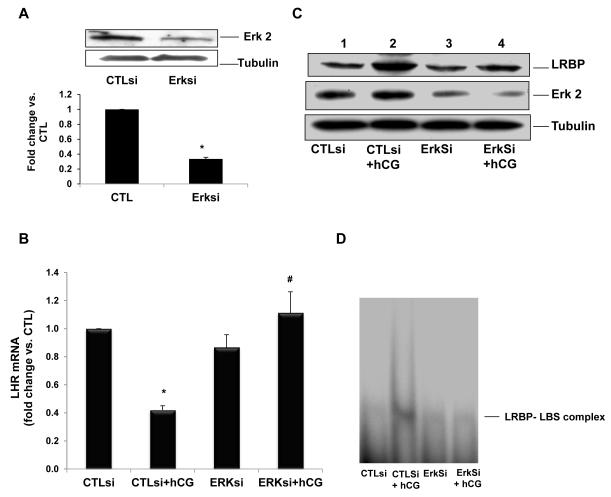
Evidence presented so far showsthat increased expression of LRBP in response to hCG treatment sets the stage for LHR mRNA down regulation. What regulates LRBP expression is an intriguing question. To address this, the signaling pathways involved in the upregulation of LRBP in response to hCG treatment were examined. It is well established that the interaction of LH/hCG with its receptor leads to activation of adenylate cyclase through stimulatory guanine nucleotide regulatory protein (Gs protein), leading to an increase in cyclic AMP levels (Marsh, 1975; Menon and Gunaga, 1974). Initial studies were conducted to determine whether the LRBP-mediated down-regulation of the LH receptor mRNA expression is mediated by cAMP. This was done by increasing the intracellular levels of cyclic AMP using rolipram, an inhibitor of type IV phosphodiesterase in the ovary (Conti, 2000). The results showed that chronic elevation of cyclic AMP in the corpus luteum by roliprammimicked LH/hCG-induced down regulation ofLHR mRNA expression (Peegel et al., 2005). LHRmRNA binding activity of LRBP in the ovarian cytosolic fractions was also found to be elevated by rolipram treatment, confirming the role of cAMP in LRBP-mediated LHR mRNA downregulation (Peegel et al., 2005). Further studies using cultured human granulosa cells revealed that inhibition of PKA resulted in the inhibition of hCG-induced increase in LRBP expression as well as LHR mRNA downregulation (Menon et al., 2011). PKA inhibition also abolished the LHR mRNA binding activity of LRBP in these cells (Menon et al., 2011). The ERK family, consisting mainly of ERK1 (p44 MAPK) and ERK2 (p42 MAPK), has been known to exert a broad regulatory influence over a wide range of processes, including LH-induced regulation of ovarian function (Fan et al., 2009; Pearson et al., 2001). LH/hCG has also been shown to stimulate the ERK pathway through PKA activation (Salvador et al., 2002; Seger et al., 2001). Therefore the role of ERK in LRBP-mediated down regulation of LHR mRNA was examined in human granulosa cells. The results showed that siRNA-mediated inhibition of ERK expression completely abolished the hCG-induced downregulation of LHR mRNA (fig 4) (Menon et al., 2011). This was accompanied by a decrease in LRBP expression as well as its LHR mRNA binding activity (fig 4) (Menon et al., 2011). ERK1/2 was also shown to undergo translocation to the nucleus during LH/hCG induced downregulation,suggesting the role of nuclear events in the induction of LRBP preceding LHR mRNA downregulation (Menon et al., 2011). These studies provide evidence that PKA-dependent ERK activation plays a pivotal role in the hCG-mediated post-transcriptional regulation of LH receptor mRNA expression by regulating the expression of LRBP.This increase in the expression of LRBP and the subsequent increase in its LHR mRNA binding activity lead to the formation of an untranslatable ribonucleoprotein complex resulting in LHR mRNA decay.
Fig 4.
ERK1/2 silencing inhibits hCG-induced decrease in LHR mRNA levels and increases in LRBP protein expression and binding activity.
Granulosa cells were transfected with either control siRNA (CTLsi) or ERK 1/2siRNA (ERKsi) and cultured for 48 h. After serum-starving for another 24 h, cells were treated with hCG (10 IU/ml) for 12 h and processed for total RNA isolation, for Western blot analysis, or for REMSA. A, ERK1/2 silencing was confirmed by the Western Blot analysis of cell lysates using total ERK2 antibody. B, Total RNAs were reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using LHR-specific primers and probes. The graph represents changes in mRNA levels normalized to 18S rRNA and are shown as fold change vs. control. Error bars, mean ± se. *, P< 0.05 vs. CTL; #, P< 0.05 vs. hCG; n = 3. C, Cell lysates were subjected to Western blot analysis to detect LRBP using specific antibody. The same membranes were then stripped and reprobed for ERK2 and β-tubulin. The blot shown is a representative of three independent experiments. D, Gel mobility shift analysis was performed with [32P]-labeled rat LBS (1.5 × 105 c.p.m) and S100 fractions containing equal amounts of total protein extracted from the different treatment groups. The autoradiogram shown is representative of three independent experiments. Copyright 2011, The Endocrine Society.
