Abstract
Clinical evidence indicates a frequent co-morbidity of nicotine and alcohol abuse and dependence. The posterior ventral tegmental area (pVTA) appears to support the reinforcing and dopamine-stimulating effects of both drugs. The current study tested the hypothesis that repeated exposure of the pVTA to one drug would increase the sensitivity of local dopamine neurons to the stimulating effects of the other drug. Female Wistar rats received repeated daily microinjections of either 100 μM nicotine or vehicle directly into the pVTA for 7 days. On the 8th day, rats received microinjections of either vehicle or ethanol (100 or 200 mg%) into the pVTA while extracellular dopamine samples were collected from the ipsilateral nucleus accumbens shell (NACsh) with microdialysis. Another experiment tested the effects of challenge microinjections of 200 μM nicotine in the pVTA on extracellular dopamine levels in the NACsh following 7 daily pretreatments with 200 mg% ethanol in the pVTA. Nicotine pretreatments increased the dopamine-stimulating effects of ethanol in the pVTA (100 mg% ethanol: 115% vs 160% of baseline in the vehicle and nicotine groups, respectively, p < 0.05; 200 mg% ethanol: 145% vs 190% of baseline in the vehicle and nicotine groups, respectively, p < 0.05). In contrast, ethanol pretreatments did not alter the stimulating effects of nicotine in the pVTA. The results suggest that repeated exposure of the pVTA to nicotine increased the response of local dopamine neurons to the stimulating effects of ethanol, whereas repeated exposure of the pVTA to ethanol did not alter the responses of pVTA dopamine neurons to nicotine.
Keywords: dopamine, ethanol, microdialysis, nicotine, nucleus accumbens, ventral tegmental area
Introduction
Alcohol drinking and tobacco smoking have been frequently reported to be co-used and/or co-abused in humans. The rates of alcohol abuse or dependence were two to three times more in regular smokers or nicotine dependent individuals than in the general population, and increased with the number of cigarettes smoked (Grant et al., 2004; John et al., 2003). Approximately 90% of individuals diagnosed with alcohol dependence reported cigarette smoking and/or nicotine dependence, a rate significantly higher than that in the general population (Batel et al., 1995; Burling and Ziff, 1988; Grant et al., 2004). The amount of cigarette smoking in alcoholics is positively correlated with the amount of alcohol consumed and severity of alcohol dependence (Batel et al., 1995; Burling and Ziff, 1988; Dawson, 2000). Furthermore, concurrent dependence on alcohol and nicotine reduced the likelihood of cessation from tobacco smoking or alcohol drinking (DiFranza and Guerrera, 1990; Miller et al., 1983).
Research in rodents also indicates an interrelationship between alcohol and nicotine. Cross tolerance developed between ethanol and nicotine (Burch et al., 1988; Collins et al., 1988). The sensitivity to and preference for ethanol in animals appear to be correlated with responsiveness to nicotine. Rodents selectively bred for high sensitivity to ethanol stimulation were also more responsive to the effects of nicotine on locomotor activity (Bergstrom et al., 2003; de Fiebre et al., 2002). Alcohol-preferring P rats self-administered greater amounts of nicotine and exhibited more robust nicotine-seeking behavior than alcohol non-preferring NP rats (Le et al., 2006). High alcohol drinking C57BL/6 mice also showed greater preference for and consumed more nicotine solutions than the low alcohol drinking DBA/2 mice (Meliska et al., 1995). Chronic voluntary drinking of ethanol by Wistar rats enhanced nicotine-induced locomotor stimulation (Blomqvist et al., 1996). On the other hand, repeated exposure to nicotine increased voluntary ethanol drinking and preference (Blomqvist et al., 1996), whereas administration of a nicotinic receptor antagonist or partial agonist reduced ethanol drinking (Blomqvist et al., 1996; Kamens et al., 2010). Mice lacking the α7 subunit of the nicotinic receptor consumed less ethanol than wild type mice (Kamens et al., 2010). Repeated exposure to nicotine also increased ethanol-induced locomotor activation, as well as dopamine release and turnover in the limbic forebrain (Blomqvist et al., 1996; Johnson et al., 1995). Furthermore, a recent study indicated that an acute injection of nicotine 4 hr prior to testing significantly increased ethanol seeking behavior and relapse-like drinking in P rats (Hauser et al., 2011).
