Abstract
Antimicrobial peptides are generated in insects exposed to pathogens for combating infection. Gloverin is a small cationic antibacterial protein whose expression is induced in the hemocytes and fat body cells of Trichoplusia ni larvae exposed to bacteria. The purpose of this study was to determine the role of gloverin during baculovirus infection. We found that gloverin expression is induced in T. ni systemically infected with the baculovirus Autographa californica M nucleopolyhedrovirus (AcMNPV). Two gloverin genes were cloned using RNA isolated from the hemocytes of T. ni larvae that were systemically infected AcMNPV budded virus (BV) and C-terminal 6x-His and V5 epitope tags were incorporated to facilitate gloverin isolation, detection and functional studies. The supernatants of Sf9 cells stably transfected with the two gloverin expression plasmids and affinity purified gloverin proteins reduced the quantity of infectious AcMNPV BV as measured in vitro by plaque assay with untransfected Sf9 cells. Nanomolar concentrations of affinity column purified gloverin protein caused calcein to be rapidly released from unilamellar vesicles comprised of phosphatidylglycerol, but not from vesicles made up of phosphatidylcholine, suggesting that gloverin interaction with membranes is rapid and affected by membrane charge. Both the BV inactivation and calcein release activities of gloverin increased with higher concentrations of gloverin. These results demonstrate that gloverin is an antiviral protein that interacts with vesicle membranes to cause the contents to be released.
Keywords: antiviral, gloverin, baculovirus, AcMNPV, Autographa californica M nucleopolyhedrovirus
1. Introduction
Antimicrobial proteins are typically comprised of less than 200 amino acids and are key factors of insect innate immunity that function to combat infectious bacteria and fungi (Jiang and Vilcinskas, 2011). The majority of these small proteins are amphipathic and have a high number of positively charged amino acids that interact with the negatively charged phospholipids of microbial membranes (Peters et al. 2010). Once bound, the peptides increase membrane permeability and permit the efflux of ions to cause cell death (Ganz, 2003). Antimicrobial proteins produced by insects have been isolated from the hemolymph (blood plasma) of lepidopterans (Lavineet al. 2005) and include cecropin (Ourth et al. 1994), moricin (Hemmiet al. 2002), lebocin (Liuet al. 2000), melittin, tenecin 4 (Chae et al. 2011), attacin (Carlssonet al. 1991; Hultmark et al. 1983) and gloverin (Axen et al. 1997; Lundström et al. 2002; Mackintoshet al. 1998). Of these, cecropin and melittinexert an antiviral activity in cultured human cells infected with human immunodeficiency virus 1 (HIV-1) by suppressing viral gene expression (Wachinger et al. 1998). Gloverin expression is upregulated in Plutellaxylostella parasitized by an ichneumonid wasp harboring a polydnavirus (Etebari et al., 2011). However, there is little known of the roles antimicrobial proteins play during viral infection in insects.
The expression of antimicrobial peptides is upregulated in lepidopteran larvae exposed to a wide range of pathogens (Brown et al. 2009; Freitaket al. 2007; Huang et al., 2009; Silva et al. 2010; Wang et al. 2010a). Gloverin is produced by the fat body and hemocytes of Trichoplusia ni larvae as a pro-protein that is proteolytically processed in the hemolymph, removing a short signal peptide from the N-terminus to form the mature protein (Lundström et al. 2002). Micromolar concentrations of the pro- and mature gloverin proteins potently inhibit growth of the bacteria Escherichia coli (Axen et al. 1997; Lundström et al., 2002). Of note, a gloverin-like protein in Helicoverpa armigeria is upregulated 3 h after the larvae are systemically infected with the budded virus (BV) of the baculovirus Autographa californica M nucleopolyhedrovirus (AcMNPV) (Wang et al. 2010a). There are two gloverin genes reported for T. ni which are highly expressed in larvae exposed to bacteria and encodeproteins having predicted molecular weights of 18.9 kDa (Lundström et al. 2002) and 17.5 kDa (Freitak et al. 2007). To study the role of gloverin during baculovirus infection, both gloverin genes were cloned from RNA isolated from the hemocytes of AcMNPV-infected T. ni and the proteins were expressed in cultured Sf9 cells. The gloverin proteins permeabilized vesicles and reduced the infectivity of AcMNPV BV, suggesting that gloverin is an antiviral protein that may act by disrupting the viral envelope.
2. Materials and Methods
2.1 Overview of Study Design
The experiments described herein were designed to study the potential antiviral activities of gloverin protein. Briefly, two gloverin genes were cloned from cDNA generated from RNA isolated from Trichoplusia ni larvae systemically infected with AcMNPV. To produce and isolate gloverin protein from Sf9 cells, the resulting gloverin genes were epitope tagged and inserted into protein expression vectors. The antiviral activity of the purified gloverin protein was assessed by exposing AcMNPV budded virus to gloverin protein and subsequently quantifying the infectivity of the virus using a plaque assay with untransfected Sf9 cells. To determine whether gloverin interacted with membranes and affected membrane permeability, calcein-encapsulated vesicles were incubated with purified gloverin protein and the amount of fluorescent calcein released from the vesicles was quantified.
2.2 Primer Design
PCR primers were designed to amplify two T. ni gloverin genes (GenBank accession numbers and AF233590.1 (TnGlv1) and EU016389.1 (TnGlv2); Table 1). Because of concerns with low levels of TnGlv1 expression in Sf9 cells, a Kozak translation initiation sequence was produced immediately after the start codon for the forward TnGlv1 primer to increase protein expression in insect Sf9 cells, as described in the pIB/V5-His vector manual (Invitrogen). This resulted in a nonsynonymous amino acid substitution of glutamine for valine in the second amino acid of TnGlv1. Reverse PCR primers were designed to remove the stop codon while maintaining the sequence in frame for fusion of a C-terminal V5 epitope and 6x-His tag.
Table 1.
Primers used for cloning.
| Gene | Primer name | Bases | Sequence |
|---|---|---|---|
| TnGlv1 | Glv1Q2V-L | 25 | CACCATGGTGTCGTCTATTTTATTA |
| TnGlv1 | Glv1-R | 24 | AAAATCATGTTCAATTTTCCCTTG |
| TnGlv1 | Glv1-L | 24 | CACCATGCAGTCGTCTATTTTATT |
| TnGlv2 | Glv2-L | 22 | CACCATGCAGTTATCAACCATC |
| TnGlv2 | Glv2-R | 24 | TTTAAAATCATGTTCAATTTTCGC |
| GAPDH | GAPDH-L | 22 | GACGGACCCTCTGGAAAACTG |
| GAPDH | GAPDH-R | 23 | ACCAGCTGATGAGCTTGACGAA |
2.3 RNA Isolation, RT-PCR and PCR
Total RNA was isolated using Trizol (Invitrogen) from the hemocytes of T. ni larvae intrahemocoelically inoculated as fourth instars with AcMNPV-hsp70lacZ BV or media vehicle (complete TNM-FH cell culture medium containing 10 % FBS) and subsequently treated with DNase (Sigma Aldrich), according the manufacturer product inserts. To produce cDNA from the isolated RNA, a reverse transcription polymerase chain reaction (RT-PCR) was performed using the Titan One-tube kit (Roche) and GAPDH- or TnGlv2-specific primers (30 cycles; primer sequences in Table 1), as described in the product insert. To determine whether viral or cellular DNA was present in the RNA isolated from T. ni larvae, RT-PCR was performed as described above using GoTaq Flexi DNA polymerase (Promega) or Titan One-tube kit enzyme that was heat inactivated (94 °C for 2 min). PCR products were analyzed using standard gel electrophoresis methods, visualized with a GelDoc XR gel documentation system (Bio-Rad) and analyzed using Quantity One software (Bio-Rad). The predicted molecular weight of the PCR products was 345 bp for GAPDH and 665 bp for TnGlv2. The band intensities for the GAPDH and TnGlv2 RT-PCR products were quantified using Quantity One software (Bio-Rad) and fold difference in TnGlv2 expression for vehicle and BV-injected samples calculated relative to the GAPDH expression in each sample. High Fidelity Platinum Taq polymerase (Invitrogen) was used to PCR amplify reverse transcribed cDNA for cloning TnGlv1 and TnGlv2. Prior to cloning, PCR Clean-Up spin columns (Promega) were used to remove primers and unincorporated nucleotides.