11. Conclusion
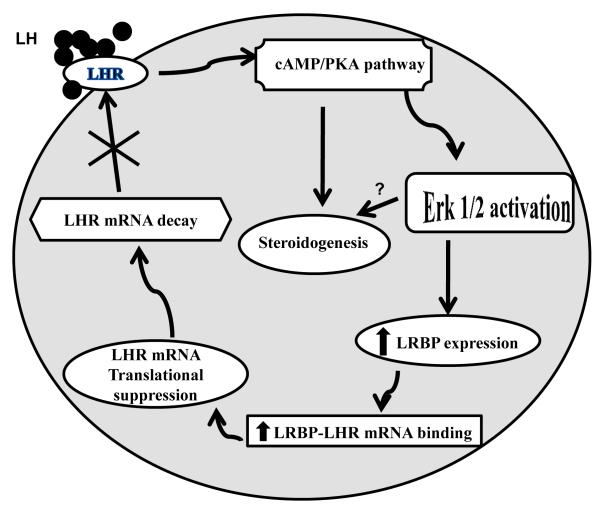
The studies presented here show the post-transcriptional mechanism involved in the changes in the steady state levels of LHR mRNA expression during the ovarian cycle. In addition to the expected transcriptional control, the ovarian cells utilize an efficient mechanism to regulate the steady state levels of LHR in the ovary in response to a constantly changing pituitary hormone environment. This allowsa means to control the steady state levels without the necessity to continuously alter the complex transcriptional assembly. As summarized in Fig 5, in response to preovulatory surge of LH, the down regulation of LHR mRNA expression is initiated by cyclic AMP-ERK mediated induction of LRBP. Since the ovary remains refractory during this period, steroidogenesis is transiently suppressed. During this period, LRBP functions as an LHR mRNA binding protein and binds LHR mRNA tosuppress translation. The untranslatable LHR mRNA-LRBP complex is then targeted to p-bodies where it undergoes decapping and degradation. Our recent finding,using yeast 2 cell hybrid screening (Wang et al., 2010) that LRBP interacts with proteins involved in mRNA degradation further supports this notion. LHR mRNA degradation causes a depletion of cell surface LHR expression preventing ovarian hyperstimulation in the presence of high levels of LH seen during this period. With the resumption of steroidogenic activity, LRBP switches its function as an enzyme involved in cholesterol biosynthesis terminating its role as LHR mRNA binding protein.
Fig 5.
Schematic model depicting the proposed signaling pathway in LH/hCG-induced LHR mRNA down-regulation.
Binding of ligand to LH receptor induces activation of ERK1/2 through the cAMP/PKA pathway. This leads to an increase in the expression of LRBP and thereby its’ LHR mRNA binding activity, which ultimately results in LHR mRNA degradation. Copyright 2011, The Endocrine Society.
Acknowledgments
Supported by NIH grant R37 06656
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah MA, Lei ZM, Li X, Greenwold N, Nakajima ST, Jauniaux E, Rao Ch V. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J. Clin. Endocrinol. Metab. 2004;89:952–956. doi: 10.1210/jc.2003-030917. [DOI] [PubMed] [Google Scholar]
- Achrekar SK, Modi DN, Meherji PK, Patel ZM, Mahale SD. Follicle stimulating hormone receptor gene variants in women with primary and secondary amenorrhea. J. Assist. Reprod. Genet. 2010;27:317–326. doi: 10.1007/s10815-010-9404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- Angelova K, de Jonge H, Granneman JC, Puett D, Bogerd J. Functional differences of invariant and highly conserved residues in the extracellular domain of the glycoprotein hormone receptors. J. Biol. Chem. 2010;285:34813–34827. doi: 10.1074/jbc.M110.148221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova K, Fanelli F, Puett D. A model for constitutive lutropin receptor activation based on molecular simulation and engineered mutations in transmembrane helices 6 and 7. J. Biol. Chem. 2002;277:32202–32213. doi: 10.1074/jbc.M203272200. [DOI] [PubMed] [Google Scholar]
- Apaja PM, Harju KT, Aatsinki JT, Petaja-Repo UE, Rajaniemi HJ. Identification and structural characterization of the neuronal luteinizing hormone receptor associated with sensory systems. J. Biol. Chem. 2004;279:1899–1906. doi: 10.1074/jbc.M311395200. [DOI] [PubMed] [Google Scholar]
- Arnhold IJ, Lofrano-Porto A, Latronico AC. Inactivating mutations of luteinizing hormone beta-subunit or luteinizing hormone receptor cause oligo-amenorrhea and infertility in women. Horm. Res. 2009;71:75–82. doi: 10.1159/000183895. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Puett D. The gonadotropin hormones and their receptors. In: Strauss JF 3rd, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology. Physiology, Pathophysiology and Clinical Management. Elsevier, Amsterdam: 2009. pp. 35–55. [Google Scholar]
- Ascoli M, Segaloff DL. On the structure of the luteinizing hormone/chorionic gonadotropin receptor. Endocr. Rev. 1989;10:27–44. doi: 10.1210/edrv-10-1-27. [DOI] [PubMed] [Google Scholar]
- Azhar S, Menon M, Menon KMJ. Receptor mediated gonadotropin action in the ovary. Modulation of progesterone response in isolated rat ovarian cells by gonadotrophin, cholera enterotoxin and cyclic nucleotides: requirement for RNA and protein synthesis. Acta. Endocrinol. (Copenh) 1980;95:528–539. [PubMed] [Google Scholar]
- Bacich DJ, Rohan RM, Norman RJ, Rodgers RJ. Characterization and relative abundance of alternatively spliced luteinizing hormone receptor messenger ribonucleic acid in the ovine ovary. Endocrinology. 1994;135:735–744. doi: 10.1210/endo.135.2.7518389. [DOI] [PubMed] [Google Scholar]
- Bause E, Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem. J. 1981;195:639–644. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PL, Herrick DJ, Prokipcak RD, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6:642–654. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- Bhowmick N, Huang J, Puett D, Isaacs NW, Lapthorn AJ. Determination of residues important in hormone binding to the extracellular domain of the luteinizing hormone/chorionic gonadotropin receptor by site-directed mutagenesis and modeling. Mol. Endocrinol. 1996;10:1147–1159. doi: 10.1210/mend.10.9.8885249. [DOI] [PubMed] [Google Scholar]