The mesolimbic dopamine system appears to be a common substrate mediating the action of alcohol or nicotine. Systemic administration of either drug preferentially increased dopamine release in the nucleus accumbens (NAC; Di Chiara and Imperato, 1988), one region closely involved in mediating the reinforcing and rewarding effects of drugs of abuse (Chao and Nestler, 2004; Koob and Volkow, 2010). Activation of dopamine neurons in the ventral tegmental area (VTA) appeared to mediate the dopamine stimulating effects of either ethanol (Brodie et al., 1990; Ding et al., 2009, 2011) or nicotine (Nisell et al., 1994a, b; Sziraki et al., 2002). Nicotine is rewarding within the VTA as indicated by conditioned place preference induced by intra-VTA administration of nicotine (Laviolette and van der Kooy, 2003). Ethanol or nicotine can be self-infused into the VTA, specifically the posterior VTA (pVTA), indicating the pVTA as an anatomical substrate supporting the reinforcing effects of both nicotine and ethanol (Ikemoto et al., 2006; Rodd-Henricks et al., 2000). Furthermore, acute exposure to ethanol or nicotine produced similar synaptic plasticity in the VTA, involving increased glutamate synaptic function onto dopamine neurons (Saal et al., 2003). Therefore, the mesolimbic dopamine system originating from the pVTA and projecting to the NAC could be a neural substrate underlying the interaction between alcohol and nicotine.
Given these findings, it is possible that prior exposure of one drug could influence the effects of the other drug on the mesolimbic dopamine system. One recent study using a combined microinjection-microdialysis technique demonstrated that repeated exposure of the pVTA to ethanol sensitized the responses of local dopamine neurons to the stimulating effects of a subsequent ethanol challenge (Ding et al., 2009). Therefore, the current study utilized the same technique and investigated the interaction between ethanol and nicotine on the mesolimbic dopamine system. The hypothesis to be tested was that prior exposure of the pVTA to one drug would increase the sensitivity of local dopamine neurons to the simulating effects of the other drug.
Methods and Materials
Animals
Adult female Wistar rats (weight 270 to 320 g, Harlan, Indianapolis IN, USA) were housed in temperature- and humidity-controlled rooms maintained on a reversed 12-hr light cycle (light on at 9:00 p.m.). Food and water were available ad libitum. Female rats were used because these rats maintain their head size better than male rats for more accurate stereotaxic placements and have been used in previous studies requiring precise cannula placements (Ding et al., 2009; Rodd-Henricks et al., 2000). The estrous cycle was not monitored in the present study. However, counterbalanced experiments were conducted on different days so that any effect of a given phase of the estrous cycle was distributed across experimental conditions. Protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were conducted in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Cannula and probe insertion
Stereotaxic surgery for insertion of cannulae and probes followed the procedure described previously (Ding et al., 2009). Briefly, one guide cannula was aimed 1 mm above the pVTA (−5.6 mm, ML +2.1 mm, DV −9.0 mm) for microinjections, and one was aimed 3 mm above the ipsilateral NAC shell (NACsh, AP +1.7 mm, ML +2.3 mm, DV −8.2 mm) for microdialysis, according to the brain atlas of Paxinos and Watson (1998). Stylets were inserted into cannula with a 0.5 mm extension beyond the guide cannula when no experiments were being conducted. Rats were allowed to recover for at least 5 days before the pretreatment. Animals were handled and habituated to the removal and re-insertion of stylets on a daily basis before the microdialysis. Loop-style dialysis probes with active membrane length 1.5 mm were inserted into the NACsh. Perfusion of probes started on the microdialysis day approximately 16–18 hr after probe insertion, as previously described (Ding et al. 2009).
Pretreatment and microdialysis procedure
The desired concentrations of ethanol (McCormick Distilling, Weston, MO) and nicotine ((−)-Nicotine hydrogen tartrate salt, Sigma) were mixed with artificial cerebrospinal fluid (aCSF: 120 mM NaCl, 4.75 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 2.5 mM CaCl, 10 mM d-glucose, pH 7.2–7.4). The pretreatment followed the procedure described previously (Ding et al., 2009). Briefly, an electrolytic microinfusion transducer (EMIT) system (Goeders and Smith, 1987) utilized in intracranial self-administration experiments was adapted for the microinjection. It consists of a current generator, a lead with two electrodes attached to, and a gas-tight drug reservoir (28 mm in length × 6 mm in diameter) attached with an injector cannula (28-gauge) inserted into the pVTA with 1 mm extension beyond the guide cannula. The EMIT system produces an electric current between the two electrodes immersed in the drug reservoir, which generates hydrogen gas and forces a 100 nl solution out of the reservoir over a 5-sec infusion period, as previously described (Gatto et al., 1994; Ding et al., 2009). A cable and swivel connecting the current generator and lead allowed free movement of animals during experiments. During each pretreatment day, vehicle or selected drug (100 μM nicotine or 200 mg% ethanol) were injected into the pVTA at a rate of three 5-sec infusions per min over a 10-min period. The pretreatment was repeated once a day for seven days, as previously described (Ding et al., 2009). During pretreatments, the insertion and removal of microinjection cannula did not elicit obvious behavioral changes.
