Abstract
Aneurysmal subarachnoid hemorrhage (aSAH) is a medical emergency that accounts for 5% of all stroke cases. Individuals affected are typically in the prime of their lives (mean age 50 years). Approximately 12% of patients die before receiving medical attention, 33% within 48 hours and 50% within 30 days of aSAH. Of the survivors 50% suffer from permanent disability with an estimated lifetime cost more than double that of an ischemic stroke. Traditionally, spasm that develops in large cerebral arteries 3-7 days after aneurysm rupture is considered the most important determinant of brain injury and outcome after aSAH. However, recent studies show that prevention of delayed vasospasm does not improve outcome in aSAH patients. This finding has finally brought in focus the influence of early brain injury on outcome of aSAH. A substantial amount of evidence indicates that brain injury begins at the aneurysm rupture, evolves with time and plays an important role in patients’ outcome. In this manuscript we review early brain injury after aSAH. Due to the early nature, most of the information on this injury comes from animals and few only from autopsy of patients who died within days after aSAH. Consequently, we began with a review of animal models of early brain injury, next we review the mechanisms of brain injury according to the sequence of their temporal appearance and finally we discuss the failure of clinical translation of therapies successful in animal models of aSAH.
1. Introduction
“When persons in good health are suddenly seized with pains in the head, and straightway are laid down speechless, and breathe with stertor, they die in seven days.” Hippocrates 460-37-BC, Aphorisms on Apoplexy (Clarke, 1963).
Hippocrates recognized the presentation of spontaneous subarachnoid hemorrhage followed by subsequent delayed neurological deterioration more than 2400 years ago. It was named for the rupturing of an intracranial aneurysm leading to arterial blood filling up the subarachnoid space. Today, despite the time lapse, diagnosis of aneurysmal subarachnoid hemorrhage (aSAH) continues to present daunting challenges for patients and their physicians. Becker’s study estimated that in the North America approximately 30,000 people suffer from non-traumatic, spontaneous SAH due to a ruptured aneurysm each year (Becker, 1998). This accounts for 5% of all stroke cases (Le Roux and Winn, 1998). The early mortality rate after aSAH remains high at 40%, 10-20% of whom never reach medical attention or die during transportation (Huang and van Gelder, 2002). Moreover, most victims of aSAH are in the prime of their lives; mean age 50 years (Nieuwkamp et al., 2005). The proportion of years of potential life lost due to aSAH (approximately 25%) is comparable with ischemic stroke and intracranial hemorrhage (Hop et al., 1997; Huang et al., 1990; Johnston et al., 1998; Sudlow and Warlow, 1997).
Approximately 85% of aSAH episodes are caused by rupturing of an intracranial aneurysm (Wirth, 1986), 10% fit into the pattern of the so-called perimesencephalic hemorrhage of unknown etiology, and the remaining 5% into various rare entities of congenital and acquired lesions of cerebral arteries and systemic disorders such as sickle cell disease, coagulopathies, tumors, and cocaine abuse (van Gijn et al., 2007).
Even though the clinical syndrome of aSAH varies in severity, few physicians will fail to recognize the classic and dramatic presentation of a 50-year-old female who collapses at home with sudden onset of the “worst headache of my life”, subsequently vomits, briefly loses consciousness, and is noted to have subhyaloid ocular hemorrhages (Terson syndrome) and a rigid neck. These are the symptoms of a ruptured cerebral aneurysm that violently ejects blood into the subarachnoid basal cisterns; a rigid non-expandable space restricted by the bony skull, causing severe elevation of intracranial pressure, which may exceed the blood pressure, diminish cerebral blood flow and lead to transient global arrest of intracranial circulation. Although reduced blood flow promotes hemostasis, if continued can lead to loss of consciousness and death.
The first choice diagnostic modality for patients suspected of aSAH is computed tomography without contrast enhancement, which, when patient is evaluated within the first few days after aSAH, detects blood in the subarachnoid space in over 95% of cases (Adams et al., 1985; Kassell and Torner, 1984). However, as aging blood become isodense with brain tissue, computed tomography fails to diagnose SAH in patients whose first evaluation occurs several days after a suggestive headache. Lumbar puncture with evidence of red blood cells or xanthochromia works best for diagnosing a days-old SAH (Frontera et al., 2009).
Two major complications significantly worsen the prognosis of aSAH; aneurismal rebleeding and delayed cerebral vasospasm with or without delayed ischemic neurological deficits (DINDs). Rebleeding is an early complication and occurs within the first 72-hours whereas DIND is a delayed secondary brain injury which manifests between day 3 to 12 post aSAH (Frontera et al., 2009). Other medical complications that negatively affect overall morbidity and mortality include cardiac arrhythmias and neurogenic pulmonary edema (for review, see (Bruder and Rabinstein, 2011).
Approximately 8% to 23% of ruptured aneurysms rebleed (Ando et al., 1989; Fujii et al., 1996; Gruber et al., 1997; Hillman et al., 1988; Inagawa et al., 1987; Kitsuta et al., 2006; Naidech et al., 2005; Ohkuma et al., 2001). Rebleeding occurs early and contributes to early mortality (first 72 hours, 40% to 80%) (Fujii et al., 1996). DIND remains the leading cause of delayed mortality and morbidity (Dorsch, 2002); it kills 7% patients, causes severe morbidity in another 7% (Kassell et al., 1985) and poor outcome in one third of all SAH patients (Haley et al., 1992; Tettenborn and Dycka, 1990).
DIND is a clinical diagnosis that was proposed by Vergouwen and colleagues in the consensus report in 2010 and was later refined by Wong et al. in the IMASH trial (Vergouwen et al., 2010; Wong et al., 2010b). DIND is defined as a “acute or sub-acute new focal neurological deficit (motor or speech deficit) that had developed after aSAH or a decrease on Glasgow coma score of ≥2 points lasting >6 hours that is not related to treatment (coiling or clipping) complications, re-bleed, progressive hydrocephalus, electrolyte or metabolic disturbance, or infection” (Vergouwen et al., 2010; Wong et al., 2010b). This is a subjective exclusion diagnosis that implies worsening prognosis of unknown etiology despite favorable or good initial presentation. DIND can be difficult to assess in poor-grade, comatose patients, where variations in examination may be subtle or imperceptible.
The pathogenesis of DINDs is poorly understood and to date no single mechanism by itself or in combination with others is identified as its source. Delayed vasospasm is present in some but not all SAH patients with DIND. The spasm of large cerebral arteries of the circle of Willis was first noted in 1951 by Ecker and Riemenschneider who, while reviewing the angiograms of aSAH patients, observed that “spasm was maximal at the lesion but extended several cm along adjacent arteries in lesser degree” (Ecker and Riemenschneider, 1951). Although Ecker and Riemenschneider did not correlate arterial spasm with clinical deterioration they noted that spasm disappeared after a few weeks in patients who survived and suggested that it may play a considerable role in the production of intra-aneurismal thrombosis and may produce unfavorable effects by impairing the blood flow to the area of brain supplied by the affected artery (Ecker and Riemenschneider, 1951). Since then the advanced radiological technology, a digital subtraction cerebral angiography, has confirmed that delayed vasospasm appears in approximately 70% of aSAH survivors 3 to 12 days after the initial hemorrhagic event (Alaraj et al., 2009; Eddleman et al., 2009). As the time of vasospasm development coincides with the period of DINDs, DINDs have traditionally been considered the direct result of delayed vasospasm. Consequently, majority of basic and clinical research has been directed towards finding strategies against delayed vasospasm with hope to prevent DINDs and to improve outcome. However, a limited and often controversial positive effect of such therapies in preventing DINDs proves that this approach does not provide expected results (explained below and for review see (Sehba et al., 2011)).
A search of aSAH literature (animal and clinical) provides a large body of evidence that suggests that presence of delayed vasospasm is not a prerequisite for DINDs and poor outcome. Early research in this area was pioneered mostly by Weir and colleagues and was carried out on two non-human primate models of aSAH. The first model mimicked SAH by injecting autologous blood into the cisterna magna and the second by placing blood clot around major arteries of circle of Wills (Weir et al., 1970). The blood injection produced early hemodynamic changes associated with aSAH (explained below) including severe elevation in intracranial pressure, immediate reduction in cerebral blood flow, and cerebral perfusion pressure (Rothberg et al., 1980), as well as a moderate vasospasm with high mortality and severe neurological deficits (Boisvert et al., 1978; Echlin, 1971; Rothberg et al., 1980; Weir et al., 1970). Furthermore, Weir and colleagues found that none of the early hemodynamic changes occurred upon clot placement and whereas majority of animals developed severe vasospasm, only few 6.7 to 33% of the animals developed mild neurological deficits and mortality remained low; below 14% (Espinosa et al., 1984; Handa et al., 1987; Nosko et al., 1987; Stoodley et al., 2000). Others report similar results (Zhang et al., 2001). n their own words “the degree of vasospasm in the animals which were dead the following day and the animals which were sitting up and eating normally was identical in the post-SAH angiograms” (Weir et al., 1970). Similar observations are made in other species using other aSAH models. Landau et al. in a rabbit puncture model observed that some animals developed severe spasm yet did not display any obvious neurological deficits whereas others that developed neurological deficit were without vasospasm (Landau and Ransohoff, 1968). Weir et al., further showed that removal of blood clot could prevent and reverse delayed vasospasm (Handa et al., 1987; Nosko et al., 1987; Stoodley et al., 2000; Zhang et al., 2001). These findings are in congruence with clinical studies where the occurrence of angiographic vasospasm correlates with the amount of blood present in the basal cisterns (Dupont et al., 2009; Fisher et al., 1980).
Clinical studies also support dissociation between DIND and vasospasm. Wilkins et al. reported no difference in responsiveness and hospital mortality in aSAH patients with and without vasospasm (Wilkins et al., 1968). In fact, they noted that in many cases vasospasm was present in the presence of clinical improvement (Wilkins et al., 1968). Other investigators have also found that delayed vasospasm does not necessarily lead to cerebral infarction after SAH (Rabinstein et al., 2004), as cerebral infarcts occur even in the absence of vasospasm (Carlson and Yonas, 2009; Dankbaar et al., 2009; Frontera et al., 2009; Parsons et al., 2007; Stein et al., 2006b). Over all, vasospasm literature indicates that of the 70% of aSAH survivors that develop delayed vasospasm, only 20-30% actually suffer from DINDs (Alaraj et al., 2009; Eddleman et al., 2009). This dissociation between the presence of vasospasm and development of a delayed ischemic injury is also found in clinical trials. Agents such as Nimodipine (a calcium channel blocker) which reduce the incidence and severity of delayed ischemic injury and improve neurological outcome in aSAH patients do not relief angiographic vasospasm (Biondi et al., 2004; Deshaies et al., 2009; Petruk et al., 1988; Philippon et al., 1986; Pickard et al., 1989). In contrast, agents such as Clazosentan, an ET-1A antagonist, which reduce the incidence of vasospasm do not improve neurological outcomes (Kramer and Fletcher, 2009; Macdonald et al., 2011; Macdonald et al., 2008; Nogueira et al., 2007; Shaw et al., 2000; Vajkoczy et al., 2005; Vergouwen, 2009). This failure in part may involve deleterious side effects associated with most drugs (pulmonary complications for Clazosentan) that counterbalance their therapeutic benefits (Macdonald et al., 2011) or aggressive use of rescue therapy that may dilute the overall results (Macdonald et al., 2011). Rescue therapies (intravenous vasopressor with or without fluid therapy, or intra-arterial vasodilator or balloon angioplasty) are associated with significant morbidity and can have a considerable effect on the large-vessel component of angiographic vasospasm. Consequently, a drug that minimizes the need and amount of rescue therapy is desirable.
All of the above findings indicate that pathophysiology of DINDs is more complicated than previously assumed. Furthermore, recent studies suggest that genetic variations may predispose some patients to development of vasospasm and DIND while protect others from it. For example; aSAH patients with polymorphisms in apolipoprotein E (APOE; neurotrophic and neuroprotective) and endothelial nitric oxide synthase (eNOS; synthesis nitric oxide; a potent vasodilator) are at greater risk of vasospasm and worse functional outcome (Alexander et al., 2009; Ko et al., 2008; Kokubo et al., 2000; Lanterna et al., 2005; Leung et al., 2002; Starke et al., 2008). Whereas a gain-of-function; reduced risk of DIND, is observed in aSAH patients with polymorphisms of the cystathionine β-synthase (metabolizes homocysteine to hydrogen sulfide; a vasodilator, regulator of neuronal ion channels and intracellular signaling pathways) (Grobelny et al., 2011). Another factor that is gaining recognition in pathogenesis of DIND is brain injury that occurs during the early phase of SAH. Increasing number of studies indicate that mechanisms deleterious to brain activate at aneurysm rupture, evolve with time and contribute to overall outcome of aSAH (Inagawa, 1997; Nau et al., 2002; Stein et al., 2006a; Stoltenberg-Didinger and Schwartz, 1987).