2.4 Cloning TnGlv1 and TnGlv2
TnGlv1 and TnGlv2 cDNA was ligated into the pENTR3D-TOPO TA vector as described by the manufacturer (Invitrogen). One Shot TOP10 chemically competent E. coli (Invitrogen) were transformed with the resulting plasmids to generate the entry clones pENTR-TnGlv1 and pENTR-TnGlv2. Chemically transformed cells were plated onto solid Luria broth (LB) media containing kanamycin (50 μg/ml), incubated overnight at 37 °C, colonies selected and cultured overnight in liquid LB containing kanamycin (50 μg/ml). Plasmid DNA was isolated from liquid cultures using QIAprep Spin Mini Prep Kit (Qiagen) and screened for the TnGlv1 or TnGlv2 inserts using PCR with the M13 forward primer and TnGlv1 or TnGlv2 specific reverse primers. Positive clones with inserts of the correct size for TnGlv1 or TnGlv2 were sequenced. Entry clones with the highest similarity (> 97 %) to the published GenBank sequences were selected for recombination with pIB/V5-His-DEST vector (Invitrogen). One Shot TOP10 Chemically Competent E. coli (Invitrogen) cells were transformed as described by the manufacturer to produce the expression vectors pIB/V5-His-DEST-TnGlv1 and pIB/V5-His-DEST-TnGlv2. Colonies were selected using LB media containing ampicillin (100 μg/ml), screened using PCR with OpIE2 forward primers and reverse primers specific for TnGlv1 or TnGlv2. The resulting positive clones, pIB/V5-His-DEST-TnGlv1 cl12.c and pIB/V5-His-DEST-TnGlv2 cl10.a, were sequenced to ensure the gloverin expression plasmids were correctly constructed and did not contain any unintended mutations.
2.5 Transfection and gloverin expression
Sf9 cells cultured in complete TNM-FH media containing 10 % FBS (Lonza) were transfected with pIB/V5-His-DEST-TnGlv1 cl12.c orpIB/V5-His-DEST-TnGlv2 cl10.a using Cellfectin II Reagent (Invitrogen) and stably transfected cells selected and maintained using Blasticidin S (InvivoGen), as described by the manufacturer. TnGlv1 and TnGlv2 protein expression was analyzed using standard SDS-PAGE and western blot methods. Molecular weight markers were ProSieveQuadColor Protein Marker (Lonza) and/or Precision Plus Protein WesternC Standards (Bio-Rad). Mini Protean TGX precast 4–20 % gels (Bio-Rad) were run at 180 V for 30 min, the proteins transferred to Immobilin Transfer Membranes (Millipore) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad) run at 20 V for 35 min. The resulting blots were stained with Ponceau S to visualize the quantity of protein transferred. The membranes were subsequently destained and probed using the Anti-V5 Epitope Tag Mouse Monoclonal Antibody (Pierce) and Goat Anti-Mouse IgG (H+L) HRP Human Adsorbed Antibody (Southern Biotech). HRP-reactive bands were developed using the chemiluminescence substrate SuperSignal West Pico (Pierce) and the blot imaged using a Nikon D700 digital single-lens reflex camera, as previously described (Khoury et al. 2010). The colors of the resulting images were inverted to generate black-colored bands with a white background, straightened and cropped using Adobe Lightroom 3 software. No other modifications were made to the blot images. The relative quantity of His-tagged protein present in blots was assessed by measuring the intensity and area of chemiluminescent bands using Quantity One software (Bio-Rad), as described by the manufacturer.
2.6 Gloverin protein purification
Sf9 cells stably transfected with pIB/V5-His-DEST-TnGlv1 cl12.c and pIB/V5-His-DEST-TnGlv2 cl10.a were cultured for 96 h in a shaking incubator (125 rpm, 28 °C, 300 ml, 1–2 × 106 cells/ml) and subsequently frozen at −80 °C. Immediately after thawing, the serine protease inhibitor PMSF was added (1 mM), the culture media centrifuged (3000 × g, 30 min), the supernatant (SN) decanted, the cell pellet suspended in 5 ml of PBS and solubilized for 10 min with cell lysis buffer (20 mM Tris pH 8.0, 137 mM NaCl, 10 % glycerol, 1 % NP-40), the lysed cells centrifuged (10,000 × g, 15 min) and the resulting SN combined with the culture media SN. The combined SN was concentrated 20-fold at 4 °C using a Minimate TFF Omega 3 kDa MWCO Membrane Filter (Pall Corporation) and Varistaltic Pump Plus (Vera). His-tagged gloverin protein was isolated from the concentrated SN (cSN) using His-Pure Ni-NTA 3mL Spin Columns (Thermo Scientific). Proteins not tightly bound to the Ni-NTA resin were removed with three sequential washes of two resin-bed volumes of 25 mM imidazole. His-tagged proteins that remained bound to the column were eluted twice with one resin-bed volume of 250 mM imidazole and a third time with 300 mM imidazole. The resulting elutions were combined, dialyzed overnight at 4 °C in PBS using 2 kDa MWCO Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific), concentrated 10-fold further using 3 kDa MWCO Ambicon centrifugal filter devices (Millipore) and filtered using a 0.45 μm syringe filter. Protein concentration was quantified using the BCA assay (Thermo Scientific), as described by the manufacturer. Western blot was used as described above to assess the quantity of His-tagged protein present in the material that flowed through the Ni-NTA columns after applying the concentrated supernatant, washes and elutions.
2.7 Virus inactivation assay
The antiviral activity of TnGlv1 and TnGlv2 protein was quantified using standard plaque assay with untransfected Sf9 cells. AcMNPV-hsp70/lacZ BV was serially diluted in TNM-FH media containing 10 % FBS (vehicle), the cell supernatants of Sf9 cells transfected with pIB/V5-His-DEST-TnGlv1 cl12.c or pIB/V5-His-DEST-TnGlv2 cl10.a, or affinity column purified TnGlv1 or TnGlv2 protein. The diluted BV was incubated for 3 h at 28 °C and plaque assayed using untrasfected Sf9 cells. Briefly, 10 μl of the incubated BV was added to the wells of a 96-well plate containing a confluent layer of Sf9 cells and 40 μl of TNM-FH media (4–6 wells per BV dilution). After incubation for 1 h at 28 °C, the inocula was removed, methyl cellulose (1.5 g/ml) dissolved in TNM-FH media added to each well and the plate incubated in a humidified chamber at 28 °C. After 3 days, the cells were fixed with formaldehyde, rinsed three times with PBS, processed using X-gal to develop the blue lacZ-positive plaques, as previously described (Chikhalya et al., 2009) and the plaques counted using a Leica MZ12.5 stereomicroscope. This experiment was repeated at least three times on separate days and the resulting data combined to calculate the mean and SEM for each treatment. For one replicate experiment, the wells of the X-gal developed plate were imaged using a MicroPublisher 3.3 cooled CCD digital color camera (QImaging) mounted to a Leica MZ12.5 stereomicroscope and QCapture Suite Imaging software (QImaging). To evaluate the concentration dependent activity of gloverin protein, decreasing concentrations of Ni-NTA purified TnGlv1 or TnGlv2 protein or vehicle (complete TNM-FH media) was incubated for 3 h in the presence of AcMNPV-hsp70/lacZ BV (105 PFU) and the resulting PFU quantified using plaque assay as described above. Statistical analysis was conducted using Prism 5.0d (GraphPad).
2.8 Calcein release from vesicle assay
The calcein release from vesicle assay employs a self-quenching dye (i.e. calcein) to quantify vesicle leakage. It is a long-standing method for measuring pore-formation and the permeabilizing activity of antimicrobial peptides (Matsuzaki et al., 1995; Prenneret al., 2001; Patel et al., 2009). Five mg of egg phosphatidylglycerol (PG) or egg phosphatidylcholine (PC) (Avanti Polar Lipids Inc.) was dissolved in 0.5 mL 3:1 chloroform/methanol (v/v) in phosphate-free glass tubes washed with Contrad 70 (Decon Labs) and dried with a stream of N2 gas. The lipid film was suspended in Buffer A (20 mM Tris-HCl, 150 mM NaCl, 0.5 mM EDTA, pH 7.4) containing 100 mM calcein (Sigma-Aldrich). After rigorous vortexing for 1 min, the lipid suspension was extruded with a mini extruder (Avanti Polar Lipids Inc) at 45 °C through a membrane with a pore size of 100 nm to form unilamellar vesicles with encapsulated calcein. The vesicles containing trapped calcein were then separated from free calcein using a PD-10 desalting column (GE Healthcare). Phospholipid concentrations were determined using the Ames phosphate assay (Ames, 1966). Changes in the fluorescence emission intensity, resulting from membrane leakage of entrapped calcein, was measured at 0.01 min intervals using a LS 55 Fluorescence Spectrometer (PerkinElmer). The excitation and emission wavelength were set at 490 and 520 nm, respectively (slit width 5 nm). The fluorescence emission intensity was continuously monitored for 12 min. Vesicles (10 μM) were equilibrated for 2 min in a 1 mL reaction cuvet containing Buffer A at 37 °C, after which the protein was added at varying lipid-protein molar ratios (100:1 to 10,000:1), which corresponded to 1 – 100 nM of protein. The percentage of protein-mediated calcein release from vesicles was quantified by measuring the fluorescence intensity change after gloverin addition, and correction by substracting the baseline fluorescence intensity at 2 min. Maximum calcein release was determined by the fluorescence intensity change after addition of detergent (Triton-X-100, 0.1 % final concentration). This resulted in the complete solubilisation of the phospholipid vesicles and the fluorescence intensity is then associated with fully dequenchedcalcein. The following formula was used to calculate the calcein released from the vesicles as a percentage of the maximum: R = [(Ip − I2)/(Imax − I2) *100]. R is calcein released from the vesicles as a percentage of the maximum; Ip is the fluorescent intensity of protein-mediated calcein release, I2 is the calcein fluorescence intensity at 2 min, before protein was added; Imax is the calcein fluorescence intensity at 10 min, after the detergent was added. Each experiment was conducted at least three times to generate the mean value ± standard deviation.