Although tissue damage was foreseeable, both aCSF- and nicotine (ethanol)-pretreated rats should not exhibit differences in damage given the same number of injections received by these groups. The previous study (Ding et al., 2009) indicated that ethanol challenge injections stimulated dopamine release in both groups, suggesting that pVTA dopamine neurons were still viable after the seven-day pretreatment. Furthermore, previous studies indicated that ethanol, as well as other drugs of abuse, elicited robust reinforcing behavior after 7–10 intracranial self-administration sessions (Gatto et al., 1994; Ikemoto et al., 2006; Rodd-Henricks et al., 2000), further supporting the viability of local dopamine neurons.
Microdialysis started approximately 16–18 hr after the last pretreatment, following the procedure described previously (Ding et al., 2009). Briefly, on microdialysis day, rats were placed into Plexiglas chambers and connected to a Harvard pump with PE20 tubing. Microdialysis aCSF (140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4·7H2O, 1.0 mM MgCl2, pH 7.2–7.4) was perfused through probes at a rate of 1.0 μl/min. Baseline samples were collected following a 90-min washout period, Rats received challenge microinjections with either vehicle or a selected drug of ethanol (100 or 200 mg%) or nicotine (200 μM) for 10 min following the procedure applied for pretreatments. Samples were collected at 20-min intervals, and were frozen immediately on dry ice before being stored at −70 °C.
Sample analysis
Dopamine in samples was analyzed with a reversed-phase high performance liquid chromatography system with electrochemical detection, as described previously (Engleman et al., 2006). Briefly, samples were loaded onto an analytical column (BDS Hypersil C18, 3 μm, 150 mm × 2 mm, Keystone Scientific, Bellefonte, PA) with mobile phase (sodium phosphate 9.0 g/l, EDTA 190 mg/l, sodium octyl-sulfate 350 mg/l, and 10% acetonitrile at pH 3.0). Two 3-mm dual glassy carbon electrodes were connected in series (oxidizing potentials set at +720 and +100 mV, respectively) and coupled to an amperometric detector (EG&G Princeton Applied Research, Princeton, NJ). DA was detected at the second electrode with sensitivity set at 0.5 nA/V. The lower limit for DA detection was approximately 0.1 nM.
Histology
At the end of each experiment, rats were euthanized with an overdose of CO2 inhalation; 1% bromophenol blue was then perfused through probes in the NACsh and also injected into the pVTA. Brains were removed quickly and frozen immediately on dry ice and stored at −20 °C. Sections (40 μm) were sliced on a cryostat microtome and stained with cresyl violet for verification of injection sites and placements of probes with reference to the rat brain atlas of Paxinos & Watson (1998).
Statistical analysis
The last three baseline samples prior to challenge microinjections were averaged and used to normalize data. ANOVAs with repeated measures on time were employed on normalized time-course data. If significant differences were detected with ANOVAs (p < 0.05), post-hoc tukey’s b tests were performed to determine the individual differences.
Results
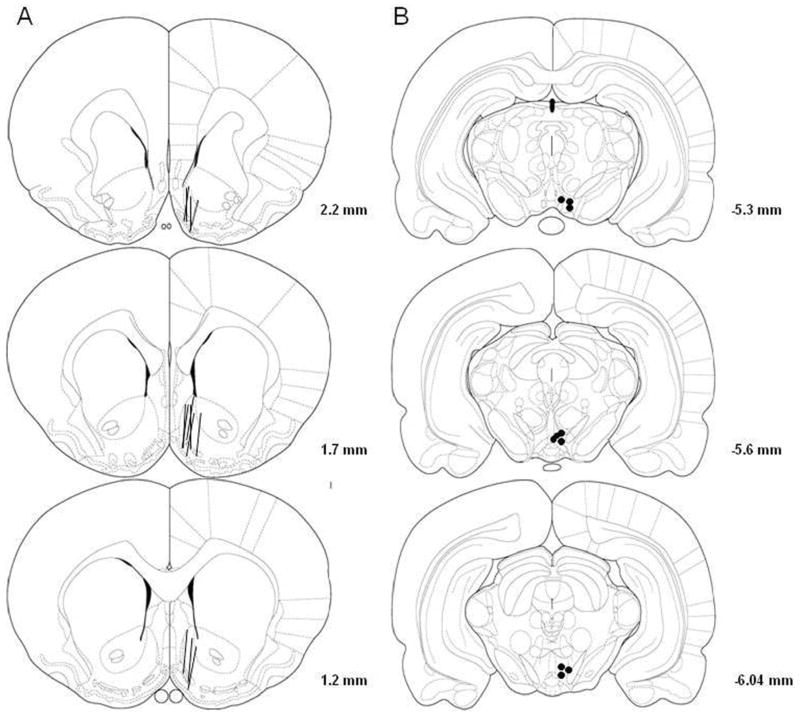
Fig. 1 shows the representative placements of probes and injection sites. The probes were mainly in the NACsh (at least 75% of the active membrane). Some probes also covered a portion of the olfactory tubercle. The injection sites were mainly inside the pVTA at coronal sections from 5.3 mm to 6.0 mm posterior to bregma (Rodd-Henricks et al., 2000). Approximately 80% of rats had correct placements and were included in the analysis.