2. Animal Models of Acute (Early) aSAH
Controllable and reproducible animal models that simulate human condition closely are essential for studying the pathophysiology and developing a treatment for any disease. Unfortunately, the nature of the aSAH (aneurysm rupture) is a sudden, unpredictable phenomenon and consequently most information on events that occur at clinical aSAH comes from observations made during rebleeds in patients. A number of investigators have used this information to develop and characterize animal models of aSAH (Barry et al., 1979; Bederson et al., 1995; Delgado-Zygmunt et al., 1992; Honma et al., 1989; Kader et al., 1990; Khajavi et al., 1997; Ram et al., 1991; Solomon et al., 1985; Veelken et al., 1995; Wanebo et al., 1998). These animal models are accepted as mimics of clinical aSAH and are widely used to study early and delayed brain injury after aSAH (Lee et al., 2009b; Megyesi et al., 1997; Prunell et al., 2003). Broadly these models can be divided into two categories: an injection model and a vascular perforation model. Below we discuss them individually.
2.1. The Injection Model
Blood released upon aneurysm rupture at SAH fills subarachnoid cisterns enveloping and compressing major conductive arteries (Figure-1A and B). Based on this fact, an injection model mimics aSAH by introducing autologous fresh blood under adequate pressure into the subarachnoid space. Since its introduction, an injection model has been adapted and modified in number of ways to ensure that injury induced is reproducible, is of desired intensity, and is similar to human aSAH. The modifications of injection model have used fresh blood, blood products, and blood clots for injection (Echlin, 1971; Peterson et al., 1990b). The most common site for blood injection is the cisterna magna (Ram et al., 1991; Solomon et al., 1985). Other sites include prechiasmatic cistern (Hansen-Schwartz et al., 2003), vicinity of an intracranial (Tsuji et al., 1996) or extracranial artery (Megyesi et al., 1997; Pickard et al., 1984). The volume of blood and infusion pressure is preselected and kept constant to ensure reproducibility of hemorrhage intensity (Hansen-Schwartz et al., 2003; Matz et al., 2000). To examine consequences of acute SAH, a single injection is sufficient. In contrast, to study delayed vasospasm double injection is necessary, in which the same volume of blood is injected twice through the same injection site 24 or 48 hours apart (Gules et al., 2002; Meguro et al., 2001b). The injection model has been modified by many investigators. One modification presented previously in cats by Trojanowski and colleagues and more recently in rabbit by Marbacher and colleagues creates aSAH by extracranial-intracranial shunting of blood from the subclavian artery into the cistern magna. Bleeding is stop by closing the three way stopcock when the intracranial pressure stabilizes (Marbacher et al., 2010; Trojanowski, 1982a). This modified model is considered more appropriate for studying a delayed and not acute SAH.
Figure 1. Experimental SAH.
A shows an image of rat brain post SAH. Note thick blood clot around circle of Willis. B shows quantitative analysis of blood distribution across brain after SAH. Note most blood accumulates around base of the cortex (BC). CC: convexity cortex, IH: interhemispheric space (adapted from (Schwartz et al., 2000a), LV: lateral ventricle. C represents a typical physiological recording of SAH. Note intracranial pressure (ICP) increases and cerebral blood flow (CBF) fall at SAH. Mean arterial blood pressure (MAP) fluctuates at SAH but returns to basal values soon after. L-CBF: left CBF, R-CBF: right CBF.
Advantages of the injection model are an easy control of hemorrhage intensity and the use of saline injection for the control group. Disadvantage is a lack of arterial stress that a rupture of aneurysm creates in human aSAH. There is also a possibility that blood injected would not remain in the subarachnoid space and get dispersed in the intracranial space and in the spinal canal diluting blood and diminishing deleterious effects of the clot presence in the subarachnoid cisterns. However, this can be addressed by tilting the head of the animal during and after blood injection to ensure that blood pools in the subarachnoid space. The angle and the time of head tilt vary among species.
Hemodynamic changes upon blood injection include increase in ICP and fall in CBF (see Figure-1C and below). The intensity of SAH in this model however, is of lesser degree compared with endovascular model (Gules et al., 2002; Prunell et al., 2003). Nevertheless, the ability to have a proper saline-injected control and investigator control of hemorrhage intensity has made this model quite popular and extensively used.
2.2. The Arterial Puncture Model
The rupture of an intracranial aneurysm is a key event of aSAH. The arterial puncture model mimics this initial event. SAH is created by puncturing an intracranial artery. The arteries commonly ruptured to create aSAH include the basilar artery (Barry et al., 1979; Kader et al., 1990) and the bifurcation of internal carotid artery (Bederson et al., 1995; Veelken et al., 1995). A puncture model has been used to study both acute and delayed effects of SAH. Although frequently used, a puncture model suffers from the major drawback of poor control of hemorrhage intensity leading to wide variation of data making interpretation of results challenging and requiring significant number of animals to assure statistical power for a study.
The size of filament and force used to rupture an artery play important role in SAH intensity (Schwartz et al., 2000a). Studies show that SAH intensity is proportionate to filament size; the smaller the diameter of filament (such as 3′O) the smaller the intensity. A complication that can associate with the puncture model is a superimposed regional ischemia. This problem usually arises when the filament is left in the artery for some time obstructing the normal arterial perfusion. Control group in this model consists of sham-operated animals, which undergo the same surgery as SAH animals including insertion of a filament into the intracranial artery with the exception of perforation. However, a lack of saline injection that helps isolating the effects of blood from those from ICP elevation has led to questioning the adequacy of this control (Schwartz et al., 2000a)
A number of investigators have compared SAH models to find the one that best mimics the human aSAH (Lee et al., 2009b; Prunell et al., 2003). There is an overall agreement that whereas injection model is easy to perform, allows better control of SAH intensity and has low mortality rate, perforation model fits the human condition the best and is better suited for research investigating early injury (Lee et al., 2009b).
3. Early Brain Injury after aSAH (first 72 hours)
A large body of animal and significantly smaller human autopsy data establishes that brain injury initiates within minutes after the initial bleed (Bederson et al., 1998; Friedrich et al., 2010a; Inagawa, 1997; Nau et al., 2002; Stein et al., 2006a; Stoltenberg-Didinger and Schwartz, 1987). Since in typical clinical scenarios there is a delay in patients reaching medical attention after aSAH, most of the information about the first hours comes from animal studies.
The nature of early brain injury after aSAH appears to be ischemic (Cahill et al., 2006b; Sehba and Bederson, 2006b; Trojanowski, 1982b). Microdialysis studies indicate that cerebral ischemia starts early after aSAH and is associated with decreased survival. In both blood injection and vessel perforation rat models, an increase in cerebral lactate/pyruvate ratio and glutamate concentration occurs within 15 minutes after aSAH (Gewirtz et al., 1999; Schubert et al., 2008a). In patients, similar findings were reported 24-48 hours after aSAH (Enblad et al., 1996; Samuelsson et al., 2009a; Schulz et al., 2000) and often preceded delayed vasospasm and neurologic deterioration (Sarrafzadeh et al., 2002). Interestingly, patients who remain asymptomatic after aSAH do not develop significant increase in cerebral ischemia-related metabolites (Sarrafzadeh et al., 2002). Hence, early detection of cerebral ischemia may prognosticate the course of aSAH and help individualize therapeutic strategy to prevent early mortality and development of delayed ischemic injury.
Below we review events that occur within the first 72 hours after SAH (Figure-2).
Figure 2. Early alterations after SAH.
Events that occurs after SAH contribute to over all outcomes are listed.
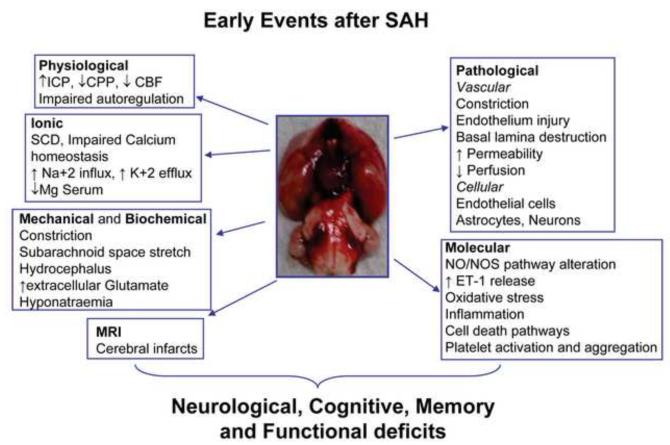
4. Early Events after aSAH
4.1. Physiological Changes
Rapid changes in intracranial pressure (ICP), cerebral perfusion pressure (CPP), and cerebral blood flow (CBF) occur after aSAH and are closely followed by impairment of CBF autoregulation (see Figure-1C) (Bederson et al., 1995; Bederson et al., 1998; Kamiya et al., 1983; Rasmussen et al., 1992; Travis and Hall, 1987; Trojanowski, 1982b).
4.1.1. Intracranial Pressure (ICP)
ICP rises as blood is released upon aneurysmal rupture and results in what most patients describe as the “the worst headache of my life” (Nornes and Magnaes, 1972). Experimental studies show that ICP peaks to a value near diastolic blood pressure and then falls and settles to a value that is near but above the baseline (Bederson et al., 1995; Trojanowski, 1982b; Voldby, 1988). In some cases, ICP remains elevated, possibly due to mass effect from enlarging hematoma or the development of acute hydrocephalus (Asano and Sano, 1977; Kamiya et al., 1983; Kuyama et al., 1984; Voldby, 1988). Animal and clinical studies link ICP increase to the hemorrhage volume, obstruction of CSF outflow, partial and/or diffuse vasoparalysis, and distal cerebral arteriolar vasodilation (Brinker et al., 1990; Grote and Hassler, 1988; Kosteljanetz, 1984; Le Roux et al., 1996; Nornes, 1973). In most cases the severity of increase in ICP can be correlated with the outcome (Heuer et al., 2004; Nagel et al., 2009a; Pereira et al., 2007; Westermaier et al., 2009). Severe ICP increase is also associated with changes in cerebral metabolism (Samuelsson et al., 2009b; Sarrafzadeh et al., 2005), inflammation (Graetz et al., 2010; Sehba et al., 2008), a fall in cerebral blood flow (Fukuhara et al., 1998; Hayashi et al., 2000; Losiniecki and Zuccarello, 2008), and development of early and delayed cerebral ischemia (Gambardella et al., 1998; Miranda et al., 2006; Soehle et al., 2007). CSF drainage with the goal of controlling the increased ICP is used to manage high-grade aSAH patients. More recently, decompressive craniectomy has been advocated to control the increased ICP in aSAH patients; however, its benefit remains to be determined (Burger et al., 2008; Jaeger et al., 2003; Nagel et al., 2009b).
4.1.2. Cerebral Perfusion Pressure (CPP)
CPP falls profoundly during, and immediately after aSAH (Fisher, 1975; Nornes, 1973, 1978). Decreased CPP contributes to early ischemic brain injury but is not solely responsible for it (Bederson et al., 1995). Experimental studies indicate that decrease in CPP at the onset of aSAH is not sufficient to cause perfusion arrest (Dorsch et al., 1989; Kuyama et al., 1984; Steiner et al., 1975). In addition, CPP reductions in animals and in humans are not always associated with poor neurological outcome after aSAH (Heuer et al., 2004; Jakubowski et al., 1982).
4.1.3. Cerebral Blood Flow (CBF)
Animal studies demonstrate that CBF falls after aSAH and may or may not recover depending upon the severity of the bleed (Bederson et al., 1995). Aneurismal SAH patients who are conscious at admission display a slight reduction in CBF while patients who are unconscious exhibit severe global hypoperfusion (Jakobsen, 1992). In the rat arterial puncture model, CBF reduction is accompanied by constriction of large cerebral blood vessels that normally are 1500 to 500mm in diameter (Bederson et al., 1998; Sehba et al., 1999). In humans, cerebral arteriography shows little evidence of acute arterial spasm (Grosset et al., 1993; Weir et al., 1978). Hence, in humans, initial fall in CBF is attributed to a period of “no-reflow”, due to elevation of ICP (Brinker et al., 1992; Grote and Hassler, 1988). The term “no-reflow” was coined by Ames in 1968 to describe a period of lack of blood filling the vessels directly after ischemia (Ames et al., 1968) and was first used by Asano and Sano in 1977, to describe early perfusion deficits due to increased ICP in SAH animals (Asano and Sano, 1977). Other factors that contribute to the initial CBF fall in humans include presence of subarachnoid blood (Clower et al., 1994; Ebel et al., 1996; Solomon et al., 1985; Umansky et al., 1983), hypovolemia caused by cerebral salt wasting and excessive urinary output (Solomon et al., 1988), and disturbed autoregulation (Ebel et al., 1996; Jakubowski et al., 1982; Kamiya et al., 1983; Rasmussen et al., 1992). The early CBF reduction after aSAH is accompanied by reduced cerebral metabolic rate of oxygen (Frykholm et al., 2004; Hayashi et al., 2008; Hayashi et al., 2000; Jakobsen et al., 1990; Kawamura et al., 2000) and signs of clinical deterioration (Kobayashi et al., 1979; Miranda et al., 2006).