2.9 Illustration Software
Illustrator and Photoshop CS5 software (Adobe) was used to construct the illustration of the hypothesized mechanisms for the antiviral activity of gloverin.
3. Results
3.1 Cloning TnGlv1 and TnGlv2 for protein expression
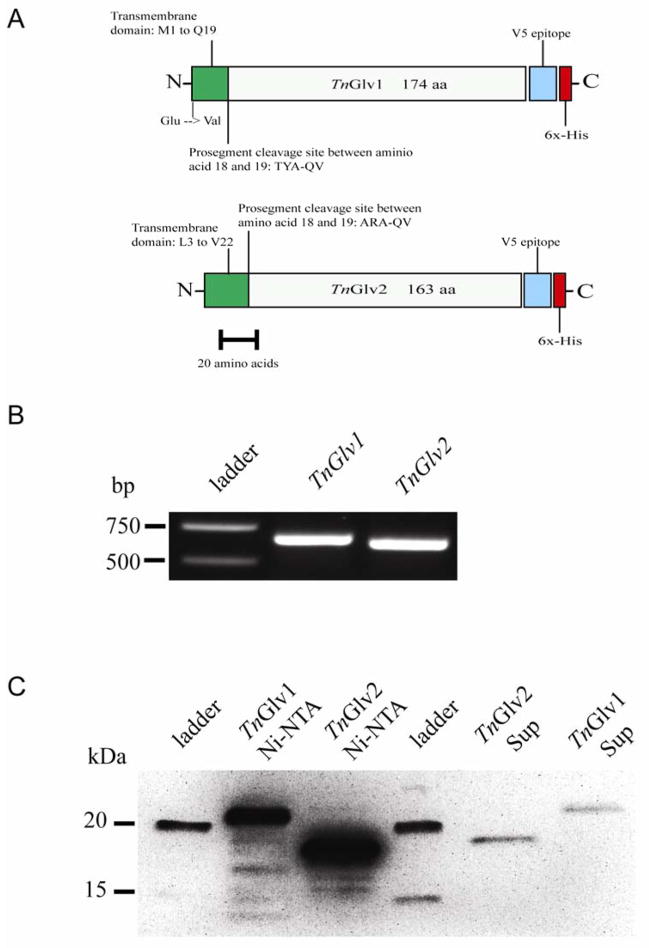
To study the role of gloverin during baculovirus infection, two gloverin genes were cloned in top ENTR3D-TOPO TA vectors using cDNA generated in a RT-PCR reaction from RNA isolated from the hemocytes of T. ni larvae infected with AcMNPV-hsp70/lacZ BV. These two gloverin genes are distinct (Figure 1A), however the gene names submitted to GenBank do not easily distinguish between the two. Therefore, we refer to GenBank accession number AF233590.1 as TnGlv1 and EU016389.1 as TnGlv2. PCR analysis of the resulting vectors (pENTR-TnGlv1 cl12 and pENTR-TnGlv2 cl10) using M13 forward and reverse primers showed single bands near the predicted sizes of 702 bp and 665 bp, respectively (Figure 1B), indicating that TnGlv1 and TnGlv2 were correctly inserted into the pENTR3D-TOPO TA vectors. To generate gloverin-containing vectors for protein expression in insect cells and to fuse V5 and 6x-His tags to the cloned TnGlv1 and TnGlv2, pENTR-TnGlv1 cl12 and pENTR-TnGlv2 cl10 were recombined with pIB/V5-His-DEST vectors to generate pIB/V5-His-DEST-TnGlv1 cl12.c and pIB/V5-His-DEST-TnGlv2 cl10.a.
Figure 1. Cloning and expression of TnGlv1 and TnGlv2.
(A) Diagram of TnGlv1 (top) and TnGlv2 (bottom) with the N-terminal signal peptide, putative transmembrane domains noted, and locations of C-terminal 6x-His and V5 epitope tags. (B) PCR analysis of the cloned TnGlv1 and TnGlv2 inserted into expression vectors. Bands of the predicted molecular weights were observed for TnGlv1 and TnGlv2 inserted in top ENTR-TnGlv1 cl12 and pENTR-TnGlv2 cl10 TA vectors (702 bp and 665 bp, respectively) using PCR with M13 forward and reverse primers. (C) Western blot of TnGlv1 and TnGlv2 protein present in the supernatants of Sf9 cells stably transfected with gloverin expression vectors (TnGlv1 Sup, TnGlv2 Sup) and of Ni-NTA affinity column purified gloverin protein (TnGlv1 Ni-NTA and TnGlv2 Ni-NTA; 60 s exposure).
Computational analysis of the predicted amino acid sequences of TnGlv1 and TnGlv2 using the Membrane Protein Explorer (MPEx) algorithm (http://blanco.biomol.uci.edu/mpex/) (Snider et al. 2009) indicate that both proteins may have a transmembrane domain contained within the putative signal peptide near the N-terminus (Figure 1A). The predicted molecular weights of the TnGlv1 and TnGlv2 protein fused to the V5/6x-His tags are 25.2 kDa and 23.5 kDa, respectively.
PCR analysis and sequencing of pIB/V5-His-DEST-TnGlv1 cl12.c and pIB/V5-His-DEST-TnGlv2 cl10.a demonstrated that: TnGlv1 and TnGlv2 were inserted in the correct orientation in top IB/V5-His-DEST, the 6x-His and V5 tags were correctly fused, the stop codons prior to the 6x-His and V5 tags were removed, greater than 97 % of the nucleotides of TnGlv1 and TnGlv2 were unchanged from the sequences reported in NCBI. Additionally, sequence analysis demonstrated that there were no unintended nonsynonymous base substitutions that changed the predicted amino acid sequence (not shown).
We attempted to express TnGlv1 and TnGlv2 protein in bacteria by recombining pENTR-TnGlv1 cl12 and pENTR-TnGlv2 cl10 with the arabinose-inducible pBAD-DEST49 bacteria expression vector. While bacteria were stably transformed with the resulting gloverin expression vectors and could be cultured in the absence of arabinose, the transformed bacteria did not grow robustly under standard inducing conditions, suggesting that TnGlv1 and TnGlv2 may be toxic to bacteria (not shown).
3.2 Isolating TnGlv1 and TnGlv2 protein
TnGlv1 and TnGlv2 protein was isolated from the supernatant and solubilized cell pellet of Sf9 cells stably transfected with pIB/V5-His-DEST-TnGlv1 cl12.c or pIB/V5-His-DEST-TnGlv2 cl10.a. The highest quantity of gloverin was present in the cell culture supernatant (not shown), suggesting that the V5-His-tagged gloverin proteins were secreted from transfected Sf9 cells. While gloverin-expressing Sf9 cells cultured in serum-free SFX medium grew robustly to high densities, high quantities of gloverin were not detected in the cell pellets or supernatants (not shown). This suggests that complete TNM-FH culture medium containing 10 % FBS was required for high expression and/or stability of TnGlv1 and TnGlv2 in transfected Sf9 cells.