Figure 1.
Representative placements of microdialysis probes in the nucleus accumbens shell (NACsh) and microinjection sites in the posterior ventral tegmental area (pVTA). Overlapping probes and injection sites are not shown for clarity purposes. 1A: The lines represent the 1.5-mm length of microdialysis probes in the NACsh. 1B: The open and filled circles represent microinjection sites within the pVTA.
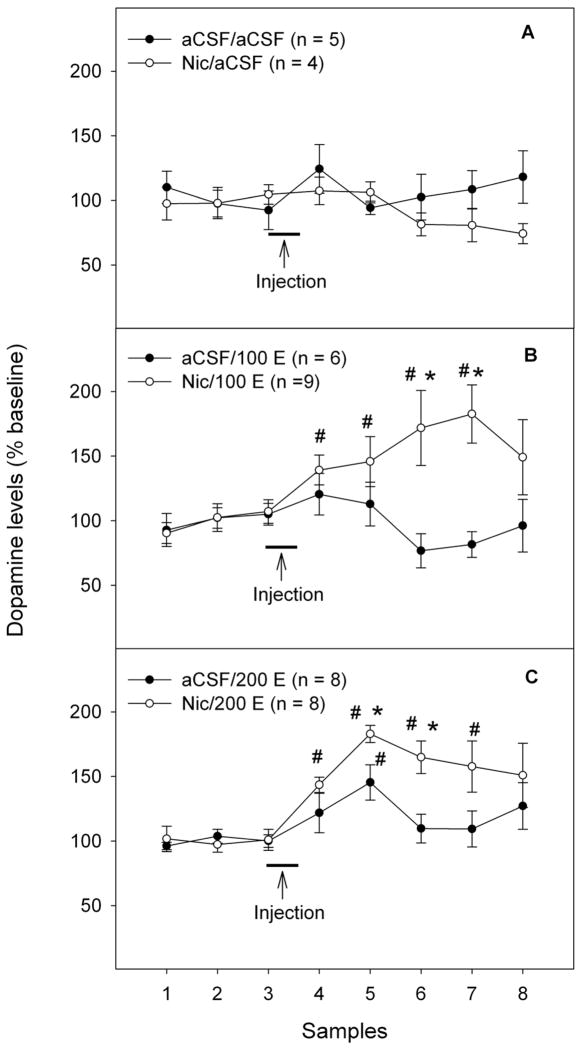
Figure 2 shows the time-course effects of challenge microinjections of aCSF or ethanol in the pVTA on extracellular DA levels in the NACsh following the 7-day pretreatment with either 100 μM nicotine or aCSF. There were no significant differences in basal extracellular dopamine levels among all groups (Table 1, F5, 34 = 0.6, p > 0.05). Using repeated measures ANOVA with ‘pretreatment’ and ‘challenge’ as between subject factors and ‘time’ as within subject factor revealed significant effects of ‘pretreatment’, ‘challenge’, ‘time’ and interaction among ‘pretreatment’ × ‘challenge’ × ‘time’ (all F values > 2.3, all p values < 0.05). Challenge injections of aCSF in either pretreatment group did not alter extracellular dopamine levels in the NACsh (Fig. 2A, p > 0.05). Challenge injections of 100 mg% ethanol (Fig. 2B) induced a non-significant increase in the aCSF-treated rats (approximately 120% of baseline, p > 0.05), but a significant increase in the nicotine-treated rats (approximately 180% of baseline, p < 0.05). Challenge injections of 200 mg% ethanol (Fig. 2C) significantly increased dopamine release in the NACsh in both aCSF- (145% of baseline, p < 0.05) and nicotine-treated rats (185% of baseline, p < 0.05). Furthermore, both doses of ethanol produced a significantly greater dopamine increase in nicotine-treated than aCSF-treated rats (Fig. 2, p < 0.05).
Figure 2.