4.1.4. CBF Autoregulation
CBF autoregulatory mechanisms are frequently impaired after aSAH (Ebel et al., 1996; Jakubowski et al., 1982; Kamiya et al., 1983; Rasmussen et al., 1992). In patients, this impairment is most pronounced during the first 72 hours after aSAH, correlates well with the severity of aSAH and affects both aspects of CBF autoregulation; the pressure autoregulation (response to change in systemic blood pressure) and chemoregulation (response to change in partial pressure of carbon dioxide) (Schmieder et al., 2006). There is some evidence that indicates that impairment of CBF autoregulation post aSAH may have dissociative characteristics; i.e. chemoregulation remains impaired even when pressure autoregulation has recovered (Schatlo et al., 2008). It is interesting to note that patients with initially preserved autoregulation are at less risk of developing DINDs compared with patients with an initially disturbed autoregulation (Lam et al., 2000; Ratsep and Asser, 2001). In many cases autoregulation impairment precedes vasospasm (Lang et al., 2001) and worsens in the presence of vasospasm (Lam et al., 2000; Lang et al., 2001). Disturbance in autoregulation after aSAH may result from acidic cerebral environment (Voldby et al., 1985), hydrocephalus (Heilbrun et al., 1972; Kamiya et al., 1983), and impaired endothelium-dependent control of vessel diameter, all of which are present during the early phase of aSAH (Gewirtz et al., 1999; Kamiya et al., 1983; Park et al., 2001; Sehba et al., 1999; Sugi et al., 1975).
4.2. Ionic Changes
Ionic distribution within and across brain cell is rapidly impaired after aSAH and promotes disturbance in brain electrical activity.
4.2.1. Cortical Spreading Depolarization (CSD)
Cortical spreading depolarization (CSD) is a wave of mass neuronal depolarization in the cortex associated with the progressive breakdown of ion homeostasis; massive neuronal of sodium and calcium influx. The increasing body of evidence from experimental and human aSAH studies indicate that changes in ionic contents of neurons leading to CSDs occur early and late after aSAH, and contribute to acute pathophysiology and the later occurring DINDs (Dreier et al., 2000; Dreier et al., 1998; Dreier et al., 2006; van den Bergh et al., 2002).
Depression of cortical activity upon placement of blood or blood products in the subarachnoid space of cats was reported by Levitt et al. in 1971 (Levitt et al., 1971). However, occurrence of CSDs after SAH was first described by Hubschmann and colleagues who identified self-propagating waves of cellular depolarization over cerebral cortex upon placement of blood or blood products in the subarachnoid space of cats (Hubschmann and Kornhauser, 1980, 1982). The same group later reported that cortical depolarization is accompanied by a profound decrease in extracellular calcium, accumulation of extracellular potassium and a transient depression of spontaneous electro-cortical activity and speculated that it may play an important role in the development of vascular spasm (Hubschmann, 1987). More recently, Dreier et al. used artificial CSF that mimicked the composition of SAH-CSF to generate CSDs in rats and noted that the hemodynamic response to CSD was changed in presence of subarachnoid erythrocyte products. CSD caused spreading ischemia (inverse hemodynamic response) in presence of SAH-CSF instead of spreading hyperemia (normal hemodynamic response) under physiological conditions (Dreier et al., 1998). Such spreading ischemias led to cortical infarction in contrast to normal CSDs that associate with spreading hyperemia (Dreier et al., 2000). In human SAH, CSDs can occur as clusters or as isolated events (Dreier et al., 2009). The Cooperative Study on Brain Injury Depolarization (COSBID) group in their initial studies on aSAH patients performed after craniotomy noted that clustered CSDs occurred at the start of neurological deterioration (Dreier et al., 2006). More recently this group examined cortical electrical activity, regional blood flow, and measured tissue oxygenation in 13 aSAH patients for two weeks after surgery and found that CSD clusters are located in close proximity to the injured brain area and are associated with prolong hypoperfusion and ischemia (Dreier et al., 2009). Electro-cortical and regional cerebral blood flow recordings provided evidence of three different neurovascular responses to CSD in SAH patients similar to the findings in animals: (1) the normal response, (2) the inverse response and (3) neurovascular uncoupling (Dreier et al., 2009). Some of mechanisms implicated in a development of CSDs after aSAH include subarachnoid presence of oxyhemoglobin (Petzold et al., 2003) and hemolyzed blood products, elevated extracellular potassium (Dreier et al., 2002; Hubschmann and Kornhauser, 1980, 1982; Levitt et al., 1971; Petzold et al., 2008), reduced cerebral NO (Petzold et al., 2008; Windmuller et al., 2005), increased glutamate receptor activity (Petzold et al., 2005b), and increased endothelin-1 concentration (Petzold et al., 2003). The exact contribution of these mechanisms in development of CDS remains to be determined.
4.2.2. Impaired Calcium Homeostasis in Cerebral Vessels
Cellular calcium homeostasis is impaired in brain parenchyma and in cerebral endothelial and smooth muscle cells early after aSAH (Hubschmann, 1987; Hubschmann and Kornhauser, 1982; Kohno et al., 1991; Sakaki et al., 1989). Calcium homeostasis is essential for physiological cell function and depends on adequate supply of adenosine triphosphate (ATP) for maintaining ionic gradients across the cell membrane. Experimental studies suggest that a pathological rise in intracellular calcium concentration in both endothelial and smooth muscle cells of cerebral vessels occur early after aSAH (Ishiguro et al., 2008; Kohno et al., 1991; Meguro et al., 2000; Minato et al., 1996; Wang et al., 1994). For example Kohno et al. using a blood injection canine model found that intracellular calcium concentration in the smooth muscle cell of basilar artery increase 15 minutes after aSAH (Kohno et al., 1991). The mechanisms involved in early calcium rise are studied and include: a marked influx of calcium via voltage sensitive calcium channels opened during membrane depolarization (Ishiguro et al., 2008), activation of NMDA receptor by glutamate released during ischemia leading to excessive release of calcium ions from endoplasmic reticulum and from mitochondria, increased calcium influx through agonist dependent calcium channels, rapid depletion of ATP stores during global ischemia (Enblad et al., 1996; Gewirtz et al., 1999; Schubert et al., 2008a; Schulz et al., 2000) leading to a depletion of energy for ATPase-dependent sodium and calcium efflux and potassium influx (Hubschmann and Kornhauser, 1980, 1982; Kohno et al., 1991; Wang et al., 1994). Clinical and experimental studies show that early ionic disturbances can last for days after aSAH (von Holst and Mathiesen, 1990; Wang et al., 1994). Moreover, experimental studies suggest that the pathological rise in intracellular calcium can promote persistent contraction of cerebral arteries, release of neurotransmitters including glutamate, activation of various enzymes including those that are detrimental to cell such as iNOS and enzymes mediating cell death (Debdi et al., 1993; Hubschmann, 1987; Meguro et al., 2000; Minato et al., 1996; Sakaki et al., 1989). Hence, calcium channel blockers (such as Nimodipine) are frequently used after surgical management of ruptured aneurysm to prevent severity of ischemic deficits in aSAH patients (Tomassoni et al., 2008).
4.2.3 Decreased Serum Magnesium
In 1982 Altura and Altura suggested that a magnesium loss may occur and contribute to traumatic and non traumatic brain injury (Altura and Altura, 1982). Since then the same group and others have found that serum and CSF magnesium level decreases after experimental and clinical aSAH (Altura et al., 1995; Altura et al., 1997; Miura, 1988; van den Bergh et al., 2003). It is found that the total serum magnesium level remains unchanged and the biologically active free ionized form of magnesium falls upon brain injury (Memon et al., 1995). Decrease in free magnesium occurs within 30 minutes after subarachnoid bleeding in animals (Altura et al., 1995) and 1-8 hours after hemorrhagic strokes in humans (Altura et al., 1997). Approximately 38% of patients admitted within 48 hours after aSAH exhibit abnormally low serum magnesium (van den Bergh et al., 2003). Magnesium is a physiological antagonist of calcium and plays an important role in maintaining intracellular calcium concentration. In addition, it maintains intracellular calcium level by keeping a block on NMDA receptor activation. The pharmacological actions of magnesium involve vasodilation, inhibition of platelet aggregation, inhibition of excitatory amino-acids release and inhibition of ET-1 synthesis (Berthon et al., 2003; McLean, 1994; van den Bergh et al., 2004). Magnesium mediated vasodilation involves the release of endothelial NO (Yang et al., 2000), increase in synthesis and release of prostacyclin (Nadler et al., 1987), and reduction in calcium influx and competition for calcium binding sites at calmodulin, rendering calmodulin unable to stimulate myosin light chain kinase to promote contraction (McLean, 1994). Consequently, decrease in magnesium after aSAH can lead to unchecked increase in intracellular calcium, increase in neurotransmitters release, activation of calcium dependent enzymes, vasoconstriction and neuronal damage (Miura, 1988; van den Bergh et al., 2004).
The effect of increasing serum magnesium levels against early brain injury after aSAH has been examined (Altura et al., 1995; Miura, 1988; Pyne et al., 2001; van den Bergh et al., 2002). Whereas pilot studies showed that increasing magnesium in serum and CSF of aSAH patients is safe and well tolerated, clinical trial failed to demonstrate any clinical benefits of this treatment (Wong et al., 2010a). A low CSF penetration of peripherally infused magnesium or earlier administration may be required to obtain benefits of magnesium therapy post aSAH.
4.2.4. Hyponatremia
Hyponatremia is a biochemical change that either present in aSAH patients at admission or develops in 1-2 days from ictus (Berendes et al., 1997). Approximately 10% to 30% of aSAH patients suffer from hyponatremia (Naval et al., 2006; Wartenberg et al., 2006). Hyponatremia in aSAH patients is difficult to treat and is associated with the risk of developing cerebral ischemia and infarctions (Hasan et al., 1990; Wijdicks et al., 1985). The exact mechanism underlying aSAH-related hyponatremia is not fully understood, however, a role of cerebral salt-wasting syndrome (CSWS) and inappropriate secretion of anti-diuretic hormone (SIADH) is suggested (Bruder et al., 2009; Doczi et al., 1981).
CSWS causes fluid depletion and compensatory hypersecretion of ADH. Many studies report an early increase in humoral (such as brain natriuretic peptide and atrial natriuretic peptide) factor-induced natriuresis in patients after aSAH (Audibert et al., 2009; Berendes et al., 1997; Espiner et al., 2002; Isotani et al., 1994; Nakamura et al., 2009; Tomida et al., 1998). In SIADH, on the other hand, water retention results in hypertonic urine, hypo-osmolar serum, and apparent euvolemia without renal, adrenal, or thyroid diseases (Kao et al., 2009). In a study consisting of 179 aSAH patients, Sherlock et al. found that in 62% of aSAH patients, hyponatremia was related to SIADH, and in 6.5% to CSWS. They concluded that SIADH is the most common cause of hyponatremia (Sherlock et al., 2006).
Distinguishing CSWS from SIADH as the source of hyponatremia can be difficult since they share many biochemical parameters, including elevated serum ADH (Kao et al., 2009). However, this distinction is crucial for formulating a rational treatment strategy, which goes in two opposite directions: fluid and sodium restrictions for CSWS, large sodium intake for SIADH. The volume of blood may help distinguish between these two situations; hypovolemia for CSWS and normal or increased volemia for SIADH (Audibert et al., 2009; Ellison and Berl, 2007).
4.3. Mechanical and Biochemical Changes
Mechanical stress and biochemical changes occur at aSAH and influence the outcome. These changes are as follows:
4.3.1. Mechanical Stress
Mechanical stress is probably the first stress exert on brain upon the aneurysm rupture. Animal studies indicate that stress constricts the artery as its wall is ruptured and stretches the subarachnoid space due to pooling of blood (Arutiunov et al., 1970; Kapp et al., 1968; Simeone et al., 1968). The stretching of the subarachnoid space is mechanically transferred to the nearby vessels and promotes constriction of the arteries with normal walls (Arutiunov et al., 1974). Over the course of its presence subarachnoid blood clot associates with the early brain injury in animals (Schwartz et al., 2000a) and with the severity of the delayed spasm in aSAH patients (Fisher et al., 1980). Hence, immediate events involved by aSAH-induced mechanical trauma have early and delayed consequences.
4.3.2. Hydrocephalus
Hydrocephalus is one of the most common mechanical complications after aSAH (Diringer, 2009). In animals, signs of hydrocephalus are reported as early as 60 minutes after aSAH and are associated with the intensity of CBF reduction and ischemia (Kamiya et al., 1983; Kuyama et al., 1984; Milhorat, 1987). Patients with aSAH who develop hydrocephalus are at greater risk of neurologic impairment and mortality than patients without hydrocephalus (Suarez-Rivera, 1998).