Because the Sf9 cells efficiently produced stable TnGlv1 and TnGlv2 protein only when cultured in complete media, the concentrated supernatant (cSN) contained high quantities of serum proteins, as indicated by the dark red-stained spots on the Ponceau S-stained blot (Supplementary Figure 1A). As a result, we could not detect the V5-tagged TnGlv2 on the blot with the anti-V5 antibody in the cSN (Supplementary Figure 1A 1B). Similar results were observed for TnGlv1 (not shown). When Ni-NTA columns were used to isolate the 6X-His tagged gloverin proteins, the serum proteins were removed by the third wash of the column (W3) as indicated by the lack of dark-red spots in the W3 lane of the Ponceau S-stained blot (Supplementary Figure 1A). A high quantity of TnGlv2 was observed in the first two elutions of the column (E1 and E2; Supplementary Figure 1B). Multiple distinct low molecular weight bands that were reactive with the mouse anti-V5 antibody were detected in the eluted TnGlv2 (Supplementary Figure 1B) and TnGlv1 (not shown), suggesting that some protein was degraded. To estimate the relative quantity of degradation in the eluted protein, densitometry was used to quantify the chemiluminescence and area of each band on the blot containing the eluted gloverin protein (Supplementary Figure 1B). We found that the large band of the molecular weight expected for V5/His-tagged TnGlv2 (23.5 kDa) comprised 32 % of the epitope-tagged protein for elution 1 (E1) and 44 % for elution 2 (E2). Very little epitope-tagged protein was detected in elution 3 (E3). Because a serine protease inhibitor (PMSF) was added at each step of protein isolation, these low molecular weight bands may represent gloverin that was cleaved by proteases while the transfected cells were cultured or during protein isolation by non-serine proteases that were not inhibited by PMSF.
A western blot with known quantities of Ni-NTA affinity column purified gloverin was used to estimate the concentrations of TnGlv1 and TnGlv2 in the serum-containing supernatants (Figure 1C). Comparing the chemiluminescence intensity and area of the gloverin bands for each sample (Figure 1C) with the known loaded quantity of Ni-NTA column purified gloverin (85 μg/ml of TnGlv1 and 525 μg/ml of TnGlv2), there was approximately 22 μg/ml of TnGlv1 and 40 μg/ml of TnGlv2 in the serum-containing supernatants. While gloverin protein isolated with the Ni-NTA column may have been degraded as it was produced by Sf9 cells or during protein isolation with affinity chromatography (Supplementary Figure 1B), band densitometry demonstrated that the final dialysis step used to purify gloverin removed most of the smaller molecular weight epitope-tagged protein (Figure 1C; 86 % and 77 % of TnGlv1 and TnGlv2, respectively, was of the predicted molecular weight for gloverin that was not degraded). Both TnGlv1 and TnGlv2 protein was present on the western blot at molecular weights that were approximately 2 kDa less than what was predicted from the amino acid sequences (Figure 1C).
3.3 Inactivation of BV by TnGlv1 and TnGlv2
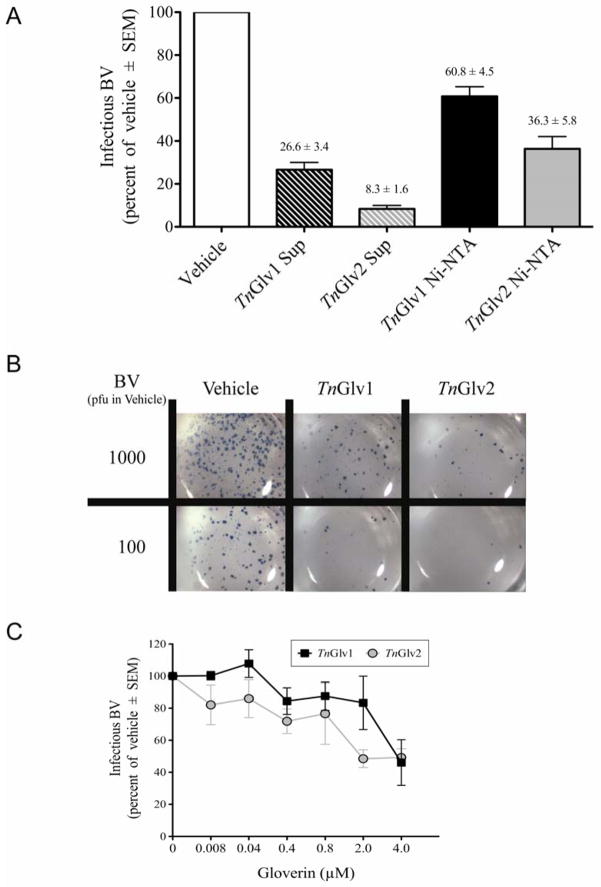
To determine if TnGlv1 or TnGlv2 could inactivate baculovirus, AcMNPV-hsp70/lacZ BV was incubated for 3 h in the presence of supernatants containing unpurified TnGlv1 (1 μM) or TnGlv2 (2 μM) or Ni-NTA affinity column purified TnGlv1 (4 μM) or TnGlv2 (4 μM). Approximately 0.03 mg/ml of gloverin (2.17 μM) was isolated from H. gloveri pupae injected with gram-negative bacteria (Axen et al., 1997), which is similar to or greater than the gloverin concentrations used for these studies. To aggregate results from multiple experiments (N ≥ 3), the observed BV plaque forming units (PFU) for each treatment was normalized to the vehicle treatment for each experiment, which was set to 100% infectious BV (Figure 2A). When BV was incubated with gloverin-containing supernatants, the quantity of infectious BV, as measured by PFU, was reduced to 26.6 ± 3.4 % and 8.3 ± 1.6 % for TnGlv1 and TnGlv2, relative to that of BV incubated in the presence of vehicle (Figure DAB). A higher quantity of infectious virus was detected for BV incubated in the presence of TnGlv1 and TnGlv2 purified using Ni-NTA affinity columns (60.8 ± 4.5 % and 36.3 ± 5.8 % of vehicle, respectively; Figure 2A). These results suggest that TnGlv1 and TnGlv2 inactivate baculovirus BV in vitro and that TnGlv2 has greater antiviral activity than TnGlv1. The observation that purified gloverin protein had lower activity relative to unpurified gloverin present in cell culture supernatants suggests that the protein purification process may have affected gloverin activity or that additional elements in the supernatants may exert antiviral activity.
Figure 2. TnGlv1 and TnGlv2 reduced the quantity of infectious AcMNPV-hsp70/lacZ BV.
(A) Infectious BV was reduced when incubated in the presence of Sf9 cell culture supernatants containing TnGlv1 (TnGlv1 Sup), TnGlv2 (TnGlv2 Sup) or Ni-NTA affinity column purified TnGlv1 (TnGlv1 Ni-NTA) or TnGlv2 (TnGlv2 Ni-NTA). The results from replicated plaque assay experiments were normalized to the vehicle treatment (TNM-FH media) for each experiment, which was set to 100 % infectious BV, and the change in the quantity of infectious BV reported as the percent of the vehicle treatment. Means and SEM were calculated from N ≥ 3 replicates per treatment. (B) Plaque assay of Sf9 cells showing blue LACZ-positive plaques of AcMNPV-hsp70/lacZ BV incubated with cell culture supernatants containing TnGlv1, TnGlv2, or vehicle. (C) Concentration dependent antiviral activity of TnGlv1 and TnGlv2 on AcMNPV BV. Plaque assays of AcMNPV-hsp70/lacZ BV incubated with increasing concentrations of affinity column purified TnGlv1 and TnGlv2 protein was correlated with decreasing quantities of infectious BV relative to vehicle treatments (BV incubated with TNM-FH media; N = 4 replicates per treatment).
To determine whether the activity of gloverin was concentration dependent, increasing concentrations of affinity column purified TnGlv1 or TnGlv2 were incubated for 3 h with AcMNPV-hsp70/lacZ BV (105 PFU) and the quantity of infectious BV quantified using plaque assay. The results were normalized to the quantity of infectious BV present when AcMNPV-hsp70/lacZ BV (105 PFU) was incubated for 3 h in the presence of vehicle (TNM-FH media). While there was no observable difference between vehicle and low concentrations of TnGlv1 (0.008 μM and 0.4 μM; Figure 2C), low concentrations of TnGlv2 moderately reduced the quantity of infectious BV (82.0 ± 12.3 % for 0.008 μM and 85.0 ± 11.8 % for 0.04 μM; Figure 2C). In contrast, higher concentrations of TnGlv1 and TnGlv2 produced substantial reductions (TnGlv1: 46.1 ± 14.3 % for 4 μM and 87.5± 8.8 % for 0.8 μM; TnGlv2: 49.2 ± 5.5 % for 4 μM and 76.5 ± 19.1 % for 0.8 μM; Figure 2C). As the concentration of TnGlv1 or TnGlv2 was increased, the quantity of infectious BV decreased (Figure 2C), suggesting that the antiviral activity of gloverin was concentration dependent.