- Blume JE, Shapiro DJ. Ribosome loading, but not protein synthesis, is required for estrogen stabilization of Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1989;17:9003–9014. doi: 10.1093/nar/17.22.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot AM, Lumbroso S, Verhoef-Post M, Richter-Unruh A, Looijenga LH, Funaro A, Beishuizen A, van Marle A, Drop SL, Themmen AP. Mutation analysis of the LH receptor gene in Leydig cell adenoma and hyperplasia and functional and biochemical studies of activating mutations of the LH receptor gene. J. Clin. Endocrinol. Metab. 2011;96:E1197–1205. doi: 10.1210/jc.2010-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury FA, Menon KMJ. Evidence that constitutively active luteinizing hormone/human chorionic gonadotropin receptors are rapidly internalized. Biochemistry. 1999;38:8703–8712. doi: 10.1021/bi990169t. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Atwood CS, Zhu X, Hartzler AW, Webber KM, Perry G, Bowen RL, Smith MA. Evidence for the role of gonadotropin hormones in the development of Alzheimer disease. Cell Mol. Life Sci. 2005;62:293–298. doi: 10.1007/s00018-004-4384-0. [DOI] [PubMed] [Google Scholar]
- Charest PG, Bouvier M. Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J. Biol. Chem. 2003;278:41541–41551. doi: 10.1074/jbc.M306589200. [DOI] [PubMed] [Google Scholar]
- Conti M. Phosphodiesterases and cyclic nucleotide signaling in endocrine cells. Mol Endocrinol. 2000;14:1317–1327. doi: 10.1210/mend.14.9.0534. [DOI] [PubMed] [Google Scholar]
- Costagliola S, Urizar E, Mendive F, Vassart G. Specificity and promiscuity of gonadotropin receptors. Reproduction. 2005;130:275–281. doi: 10.1530/rep.1.00662. [DOI] [PubMed] [Google Scholar]
- Davis D, Liu X, Segaloff DL. Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in FSH receptor function. Mol. Endocrinol. 1995;9:159–170. doi: 10.1210/mend.9.2.7776966. [DOI] [PubMed] [Google Scholar]
- Davis DP, Rozell TG, Liu X, Segaloff DL. The six N-linked carbohydrates of the lutropin/choriogonadotropin receptor are not absolutely required for correct folding, cell surface expression, hormone binding, or signal transduction. Mol. Endocrinol. 1997;11:550–562. doi: 10.1210/mend.11.5.9927. [DOI] [PubMed] [Google Scholar]
- De Leener A, Caltabiano G, Erkan S, Idil M, Vassart G, Pardo L, Costagliola S. Identification of the first germline mutation in the extracellular domain of the follitropin receptor responsible for spontaneous ovarian hyperstimulation syndrome. Hum. Mutat. 2008;29:91–98. doi: 10.1002/humu.20604. [DOI] [PubMed] [Google Scholar]
- Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vitam. Horm. 2002;64:249–322. doi: 10.1016/s0083-6729(02)64008-7. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bolz M, Reimer T, Costagliola S, Gerber B. Two different entities of spontaneous ovarian hyperstimulation in a woman with FSH receptor mutation. Reprod. Biomed Online. 2010;20:751–758. doi: 10.1016/j.rbmo.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, Aittomaki K. A Novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J. Clin. Endocrinol Metab. 2002;87:1151–1155. doi: 10.1210/jcem.87.3.8319. [DOI] [PubMed] [Google Scholar]
- Dufau ML. The luteinizing hormone receptor. Annu. Rev. Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Comparative structural analysis of the binding domain of follicle stimulating hormone receptor. Proteins. 2008;72:393–401. doi: 10.1002/prot.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Kenna P, Jordan SA, Kumar-Singh R, Humphries MM, Sharp EM, Sheils D, Humphries P. Autosomal dominant retinitis pigmentosa: a novel mutation at the peripherin/RDS locus in the original 6p-linked pedigree. Genomics. 1992;14:805–807. doi: 10.1016/s0888-7543(05)80193-4. [DOI] [PubMed] [Google Scholar]
- Fields MJ, Shemesh M. Extragonadal luteinizing hormone receptors in the reproductive tract of domestic animals. Biol. Reprod. 2004;71:1412–1418. doi: 10.1095/biolreprod.104.027201. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Drummond AE. Regulation of the FSH Receptor in the Ovary. Trends Endocrinol. Metab. 1999;10:183–188. doi: 10.1016/s1043-2760(98)00144-1. [DOI] [PubMed] [Google Scholar]
- Gavel Y, von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990;3:433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimova T, Thanasoula MN, Zattas D, Seli E, Sakkas D, Lalioti MD. Identification and in vitro characterization of follicle stimulating hormone (FSH) receptor variants associated with abnormal ovarian response to FSH. J. Clin. Endocrinol. Metab. 2010;95:529–536. doi: 10.1210/jc.2009-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh DK, Peegel H, Dunham WR, Sands RH, Menon KMJ. Modulation of progesterone synthesis and cytochrome P450 levels in rat luteal cells during human chorionic gonadotropin-induced desensitized state. Endocrinology. 1988;123:514–522. doi: 10.1210/endo-123-1-514. [DOI] [PubMed] [Google Scholar]