In humans, three phases of aSAH-related hydrocephalus are recognized. These phases are separated by time of presentation from ictus; acute (≤3 days), subacute (4–13 days), and chronic (≥14 days) (Demirgil et al., 2003; Vale et al., 1997). Approximately 20% to 30% aSAH patients suffer from acute phase hydrocephalus (Diringer, 2009; Milhorat, 1987). Most cases of aSAH complicated by acute hydrocephalus have large bleeds, poor cerebral perfusion, reduced CBF (van Asch et al., 2010) and present with poor clinical grade and higher Fisher Scale scores on admission (Brisman and Berenstein, 2004; Dorai et al., 2003). Milhorat studied division of clinical status in aSAH patients with acute hydrocephalus and found Grade I in 3%; Grade II in 5%; “Good” Grade III in 21%, “Bad” Grade III in 40%, Grade IV in 42%, and Grade V in 26% (Milhorat, 1987). Risk factors of acute hydrocephalus post aSAH are studied and include presence of blood in intraventricular space (Dorai et al., 2003; Suarez-Rivera, 1998), hemorrhage from posterior circulation aneurysms, diffuse spread of subarachnoid blood (Graff-Radford et al., 1989), rebleeding, hypertension (Mehta et al., 1996) and increased sympathetic activity (Jadhav et al., 2008; Lambert et al., 2002).
The exact mechanism underlying the development of acute hydrocephalus after aSAH is not established, however, sudden obstruction of cerebrospinal fluid circulation is considered an important contributor (Graff-Radford et al., 1989; Milhorat, 1987). Majority of patients with acute hydrocephalus exhibit clinical improvement after ventricular drainage (Bederson et al., 2009).
4.3.3. Increase in Extracellular Glutamate
In the arterial puncture rat model, cerebral glutamate level increases within minutes after aSAH and reaches a stable peek in approximately 40 minutes (Bederson et al., 1998; Sehba et al., 1999). This biochemical change found in both clinical and experimental studies is associated with the intensity of initial insult (Bederson et al., 1998; Enblad et al., 1996; Samuelsson et al., 2007; Sarrafzadeh et al., 2002; Schubert et al., 2008a; Schulz et al., 2000) and correlates well with clinical status and outcome of aSAH patients (Hutchinson et al., 2002; Nilsson et al., 1996; Sarrafzadeh et al., 2002; Sarrafzadeh et al., 1998; Saveland et al., 1996; Schulz et al., 2000; Skjoth-Rasmussen et al., 2004; Staub et al., 2000). Elevated interstitial glutamate concentration is considered one of the markers of excitotoxicity (Hillered et al., 2005) and is linked to cellular leakage, altered synaptic transmission, blood–brain barrier disruption, and inhibited glutamate uptake (Hillered et al., 2005). Mechanisms of glutamate mediated toxicity include excessive activation of N-methyl-D-aspartate (NMDA) receptor causing massive calcium influx and subsequent necrosis and apoptotic cell death (McCulloch, 1992; Owens et al., 1997). Experimental studies indicate that the early inhibition of glutamate receptors prevents aSAH associated blood-brain barrier leakage (Palmer et al., 1995) and development of delayed vasospasm (Zuccarello et al., 1994). A number of investigators have used magnesium to block NMDA receptor activity in attempt to prevent the development of delayed vasospasm and DINDs in aSAH patients (Dorhout Mees et al., 2010; Wong et al., 2006). These studies have met little success (Dorhout Mees et al., 2010; Wong et al., 2006).
4.4. Magnetic Resonance Imaging (MRI) Changes
Experimental studies indicate that early cerebral changes after aSAH can be detected by MRI (Busch et al., 1998; Jadhav et al., 2008; Piepgras et al., 2001; Schubert et al., 2008a; van den Bergh et al., 2002). Busch et al. used MRI with diffusion weight imaging (DWI) in a rat aSAH model and found decrease in apparent diffusion coefficient (ADC) interpreted as acute cytotoxic edema within 2 min after aSAH (Busch et al., 1998). In addition, they noted DWI changes representing spreading depression after a delay of 1–3 min (Busch et al., 1998). On whole, experimental studies suggest that decrease in ADC 3 hours after aSAH is accompanied by ischemia (indicated by changes in cerebral energy metabolites) and can be reversed by hypothermia (Piepgras et al., 2001; Schubert et al., 2008b). Ischemic ADC changes are known to precede persistent neuronal death (Rojas et al., 2006). Indeed, using a canine aSAH model, Zhang et al. found delayed (7 days) neuronal injury in animals that had displayed ADC changes 48 hours after aSAH (Jadhav et al., 2008). They concluded that MRI is useful for a non-invasive study of early cerebral injury after aSAH (Jadhav et al., 2008).
In aSAH patients’ the often lack of availability of MRI and risks involved in scanning unstable patients have limited the use of early MRI (Bederson et al., 2009; Fiebach et al., 2004; van Gijn and Rinkel, 2001) and as the results early MRI data in aSAH patients in scarce. One early clinical MRI study in patients who were diagnosed by computed tomography within 6 hours of aSAH showed no perfusion deficits (Fiebach et al., 2004). However, as patients enrolled in this study were of low-grade aSAH (low Hunt and Hess grades 1 or 2) and had good recovery, this study may have limited value. At least three investigators have reported the early MRI detecting cerebral infarct after SAH (Hadeishi et al., 2002; Shimoda et al., 2001; Weidauer et al., 2008). Weidauer et al. reported that MRI detected cortical infarcts in grade 3 aSAH patients with mild angiographic vasospasm within 72 hours from the ictus (Weidauer et al., 2008). Shimoda et al. and Hadeishi et al. report similar findings (Hadeishi et al., 2002; Shimoda et al., 2001). Hence, it appears that MRI when used early after aSAH can provide information about presence of cerebral injury. However, the benefits of using MRI early in the course of disease remain to be examined.
4.5. Pathological Changes
Vascular and non-vascular cerebral structures endure pathological changes early after aSAH (Figures 3 and 4).
Figure 3. Factors promoting cerebral vessels constriction after SAH.
Large and small cerebral vessels constrict after SAH. Major contributors of this constriction are listed.
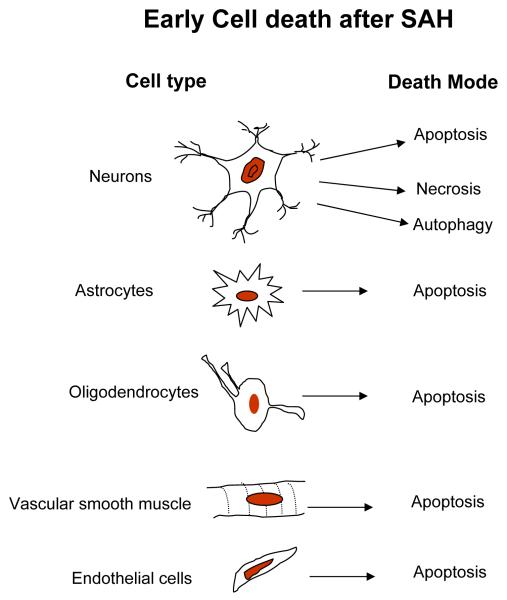
Figure 4. Early cell death after SAH.
Lists the identity and the mode of death cells early after SAH.
4.5.1. Cerebral Vessels
Experimental studies have shed light on the early response of large and small parenchymal vessels to aSAH. The effect of aSAH on parenchymal vessels appears to be comparatively greater than on large cerebral vessels (Bederson et al., 1998; Debdi et al., 1992, 1993; Sehba et al., 2010; Sehba et al., 2007b). Most of data on early vascular changes come from animal models; however, some human studies report similar findings (Bevan et al., 1998; Hatake et al., 1992; Hoelper et al., 2003; Pennings et al., 2004; Uhl et al., 2003).
Animal studies demonstrate that large and small cerebral vessels constrict within minutes after aSAH (Bederson et al., 1998; Sehba et al., 1999; Sehba et al., 2007b). Two phases of constriction are recognized in large vessels and in most cases accompany CBF reduction and perfusion deficits. The first phase is present as early as 10 minutes after aSAH and persists for at least 6 hours (Alkan et al., 2001; Bederson et al., 1998; Clower et al., 1994; Ono et al., 1997; Ono et al., 2003; Sehba et al., 2007b) and the second phase appears 48-72 hours later (Ohkuma et al., 1997; Ono et al., 2003; Yoshimoto et al., 1993; Zubkov et al., 2000; Zubkov et al., 2002b). Constriction of parenchymal vessels is also noted in patients during surgery for aneurysm repair within first 72 hours after aSAH (Pennings et al., 2004; Uhl et al., 2003).
Endothelial dysfunction is considered one of the key factors in early vasoconstriction and in delayed vasospasm after aSAH (Iuliano et al., 2004; Jung et al., 2004; Kassell et al., 1985; Miller et al., 2010; Park et al., 2001; Sobey and Faraci, 1998). In normal physiology, endothelium controls vascular tone and blood flow by releasing various contractile (such as Endothelin-1) and relaxant agents (such as nitric oxide, prostaglandin-I(2) and others) (Andresen et al., 2006). Animal studies show that morphological and functional changes occur in vascular endothelium post aSAH. Morphological changes include corrugation of endothelium membrane, appearance of endothelial cytoplasmic flaps or microvilli that extend to the vessel lumen and are characteristic of cerebral ischemia and local endothelial denudation (Clower et al., 1994; Friedrich et al., 2010a; Ono et al., 1997; Sehba and Friedrich, submitted). Functional changes include decrease in response of vasodilators that require a functional endothelium for eliciting their effect; such as acetylcholine thrombin, and bradykinin (Hongo et al., 1988; Nakagomi et al., 1987) or due to inhibition of endothelium-based vasodilation (ADMA) (Iuliano et al., 2004; Jung et al., 2004). Consequently, cerebral arteries become hypersensitive to contractile agents (such as serotonin, norepinephrine and others) after aSAH (Debdi et al., 1992). Decreased dilation by agents requiring functional endothelium and hypersensitivity to contractile agents is also found in arterial specimens acquired from patients who died within the first 72 hours post aSAH (Bevan et al., 1998; Hatake et al., 1992). Apoptotic death of endothelial cells of large cerebral arteries is observed 3 days after aSAH (Zubkov et al., 2002b). Parenchymal vessels display earlier and more severe morphological changes compared with large vessels. The endothelium lining of the parenchymal vessels is disrupted and detached from the basal lamina layer within 10 minutes (Friedrich et al., 2010a) and apoptotic enzymes are activated in endothelial nuclei within 3 hours after aSAH (Friedrich et al., in press). Hence, it is not surprising that endothelium of small parenchymal vessels becomes dysfunctional much earlier, within 20 minutes after aSAH (Park et al., 2001). Another morphological change that to date is found limited to parenchymal vessels only (at least in the initial hours after aSAH) is the destruction of basal lamina. This phenomenon is a frequent finding in animal studies but is yet to be established in clinical aSAH. Animal studies demonstrate that degradation of major proteins of basal lamina starts within minutes after aSAH and persists for at least 24 hours (Guo et al., 2010; Scholler et al., 2007; Sehba et al., 2004b; Yatsushige et al., 2007 ). It has been suggested that this degradation may represent the initiation of compensatory, yet clinically inefficient angiogenesis in response to hypoxia (Josko et al., 2001). Regardless of the cause, pathological consequence of basal lamina degradation on parenchymal vasculature is destabilization of microcirculation, increase of vascular permeability and edema (Hamann et al., 1995). Indeed, a marked increase in permeability of cerebral microvessels is documented both in animal and human studies (Doczi et al., 1986a; Doczi et al., 1986b; Friedrich et al., 2010b; Germano et al., 2000). Moreover, this increase correlates with the development of DINDs (Doczi, 1985; Doczi et al., 1986a; Germano et al., 1992; Germano et al., 2000; Imperatore et al., 2000; Symon, 1978) and poor clinical outcome in aSAH patients (Doczi et al., 1986a; Scholler et al., 2007; Smith et al., 1997; Yatsushige et al., 2006).
4.5.2. Cell Death (necrosis, apoptosis and autophagy)
Except for few early autopsy cases almost all first hand information on the early cell death after aSAH comes from animal studies. These studies demonstrate that cell death starts within 24 hours after aSAH. Serum levels of neuron specific enolase, a marker of neuronal injury, is elevated in patients and associated with the amount of subarachnoid blood and poor neurological status on admission, as well as it correlates with the development of delayed ischemic neuronal damage (Cunningham et al., 1994; Kuroiwa et al., 1994; Mabe et al., 1991). In addition, serum concentration of S100-B, a marker of glial injury, is increased in patients within 3 days after aSAH (Oertel et al., 2006). Consequently, it appears that although neurons are experiencing deleterious effects of aSAH very early, they are not the only target of cell death pathways. Indeed, Prunal et al. using animal aSAH models have found that in addition to neurons, astrocytes, and oligodendrocytes also undergo apoptosis 24 hr after aSAH (Prunell et al., 2005). Other investigators report apoptosis of smooth muscle and endothelial cells 24 - 72 hours after aSAH (Cahill et al., 2006a; Friedrich et al., in press; Park et al., 2004; Yatsushige et al., 2007).