3.4 Vesicle lysis by gloverin
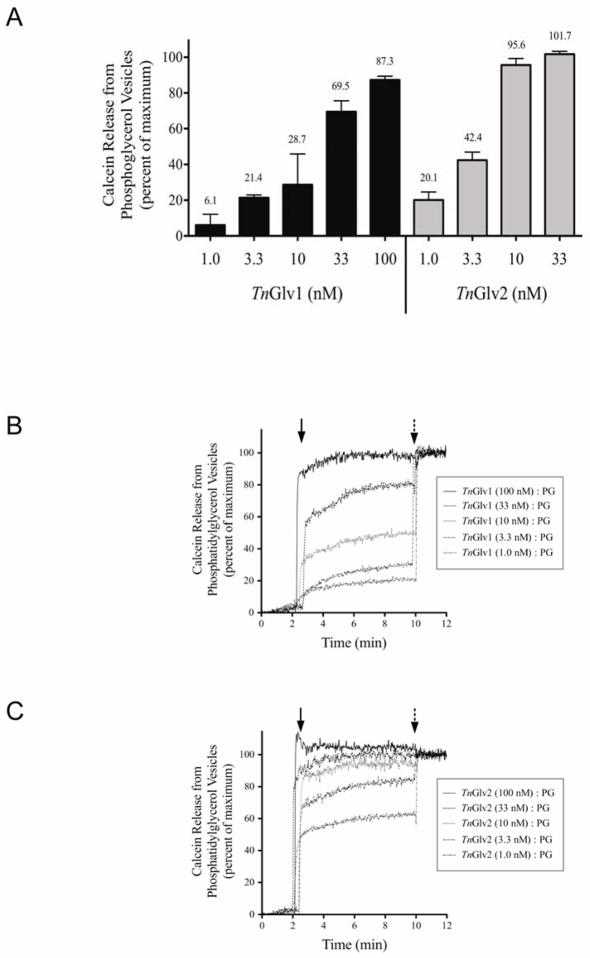
To determine whether gloverin could interact with membranes and induce changes in membrane permeability, unilamellar vesicles encapsulated with the fluorescent probe calcein were incubated with affinity column purified TnGlv1 or TnGlv2 and the amount of calcein released quantified. The maximum release of calcein from the vesicles was determined by solubilizing the vesicles with detergent 10 min after gloverin addition. This is a long-standing method to assess the pore-forming activity of antimicrobial peptides (Matsuzaki et al., 1995; Prenneret al., 2001; Patel et al., 2009). Phosphatidylglycerol (PG) vesicles were used to evaluate the interaction of gloverin with negatively charged membranes. Nanomolar concentrations of TnGlv1 and TnGlv2 caused greater than 85 % of the encapsulated calcein to be released from PG vesicles within two minutes of being added to the reaction cuvette (TnGlv1: 87.3 ± 2.1 % for 100nM; TnGlv2: 95.6 ± 3.7 % for 10nM; Figure 3). Decreasing the concentrations of gloverin resulted in proportional decreases in calcein released from the PG vesicles (TnGlv1: 69.5 ± 6.1 % for 33 nM and 21.4 ± 1.5 % for 3.3 nM; TnGlv2: 42.4 ± 4.5 % for 3.3 nM and 20.1 ± 4.5 % for 1.0 nM; Figure 3A), suggesting that the vesicle perforation activity of gloverin was concentration dependent. When the quantity of calcein released from PG vesicles was compared for similar concentrations of TnGlv1 and TnGlv2 (ex. 3.3 nM), two to three times more calcein was released from vesicles incubated with TnGlv2 (Figure 3A). The release of calcein from PG vesicles was almost completed within 30 s after addition of TnGlv1 or TnGlv2 and was close to maximum within 2 min after addition of protein (Figure 3B 3C), suggesting that the interaction between gloverin and PG vesicles occurred rapidly and with high efficiency. The higher pore-forming activity of TnGlv2 relative to TnGlv1 (Figure 3A) correlated with the relatively higher antiviral activity of TnGlv2 (Figure 2A).
Figure 3. Gloverin-mediated release of calcein from phosphatidylglycerol (PG) or phosphatidylcholine (PC) vesicles.
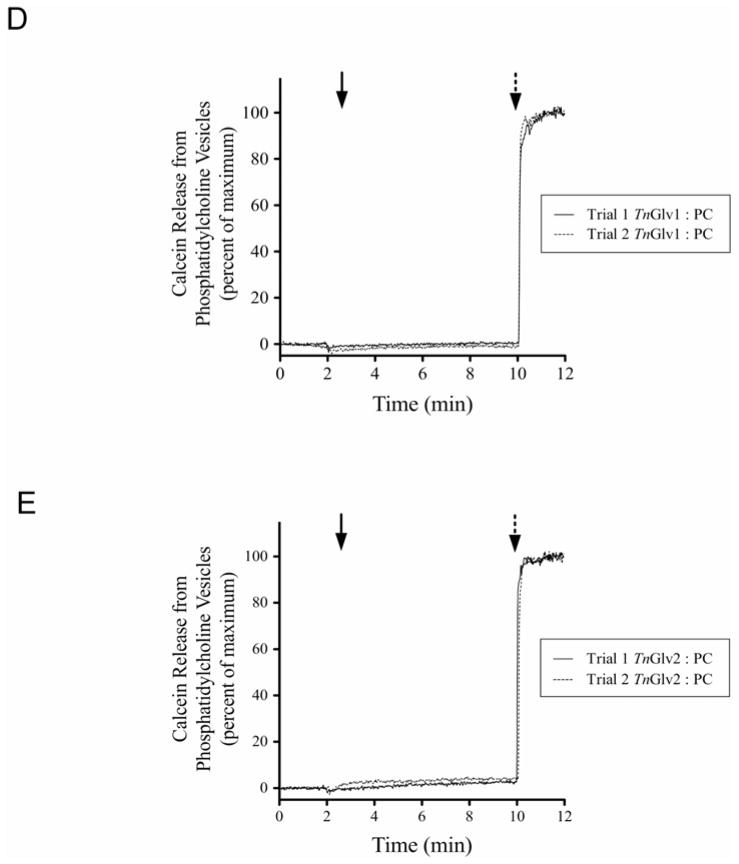
(A) Unilamellar vesicles comprised of PG and encapsulated with calcein were incubated with nanomolar concentrations of affinity purified TnGlv1 or TnGlv2 and the quantity of calcein released quantified using a fluorescence spectrophotometer (N = 3 replicates per concentration and treatment). Increasing concentrations of TnGlv1 or TnGlv2 protein resulted in concomitant increases in calcein released from the PG vesicles. (B and C) Individual trials showing the release of calcein over time from PG vesicles incubated with multiple concentrations of TnGlv1 (B) or TnGlv2 (C). Maximum gloverin-mediated calcein release from PG vesicles occurred within 2 min of the gloverin addition to the reaction cuvette. (D and E) TnGlv1 and TnGlv2 (100 nM) did not cause calcein release from PC vesicles after an 8 min incubation period. Solid arrows indicate the time that test proteins were added to the reaction cuvettes and broken arrows indicate the time that detergent was added to each reaction cuvette to cause full release of the encapsulated calcein from the vesicles.
Vesicles comprised of phosphatidylcholine (PC) and encapsulated with calcein were used to determine if the phospholipid charge affected the interaction between gloverin and the membrane. In contrast to PG, the PC impart a neutral charge to the vesicles. Three separate trials using high concentrations of TnGlv1 and TnGlv2 (100nM) incubated with PC vesicles showed no or less than 2 % of the calcein released during the 8 min incubation period (Figure 3D 3E).
3.5 TnGlv2 expression in T. nilarvae
Expression of TnGlv2 in hemocytes isolated from T. ni 12 h after inoculation with 500 PFU of AcMNPV-hsp70/lacZ BV or vehicle (TNM-FH media) was analyzed using semi-quantitative RT-PCR. When normalized to GAPDH expression, TnGlv2 expression was increased 5.7-fold in hemocytes isolated from larvae inoculated with BV relative to vehicle-inoculated larvae (Figure 4). Control amplifications using only Taq DNA polymerase or heat-inactivated reverse transcriptase and GAPDH or TnGlv2 primers did not amplify the template (Supplementary Figure 2) demonstrating the absence of contaminating DNA in the RNA isolated from T. ni hemocytes.
Figure 4. Transcriptional analysis of gloverin in hemocytes isolated from AcMNPV-infected T. ni larva.