- Goetz TL, Lloyd TL, Griswold MD. Role of E box and initiator region in the expression of the rat follicle-stimulating hormone receptor. J. Biol. Chem. 1996;271:33317–33324. doi: 10.1074/jbc.271.52.33317. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J. Clin. Endocrinol. Metab. 2000;85:2281–2286. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Gudermann T, Nieschlag E. Molecular cloning of a truncated isoform of the human follicle stimulating hormone receptor. Biochem. Biophys. Res. Commun. 1992;188:1077–1083. doi: 10.1016/0006-291x(92)91341-m. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Partsch CJ, Simoni M, Nordhoff V, Sippell WG, Nieschlag E, Saxena BB. A mutation in the first transmembrane domain of the lutropin receptor causes male precocious puberty. J. Clin. Endocrinol. Metab. 1998;83:476–480. doi: 10.1210/jcem.83.2.4579. [DOI] [PubMed] [Google Scholar]
- Heckert LL, Daggett MA, Chen J. Multiple promoter elements contribute to activity of the follicle-stimulating hormone receptor (FSHR) gene in testicular Sertoli cells. Mol. Endocrinol. 1998;12:1499–1512. doi: 10.1210/mend.12.10.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL, Sawadogo M, Daggett MA, Chen JK. The USF proteins regulate transcription of the follicle-stimulating hormone receptor but are insufficient for cell-specific expression. Mol. Endocrinol. 2000;14:1836–1848. doi: 10.1210/mend.14.11.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Hornbaker K, Rice DA, Sawadogo M, Heckert LL. In vivo regulation of follicle-stimulating hormone receptor by the transcription factors upstream stimulatory factor 1 and upstream stimulatory factor 2 is cell specific. Endocrinology. 2008;149:5297–5306. doi: 10.1210/en.2007-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkin RW, Liu X, Ascoli M. Truncation of the C-terminal tail of the follitropin receptor does not impair the agonist- or phorbol ester-induced receptor phosphorylation and uncoupling. J. Biol. Chem. 1995;270:26683–26689. doi: 10.1074/jbc.270.44.26683. [DOI] [PubMed] [Google Scholar]
- Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–393. doi: 10.1210/endo-128-1-388. [DOI] [PubMed] [Google Scholar]
- Horiuchi A, Nikaido T, Yoshizawa T, Itoh K, Kobayashi Y, Toki T, Konishi I, Fujii S. HCG promotes proliferation of uterine leiomyomal cells more strongly than that of myometrial smooth muscle cells in vitro. Mol. Hum. Reprod. 2000;6:523–528. doi: 10.1093/molehr/6.6.523. [DOI] [PubMed] [Google Scholar]
- Houde A, Lambert A, Saumande J, Silversides DW, Lussier JG. Structure of the bovine follicle-stimulating hormone receptor complementary DNA and expression in bovine tissues. Mol. Reprod. Dev. 1994;39:127–135. doi: 10.1002/mrd.1080390202. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Jungmann R, Derda D, Birnbaumer L. LH-induced desensitization of the adenylyl cyclase system in ovarian follicles. Adv. Exp. Med. Biol. 1979;112:27–44. doi: 10.1007/978-1-4684-3474-3_3. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Nakamura K, Kogure K, Omori Y, Yamashita S, Kubota K, Mizutani T, Miyamoto K, Minegishi T. Effect of estrogen on the expression of luteinizing hormone-human chorionic gonadotropin receptor messenger ribonucleic acid in cultured rat granulosa cells. Endocrinology. 2008;149:1524–1533. doi: 10.1210/en.2007-1163. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Bickel M, Pluznik DH, Cohen RB. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3′-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J. Biol. Chem. 1991;266:17959–17965. [PubMed] [Google Scholar]
- Jacobson A, Peltz SW. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu. Rev. Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- Jia XC, Oikawa M, Bo M, Tanaka T, Ny T, Boime I, Hsueh AJ. Expression of human luteinizing hormone (LH) receptor: interaction with LH and chorionic gonadotropin from human but not equine, rat, and ovine species. Mol. Endocrinol. 1991;5:759–768. doi: 10.1210/mend-5-6-759. [DOI] [PubMed] [Google Scholar]
- Jiang X, Dreano M, Buckler DR, Cheng S, Ythier A, Wu H, Hendrickson WA, el Tayar N. Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone-receptor interactions. Structure. 1995;3:1341–1353. doi: 10.1016/s0969-2126(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Kajava AV, Vassart G, Wodak SJ. Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure. 1995;3:867–877. doi: 10.1016/S0969-2126(01)00222-2. [DOI] [PubMed] [Google Scholar]
- Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol. Endocrinol. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mrna binding during receptor down-regulation. J. Biol. Chem. 1998;273:10658–10664. doi: 10.1074/jbc.273.17.10658. [DOI] [PubMed] [Google Scholar]
- Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999;38:16889–16897. doi: 10.1021/bi9915770. [DOI] [PubMed] [Google Scholar]
- Kawate N, Kletter GB, Wilson BE, Netzloff ML, Menon KMJ. Identification of constitutively activating mutation of the luteinising hormone receptor in a family with male limited gonadotrophin independent precocious puberty (testotoxicosis) J. Med. Genet. 1995;32:553–554. doi: 10.1136/jmg.32.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate N, Menon KMJ. Palmitoylation of luteinizing hormone/human choriogonadotropin receptors in transfected cells. Abolition of palmitoylation by mutation of Cys-621 and Cys-622 residues in the cytoplasmic tail increases ligand-induced internalization of the receptor. J. Biol. Chem. 1994;269:30651–30658. [PubMed] [Google Scholar]