Most animal studies find necrosis and apoptosis to be the modes of cell death post aSAH (Akpinar et al., 2005; Cahill et al., 2006a; Dreier et al., 2000; Matz et al., 2001; Prunell et al., 2005; Zubkov et al., 2002b). More recently, Lee et al. have found autophagic death of neurons 24 hours after aSAH (Lee et al., 2009a). It appears that more than one mode of cell death is active at any given time after aSAH (Dreier et al., 2000; Friedrich et al., in press; Lee et al., 2009a; Matz et al., 2001). Dreier et al. reported necrotic and apoptotic cell death and cerebral infarction in animals 24 hours after aSAH (Dreier et al., 2000). Similarly, Matz et al. found necrosis and apoptosis at 24 hours in mice after heme injection (Matz et al., 2001). More recently, Lee et al. reported neuronal death via apoptosis in the superficial layers of the fronto-basal cortex, and via autophagy in deep cortical structures in animals 24 hours after aSAH (Lee et al., 2009a). Human autopsy studies involving patients who died 24 hours to 10 days after aSAH have found neuronal apoptosis in dentate gyrus (Nau et al., 2002).
Animal studies indicate that apoptotic cell death after aSAH is evoked via extrinsic and intrinsic mechanisms (Cheng et al., 2009; Meguro et al., 2001a; Park et al., 2004). Intrinsic mechanisms appear to be mainly caspase dependent (Cheng et al., 2009; Meguro et al., 2001a); however, some evidence of caspase independent intrinsic mechanisms involving free radicals mediated apoptosis exists (Endo et al., 2007; Satoh et al., 2001).
Caspase dependent intrinsic pathway activates upon pathological rise in intracellular calcium concentration (Broughton et al., 2009). Its main events include activation of calcium-activated proteases (calpains), cleavage of Bcl-2 interacting domain (BID) to the truncated active form (tBID), and activation of proapoptotic proteins including Bak, Bax, Bad, and Bcl-XS and release of pro-apoptotic proteins by tBID to activate caspase dependent apoptosis (Broughton et al., 2009). A number of studies suggest that caspase dependent intrinsic pathway is activated early after aSAH (Gules et al., 2003; Yamaura et al., 1993; Zhou et al., 2004; Zubkov et al., 2002a). For example, Yamaura et al. demonstrated that calpain (proteolytic enzyme that hydrolysis its substrate resulting in apoptosis) activates within 40 minutes in canine basilar artery and contributes to vasoconstriction that can be inhibited by calphostin, an intrinsic inhibitor of calpain (Yamaura et al., 1993). Other studies demonstrate that calpain inhibitors used early after aSAH prevent the BBB opening and neurological deficits (Germano et al., 2002), and attenuate cerebral vasospasm (Cappelletto et al., 1997; Fujikawa et al., 1999). Similar benefits are reported upon inhibition of caspase activity after aSAH (Gules et al., 2003; Zhou et al., 2004; Zubkov et al., 2002a). Caspases involved in apoptosis after aSAH are caspase-3, 8 and 9 (Park et al., 2004; Prunell et al., 2005; Zhou et al., 2004).
Extrinsic mechanisms of apoptosis commonly called “death receptor pathway” involve the death receptors located on the cell surface (Broughton et al., 2009). These receptors belong to the tumor necrosis factor receptor (TNFR) superfamily, and include TNFR-1, Fas, and p75NTR (Loh et al., 2006) and mediate apoptosis via caspase-3 activation (Sugawara et al., 2004). Fas-associated death domain protein (FADD) is a component of the death-inducing signaling complex and is recruited to the signaling complex in response to death receptor-mediated signaling. Jayaraman et al. found that FADD is up-regulated in the wall of human ruptured and unruptured aneurysms indicating that this pathway contributes to aneurysm formation and growth (Jayaraman et al., 2005). In animals, the only report of apoptosis occurring via extrinsic mechanism after aSAH comes from Zhou and colleagues who show co-localization of TUNEL immunostaining with caspase-3 and TNFR1 in endothelial cells of canine basilar arteries 7 days after aSAH (Zhou et al., 2004). Hence, extrinsic mechanisms of apoptosis appear to contribute to aneurysm formation and in late phase cell death after aSAH and their importance in the early phase cell death after aSAH remains to be elucidated.
4.6. Molecular Changes
4.6.1. Nitric oxide/Nitric Oxide Synthase Pathway
Pathological alteration in nitric oxide (NO)/nitric oxide synthase (NOS) pathway occurs early after aSAH and contributes to early ischemic brain injury (Schwartz et al., 2000b; Sehba et al., 1999; Sehba et al., 2000) and to the pathogeneses of delayed vasospasm and DINDs (Afshar et al., 1995; Durmaz et al., 2008; Edwards et al., 1992; Khaldi et al., 2001; Ng et al., 2001; Pluta et al., 1997b; Suzuki et al., 1994; Woszczyk et al., 2003). Animal studies demonstrate that cerebral NO level decreases within 10 minutes (Sehba et al., 2000) and increases above basal level at 24 hours after SAH (Yatsushige et al., 2006). In humans, increased cerebral NO level is found 24 hours after aSAH and is associated with poor outcome (Durmaz et al., 2008; Khaldi et al., 2001; Ng et al., 2001). Mechanisms underlying alteration in cerebral NO level are investigated and it is suggested that initial decrease in cerebral NO involves scavenging by hemoglobin, (Afshar et al., 1995; Kajita et al., 1994; Watkins, 1995), free radicals (Sobey and Faraci, 1998), and vascular neutrophils (Friedrich et al., 2011; Provencio and Vora, 2005) or nitrite reduction” (Pluta et al., 2005) rather than impairment of NO synthesis because the overall NOS activity remains unchanged during the first 90 minutes after aSAH (Sehba et al., 2004a). The temporary recovery and increase NO above the basal level appears to involve saturation of scavenging mechanisms and/or an increase in NOS expression and activity (Sehba and Bederson, 2006b; Sehba et al., 2004a).
An active NO/NOS pathway is crucial in the regulation of cerebral blood flow and blood pressure (Sobey and Faraci, 1998). In addition, NO plays an important role in smooth muscle cell proliferation, inhibition of platelet aggregation, and adherence of leukocytes to the endothelium in responses to vessel injury (Cooke and Dzau, 1997). Hence, it is not surprising that constriction of large and small cerebral vessels and luminal aggregation of platelets occurs within minutes after aSAH (Bederson et al., 1998; Sehba et al., 2005); the time when cerebral NO is reduced (Sehba et al., 2000). Since the capacity of arteries to synthesize cGMP (involved in NO mediated vasodilatation) and dilate in response to an NO donor remains unchanged during this early period, many investigators have used NO donors to dilate arteries and recover CBF and prevent early ischemic injury after experimental SAH (Park et al., 2001; Sehba et al., 1999; Sehba et al., 2007b; Sobey and Faraci, 1998).
Large increase in cerebral NO at the time when its vascular response is no longer needed can also be devastating to brain (Iadecola, 1997); i.e. a pathological rise in cerebral NO level beyond baseline 24 hour after aSAH has been proved detrimental (Ayer and Zhang, 2008; Petzold et al., 2005a; Sehba and Bederson, 2006b). In this setting, NO acts as a free radical itself and in the form of peroxynitrite (a powerful oxidant) attacks cell membrane causing pathological changes in the endothelium and smooth muscle cell structures (Beckman et al., 1990). Putative mechanisms of NO-mediated cell injury involve activation of poly(ADP-ribose) synthase and subsequent depletion of cellular β-nicotinamide adenine dinucleotide and ATP (cellular energy depletion) leading to cell death (Carson et al., 1986; Szabo and Dawson, 1998), mitochondria damage (Higuchi et al., 1996; Iadecola, 1997; Leist and Nicotera, 1998), and changes in ion flux of sodium, potassium, and calcium channels leading to axonal degeneration (Petzold et al., 2005a). Most of these mechanisms are found active in animals and in humans early after aSAH (Ayer and Zhang, 2008; Petzold et al., 2005a; Petzold et al., 2008) and are associated with early brain injury, pathogenesis of DINDs, and poor clinical outcome (Durmaz et al., 2008; Jung et al., 2007; Khaldi et al., 2001; Medele et al., 1996; Ng et al., 2001; Sayama et al., 1999; Woszczyk et al., 2003; Yamamoto et al., 1997).
Over all it appears that whereas increasing cerebral NO level few hours after aSAH preserves brain functions, beyond this time, vigilant monitoring of cerebral NO level is warranted to not exceed past physiological level.
4.6.2. Endothelin-1 (ET-1)
Animal studies show that CSF level of ET-1 increases within minutes after aSAH (Josko et al., 1998; Wang et al., 1995). In aSAH patients, increase in CSF and plasma ET-1 is observed 24 hours from ictus (Kobayashi et al., 1995), and is associated with the occurrence of delayed vasospasm (Gruber et al., 2000a). Animal studies indicate that the increase in cerebral ET-1 after aSAH results from excessive release by astrocytes during the period of initial ischemia (Pluta et al., 1997a). It is suggested that the early increase in ET-1 level along with decease in cerebral NO (above) after aSAH disturbs the delicate balance between vasoconstrictive and vasodilatory forces necessary to maintain physiological vessel tone and flow and leads to unopposed constriction via activation of ET-1 receptors (Afshar et al., 1995). Consequently, it is possible to inhibit vascular constriction post aSAH by increasing cerebral NO; such as by an NO donor, and/or by inhibiting ET-1 activity such as by ET-1 antagonism (Agrawal et al., 2009; Clozel and Watanabe, 1993; Macdonald et al., 2008; Pluta et al., 2005; Sehba et al., 1999).
ET-1 is a peptide secreted in the brain by vascular endothelium, neurons, astrocytes and macrophages (Levin, 1995). It acts through three receptors: ET-A, ET-B1 and ET-B2 receptors (Rothoerl and Ringel, 2007). ET-A receptor is expressed in vascular smooth muscle cells and mediates vasoconstriction; ET-B1 receptor is expressed in vascular endothelial cells and mediates endothelium-dependent vasodilation and ET-B2 receptor is expressed in smooth muscle cells and mediates vasoconstriction (Levin, 1995). Studies show that expression of ET-1 receptors increases 24 to 48 hours after aSAH (Hansen-Schwartz et al., 2003; Vikman et al., 2006). In normotensive animals, intracisternal administration of ET-1 causes widespread long lasting vasoconstriction and profound cerebral ischemia (Asano et al., 1989; Macrae et al., 1991).
One key finding made in animals and in humans that points at ET-1 as the dominant culprit in the pathogenesis of delayed vasospasm after aSAH is that it produces long-lasting constriction (Kobayashi et al., 1991; Papadopoulos et al., 1990). Additional factors establishing importance of ET-1 in delayed vasospasm include: (1) ET-1 is increased early in CSF and plasma after aSAH (Josko et al., 1998; Kobayashi et al., 1995; Wang et al., 1995), (2) agents that promote ET-1 release in CSF and plasma (thrombin and oxyhemoglobin) increase early after aSAH, and (3) ET-1 produces degenerative morphological changes in the vascular wall that are similar to those observed after aSAH (Asano et al., 1990; Kasuya et al., 1993; Kobayashi et al., 1991; Peltonen et al., 1997).
Connecting delayed vasospasm to DINDs a number of investigators have attempted to use ET-1 receptor antagonists to prevent delayed vasospasm and cortical infarctions after aSAH. These agents successfully reduced the incidence and intensity of vasospasm but had little effect on DINDs and on the long-term outcome (Kramer and Fletcher, 2009; Macdonald et al., 2011; Macdonald et al., 2008; Nogueira et al., 2007; Shaw et al., 2000; Vajkoczy et al., 2005; Vergouwen, 2009).
4.6.3 Oxidative and Nitrosative Stress
Substantial amount of data supports early generation of oxygen free radicals (ROS) and oxidative stress after aSAH (Gaetani et al., 1990b; Gaetani et al., 1994; Marzatico et al., 1993; Marzatico et al., 1998; Sano, 1994; Schulz et al., 2000) and their association with early brain injury and pathogenesis of delayed vasospasm and/or DINDs (Asaeda et al., 2005; Gaetani et al., 1997; Imperatore et al., 2000; Kamezaki et al., 2002; Liu et al., 2007; Marzatico et al., 1998; Pyne-Geithman et al., 2009; Sano, 1994; Shin et al., 2003). Animal studies show that activities of enzymatic and non-enzymatic antioxidant systems decrease within 60 minutes (Marzatico et al., 1993), and the products of lipid peroxidation increase 1-6 hours after aSAH (Gaetani et al., 1990b). In humans, decrease in antioxidant systems (Gaetani et al., 1997; Gaetani et al., 1998; Lin et al., 2006; Marzatico et al., 1998), and increase in lipid peroxidation products is found within 72 hours from ictus and correlates well with poor clinical status and outcome (Asaeda et al., 2005; Gaetani et al., 1997; Hsieh et al., 2009; Kamezaki et al., 2002; Polidori et al., 1997).