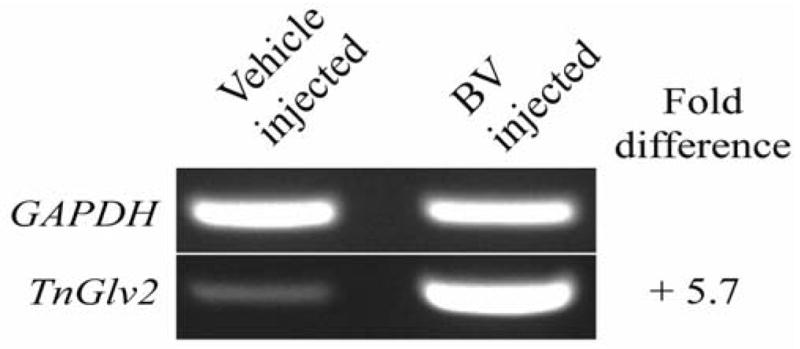
Gene expression was analyzed using semi-quantitative RT-PCR for hemocytes isolated from T. ni 12 h after inoculation with AcMNPV-hsp70/lacZ BV or vehicle (TNM-FH). Relative to GAPDH expression, TnGlv2 was upregulated 5.7-fold.
4. Discussion
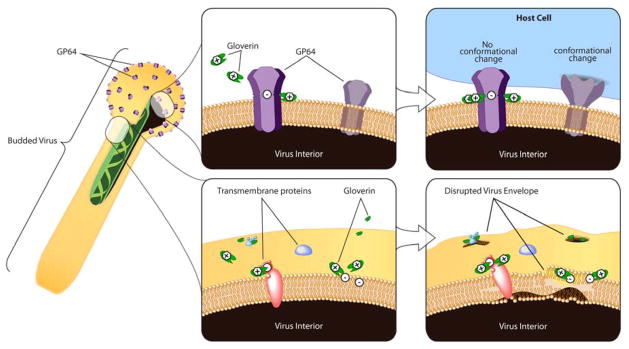
Although the antibacterial activity of gloverin is well appreciated, understanding its impact on viruses is of interest because gloverin expression is upregulated in lepidopteran larvae systemically infected with baculoviruses (Wang et al., 2010a). In the present study, we demonstrate that gloverin reduced the infectivity of baculovirus BV in vitro and that gloverin perforated vesicles. Together, these results suggest that gloverin inactivates BV by disrupting the viral membrane surrounding the nucleocapsid (Figure 5).
Figure 5. Hypothetical mechanisms of action for the observed antiviral activities of gloverin.
Top panels. GP64 is the attachment and fusion protein of AcMNPV BV. The overall negative charge of GP64 or clusters of negatively charged amino acids localized near the transmembrane domain of GP64 may attract the positively charged amino acids of gloverin to the surface of BV. Gloverin may subsequently hinder the conformational changes of GP64 needed to initiate an infection. Bottom panels. Gloverin may interact with negatively charged amino acids of other proteins localized to the envelope or with negatively charged phospholipids of the envelope membrane. Accumulated gloverin on the surface of BV may cause membrane strain or pores to form that disrupt the BV envelope. Illustration credit: Emily L. Ling.
Gloverin proteins are small, less than 200 amino acids in size, and their expression is potently stimulated in several species of lepidopteran larvae and pupae infected with bacteria (Axen et al., 1997; Brown et al., 2009; Kawaoka et al., 2008; Lundström et al., 2002; Mackintosh et al., 1998; Silva et al., 2010; Zhou et al., 2008). The induction of gloverin expression is higher in larvae inoculated intrahemocoelically with the Gram-negative bacteria E. coli, compared to challenge with the fungus Candida albicans (Kawaoka et al., 2008). Gloverin and gloverin-like proteins inhibit growth of E. coli (Lundström et al., 2002) but have little effect on Gram-positive bacteria or fungi (Mackintosh et al., 1998). Fat body cells and hemocytes mediate essential immune system functions and both express gloverin in response to infection by bacteria (Lundström et al., 2002; Tamezguerra et al., 2008). Although a high quantity of gloverin is present in the hemolymph of insects (Axen et al., 1997) and in the medium of Sf9 cells transfected with gloverin-expressing plasmids (Figure 1C), this does not appear to negatively affect cell growth.
As with many antimicrobial proteins, gloverin is produced as a pro-protein that contains a N-terminal signal peptide that is proteolytically removed to produce the mature form (Axen et al., 1997). Computational analysis of TnGlv1 and TnGlv2 suggest that the signal peptide of these proteins contain a transmembrane domain (Figure 1A). When expressed in Sf9 cells, the N-terminal signal peptide of gloverin is not removed (Lundström et al., 2002). However, gloverin cloned from T. ni and expressed in Sf9 cells displays a high antibacterial activity that is similar to the mature form isolated from Hyalophora gloveri larvae (Lundström et al., 2002), suggesting that removing the signal peptide is not essential for the antibacterial activity. When the total peptide content of cell-free hemolymph isolated from Galleria mellonella was analyzed using liquid-chromatography mass spectrometry, the region containing the N-terminal signal peptide was detected (Brown et al., 2009), suggesting that the pro-protein is secreted into the hemolymph and may be active during the immune response against bacteria.
The 6x-His and V5 epitope tags that were engineered into the C-termini of TnGlv1 and TnGlv2 are widely employed individually or in combination to isolate proteins for functional studies. The 6x-His tag is comprised of 6 histidine amino acids. When incorporated into the C- or N-termini of proteins, the His tag is reported to not significantly affect protein structure (Carson et al., 2007). The V5 epitope sequence is formed from 14 amino acids of the RNA polymerase subunit from simian virus 5 (Southern et al. 1991). Although inserting the V5 epitope tag into the middle of the coding region of polymerases can reduce the polymerase activity (Shi and Elliott, 2009), the V5 epitope has been successfully engineered into the C- and N-termini of proteins without affecting protein function (Alarcon-Chaidez et al. 2003; Morais and Costa, 2003; Oezbey et al. 2007). However, because there is no means for removing the V5/6x-His epitope tag from the gloverin proteins that were expressed in Sf9 cells, it remains possible that the biological activity of the proteins was affected.
The predicted amino acid sequences of TnGlv1 and TnGlv2 show that the proteins have 27 and 29 positively charged arginine or lysine amino acids, respectively, which impart an overall net positive charge to the proteins. The outer membranes of Gram-negative bacteria, including E. coli, contain lipopolysaccharide (LPS) that increases the negative charge of the outer membrane. The specificity of gloverin to Gram-negative bacteria is hypothesized to result from electrostatic interactions between the positively charged gloverin and negatively charged LPS (Axen et al., 1997). Once attached to the outer membrane of E. coli, gloverin is reported to stimulate a reduction in the biosynthesis of the outer membrane proteins OmpA and OmpF without affecting the production of other proteins (Axen et al., 1997). OmpA is a multifunctional protein that aids in maintaining membrane structure and normal cell shape (Sonntaget al., 1978) while also contributing to the initial steps of bacterial adhesion and invasion into tissues (Prasadarao et al., 1996). OmpF is a porin that forms a large beta-barrel to allow passive diffusion of molecules across the outer membrane (Nikaido, 2003). However, the down regulation of OmpF and OmpA observed for E. coli exposed to gloverin (Lundström et al., 2002) may instead be a response initiated by the microbe to limit the efflux of intracellular contents and prevent cell lysis.
The work presented in Axen et al (1997) is consistent with hypothesis that gloverin binds to LPS and inhibits outer membrane protein synthesis to increase membrane permeability. However, our results demonstrate that gloverin reduces the infectivity of baculovirus BV in vitro and acts alone to permeabilize membranes. The BV membrane is generated from the plasma membrane of an infected cell as the virus buds and is released. Because BV is metabolically inactive and eukaryotic cells lack known homologs of OmpA and OmpF, it is unlikely that antiviral activity of gloverin only results from an inhibition of protein synthesis. Many antibacterial proteins, including defensins produced by vertebrates (Wimley et al. 1994) and cecropins produced by insects (Christensen et al. 1988), act against microbes by disrupting membranes and forming pores (Peters et al. 2010). Although gloverin contains a transmembrane domain, it is removed if the signal peptide is cleaved from gloverin. However, the transmembrane domain of gloverin may not be essential to form pores because defensin binds to the outer membrane of microbes to cause membrane strain and pores to form that permit molecules to leak across the membrane (Ganz, 2003). Additionally, the human alpha defensin HNP-1 lacks a transmembrane domain, yet it inactivates several enveloped viruses (Daher et al. 1986). Whether or not gloverin retains the transmembrane signal peptide, the overall positive charge of gloverin may allow it to accumulate on the surface of microbes or enveloped viruses to cause membrane strain and pores to form. Pores in the envelope of baculovirus BV may allow small proteins or molecules to leak across the viral envelope or disrupt the assemblage of envelope-associated proteins (Figure 5).