- Kawate N, Okuda K. Coordinated expression of splice variants for luteinizing hormone receptor messenger RNA during the development of bovine corpora lutea. Mol. Reprod. Dev. 1998;51:66–75. doi: 10.1002/(SICI)1098-2795(199809)51:1<66::AID-MRD8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Kremer H, Mariman E, Otten BJ, Moll GW, Jr., Stoelinga GB, Wit JM, Jansen M, Drop SL, Faas B, Ropers HH, et al. Cosegregation of missense mutations of the luteinizing hormone receptor gene with familial male-limited precocious puberty. Hum. Mol. Genet. 1993;2:1779–1783. doi: 10.1093/hmg/2.11.1779. [DOI] [PubMed] [Google Scholar]
- Kudo M, Osuga Y, Kobilka BK, Hsueh AJ. Transmembrane regions V and VI of the human luteinizing hormone receptor are required for constitutive activation by a mutation in the third intracellular loop. J Biol Chem. 1996;271:22470–22478. doi: 10.1074/jbc.271.37.22470. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Hunzicker-Dunn M. Phosphorylation-independent desensitization of the luteinizing hormone/chorionic gonadotropin receptor in porcine follicular membranes. Mol. Endocrinol. 1994;8:1537–1546. doi: 10.1210/mend.8.11.7877622. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277–3279. doi: 10.1210/endo-126-6-3277. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Tilly JL, Aihara T, Nishimori K, Hsueh AJ. Gonadotropin-induced up- and down-regulation of ovarian follicle-stimulating hormone (FSH) receptor gene expression in immature rats: effects of pregnant mare’s serum gonadotropin, human chorionic gonadotropin, and recombinant FSH. Endocrinology. 1992;130:1289–1295. doi: 10.1210/endo.130.3.1537292. [DOI] [PubMed] [Google Scholar]
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, Morgan FJ, Isaacs NW. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Segaloff DL. Naturally occurring mutations of the luteinizing-hormone receptor: lessons learned about reproductive physiology and G protein-coupled receptors. Am. J. Hum. Genet. 1999;65:949–958. doi: 10.1086/302602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue L, Chan WY, Hsueh AJ, Kudo M, Hsu SY, Wu SM, Blomberg L, Cutler GB., Jr. Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc. Natl. Acad. Sci. U S A. 1995;92:1906–1910. doi: 10.1073/pnas.92.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue L, Wu SM, Kudo M, Hsueh AJ, Cutler GB, Jr., Griffin JE, Wilson JD, Brain C, Berry AC, Grant DB, et al. A nonsense mutation of the human luteinizing hormone receptor gene in Leydig cell hypoplasia. Hum. Mol. Genet. 1995;4:1429–1433. doi: 10.1093/hmg/4.8.1429. [DOI] [PubMed] [Google Scholar]
- Lazari MF, Bertrand JE, Nakamura K, Liu X, Krupnick JG, Benovic JL, Ascoli M. Mutation of individual serine residues in the C-terminal tail of the lutropin/choriogonadotropin receptor reveal distinct structural requirements for agonist-induced uncoupling and agonist-induced internalization. J. Biol. Chem. 1998;273:18316–18324. doi: 10.1074/jbc.273.29.18316. [DOI] [PubMed] [Google Scholar]
- Leibold EA, Munro HN. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc. Natl. Acad. Sci. U S A. 1988;85:2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parsels LA, Voeller DM, Allegra CJ, Maley GF, Maley F, Chu E. Characterization of a cis-acting regulatory element in the protein coding region of thymidylate synthase mRNA. Nucleic Acids Res. 2000;28:1381–1389. doi: 10.1093/nar/28.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DL, Peegel H, Mosier SM, Menon KMJ. Loss of lutropin/human choriogonadotropin receptor messenger ribonucleic acid during ligand-induced down-regulation occurs post transcriptionally. Endocrinology. 1993;132:235–240. doi: 10.1210/endo.132.1.8419125. [DOI] [PubMed] [Google Scholar]
- Madhra M, Gay E, Fraser HM, Duncan WC. Alternative splicing of the human luteal LH receptor during luteolysis and maternal recognition of pregnancy. Mol. Hum. Reprod. 2004;10:599–603. doi: 10.1093/molehr/gah076. [DOI] [PubMed] [Google Scholar]
- Marsh JM. The role of cyclic AMP in gonadal function. Adv. Cyclic Nucleotide Res. 1975;6:137–199. [PubMed] [Google Scholar]
- McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, Segaloff DL, Seeburg PH. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989;245:494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Menon B, Franzo-Romain M, Damanpour S, Menon KMJ. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol. Endocrinol. 2011;25:282–290. doi: 10.1210/me.2010-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon B, Peegel H, Menon KMJ. Evidence for the association of luteinizing hormone receptor mRNA-binding protein with the translating ribosomes during receptor downregulation. Biochim. Biophys. Acta. 2009;1793:1787–1794. doi: 10.1016/j.bbamcr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon KMJ, Clouser CL, Nair AK. Gonadotropin receptors: role of post-translational modifications and post-transcriptional regulation. Endocrine. 2005;26:249–257. doi: 10.1385/ENDO:26:3:249. [DOI] [PubMed] [Google Scholar]
- Menon KMJ, Gunaga KP. Role of cyclic AMP in reproductive processes. Fertil. Steril. 1974;25:732–750. doi: 10.1016/s0015-0282(16)40577-7. [DOI] [PubMed] [Google Scholar]
- Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod. 2004;70:861–866. doi: 10.1095/biolreprod.103.024471. [DOI] [PubMed] [Google Scholar]
- Min L, Ascoli M. Effect of activating and inactivating mutations on the phosphorylation and trafficking of the human lutropin/choriogonadotropin receptor. Mol. Endocrinol. 2000;14:1797–1810. doi: 10.1210/mend.14.11.0555. [DOI] [PubMed] [Google Scholar]