ROS generated after aSAH include superoxide anion (O2*) (Marzatico et al., 1993), hydroxyl radical (OH*), hydrogen peroxide (H2O2) (Gaetani et al., 1994), nitric oxide (NO*), and peroxynitrate (ONOO-) (Asano and Matsui, 1999; Ayer and Zhang, 2008; Lin et al., 2006; Petzold et al., 2005a). Animal studies indicate that majority of these ROS are generated during auto-oxidation of hemoglobin upon erythrocytes lysis in the subarachnoid space (Asano, 1999; Asano and Matsui, 1999; Misra and Fridovich, 1972; Sercombe et al., 2002). Other sources of post aSAH ROS include increased NOS activity (Ayer and Zhang, 2008; Petzold et al., 2005a; Sehba et al., 2004a), disrupted mitochondrial respiration (Piantadosi and Zhang, 1996), hypoxic conversion of endothelial xanthine dehydrogenase to xanthine oxidase (Kim et al., 1987; Lindsay et al., 1991; Sermet et al., 2000; von Holst and Sollevi, 1985), lipid peroxidation (Sano, 1994; Schulz et al., 2000), and up-regulation of NADPH oxidase (Liu et al., 2007). For review see Ayer and Zhang (Ayer and Zhang, 2008).
Consequences of oxidative stress after aSAH may include injury to smooth muscle and endothelium of vascular wall, disruption of the blood brain barrier, production of strong spasmogens such as leukotriene C4 and prostaglandin D2 from the lipoxygenase and cyclo-oxygenase pathways of arachidonic acid metabolism (Gaetani et al., 1990b). In addition, oxidative stress induces enzymes of apoptotic pathway including p53, caspase-3 and 9 to promote apoptotic cell death (Ayer and Zhang, 2008). Consequently, overexpression of CuZn superoxide dismutase (SOD; a potent endogenous antioxidant) in transgenic mice prevents apoptotic cell death (Matz et al., 2000), and reduces mortality (Endo et al., 2007) after aSAH. Antioxidants have successfully have been used to prevent oxidative stress and decrease early brain injury in animals (Gaetani et al., 1990a; Hall and Travis, 1988) but have met little success in improving outcome in clinical trials (Gomis et al., 2010; Zhang et al., 2010).
4.6.4. Inflammation
Numerous different inflammatory pathways are activated early after aSAH (Handa et al., 1995; Kaynar et al., 2004; Mack et al., 2002; Mocco et al., 2002; Tanriverdi et al., 2005). An early inflammation in aSAH patients is linked to poor neurological grade on admission, fever, malaise, leukocytosis, increased BBB permeability, brain edema, small vessel thrombosis, pathogenesis of vasospasm and DINDs (Barone and Feuerstein, 1999; Chaichana et al., 2010; Frijns and Kappelle, 2002; Kaynar et al., 2004; Kubo et al., 2008; Mack et al., 2002; Neil-Dwyer and Cruickshank, 1974).
Neutrophils, the cells of innate immune response, accumulate in cerebral vessels within 10 minutes after aSAH in animals and persist for at least 24 hrs (Friedrich et al., 2011). Similarly, soluble and tissue markers of inflammation increase within 24 hrs after aSAH in animals (Bavbek et al., 1998; Handa et al., 1995; Lin et al., 2005), and within the first 3 days from ictus in patients (Dumont et al., 2003; Fassbender et al., 2001; Fountas et al., 2009; Gruber et al., 2000b; Kacira et al., 2007; Mack et al., 2002; Peterson et al., 1990a; Rothoerl et al., 2006; Takizawa et al., 2001). Parenchymal migration of leukocytes, a major step in inflammation begins early after aSAH and contributes to poor outcome (Bavbek et al., 1998; Friedrich et al., 2011; Handa et al., 1995; Kaynar et al., 2004; Lin et al., 2005; Mack et al., 2002; Mocco et al., 2002; Tanriverdi et al., 2005). Leukocyte migration requires endothelial expression of adhesion molecules (vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin to aide in their endothelial adherence and subsequent transit into the brain parenchyma (Rothlein, 1997; Springer, 1994). Hence, in animals, leukocyte migration and its contribution to brain injury is established as increased endothelial expression of adhesion molecules within 24 hours after aSAH and their selective inhibition leads to improved outcome (Bavbek et al., 1998; Handa et al., 1995; Lin et al., 2005). In aSAH patients, an increase in soluble forms of adhesion molecules is found within the first 3 days and is associated with poor outcome (Kaynar et al., 2004; Mack et al., 2002; Mocco et al., 2002; Tanriverdi et al., 2005).
C-reactive protein (CRP) is another early sensitive marker of systemic inflammation (Pepys and Hirschfield, 2003). Studies find that CRP level increases in serum and CSF within 2-3 days after aSAH (Fountas et al., 2009; Kacira et al., 2007; Rothoerl et al., 2006; Takizawa et al., 2001). Moreover, in aSAH patients the elevated CRP level on admission correlates well with low GCS scores, high Hunt and Hess and Fisher grades, and the occurrence of delayed vasospasm (Fountas et al., 2009).
Pro-inflammatory cytokines (interleukin-1β (IL-1β) and interleukin-6 (IL-6), interleukin-1 receptor (IL-1Ra) and tumor necrosis factor (TNF-α)) orchestrate inflammatory cascade in response to any injury. Cytokines modulate vessel tone by inducing synthesis of vasoconstrictors such as endothelin-1 (Marsden and Brenner, 1992), by expression of adhesion molecule responsible for focal leukocyte recruitment (Handa et al., 1995), and by impairing vascular permeability (McKeating and Andrews, 1998) and the blood-brain barrier function (Holmin et al., 1998; Sozen et al., 2009). Furthermore, IL-6 contributes to intracranial hypertension (Argaw et al., 2006; Paul et al., 2003), and TNF-α in hemolysis-induced vasoconstriction (Vecchione et al., 2009). In aSAH patients, serum and CSF level of cytokines increases within 1-3 days from ictus (Dumont et al., 2003; Fassbender et al., 2001; Graetz et al., 2010; Gruber et al., 2000b; Hendryk et al., 2004; Peterson et al., 1990a) and is associated with hyperthermia, vascular spasm, and unfavorable outcome (Dumont et al., 2003; Jedrzejowska-Szypulka et al., 2009; Mathiesen et al., 1997). Although the exact source of cytokine release after aSAH is not known, endothelial cells, neutrophils, macrophages, astrocyte, microglia, and neurons are implicated (Dumont et al., 2003; Takizawa et al., 2001; Vecchione et al., 2009). Inflammasome are yet another source of pro-inflammatory cytokines (de Rivero Vaccari et al., 2009), their importance in inflammation after SAH remains to be elucidated.
4.6.5. Platelets
Experimental and clinical studies indicate that platelets activate early after aSAH (Clower et al., 1988; Denton et al., 1971; Haining et al., 1988; Hirashima et al., 2005; Ishikawa et al., 2009; Sehba et al., 2005; Stein et al., 2006a). Reduction in venous jugular platelet counts and shape change indicating sequestration and activation is observed 5 minutes after experimental (Denton et al., 1971), and 48 hours after clinical aSAH (Hirashima et al., 2005). Moreover, platelet aggregates are found lodged in major cerebral arteries at 2 hours (Clower et al., 1988; Haining et al., 1988), and in the parenchymal vessels 10 minutes after experimental aSAH (Ishikawa et al., 2009; Sehba et al., 2005). Autopsy specimen of humans died within 2 days after aSAH demonstrate micro-emboli in small arteries (Stein et al., 2006a). The aggregates lodged in parenchymal vessels may have originally formed at the site of the aneurysm rupture in a large cerebral vessel at aSAH and traveled downstream to parenchymal vessels. Alternatively, they may have formed in the vessels due to activation of endothelium and the reduction in blood flow after aSAH.
The presence of platelet aggregates in parenchymal vessels may promote the “no-reflow” phenomenon (Abumiya et al., 2000), the absence of vascular filling after a period of global cerebral ischemia (Ames et al., 1968). In addition, parenchymal platelets aggregates can stimulate or initiate events that can devastate an injured brain. Most of these events are found active within minutes after experimental aSAH and include: (1) the mechanical obstruction of vessel lumen (Friedrich et al., 2010b); (2) vasoconstriction via release of serotonin, ADP and PDGF (del Zoppo, 1997; Fukami et al., 2001; Okada et al., 1994; Reed, 2002; Sehba et al., 2007b); (3) denudation of endothelium thereby promoting further platelet aggregation (Friedrich et al., 2010a; Rosenblum, 1997; Said et al., 1993) and finally, (4) destruction of major proteins of the vessel wall by releasing collagenases such as matrix metalloproteinases-2 and 9 (MMP-2 and 9) (Fernandez-Patron et al., 1999; Friedrich et al., 2010a; Rosenberg et al., 1998; Rosenberg et al., 1992; Sehba et al., 2007a; Sehba et al., 2007b; Sehba et al., 2004b). Moreover, the recent study demonstrates that luminal platelet aggregates escape into the brain parenchyma within 10 minutes after aSAH and that this process is still active at 24 hours (Friedrich et al., 2010a). The presence of platelets in the brain parenchyma may activate additional inflammatory mechanisms and further aggravate brain injury.
4.7. Neurological, Cognitive and Functional Deficits
Majority of aSAH patients at admission present disturbed consciousness and change in cognition, together with perceptual (such as illusions and hallucinations), and emotional disturbances (such as agitation and anger) (Reijneveld et al., 2000). The Hunt and Hess, the Glasgow comma scale (GCS), and the World Federation of Neurological Surgeons (WFNS) Grading scales are routinely used to assess patient status during early phase of aSAH and to make treatment decisions (Starke et al., 2009). Studies show that the patients’ status on admission correlates well with the outcome,, i.e., patients in low grades on admission usually have poor outcome (Hutter et al., 2001). Similarly, the presence of acute focal neurological deficits on admission is also associated with non-favorable outcome (Sarrafzadeh et al., 2003). Cerebral microdialysis in aSAH patients with acute focal neurological deficits reveals low glucose, high glutamate and glycerol levels confirming the presence of ischemia, excitoxicity, and lipid peroxidation, (Kerner et al., 2007; Sarrafzadeh et al., 2003).
Animal studies present a more complete picture of behavioral changes and deficits occurring during the early phase after aSAH. These studies show decrease in appetite (Guo et al., 2010), weight (Germano et al., 2007; Germano et al., 2002), but little or no change in motor functions except some possible losses of coordination skills (Germano et al., 1994; Silasi and Colbourne, 2009; Thal et al., 2008). Germano and colleagues studied animals for coordination skills from ictus to 5 days after aSAH and found transient reduction in beam balance at 24 hours and persistent reduction in traverse beam walking ability for 4 days (Germano et al., 2002). Thal et al., however, found no significant change in animals coordination skills during the first 48 hours after aSAH using beam balance or rotarod tests (Thal et al., 2008). In contrast to coordination, the overall neurological status of animals is significantly impaired 72 hours after aSAH (Ostrowski et al., 2005; Park et al., 2004; Thal et al., 2009). Thal et al. used a 100 point neuro-score to examine general behavioral deficit, cranial nerve reflexes, motor deficit, sensory deficit, coordination and found a significant reduction in overall score of animals 24-48 hours after aSAH (Thal et al., 2009). Silasi et al. used a battery of tests to check motor and cognitive skills in animals 3-7 days after aSAH and found minor non-significant changes (Silasi and Colbourne, 2009). Taken together animal studies indicate significant neurological and behavioral impairment and some coordination impairment during the early phase of aSAH.
5. Failure to Translate Successful Animal Therapies to Clinical Settings
Although animal research has undeniably advanced our understanding of injury after aSAH, it has failed to provide a therapy (see Table 1). This research helped in elimination of compounds that are not found effective or were too toxic for clinical evaluation; nevertheless many compounds that were found promising in animals failed in clinical trials. This failure questions the value of animal research in development of an effective therapy against aSAH and its complications. A number of factors have been recognized making translation of animal research results into clinics difficult.
Table 1. Failure of Clinical trials against SAH.