The interaction of positively charged gloverin with AcMNPV BV may be driven by charge-based interactions between gloverin and the BV envelope protein GP64 (Figure 5). GP64 is one of the three most abundant proteins that are present in AcMNPV BV (Wang et al., 2010b). GP64 is responsible for attaching BV to the cell surface and mediating fusion of the BV envelope with the endosome membrane to cause entry of the virus into the cell (Blissard and Wenz, 1992). The region of GP64 displayed on the surface of BV (amino acids 21–483) has a predicted isoelectric point (pI) of 5.59, indicating that this region has an overall negative charge at a physiological pH and may interact with the positively charged gloverin. The measured pI of gloverin isolated from H. gloveri pupae is 8.5 (Axen et al., 1997) while the pH of hemolymph in lepidopteran larvae was measured at 6.45 and 6.8 (Wangand Haunerland, 1993; Wyatt, et al., 1956). The crystal structure of post-fusion GP64 places two clusters of negatively charged amino acids (aspartic acid and glutamic acid) on the solvent-exposed surface of GP64 and near the transmembrane domain (residues 114–133 and 410–418) (Kadlec et al. 2008). The negatively charged amino acids of residues 114–133 are contained within the fusion domain of GP64 (residues 67–166); the fusion domain plays a crucial role in triggering fusion between viral and cellular membranes (Kadlec et al., 2008). Gloverin binding to the fusion domain of GP64 may restrict the protein movements required for membrane fusion, thereby reducing BV infectivity. Alternatively, this charge-based interaction may direct gloverin to the surface of BV to cause membrane strain or pores to form that disrupt the BV envelope and reduce infectivity of the virus (Figure 5). Future studies of truncated gloverin and GP64 will provide detailed analysis of the mechanisms that mediate the reduced infectivity of BV exposed to gloverin.
Gloverin expression is induced in response to infection and reduces the growth of Gram-negative bacteria and the infectivity of baculovirus BV. The current models proposes that gloverin inhibits the synthesis of specific proteins localized to the outer membrane of bacteria (Lundström et al., 2002). Because gloverin also displays antiviral activity and perforates vesicles, we suggest an alternative mode of action in which gloverin strains the BV envelope to form pores (Figure 5), and in this regard, may be mechanistically similar to defensin.
Supplementary Material
(A) Ponceau S-stained blot of concentrated supernatant (cSN) from Sf9 cells transfected with the TnGlv2 expression vector (pIB/V5-His-DEST-TnGlv2 cl10.a) that was isolated using Ni-NTA affinity columns (FT is flow through the Ni-NTA column, W1-W3 are washes 1 to 3 of the Ni-NTA column, E1-E3 are elutions 1 to 3 from the Ni-NTA column). (B) The quantity of TnGlv2 isolated as shown by chemiluminescence of the blot in Supplementary Figure 1A probed with mouse anti-V5 and anti-mouse HRP-conjugated antibodies (30 s exposure).
Control amplifications of GAPDH (A) or TnGlv2 (B) using RNA isolated from T. ni inoculated with vehicle (TNM-FH) or AcMNPV-hsp70/lacZ BV. GAPDH was robustly amplified using the active Titan One enzyme mix and the RNA isolated from T. ni inoculated with vehicle or BV. Heat inactivating the Titan One enzyme mix denatures the reverse transcriptase (RT) enzyme, while the DNA polymerase remains active. Heat-inactivated Titan One enzyme mix or Taq DNA polymerase alone did not generate a detectable PCR product with primers for either GAPDH or TnGlv2. A band at the predicted molecular weight for TnGlv2 was present, but faint, when the active Titan One enzyme mix and TnGlv2 primers were used with RNA isolated from BV-inoculated larvae. No PCR product was detected when no RNA was included with active Titan One enzyme mix and primers for either GAPDH or TnGlv2.
Highlights.
Gloverin expression is upregulated in Trichoplusia ni infected with the baculovirus AcMNPV.
Gloverin protein reduced the infectivity of AcMNPV budded virus.
Vesicles comprised of phosphatidylglycerol were perforated by gloverin.
Acknowledgments
This work was funded by grants from the US Department of Agriculture (2008-03990) to E.J.HS, the National Institutes of Health (NIH) to P.M.M.W (SC3GM089564), the NIH Research Initiative for Scientific Enhancement (RISE) program (5R25GM071638), and the NIH Bridges to Baccalaureate summer research program. We thank Emily L. Ling for preparing the illustrations in Figure 5 that depict the hypothesized mechanisms of action for the antiviral activities of gloverin.
Footnotes
6. Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniela A. Moreno-Habel, Email: Daniel.Moreno@student.csulb.edu.
Ivan M. Biglang-awa, Email: Ivan.Biglangawa@student.csulb.edu.
Angelica Dulce, Email: Maria.Dulce@student.csulb.edu.
Dee DeeLuu, Email: DeeDee.Luu@student.csulb.edu.
Peter Garcia, Email: Peter.Garcia@student.csulb.edu.
Paul M. M. Weers, Email: Paul.Weers@csulb.edu.
Eric J. Haas-Stapleton, Email: Eric.Haas-Stapleton@csulb.edu.
References
- Alarcon-Chaidez FJ, Müller-Doblies UU, Wikel S. Characterization of a recombinant immunomodulatory protein from the salivary glands of Dermacentor andersoni. Parasite immunology. 2003;25(2):69–77. doi: 10.1046/j.1365-3024.2003.00609.x. [DOI] [PubMed] [Google Scholar]
- Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. In: Neufeld E, Ginsburg V, editors. Methods in enzymology. Vol. VIII: Complex Carbohydrates. 1966. pp. 115–118. [Google Scholar]
- Axen A, Carlsson A, Engström Å, Bennich H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur J Biochem. 1997;247:614–619. doi: 10.1111/j.1432-1033.1997.00614.x. [DOI] [PubMed] [Google Scholar]
- Blissard GW, Wenz JR. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem Mol Biol. 2009;39(11):792–800. doi: 10.1016/j.ibmb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Engström P, Palva ET, Bennich H. Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infection and Immunity. 1991;59:3040–3045. doi: 10.1128/iai.59.9.3040-3045.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M, Johnson DH, McDonald H, Brouillette C, Delucas LJ. His-tag impact on structure. Acta crystallographica Section D, Biological crystallography. 2007;63(Pt 3):295–301. doi: 10.1107/S0907444906052024. [DOI] [PubMed] [Google Scholar]
- Chae JH, Kurokawa K, So YI, Hwang HO, Kim MS, Park JW, Jo YH, Lee YS, Lee BL. Purification and characterization of tenecin 4, a new anti-Gram-negative bacterial peptide, from the beetle Tenebrio molitor. Dev and Comp Immunol. 2011 doi: 10.1016/j.dci.2011.09.010. In press. [DOI] [PubMed] [Google Scholar]
- Chikhalya A, Luu DD, Carrera M, La Cruz DeA, Torres M, Martinez EN, Chen T, Haas-Stapleton EJ. Pathogenesis of Autographa californica multiple nucleopolyhedrovirus in fifth-instar Anticarsia gemmatalis larvae. J Gen Virol. 2009;90(Pt 8):2023–2032. doi: 10.1099/vir.0.011718-0. [DOI] [PubMed] [Google Scholar]
- Christensen B, Fink J, Merrifield RB, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. PNAS. 1988;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60(3):1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo VK, Tsuji Y, Yasuda T, Kato T, Sakamoto N, Suzuki H, Park EY. Expression of an RSV-gag virus-like particle in insect cell lines and silkworm larvae. J Vir Methods. 2011;177(2):147–152. doi: 10.1016/j.jviromet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Etebari K, Palfreyman RW, Schlipalius D, Nielsen LK, Glatz RV, Asgari S. Deep sequencing-based transcriptome analysis of Plutella xylostella larvae parasitized by Diadegma semiclausum. BMC Genomics. 2011;12:446. doi: 10.1186/1471-2164-12-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitak D, Wheat CW, Heckel DG, Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biology. 2007;5(1):56. doi: 10.1186/1741-7007-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Rev Immunol. 2003 Sep;3(9):710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Ishibashi J, Hara S, Yamakawa M. Solution structure of moricin, an antibacterial peptide, isolated from the silkworm Bombyx mori. FEBS letters. 2002;518(1–3):33–38. doi: 10.1016/s0014-5793(02)02637-6. [DOI] [PubMed] [Google Scholar]