- Minegishi T, Nakamura K, Takakura Y, Miyamoto K, Hasegawa Y, Ibuki Y, Igarashi M, Minegish T. Cloning and sequencing of human LH/hCG receptor cDNA. Biochem. Biophys. Res. Commun. 1990;172:1049–1054. doi: 10.1016/0006-291x(90)91552-4. [DOI] [PubMed] [Google Scholar]
- Minegishi T, Nakamura K, Yamashita S, Omori Y. The effect of splice variant of the human luteinizing hormone (LH) receptor on the expression of gonadotropin receptor. Mol. Cell Endocrinol. 2007;260-262:117–125. doi: 10.1016/j.mce.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Moench SJ, Moreland J, Stewart DH, Dewey TG. Fluorescence studies of the location and membrane accessibility of the palmitoylation sites of rhodopsin. Biochemistry. 1994;33:5791–5796. doi: 10.1021/bi00185a017. [DOI] [PubMed] [Google Scholar]
- Montanelli L, Delbaere A, Di Carlo C, Nappi C, Smits G, Vassart G, Costagliola S. A mutation in the follicle-stimulating hormone receptor as a cause of familial spontaneous ovarian hyperstimulation syndrome. J. Clin. Endocrinol. Metab. 2004;89:1255–1258. [PubMed] [Google Scholar]
- Mueller S, Jaeschke H, Gunther R, Paschke R. The hinge region: an important receptor component for GPHR function. Trends Endocrinol. Metab. 2010;21:111–122. doi: 10.1016/j.tem.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Munshi UM, Clouser CL, Peegel H, Menon KMJ. Evidence that palmitoylation of carboxyl terminus cysteine residues of the human luteinizing hormone receptor regulates postendocytic processing. Mol. Endocrinol. 2005;19:749–758. doi: 10.1210/me.2004-0335. [DOI] [PubMed] [Google Scholar]
- Munshi UM, Peegel H, Menon KMJ. Palmitoylation of the luteinizing hormone/human chorionic gonadotropin receptor regulates receptor interaction with the arrestin-mediated internalization pathway. Eur. J. Biochem. 2001;268:1631–1639. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- Nagy E, Rigby WF. Glyceraldehyde-3-phosphate dehydrogenase selectively binds AU-rich RNA in the NAD(+)-binding region (Rossmann fold) J. Biol. Chem. 1995;270:2755–2763. doi: 10.1074/jbc.270.6.2755. [DOI] [PubMed] [Google Scholar]
- Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J. Biol. Chem. 2002;277:21468–21473. doi: 10.1074/jbc.M111653200. [DOI] [PubMed] [Google Scholar]
- Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J. Biol. Chem. 2004;279:14937–14944. doi: 10.1074/jbc.M309484200. [DOI] [PubMed] [Google Scholar]
- Nair AK, Menon KMJ. Regulation of luteinizing hormone receptor expression: evidence of translational suppression in vitro by a hormonally regulated mRNA-binding protein and its endogenous association with luteinizing hormone receptor mRNA in the ovary. J. Biol. Chem. 2005;280:42809–42816. doi: 10.1074/jbc.M503154200. [DOI] [PubMed] [Google Scholar]
- Nair AK, Peegel H, Menon KMJ. The role of luteinizing hormone/human chorionic gonadotropin receptor-specific mRNA binding protein in regulating receptor expression in human ovarian granulosa cells. J. Clin. Endocrinol. Metab. 2006;91:2239–2243. doi: 10.1210/jc.2005-2739. [DOI] [PubMed] [Google Scholar]
- Nakabayashi K, Kudo M, Kobilka B, Hsueh AJ. Activation of the luteinizing hormone receptor following substitution of Ser-277 with selective hydrophobic residues in the ectodomain hinge region. J. Biol. Chem. 2000;275:30264–30271. doi: 10.1074/jbc.M005568200. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hipkin RW, Ascoli M. The agonist-induced phosphorylation of the rat follitropin receptor maps to the first and third intracellular loops. Mol. Endocrinol. 1998;12:580–591. doi: 10.1210/mend.12.4.0087. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamashita S, Omori Y, Minegishi T. A splice variant of the human luteinizing hormone (LH) receptor modulates the expression of wild-type human LH receptor. Mol. Endocrinol. 2004;18:1461–1470. doi: 10.1210/me.2003-0489. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Maekawa R, Yamagata Y, Tamura I, Sugino N. A novel mutation in exon8 of the follicle-stimulating hormone receptor in a woman with primary amenorrhea. Gynecol. Endocrinol. 2008;24:708–712. doi: 10.1080/09513590802454927. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Dunkel L, Hsueh AJ, Yamoto M, Nakano R. Expression of luteinizing hormone and chorionic gonadotropin receptor messenger ribonucleic acid in human corpora lutea during menstrual cycle and pregnancy. J. Clin. Endocrinol. Metab. 1995;80:1444–1448. doi: 10.1210/jcem.80.4.7714122. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Dudley K, Rajapaksha WR. Expression of follicle stimulating hormone-receptor mRNA during gonadal development. Mol. Cell. Endocrinol. 1996;125:169–175. doi: 10.1016/s0303-7207(96)03957-3. [DOI] [PubMed] [Google Scholar]
- Pachter JS, Yen TJ, Cleveland DW. Autoregulation of tubulin expression is achieved through specific degradation of polysomal tubulin mRNAs. Cell. 1987;51:283–292. doi: 10.1016/0092-8674(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peegel H, Randolph J, Jr., Midgley AR, Menon KMJ. In situ hybridization of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994;135:1044–1051. doi: 10.1210/endo.135.3.8070346. [DOI] [PubMed] [Google Scholar]