Effects of challenge injections of ethanol (0, 100 or 200 mg%) into the posterior ventral tegmental area (pVTA) on extracellular dopamine levels in the nucleus accumbens shell following repeated exposure of the pVTA to either aCSF or nicotine (100 μM). ‘aCSF/aCSF’: group pretreated with aCSF and challenged with aCSF; ‘Nic/aCSF’: group pretreated with 100 μM nicotine and challenged with aCSF; ‘aCSF/100 E’: group pretreated with aCSF and challenged with 100 mg% ethanol; ‘Nic/100 E’: group pretreated with 100 μM nicotine and challenged with 100 mg% ethanol; ‘aCSF/200 E’: group pretreated with aCSF and challenged with 200 mg% ethanol; ‘Nic/200 E’: group pretreated with 100 μM nicotine and challenged with 200 mg% ethanol. * p < 0.05, significantly greater than levels in the corresponding aCSF-treated group. # p < 0.05, significantly greater than baseline levels.
Table 1.
Average baseline dopamine levels (nM) in each group of rats before the challenge injection (mean ± S.E.M)
| Group | Pretreatment | Challenge | n | Dopamine (nM) |
|---|---|---|---|---|
| 1 | aCSF | aCSF | 5 | 0.3 ± 0.1 |
| 2 | aCSF | 100 mg% EtOH | 6 | 0.6 ± 0.1 |
| 3 | aCSF | 200 mg% EtOH | 8 | 0.5 ± 0.1 |
| 4 | 100 μM Nicotine | aCSF | 4 | 0.4 ± 0.1 |
| 5 | 100 μM Nicotine | 100 mg% EtOH | 9 | 0.5 ± 0.1 |
| 6 | 100 μM Nicotine | 200 mg% EtOH | 8 | 0.5 ± 0.2 |
| 7 | aCSF | 200 μM Nicotine | 9 | 0.8 ± 0.2 |
| 8 | 200 mg% EtOH | 200 μM Nicotine | 9 | 1.4 ± 0.3 |
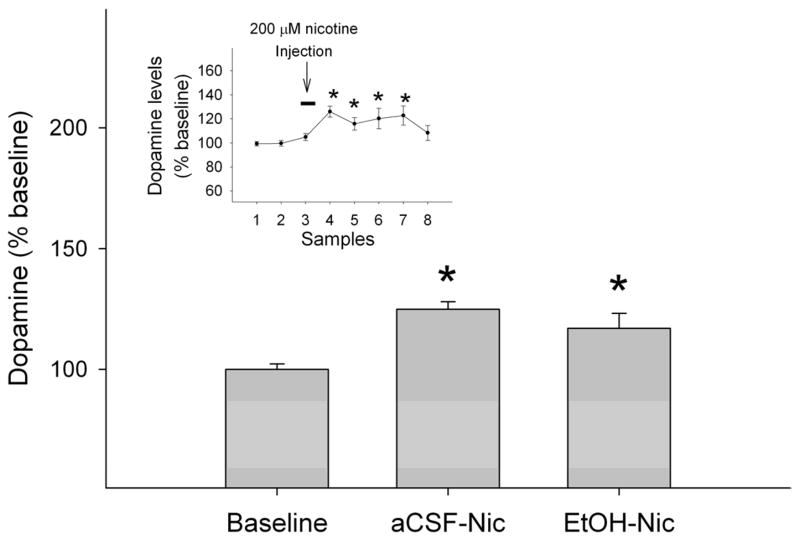
Figure 3 shows the effects of challenge injections of 200 μM nicotine in the pVTA on extracellular dopamine levels in the NACsh following the 7-day pretreatment with either aCSF or 200 mg% ethanol. There was no significant difference in the basal extracellular dopamine levels between the two groups (Table 1, t16 = 1.9, p = 0.08). Average basal extracellular dopamine levels in this study appeared to be higher than those in the above study. This might be due to the reason that these two studies were conducted at different time with microdialysis membranes from different batches that may have different recovery rates. Figure 3 shows the averaged peak values of extracellular dopamine levels in the NACsh during the 0–40 min period following the nicotine (200 μM) challenge injections. Nicotine increased extracellular dopamine levels to 125 ± 3% in the aCSF-treated group and 117 ± 6% in the ethanol-treated group (Figure 3). These levels were significantly higher than the corresponding baseline levels (p < 0.05), but were not different from each other (Fig. 3). Time-course data indicated that nicotine, microinjected into the pVTA, increased dopamine levels within the first 20 min (insert Fig. 3).
Figure 3.