We list some of the most significant clinical trail failures to date. Agents listed were beneficial against animal SAH were not found successful against human SAH.
| A) Agents Studied Against Vasospasm | |||
|---|---|---|---|
| i. Vasospasm Prevention | |||
| Agent | Mechanism of action |
Preclinical Success | Clinical success |
| Clazosentan | ET-1 receptor antagonist |
Prevents constriction and hypoperfusion (Schubert et al., 2008; Vatter et al., 2007; Vatter et al., 2005) |
Reduces the incidence vasospasm without improvement in overall outcome (Kramer and Fletcher, 2009; Macdonald et al., 2011; Vergouwen, 2009) |
| Magnesium therapy |
Recovers Serum Magnesium |
Reverses constriction, reduces duration of ischemic depolarization and ischemic brain lesions (van den Bergh et al., 2002) |
Reduces the incidence of vasospasm, some improvement in overall outcome (Westermaier et al., 2010; Wong et al., 2010) |
| Tirilazda mesylate |
Antioxidant | Protects vascular endothelium and blood- brain barrier (Hall and Travis, 1988; Smith et al., 1997; Smith et al., 1996) |
Some gender specific (male) benefits (Haley et al., 1995; Jang et al., 2009; Kassell et al., 1996; Lanzino and Kassell, 1999) |
| Statins | Inhibit HMG CoA reductase, Increase eNO synthesis |
Reduces vasospasm and improves neurological functions in severe SAH (McGirt et al., 2006; Sugawara et al., 2008) |
May reduce the incidence of vasospasm, some improvement in overall outcome (Kern et al., 2009; Kramer et al., 2008; McGirt et al., 2009; Tseng et al., 2007) |
| Erythropoietin | Erythropoietin receptor agonist |
Reduces edema, inflammation, microcirculatory impairment and neuronal death (Grasso, 2001; Murphy et al., 2008) |
May reduce the incidence of vasospasm without improvement in overall outcome (Springborg et al., 2007; Tseng et al., 2010) |
| Fasudil hydrochloride |
Rho-kinase inhibitor |
Reduces endothelial injury, arterial constriction and neuronal damage, improves cognitive deficits (Huang et al., 2008; Satoh et al., 1999; Takanashi et al., 2001) |
Reduces the incidence vasospasm, some improvement in overall outcome (Suzuki et al., 2008; Zhao et al., 2006) |
| ii. Vasospasm Reversal | |||
| Papaverine | Vasodilator | Dilates blood vessels depending upon on treatment time and vasospasm severity (Macdonald et al., 1995) |
Transient reduction in vasospasm, has serious side effects and little clinical benefits (Polin et al., 1998; Vajkoczy et al., 2001) |
| Fasudil hydrochloride |
Rho-kinase inhibitor |
Reduces endothelial injury, arterial constriction and neuronal damage, improves cognitive deficits (Huang et al., 2008; Satoh et al., 1999; Takanashi et al., 2001) |
Reduces the intensity of vasospasm, some improvement in overall outcome (Shibuya et al., 1992; Tachibana et al., 1999; Tanaka et al., 2005). |
| Nimodipine | Calcium channel inhibitor |
Improves blood supply and attenuates constriction (Bilginer et al., 2009; Sun et al., 2003) |
May reduce the intensity of vasospasm, some improvement in overall outcome, is only FDA approved drug for post SAH use (Allen et al., 1983; Bederson et al., 2009) |
| Nicardipine | Calcium channel inhibitor |
Reduces constriction (Debdi et al., 1992) | Reduces the intensity of vasospasm without improvement in overall outcome (Haley et al., 1993; Rinkel et al., 2005) |
| B) Agents Studied Against Delayed Ischemic Neurological deficits (DINDs) | |||
| 3H-therapy* (hypervolemia, hypertension, hemodilution) |
Improve CBF and brain tissue oxygenation |
Little or no improvement in CBF and brain tissue oxygenation (Dueck et al., 2001; Muench et al., 2007) |
Somewhat effective in reducing DINDs but has significant serious side effects, is commonly used against DINDs and vasospasm (Awad et al., 1987; Lee et al., 2006; Meyer et al., 2011) |
| Nimodipine | Calcium channel inhibitor |
Improves blood supply and attenuates constriction (Bilginer et al., 2009; Sun et al., 2003) |
Somewhat reduction in DINDs with little improvement in vasospasm , is only FDA approved drug for post SAH use (Allen et al., 1983; Bederson et al., 2009; Rinkel et al., 2005) |
| Statins | HMG CoA reductase inhibitors, increase eNO synthesis |
Reduces vasospasm and improves neurological functions in severe SAH (McGirt et al., 2006; Sugawara et al., 2008). |
Some reduction in DINDs and in vasospasm (Sillberg et al., 2008; Tseng et al., 2005; Vergouwen et al., 2010) |
triple H therapy is often limited to increase of blood pressure (1H) and sometimes modulation (2H) (Chittiboina et al., 2011; Treggiari, 2011).
5.1. Animal Species
Quite a few species have been used to study early and delayed injury after aSAH. This list includes non-human primates, pigs, goats, dogs, cats, and rodents (rat and mice, for review see (Sehba and Bederson, 2006a)). In recent years, rodents have become increasingly popular to study aSAH as they are relatively inexpensive, amenable to genetic alteration, and easy to manipulate in a laboratory setting. However, it is clear that though mammals, rodents are physiologically, neuroanatomically and metabolically different from humans; they lack gall bladder, process fat and cholesterol in different ways, and require greater mg/kg drug doses to produce a response similar to larger animals (Bergen and Mersmann, 2005; Mordenti and Chappell, 1989). In addition, rodent cerebral vasculature is anatomically different than humans. For instance, it lacks interadventitial space in arterial walls and has abundant collaterals (Frederickson and Low, 1969; Kader et al., 1990). Moreover, studies of focal ischemia demonstrate that a similar occlusion of middle cerebral artery causes larger infarction and more extensive cell death in rodents as compared with humans (Carmichael, 2005).
Non-human primates are closer to human in physiologically, neuroanatomy, and metabolism and more likely to produce data that could be readily translated to human condition. However, cost and ethical issues of primate research has made their wider use by most laboratories difficult, if not impossible. Despite, the recognition of role of primate research for translational medicine (Cook and Tymianski, 2011) without better funding, institutional change in animal facilities, and costs its use will remain limited and rodent models will continue to provide the predominant basic science research into the mechanisms of brain injury and its treatment. Perhaps confirmation in primate of a treatment found effective in rodents or other species may reduce the number of failures in clinical trial. However, this cannot be guaranteed either, a fact observed as the recent failure of clinical trial of stroke that was based on the robust evidence of effectiveness in a non-human primate model of stroke (Diener et al., 2008; Lees et al., 2006; Shuaib et al., 2007). A better collaboration between laboratories and a better funding mechanism may solve some of the problems. This will allow for compounds found effective in rodent models in small laboratories to be examined in primates in other research centers; large laboratories of pharmacological companies, or government supported facilities before their clinical evaluation. These solutions may result in fewer clinical disappointments.
5.2. Methodological Flaws in Animal Studies
5.2.1. Age, Health, and Gender Issues
Methodological flaws in animal experimentation can contribute to inability of clinical translation of their results. Methodological flaws can come in different forms. Critical disease specific disparities between the animal models and the clinical trials testing the treatment strategy are major flaws. Most often experiments are carried out in young, non-diseased animals and do not simulate the age or condition of patients at risk of aSAH (45-55 year old, majority hypertensive). Similarly, most experiments are performed on male animals to avoid the variability caused by female hormone cycling, whereas in reality not only more females then males suffer from aSAH but some agents (such as Tirilazad mesylate) have a gender specific activity and their effectiveness differs between sexes (Kassell et al., 1996; Kongable et al., 1996; Lanzino and Kassell, 1999).
5.2.2. Animal Allocation, Control Group, Blinded Assessment, and Statistical Power
Another methodological flaw is the lack of random allocation of animals. Not many studies indicate if animals used for particular experiment were randomized. Furthermore, quite often the experimental study does not have control group or the control group is inadequately established. Blind assessment is essential for a non-biased meaningful study. Unfortunately, not many experimental studies indicate whether assessments were performed in a blind manner. Similarly, season and time of the day is known to influence the outcome of aSAH (Gallerani et al., 1996; Hughes et al., 2010 ; Muroi et al., 2004). To our best knowledge, these elements are seldom, if ever, addressed in research models. If investigator is not blinded to the identity of the drug that an animal receives then there is possibility that its effect on the animal is overrepresented. Sample size that is sufficiently powered to allow statistical analysis provides inadequate data that is more observational and can lead to incorrect conclusions about efficacy. The bottom line is that we need more stringent requirements for reporting animal data (Hackam, 2007).
All of the above factors make it essential that systematic reviews and meta-analyses of outcomes of animal studies using the agent(s) of interest are performed before a clinical trial. Such a close analysis of all available experimental data may facilitate detection of toxicity and efficacy, and aid in the selection of the most promising compounds for clinical trials.
5.2.3. Focus of Therapy; Study Endpoints
Failure of animal studies to translate in human may also results from the difference in the end points considered important for a drug or treatment. Since delayed vasospasm has been considered the most important determinant of outcome after aSAH, most animal studies have focused on prevention and treatment of vasospasm to improve aSAH outcome. However, the results of a recent clinical trial indicate that this approach may not be proper (see table 1) and that it is time to revise treatment strategy. As discussed above, brain injury after aSAH begins at the initial bleed and plays an important role in the outcome. Although research on early brain injury after aSAH is still in its infancy and most data describing it comes from laboratory, massive brain injury observed during autopsy of patients that died early after aSAH confirms its importance in the outcome (Nau et al., 2002; Stoltenberg-Didinger and Schwartz, 1987). It is suggested that many of the early mechanisms evolve with time and contribute to the outcome of aSAH (Sehba et al., 2011). Consequently, these mechanisms and their timely addressing need to be considered while designing a therapeutic strategy against aSAH.
It is also important to define outcome measure of a drug efficacy. For many patients and their families, the quality of life is as important as prolongation of life. Consequently, maintenance and recovery of damaged neuronal circuits important for everyday life activities such as cognitive and motor functions, speech and memory could be a better measure of a drug efficacy (Chahal et al., 2011; Hutter et al., 1995; Vieira et al., 2011). Thus, for experimental compounds to become a successful therapy in humans, a therapy that goes beyond prevention of cell death and addresses the acute and delayed deficits that affect quality of life of aSAH victims is required. Perhaps neurobehavioral status is a better assessment of patient outcome and should be the focus of therapy. This requires identification of the neurobehavioral function (such as memory, life style, etc) affected by aSAH and preparation of an assessment method that would allow their proper scaling and grading. This could only be achieved by a long-term evaluation of aSAH patients, perhaps in form of a multicenter project. Better animal models that exhibit neurobiological deficits similar to those in humans post SAH are also needed, so that therapeutic strategies that ameliorate them could be identified.
6. Conclusion
Despite extensive research the patient outcome post aSAH remains poor. Findings that prevention of delayed vasospasm does not improve outcome indicate that its importance in patient outcome has been misinterpreted. More recently, early brain injury has emerged as a new frontier and requires a better understanding and consideration in devising therapeutic strategy for improving aSAH outcome. In addition, better end points such as measurements of neurobehavioral deficits endured by aSAH patients are essential and their translation to the animal models is critical in identifying a potential therapy. Relevant animal models and timely treatment focused on prevention of early brain injury may establish a therapy, which if found beneficial for animals could be successfully translated in human aSAH trials.
Highlights.
Despite extensive research patient outcome post aSAH remains poor.
Delayed vasospasm is not the sole determinant of poor outcome in aSAH patients.
Brain injury begins at aneurysm rupture and contributes to overall outcome.
Understanding mechanisms of early brain injury is essential for its prevention.
Clinically relevant animal models will help us achieve this objective.
Acknowledgments
This work was supported by American Heart Organization Grant [10GRNT4570012 (FAS)] and the National Institutes of Health National Center for Research Resources [RO1 NS050576 (FAS); NS053407] (JHZ); and by the Intramural Research Program (RMP) of the National Institute of Neurological Disorders and Stroke.