- Huang L, Cheng T, Xu P, Cheng D, Fang T, Xia Q. A Genome-Wide Survey for Host Response of Silkworm, Bombyx mori during Pathogen Bacillus bombyseptieus Infection. PloS one. 2009;4(12):e8098. doi: 10.1371/journal.pone.0008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D, Engström Å, Andersson K, Steiner H, Bennich H, Boman HG. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. The EMBO journal. 1983;2(4):571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost M. Immunity in lepidopteran insects. Invert Immun. 2011:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlec J, Loureiro S, Abrescia NGA, Stuart DI, Jones IM. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nature structural molecular biology. 2008;15(10):1024–1030. doi: 10.1038/nsmb.1484. [DOI] [PubMed] [Google Scholar]
- Kawaoka S, Katsuma S, Daimon T, Isono R, Omuro N, Mita K, Shimada T. Functional analysis of four Gloverin-like genes in the silkworm, Bombyx mori. Arch Insect Biochem Physiol. 2008;67(2):87–96. doi: 10.1002/arch.20223. [DOI] [PubMed] [Google Scholar]
- Khoury M, Parker I, Aswad DW. Acquisition of chemiluminescent signals from immunoblots with a digital single-lens reflex camera. Analyt Biochem. 2010;397:129–131. doi: 10.1016/j.ab.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine MD, Chen G, Strand MR. Immune challenge differentially affects transcript abundance of three antimicrobial peptides in hemocytes from the moth Pseudoplusia includens. Insect Biochem Mol Biol. 2005;35(12):1335–1346. doi: 10.1016/j.ibmb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Liu G, Kang D, Steiner H. Trichoplusia ni Lebocin, an Inducible Immune Gene with a Downstream Insertion Element. Biochem Biophy Res Com. 2000;269(3):803–807. doi: 10.1006/bbrc.2000.2366. [DOI] [PubMed] [Google Scholar]
- Lundström A, Liu G, Kang D, Berzins K, Steiner H. Trichoplusia ni gloverin, an inducible immune gene encoding an antibacterial insect protein. Insect Biochem Mol Biol. 2002;32(7):795–801. doi: 10.1016/s0965-1748(01)00162-x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Murase O, Miyajima K. Kinetics of pore formation by an antimicrobial peptide, magainin 2, in phospholipid bilayers. Biochemistry. 1995;3:34(39):12553–9. doi: 10.1021/bi00039a009. [DOI] [PubMed] [Google Scholar]
- Mackintosh JA, Gooley AA, Karuso PH, Beattie AJ, Jardine DR, Veal DA. A gloverin-like antibacterial protein is synthesized in Helicoverpa armigera following bacterial challenge. Dev Comp Immunol. 1998;22:387–399. doi: 10.1016/s0145-305x(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Mcintosh AH, Grasela JJ, Goodman CL, Ignoffo CM. Growth of a clonal cell line of Helicoverpa zea (Lepidoptera: Noctuidae) in suspension culture and replication of its homologous baculovirus Hz SNPV. Appl Entomol Zool. 2001;36:349–352. [Google Scholar]
- Morais VA, Costa J. Stable expression of recombinant human alpha3/4 fucosyltransferase III in Spodoptera frugiperda Sf9 cells. J biotech. 2003;106(1):69–75. doi: 10.1016/j.jbiotec.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Micro Mol Biol Rev. 2003 Dec;67(4):593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oezbey S, Stengel C, Schlötzer-Schrehardt U, Ekici A, Rautenstrauss B. Heterologous expression of wild type and mutant myocilin in High Five insect cells shows comparable effects to cultivated trabecular meshwork cells. Biomol Eng. 2007;24(3):313–317. doi: 10.1016/j.bioeng.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ourth DD, Lockey TD, Renis HE. Induction of Cecropin-Like and Attacin-Like Antibacterial but Not Antiviral Activity in Larvae. Biochem and Biophys Res Com. 1994;200(1):35–44. doi: 10.1006/bbrc.1994.1410. [DOI] [PubMed] [Google Scholar]
- Patel H, Tscheka C, Heerklotz H. Characterizing vesicle leakage by fluorescent lifetime measurements. Soft Matter. 2009;5(15):2849–51. [Google Scholar]
- Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial Peptides: Primeval Molecules or Future Drugs. PLoS Pathogens. 2010;6(10):e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadarao NV, Wass CA, Weiser JN, Stins MF, Huang SH, Kim KS. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner EJ, Lewis RN, Jelokhani-Niaraki M, Hodges RS, McElhaney RN. Cholesterol attenuates the interaction of the antimicrobial peptide gramicidin S with phospholipid bilayer membranes. Biochim Biophys Acta. 2001 Feb 9;1510(1–2):83–92. doi: 10.1016/s0005-2736(00)00337-0. [DOI] [PubMed] [Google Scholar]
- Shi X, Elliott RM. Generation and analysis of recombinant Bunyamwera orthobunyaviruses expressing V5 epitope-tagged L proteins. J Gen Virol. 2009;90(Pt 2):297–306. doi: 10.1099/vir.0.007567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JLC, Barbosa JF, Bravo JP, de Souza EM, Huergo LF, Pedrosa FO, Esteves E, Daffre S, Fernandez MA. Induction of a gloverin-like antimicrobial polypeptide in the sugarcane borer Diatraea saccharalis challenged by septic injury. Brazil J Med Biol Res. 2010;43(5):431–436. doi: 10.1590/s0100-879x2010005000010. [DOI] [PubMed] [Google Scholar]
- Snider C, Jayasinghe S, Hristova K, White SH. MPEx: A tool for exploring membrane proteins. Protein Science. 2009;18(12):2624–2628. doi: 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I, Schwarz H, Hirota Y. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136(1) doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern JA, Young DF, Heaney F, Baumgärtner WK, Randall RE. Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J Gen Virol. 1991;72(Pt 7):1551–1557. doi: 10.1099/0022-1317-72-7-1551. [DOI] [PubMed] [Google Scholar]
- Tamez-Guerra P, Valadez-Lira JA, Alcocer-González JM, Oppert B, Gomez-Flores R, Tamez-Guerra Rodríguez-Padilla C. Detection of genes encoding antimicrobial peptides in Mexican strains of Trichoplusia ni (Hübner) exposed to Bacillus thuringiensis. J Invertebr Pathol. 2008;98:218–227. doi: 10.1016/j.jip.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol. 1998;79(Pt 4):731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu Y, He HJ, Zhao XF, Wang JX. Immune responses of Helicoverpa armigera to different kinds of pathogens. BMC Immunology. 2010a;11(1):9. doi: 10.1186/1471-2172-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Deng F, Hou D, Zhao Y, Guo L, Wang H, Hu Z. Proteomics of the Autographa californica nucleopolyhedrovirus budded virions. J Virol. 2010b;84(14):7233–7242. doi: 10.1128/JVI.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Haunerland NH. Storage protein uptake in Helicoverpa zea. Purification of the very high density lipoprotein receptor from perivisceral fat body. J Biol Chem. 1993;268(22):16673–16678. [PubMed] [Google Scholar]
- Wimley WC, Selsted, Michael E, White SH. Interactions between human defensins and lipid bilayers: Evidence for formation of multimeric pores. Protein Science. 1994;3(9):1362–1373. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt GR, Loughheed TC, Wyatt SS. The chemistry of insect hemolymph; organic components of the hemolymph of the silkworm, Bombyx mori, and two other species. J Genphysiol. 1956;39(6):853–868. doi: 10.1085/jgp.39.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Yang HJ, Chen M, Lou CF, Zhang YZ, Chen KP, Wang Y, Yu ML, Yu F, Li JY, Zhong BX. Comparative Proteomic Analysis between the Domesticated Silkworm (Bombyx mori) Reared on Fresh Mulberry Leaves and on Artificial Diet. J Proteome Res. 2008;7(12):5103–5111. doi: 10.1021/pr800383r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Ponceau S-stained blot of concentrated supernatant (cSN) from Sf9 cells transfected with the TnGlv2 expression vector (pIB/V5-His-DEST-TnGlv2 cl10.a) that was isolated using Ni-NTA affinity columns (FT is flow through the Ni-NTA column, W1-W3 are washes 1 to 3 of the Ni-NTA column, E1-E3 are elutions 1 to 3 from the Ni-NTA column). (B) The quantity of TnGlv2 isolated as shown by chemiluminescence of the blot in Supplementary Figure 1A probed with mouse anti-V5 and anti-mouse HRP-conjugated antibodies (30 s exposure).
Control amplifications of GAPDH (A) or TnGlv2 (B) using RNA isolated from T. ni inoculated with vehicle (TNM-FH) or AcMNPV-hsp70/lacZ BV. GAPDH was robustly amplified using the active Titan One enzyme mix and the RNA isolated from T. ni inoculated with vehicle or BV. Heat inactivating the Titan One enzyme mix denatures the reverse transcriptase (RT) enzyme, while the DNA polymerase remains active. Heat-inactivated Titan One enzyme mix or Taq DNA polymerase alone did not generate a detectable PCR product with primers for either GAPDH or TnGlv2. A band at the predicted molecular weight for TnGlv2 was present, but faint, when the active Titan One enzyme mix and TnGlv2 primers were used with RNA isolated from BV-inoculated larvae. No PCR product was detected when no RNA was included with active Titan One enzyme mix and primers for either GAPDH or TnGlv2.