Effects of challenge injections of nicotine (200 μM) into the posterior ventral tegmental area VTA (pVTA) on extracellular dopamine levels (averaged peak values of extracellular dopamine levels during the 0–40 min period after the initiation of the challenge injection) in the nucleus accumbens shell following repeated exposure of the pVTA to either aCSF or 200 mg% ethanol. ‘aCSF-Nic’: nicotine induced dopamine release in aCSF-pretreated rats; ‘EtOH-Nic’: nicotine induced dopamine release in ethanol-pretreated rats. For simplicity, baseline levels from only one group were presented because baseline levels were almost identical in the two groups. The insert shows time-course effects of microinjections of nicotine on dopamine release in the nucleus accumbens shell after pretreatments. Since there was no significant differences between groups receiving either aCSF or 200 mg% ethanol pretreatments (F1, 16 = 2.16, p = 0.16), data were collapsed across groups. * p < 0.05, significantly greater than baseline levels.
Discussion
The major findings of the current study are that repeated exposure of the pVTA to nicotine enhanced local ethanol-stimulated dopamine release in the NACsh, suggesting that prior nicotine exposure produced neuroadaptive changes within the pVTA, leading to increased sensitivity of local dopamine neurons to the stimulating effects of ethanol. On the other hand, repeated exposure of the pVTA to ethanol did not alter the stimulating effects of nicotine on pVTA dopamine neurons projecting to the NACsh. These results suggest cross-sensitization of nicotine to the effects of ethanol in the pVTA did not occur, since a previous study (Ding et al., 2009) demonstrated that this treatment with ethanol subsequently produced increased sensitivity of the pVTA to the dopamine-stimulating effects of ethanol.
Microinjections of ethanol in vehicle-pretreated rats produced a dose-related increase of extracellular dopamine levels in the NACsh (Fig. 2). These results replicated findings from a previous study (Ding et al., 2009). In both studies, extracellular dopamine levels in the NACsh induced by 200 mg% ethanol started to increase during the first 20 min and peaked around 40 min (the second sample collected) after the microinjection following the repeated (5–7 days) vehicle-pretreatment. The mechanisms for the delayed maximal response remain unknown. Noticeably, a recent study (Ding et al. 2011) indicated that acute microinjections of 200 mg% ethanol in the pVTA produced immediate maximal increase of extracellular dopamine levels in both the medial prefrontal cortex and ventral pallidum at 10 min (the first sample collected) after the microinjection. Therefore, certain changes associated with repeated pretreatments with vehicle, e.g., tissue damage, might contribute to the delayed maximal response to ethanol observed in the current and previous studies (Fig. 2C, Ding et al., 2009). The concentrations of ethanol in the pVTA are predicted to decrease rapidly due to diffusion and clearance (Gonzales et al., 1998). Since the extent of diffusion remains unknown, the contribution of areas adjacent to the pVTA to the stimulating effects of ethanol may not be ruled out. However, previous findings indicated that microinfusions of ethanol into areas adjacent to the pVTA, including the anterior VTA and substantia nigra, did not induce self-administration behavior or dopamine release in the NACsh, (Rodd-Henricks et al., 2000; Ding et al., 2009), suggesting minimal contributions from these regions.
Ethanol has been shown to have both direct and indirect stimulating effects on VTA dopamine neurons. Direct ethanol potentiation of several receptors in dopamine neurons, including 5-HT3 (Lovinger and White, 1991) and/or nicotinic receptors (Narahashi et al., 2001), may account for the initial rise of dopamine levels in the NACsh. On the other hand, the indirect effects of ethanol on neurotransmitter inputs to the VTA that regulate dopamine neurons may also contribute to the initial rise as well as prolonged elevation of dopamine levels in the NACsh, including mechanisms involving inhibition of GABA interneurons (Stobbs et al., 2004) and/or increase of local glutamate release (Xiao et al., 2009).
Repeated microinjections of nicotine in the pVTA appeared to enhance the responses of local dopamine neurons to a subsequent administration of ethanol (Fig. 2). These results are in agreement with a previous study demonstrating an enhanced dopamine release in forebrain limbic regions induced by systemic ethanol administration following 10-day repeated s.c. injections of nicotine (Blomqvist et al., 1996). It also suggests that nicotine-sensitized responses of pVTA dopamine neurons to ethanol may contribute to enhanced ethanol-induced locomotor activation and voluntary ethanol drinking following chronic nicotine exposure (Blomqvist et al., 1996; Johnson et al., 1995; Potthoff et al., 1983).