- Peegel H, Towns R, Nair A, Menon KMJ. A novel mechanism for the modulation of luteinizing hormone receptor mRNA expression in the rat ovary. Mol. Cell Endocrinol. 2005;233:65–72. doi: 10.1016/j.mce.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Peltoketo H, Strauss L, Karjalainen R, Zhang M, Stamp GW, Segaloff DL, Poutanen M, Huhtaniemi IT. Female mice expressing constitutively active mutants of FSH receptor present with a phenotype of premature follicle depletion and estrogen excess. Endocrinology. 2010;151:1872–1883. doi: 10.1210/en.2009-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma D, Verhoef-Post M, Berns EM, Themmen AP. LH receptor gene mutations and polymorphisms: an overview. Mol. Cell Endocrinol. 2007;260-262:282–286. doi: 10.1016/j.mce.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Pioli PA, Hamilton BJ, Connolly JE, Brewer G, Rigby WF. Lactate dehydrogenase is an AU-rich element-binding protein that directly interacts with AUF1. J. Biol. Chem. 2002;277:35738–35745. doi: 10.1074/jbc.M204002200. [DOI] [PubMed] [Google Scholar]
- Puett D, Li Y, DeMars G, Angelova K, Fanelli F. A functional transmembrane complex: the luteinizing hormone receptor with bound ligand and G protein. Mol. Cell Endocrinol. 2007;260-262:126–136. doi: 10.1016/j.mce.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Han B, Liu BL, Chen X, Ru Y, Cheng KX, Chen FG, Zhao SX, Liang J, Lu YL, Tang JF, Wu YX, Wu WL, Chen JL, Chen MD, Song HD. A splice site mutation combined with a novel missense mutation of LHCGR cause male pseudohermaphroditism. Hum. Mutat. 2009;30:E855–865. doi: 10.1002/humu.21072. [DOI] [PubMed] [Google Scholar]
- Rannikki AS, Zhang FP, Huhtaniemi IT. Ontogeny of follicle-stimulating hormone receptor gene expression in the rat testis and ovary. Mol. Cell Endocrinol. 1995;107:199–208. doi: 10.1016/0303-7207(94)03444-x. [DOI] [PubMed] [Google Scholar]
- Rao CV, Lei ZM. The past, present and future of nongonadal LH/hCG actions in reproductive biology and medicine. Mol. Cell Endocrinol. 2007;269:2–8. doi: 10.1016/j.mce.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Richards JS, Hedin L. Molecular aspects of hormone action in ovarian follicular development, ovulation, and luteinization. Annu. Rev. Physiol. 1988;50:441–463. doi: 10.1146/annurev.ph.50.030188.002301. [DOI] [PubMed] [Google Scholar]
- Rodien P, Beau I, Vasseur C. Ovarian hyperstimulation syndrome (OHSS) due to mutations in the follicle-stimulating hormone receptor. Ann. Endocrinol. (Paris) 2010;71:206–209. doi: 10.1016/j.ando.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Ross J. mRNA stability in mammalian cells. Microbiol. Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Stout CD, Kaptain S, Harford JB, Klausner RD. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991;64:881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Saint-Dizier M, Chopineau M, Dupont J, Daels PF, Combarnous Y. Expression and binding activity of luteinizing hormone/chorionic gonadotropin receptors in the primary corpus luteum during early pregnancy in the mare. Biol. Reprod. 2003;69:1743–1749. doi: 10.1095/biolreprod.103.018812. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Babu PS. The tale of follitropin receptor diversity: a recipe for fine tuning gonadal responses? Mol. Cell Endocrinol. 2007;260-262:163–171. doi: 10.1016/j.mce.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143:2986–2994. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]
- Saner-Amigh K, Mayhew BA, Mantero F, Schiavi F, White PC, Rao CV, Rainey WE. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 2006;91:1136–1142. doi: 10.1210/jc.2005-1298. [DOI] [PubMed] [Google Scholar]
- Segaloff DL. Diseases associated with mutations of the human lutropin receptor. Prog. Mol. Biol. Transl. Sci. 2009;89:97–114. doi: 10.1016/S1877-1173(09)89004-2. [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Ascoli M. The gonadotrophin receptors: insights from the cloning of their cDNAs. Oxf. Rev. Reprod. Biol. 1992;14:141–168. [PubMed] [Google Scholar]
- Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol. Endocrinol. 1990;4:1856–1865. doi: 10.1210/mend-4-12-1856. [DOI] [PubMed] [Google Scholar]
- Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, 3rd, Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- Sen KK, Azhar S, Menon KMJ. Receptor-mediated gonadotropin action in the ovary. Desensitization of gonadotropin binding sites, activation of adenosine 3′:5′-cyclic monophosphate-dependent protein kinase(s), and regulation of steroidogenesis in rat ovary. J. Biol. Chem. 1979;254:5664–5671. [PubMed] [Google Scholar]
- Shenker A. Activating mutations of the lutropin choriogonadotropin receptor in precocious puberty. Receptors Channels. 2002;8:3–18. [PubMed] [Google Scholar]
- Shenker A, Laue L, Kosugi S, Merendino JJ, Jr., Minegishi T, Cutler GB., Jr. A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, Costagliola S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N. Engl. J. Med. 2003;349:760–766. doi: 10.1056/NEJMoa030064. [DOI] [PubMed] [Google Scholar]
- Tai N, Schmitz JC, Chen TM, Chu E. Characterization of a cis-acting regulatory element in the protein-coding region of human dihydrofolate reductase mRNA. Biochem. J. 2004;378:999–1006. doi: 10.1042/BJ20031396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tano M, Minegishi T, Nakamura K, Karino S, Ibuki Y, Miyamoto K. Transcriptional and post-transcriptional regulation of FSH receptor in rat granulosa cells by cyclic AMP and activin. J. Endocrinol. 1997;153:465–473. doi: 10.1677/joe.0.1530465. [DOI] [PubMed] [Google Scholar]