The current results suggest that repeated nicotine treatment produced adaptive changes in the pVTA. However, the substrates involved are unknown. Nicotinic receptors have been found in the VTA and on dopamine neurons (Clarke and Pert, 1985; Clarke et al., 1984). Activation of these receptors can stimulate dopamine neurons (Calabresi et al., 1989; Nisell et al., 1994a). Therefore, possible adaptations in VTA nicotinic receptors and various subunits may contribute to the observed effects. In support of this conclusion, pretreatment with nicotine for 5–7 days significantly increased nicotine binding sites in various brain regions, including an approximately 75% increase in the VTA (Ksir et al., 1985; Pauly et al., 1996). In addition, nicotine treatment increased the expression of different nicotinic receptor subunits, including α4, β2, α7 and α6 (Govind et al., 2009; Lajtha and Sershen, 2010), many of which are expressed in dopamine neurons (Azam et al., 2002). The α4β2 nicotinic receptor is likely to contribute to the increased sensitivity to ethanol observed in the current study, as evidence has shown that activation of these receptors in the VTA stimulates local dopamine neurons (Chen et al., 2003) and ethanol acts as a co-agonist to the α4β2 nicotinic receptor (Narahashi et al., 1999). The hypothesized up-regulation of VTA nicotine receptors may also alter nicotine regulation of VTA dopamine neurons. Although this possibility was not explored in the present study, studies from Rhaman et al. (2004) and Balfour et al. (1998) demonstrated that local application of nicotine in the VTA produced significantly greater increases of extracellular dopamine levels in both the VTA and NAC in rats receiving 5-day repeated treatment of nicotine compared to rats receiving repeated saline treatments. These two studies suggest that repeated nicotine treatment increased the sensitivity of VTA dopamine neurons to the local stimulating effects of nicotine.
Challenge injections of 200 μM nicotine produced a moderate but significant increase (approximately 125% of baseline) of dopamine release in the NACsh (Fig. 3). The magnitude of the effect is similar to previous findings (Nisell et al., 1994a; Tizabi et al., 2002). Noticeably, the nicotine dose was much lower in the current study (0. 09 μg in free base) compared to the Tizabi et al. (2002) study (0.25–1.0 μg in free base). Several factors may contribute to this difference. The administration paradigms were different. Tizabi et al. (2002) study involved a single injection paradigm (200 nl during a 2-min period), whereas the current study employed a pulse-like injection paradigm (100 nl/pulse injection, 3 pulse injections/min over 10 min). In addition, the Tizabi et al. (2002) study appeared to target more of the anterior VTA (AP: −5.0 mm from bregma), whereas the current study was mainly at the pVTA (AP: −5.6 mm). It has been shown that the pVTA is more sensitive to the acute reinforcing and dopamine-stimulating effects of nicotine (Ikemoto et al., 2006; Li et al., 2011).
In contrast to the nicotine pretreatment study, the ethanol pretreatment of the pVTA did not appear to alter the stimulating effects of nicotine on local dopamine neurons projecting to the NACsh (Fig. 3). A previous study using the same ethanol pretreatment procedure sensitized the pVTA dopamine neurons to the stimulating effects of ethanol, suggesting that certain adaptive changes occurred following ethanol pretreatment (Ding et al., 2009). These findings suggest that the ethanol-induced adaptations in the pVTA may not involve nicotinic receptors. Although inconclusive, a number of studies have shown that chronic systemic ethanol exposure altered the binding sites of nicotinic receptors in various brain regions (Booker and Collins, 1997; Robles and Sabria, 2008; Yoshida et al., 1982). It should be noted that some of these studies involved a prolonged chronic ethanol drinking procedures, e.g. 5–6 months of consumption (Booker and Collins, 1997; Yoshida et al., 1982). Therefore, it is possible that 7 daily exposures to ethanol were not sufficient to produce changes in nicotinic receptors. Indeed, several studies indicated that systemic ethanol exposure for a short period time (10–21 days) failed to alter binding sites of nicotinic receptors in mice (Burch et al., 1988; Collins et al., 1988). The current and Ding et al. (2009) studies also suggest that ethanol-induced adaptive changes are not involved in the stimulating effects of nicotine.
In summary, the present study demonstrated that the repeated exposure of the pVTA to nicotine increases the response of mesolimbic dopamine neurons to the stimulating effects of ethanol. This increased sensitivity could be one neurochemical mechanism contributing to the inter-relationship between smoking and alcohol abuse.