- Tao YX, Segaloff DL. Follicle stimulating hormone receptor mutations and reproductive disorders. Prog. Mol. Biol. Transl. Sci. 2009;89:115–131. doi: 10.1016/S1877-1173(09)89005-4. [DOI] [PubMed] [Google Scholar]
- Themmen AP. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130:263–274. doi: 10.1530/rep.1.00663. [DOI] [PubMed] [Google Scholar]
- Themmen AP, Blok LJ, Post M, Baarends WM, Hoogerbrugge JW, Parmentier M, Vassart G, Grootegoed JA. Follitropin receptor down-regulation involves a cAMP-dependent post-transcriptional decrease of receptor mRNA expression. Mol. Cell Endocrinol. 1991;78:R7–13. doi: 10.1016/0303-7207(91)90130-k. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- Tholanikunnel BG, Malbon CC. A 20-nucleotide (A + U)-rich element of beta2-adrenergic receptor (beta2AR) mRNA mediates binding to beta2AR-binding protein and is obligate for agonist-induced destabilization of receptor mRNA. J. Biol. Chem. 1997;272:11471–11478. doi: 10.1074/jbc.272.17.11471. [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem. Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Vu-Hai MT, Huet JC, Echasserieau K, Bidart JM, Floiras C, Pernollet JC, Milgrom E. Posttranslational modifications of the lutropin receptor: mass spectrometric analysis. Biochemistry. 2000;39:5509–5517. doi: 10.1021/bi992913f. [DOI] [PubMed] [Google Scholar]
- Wang L, Gulappa T, Menon KMJ. Identification and characterization of proteins that selectively interact with the LHR mRNA binding protein (LRBP) in rat ovaries. Biochim. Biophys. Acta. 2010;1803:591–597. doi: 10.1016/j.bbamcr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Nair AK, Menon KMJ. Ribonucleic acid binding protein-mediated regulation of luteinizing hormone receptor expression in granulosa cells: relationship to sterol metabolism. Mol. Endocrinol. 2007;21:2233–2241. doi: 10.1210/me.2007-0102. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hipkin RW, Ascoli M. Progressive cytoplasmic tail truncations of the lutropin-choriogonadotropin receptor prevent agonist- or phorbol ester-induced phosphorylation, impair agonist- or phorbol ester-induced desensitization, and enhance agonist-induced receptor down-regulation. Mol. Endocrinol. 1996;10:748–759. doi: 10.1210/mend.10.6.8776735. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu X, Ascoli M. Phosphorylation of the lutropin/choriogonadotropin receptor facilitates uncoupling of the receptor from adenylyl cyclase and endocytosis of the bound hormone. Mol. Endocrinol. 1997;11:183–192. doi: 10.1210/mend.11.2.9889. [DOI] [PubMed] [Google Scholar]
- Xing W, Sairam MR. Characterization of regulatory elements of ovine follicle-stimulating hormone (FSH) receptor gene: the role of E-box in the regulation of ovine FSHreceptor expression. Biol. Reprod. 2001;64:579–589. doi: 10.1095/biolreprod64.2.579. [DOI] [PubMed] [Google Scholar]
- Yano K, Hidaka A, Saji M, Polymeropoulos MH, Okuno A, Kohn LD, Cutler GB., Jr. A sporadic case of male-limited precocious puberty has the same constitutively activating point mutation in luteinizing hormone/choriogonadotropin receptor gene as familial cases. J. Clin. Endocrinol. Metab. 1994;79:1818–1823. doi: 10.1210/jcem.79.6.7527413. [DOI] [PubMed] [Google Scholar]
- Yano K, Saji M, Hidaka A, Moriya N, Okuno A, Kohn LD, Cutler GB., Jr. A new constitutively activating point mutation in the luteinizing hormone/choriogonadotropin receptor gene in cases of male-limited precocious puberty. J. Clin. Endocrinol. Metab. 1995;80:1162–1168. doi: 10.1210/jcem.80.4.7714085. [DOI] [PubMed] [Google Scholar]
- Yariz KO, Walsh T, Uzak A, Spiliopoulos M, Duman D, Onalan G, King MC, Tekin M. Inherited mutation of the luteinizing hormone/choriogonadotropin receptor (LHCGR) in empty follicle syndrome. Fertil. Steril. 2011;96:e125–130. doi: 10.1016/j.fertnstert.2011.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron Y, Schwartz D, Evans MI, Lessing JB, Rotter V. Alternatively spliced mRNA transcripts encoding the extracellular domain of the FSH receptor gene. Expression in the mouse ovary during the ovulatory cycle. J. Reprod. Med. 1998;43:435–438. [PubMed] [Google Scholar]
- Zeleznik AJ, Midgley AR, Jr., Reichert LE., Jr. Granulosa cell maturation in the rat: increased binding of human chorionic gonadotropin following treatment with follicle-stimulating hormone in vivo. Endocrinology. 1974;95:818–825. doi: 10.1210/endo-95-3-818. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Schuler HM, Reichert LE., Jr. Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology. 1981;109:356–362. doi: 10.1210/endo-109-2-356. [DOI] [PubMed] [Google Scholar]
- Zhang R, Cai H, Fatima N, Buczko E, Dufau ML. Functional glycosylation sites of the rat luteinizing hormone receptor required for ligand binding. J. Biol. Chem. 1995;270:21722–21728. doi: 10.1074/jbc.270.37.21722. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang H, Ascoli M. The lutropin/choriogonadotropin receptor is palmitoylated at intracellular cysteine residues. Mol. Endocrinol. 1995;9:141–150. doi: 10.1210/mend.9.2.7776964. [DOI] [PubMed] [Google Scholar]