Acknowledgments
This study was supported in part by NIAAA grants AA07462, AA019366 and AA012262. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
We thank Sarah R. Hall, Erin M. Larrabee, and Joseph McClaren for their skillful technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ. Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res. 2003;27:1535–1547. doi: 10.1097/01.ALC.0000091226.18969.B9. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Booker TK, Collins AC. Long-term ethanol treatment elicits changes in nicotinic receptor binding in only a few brain regions. Alcohol. 1997;14:131–140. doi: 10.1016/s0741-8329(96)00116-4. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Burch JB, de Fiebre CM, Marks MJ, Collins AC. Chronic ethanol or nicotine treatment results in partial cross-tolerance between these agents. Psychopharmacology. 1988;95:452–458. doi: 10.1007/BF00172954. [DOI] [PubMed] [Google Scholar]
- Burling TA, Ziff DC. Tobacco smoking: a comparison between alcohol and drug abuse inpatients. Addict Behav. 1988;13:185–190. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurons in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sharples TJ, Phillips KG, Benedetti G, Broad LM, Zwart R, Sher E. The nicotinic α4β2 receptor selective agonist, TC-2559, increases dopamine neuronal activity in the ventral tegmental area of rat midbrain slices. Neuropharmacology. 2003;45:334–344. doi: 10.1016/s0028-3908(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigraostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Pert CB, Pert A. Autoradiographic distribution of nicotine receptors in rat brain. Brain Res. 1984;323:390–395. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- Collins AC, Burch JB, de Fiebre CM, Marks MJ. Tolerance to and cross tolerance between ethanol and nicotine. Pharmacol Biochem Behav. 1988;29:365–373. doi: 10.1016/0091-3057(88)90170-0. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, Dawson R, Jr, de Fiebre CM. The selectively bred high alcohol sensitivity (HAS) and low alcohol sensitivity (LAS) rats differ in sensitivity to nicotine. Alcohol Clin Exp Res. 2002;26:765–772. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans prefrentially increase synaptic dopamine concentrations in the mesolimbic systeme of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, McBride WJ, Rodd ZA. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology. 2011;216:245–255. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Intracranial self-administration methodologies. Neurosci Biobehav Rev. 1987;11:319–329. doi: 10.1016/s0149-7634(87)80017-9. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, McNabb J, Yim HJ, Ripley T, Bungay PM. Quantitative microdialysis of ethanol in rat striatum. Alcohol Clin Exp Res. 1998;22:858–867. [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine Modulates Alcohol-Seeking and Relapse by Alcohol-Preferring (P) Rats in a Time-Dependent Manner. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Hill A, Rumpf HJ, Hapke U, Meyer C. Alcohol high risk drinking, abuse and dependence among tobacco smoking medical care patients and the general population. Drug Alcohol Depend. 2003;69:189–195. doi: 10.1016/s0376-8716(02)00315-0. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Blomqvist O, Engel JA, Soderpalm B. Subchronic intermittent nicotine treatment enhances ethanol-induced locomotor stimulation and dopamine turnover in mice. Behav Pharmacol. 1995;6:203–207. [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology. 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksir C, Hakan R, Hall DP, Jr, Kellar KJ. Exposure to nicotine enhances the behavioral stimulant effect of nicotine and increases binding of [3H]acetylcholine to nicotinic receptors. Neuropharmacology. 1985;24:527–531. doi: 10.1016/0028-3908(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Lajtha A, Sershen H. Nicotine: Alcohol reward interactions. Neurochem Res. 2010;35:1248–1258. doi: 10.1007/s11064-010-0181-8. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003;8:50–59. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Doyon WM, Dani JA. Acute in vivo nicotine administration enhances synchrony among dopamine neurons. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50:619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Miller WR, Hedrick KE, Taylor CA. Addictive behaviors and life problems before and after behavioral treatment of problem drinkers. Addict Behav. 1983;8:403–412. doi: 10.1016/0306-4603(83)90041-2. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Kuriyama K, Illes P, Wirkner K, Fischer W, Muhlberg K, Scheibler P, Allgaier C, Minami K, Lovinger DM, et al. Neuroreceptors and ion channels as targets of alcohol. Alcohol Clin Exp Res. 2001;25:182S–188S. doi: 10.1097/00000374-200105051-00030. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994a;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994b;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. J Pharmacol Exp Ther. 1996;278:361–369. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Potthoff AD, Ellison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall W. Local perfusion of nicotine differentially modulate somatodendritic dopamine release in the rat ventral tegmental area after nicotine preexposure. Neurochem Res. 2004;29:1687–1693. doi: 10.1023/b:nere.0000035803.64724.17. [DOI] [PubMed] [Google Scholar]
- Robles N, Sabria J. Effects of moderate chronic ethanol consumption on hippocampal nicotinic receptors and associative learning. Neurobiol Learn Mem. 2008;89:497–503. doi: 10.1016/j.nlm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D -aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Hashim A, Lajtha A. Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens. Neurochem Res. 2002;27:253–261. doi: 10.1023/a:1014844823534. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Xiao C, Shao XM, Olive MF, Griffin WC, 3rd, Li KY, Krnjevic K, Zhou C, Ye JH. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Engel J, Liljequist S. The effect of chronic ethanol administration of high affinity 3H-nicotinic binding in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1982;321:74–76. doi: 10.1007/BF00586353. [DOI] [PubMed] [Google Scholar]