List of nonstandard abbreviations
- aSAH
aneurysmal subarachnoid hemorrhage
- DIND
delayed ischemic neurological deficits
- ICP
intracranial pressure
- CPP
cerebral perfusion pressure
- CBF
cerebral blood flow
- CSD
cortical spreading depolarization
- NMDA
N-methyl-D-aspartate
- CSWS
cerebral salt-wasting syndrome
- SIADH
secretion of anti-diuretic hormone
- MRI
magnetic resonance imaging
- DWI
diffusion weight imaging
- ADC
apparent diffusion coefficient
- BID
Bcl-2 interacting domain
- tBID
truncated Bcl-2 interacting domain
- TNFR
tumor necrosis factor receptor
- FADD
Fas-associated death domain protein
- NO
nitric oxide
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- CSF
cerebral spinal fluid
- ET-1
endothelin-1
- ROS
oxygen free radicals
- BBB
blood brain barrier
- CRP
C-reactive protein
- TNF-α
tumor necrosis factor
- MMP-2 and 9
matrix metalloproteinases-2 and 9
- GCS
Glasgow comma scale
- WFNS
World Federation of Neurological Surgeons
- cGMP
cyclic guanosine 3′,5′-monophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abumiya T, Fitridge R, Mazur C, Copeland BR, Koziol JA, Tschopp JF, Pierschbacher MD, del Zoppo GJ. Integrin alpha(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31:1402–1409. doi: 10.1161/01.str.31.6.1402. discussion 1409-1410. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr., Kassell NF, Torner JC. Usefulness of computed tomography in predicting outcome after aneurysmal subarachnoid hemorrhage: a preliminary report of the Cooperative Aneurysm Study. Neurology. 1985;35:1263–1267. doi: 10.1212/wnl.35.9.1263. [DOI] [PubMed] [Google Scholar]
- Afshar JK, Pluta RM, Boock RJ, Thompson BG, Oldfield EH. Effect of intracarotid nitric oxide on primate cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1995;83:118–122. doi: 10.3171/jns.1995.83.1.0118. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Patir R, Kato Y, Chopra S, Sano H, Kanno T. Role of intraventricular sodium nitroprusside in vasospasm secondary to aneurysmal subarachnoid haemorrhage: a 5-year prospective study with review of the literature. Minim Invasive Neurosurg. 2009;52:5–8. doi: 10.1055/s-0028-1085454. [DOI] [PubMed] [Google Scholar]
- Akpinar G, Acikgoz B, Surucu S, Celik HH, Cagavi F. Ultrastructural changes in the circumventricular organs after experimental subarachnoid hemorrhage. Neurol Res. 2005;27:580–585. doi: 10.1179/016164105X48752. [DOI] [PubMed] [Google Scholar]
- Alaraj A, Charbel FT, Amin-Hanjani S. Peri-operative measures for treatment and prevention of cerebral vasospasm following subarachnoid hemorrhage. Neurol Res. 2009;31:651–659. doi: 10.1179/174313209X382395. [DOI] [PubMed] [Google Scholar]
- Alexander S, Poloyac S, Hoffman L, Gallek M, Dianxu R, Balzer J, Kassam A, Conley Y. Endothelial nitric oxide synthase tagging single nucleotide polymorphisms and recovery from aneurysmal subarachnoid hemorrhage. Biol Res Nurs. 2009;11:42–52. doi: 10.1177/1099800409334751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan T, Tureyen K, Ulutas M, Kahveci N, Goren B, Korfali E, Ozluk K. Acute and delayed vasoconstriction after subarachnoid hemorrhage: local cerebral blood flow, histopathology, and morphology in the rat basilar artery. Arch Physiol Biochem. 2001;109:145–153. doi: 10.1076/apab.109.2.145.4267. [DOI] [PubMed] [Google Scholar]
- Altura BM, Gebrewold A, Altura BT, Gupta RK. Role of brain [Mg2+]i in alcohol-induced hemorrhagic stroke in a rat model: a 31P-NMR in vivo study. Alcohol. 1995;12:131–136. doi: 10.1016/0741-8329(94)00072-7. [DOI] [PubMed] [Google Scholar]
- Altura BT, Altura BM. The role of magnesium in etiology of strokes and cerebrovasospasm. Magnesium. 1982;1:277–291. [Google Scholar]
- Altura BT, Memon ZI, Zhang A, Cheng TP, Silverman R, Cracco RQ, Altura BM. Low levels of serum ionized magnesium are found in patients early after stroke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular muscle cells. Neurosci Lett. 1997;230:37–40. doi: 10.1016/s0304-3940(97)00471-0. [DOI] [PubMed] [Google Scholar]
- Ames A. d., Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- Ando T, Sakai N, Yamada H, Iwai T, Nishimura Y, Hirata T, Funakoshi T, Takada M. Analysis of reruptured cerebral aneurysms and the prophylactic effects of barbiturate therapy on the early stage. Neurol Res. 1989;11:245–248. doi: 10.1080/01616412.1989.11739900. [DOI] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM., Jr. Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- Arutiunov AI, Baron MA, Majorova NA. Experimental and clinical study of the development of spasm of the cerebral arteries related to subarachnoid hemorrhage. J Neurosurg. 1970;32:617–625. doi: 10.3171/jns.1970.32.6.0617. [DOI] [PubMed] [Google Scholar]
- Arutiunov AI, Baron MA, Majorova NA. The role of mechanical factors in the pathogenesis of short-term and prolonged spasm of the cerebral arteries. J Neurosurg. 1974;40:459–472. doi: 10.3171/jns.1974.40.4.0459. [DOI] [PubMed] [Google Scholar]
- Asaeda M, Sakamoto M, Kurosaki M, Tabuchi S, Kamitani H, Yokota M, Watanabe T. A non-enzymatic derived arachidonyl peroxide, 8-iso-prostaglandin F2 alpha, in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage participates in the pathogenesis of delayed cerebral vasospasm. Neurosci Lett. 2005;373:222–225. doi: 10.1016/j.neulet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Asano T. Oxyhemoglobin as the principal cause of cerebral vasospasm: a holistic view of its actions. Crit Rev Neurosurg. 1999;9:303–318. doi: 10.1007/s003290050147. [DOI] [PubMed] [Google Scholar]
- Asano T, Ikegaki I, Satoh S, Suzuki Y, Shibuya M, Sugita K, Hidaka H. Endothelin: a potential modulator of cerebral vasospasm. Eur J Pharmacol. 1990;190:365–372. doi: 10.1016/0014-2999(90)94201-8. [DOI] [PubMed] [Google Scholar]
- Asano T, Ikegaki I, Suzuki Y, Satoh S, Shibuya M. Endothelin and the production of cerebral vasospasm in dogs. Biochem Biophys Res Commun. 1989;159:1345–1351. doi: 10.1016/0006-291x(89)92258-4. [DOI] [PubMed] [Google Scholar]
- Asano T, Matsui T. Antioxidant therapy against cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Cell Mol Neurobiol. 1999;19:31–44. doi: 10.1023/A:1006908422937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Sano K. Pathogenetic role of no-reflow phenomenon in experimental subarachnoid hemorrhage in dogs. J Neurosurg. 1977;46:454–466. doi: 10.3171/jns.1977.46.4.0454. [DOI] [PubMed] [Google Scholar]
- Audibert G, Steinmann G, de Talance N, Laurens MH, Dao P, Baumann A, Longrois D, Mertes PM. Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation. Anesth Analg. 2009;108:1922–1928. doi: 10.1213/ane.0b013e31819a85ae. [DOI] [PubMed] [Google Scholar]
- Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Barry KJ, Gogjian MA, Stein BM. Small animal model for investigation of subarachnoid hemorrhage and cerebral vasospasm. Stroke. 1979;10:538–541. doi: 10.1161/01.str.10.5.538. [DOI] [PubMed] [Google Scholar]
- Bavbek M, Polin R, Kwan AL, Arthur AS, Kassell NF, Lee KS. Monoclonal antibodies against ICAM-1 and CD18 attenuate cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Stroke. 1998;29:1930–1935. doi: 10.1161/01.str.29.9.1930. discussion 1935-1936. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Epidemiology and clinical presentation of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 1998;9:435–444. [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Connolly ES, Jr., Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr., Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins A. L. r., Vallabhajosyula P. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery. 1998;42:352–360. doi: 10.1097/00006123-199802000-00091. [DOI] [PubMed] [Google Scholar]
- Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, Schulte M, von Wild K, Scherer R. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997;349:245–249. doi: 10.1016/s0140-6736(96)08093-2. [DOI] [PubMed] [Google Scholar]
- Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005;135:2499–2502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- Berthon N, Laurant P, Fellmann D, Berthelot A. Effect of magnesium on mRNA expression and production of endothelin-1 in DOCA-salt hypertensive rats. J Cardiovasc Pharmacol. 2003;42:24–31. doi: 10.1097/00005344-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Bevan JA, Bevan RD, Walters CL, Wellman T. Functional changes in human pial arteries (300 to 900 micrometer ID] within 48 hours of aneurysmal subarachnoid hemorrhage. Stroke. 1998;29:2575–2579. doi: 10.1161/01.str.29.12.2575. [DOI] [PubMed] [Google Scholar]
- Biondi A, Ricciardi GK, Puybasset L, Abdennour L, Longo M, Chiras J, Van Effenterre R. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol. 2004;25:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- Boisvert DP, Overton TR, Weir B, Grace MG. Cerebral arterial responses to induced hypertension following subarachnoid hemorrhage in the monkey. J Neurosurg. 1978;49:75–83. doi: 10.3171/jns.1978.49.1.0075. [DOI] [PubMed] [Google Scholar]
- Brinker T, Seifert V, Dietz H. Cerebral blood flow and intracranial pressure during experimental subarachnoid haemorrhage. Acta Neurochir. 1992;115:47–52. doi: 10.1007/BF01400590. [DOI] [PubMed] [Google Scholar]
- Brinker T, Seifert V, Stolke D. Acute changes in the dynamics of the cerebrospinal fluid system during experimental subarachnoid hemorrhage. Neurosurgery. 1990;27:369–372. doi: 10.1097/00006123-199009000-00005. [DOI] [PubMed] [Google Scholar]
- Brisman JL, Berenstein A. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2004;54:1031. doi: 10.1227/01.neu.0000117123.32806.f9. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Bruder N, Ichai C, Gelb AW. Hyponatremia and subarachnoid hemorrhage: will that be one pinch or two of salt? Anesth Analg. 2009;108:1734–1735. doi: 10.1213/ane.0b013e3181a32872. [DOI] [PubMed] [Google Scholar]
- Bruder N, Rabinstein A. Cardiovascular and pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:257–269. doi: 10.1007/s12028-011-9598-4. [DOI] [PubMed] [Google Scholar]
- Burger R, Duncker D, Uzma N, Rohde V. Decompressive craniotomy: durotomy instead of duroplasty to reduce prolonged ICP elevation. Acta Neurochir Suppl. 2008;102:93–97. doi: 10.1007/978-3-211-85578-2_19. [DOI] [PubMed] [Google Scholar]
- Busch E, Beaulieu C, de Crespigny A, Moseley ME. Diffusion MR imaging during acute subarachnoid hemorrhage in rats. Stroke. 1998;29:2155–2161. doi: 10.1161/01.str.29.10.2155. [DOI] [PubMed] [Google Scholar]
- Cahill J, Calvert JW, Solaroglu I, Zhang JH. Vasospasm and p53-induced apoptosis in an experimental model of subarachnoid hemorrhage. Stroke. 2006a;37:1868–1874. doi: 10.1161/01.STR.0000226995.27230.96. [DOI] [PubMed] [Google Scholar]
- Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006b;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- Cappelletto B, Caner HH, Schottler F, Kwan AL, Eveleth D, Foley PL, Kassell NF, Lee KS. Attenuation of vasospasm and hemoglobin-induced constriction in the rabbit basilar artery by a novel protease inhibitor. Neurosurg Focus. 1997;3:e2. doi: 10.3171/foc.1997.3.4.5. [DOI] [PubMed] [Google Scholar]
- Carlson AP, Yonas H. Radiographic assessment of vasospasm after aneurysmal subarachnoid hemorrhage: the physiological perspective. Neurol Res. 2009;31:593–604. doi: 10.1179/174313209X455754. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DA, Seto S, Wasson DB, Carrera CJ. DNA strand breaks, NAD metabolism, and programmed cell death. Exp Cell Res. 1986;164:273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Chahal N, Barker-Collo S, Feigin V. Cognitive and functional outcomes of 5-year subarachnoid haemorrhage survivors: comparison to matched healthy controls. Neuroepidemiology. 2011;37:31–38. doi: 10.1159/000328647. [DOI] [PubMed] [Google Scholar]
- Chaichana KL, Pradilla G, Huang J, Tamargo RJ. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. Surg Neurol. 2010;73:22–41. doi: 10.1016/j.surneu.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke E. Apoplexy in the Hippocratic Writings. Bull Hist Med. 1963;37:301–314. [PubMed] [Google Scholar]
- Clower BR, Yamamoto Y, Cain L, Haines DE, Smith RR. Endothelial injury following experimental subarachnoid hemorrhage in rats: effects on brain blood flow. Anat Rec. 1994;240:104–114. doi: 10.1002/ar.1092400110. [DOI] [PubMed] [Google Scholar]
- Clower BR, Yoshioka J, Honma Y, Smith RR. Pathological changes in cerebral arteries following experimental subarachnoid hemorrhage: role of blood platelets. Anat Rec. 1988;220:161–170. doi: 10.1002/ar.1092200207. [DOI] [PubMed] [Google Scholar]
- Clozel M, Watanabe H. BQ-123, a peptidic endothelin ETA receptor antagonist, prevents the early cerebral vasospasm following subarachnoid hemorrhage after intracisternal but not intravenous injection. Life Sci. 1993;52:825–834. doi: 10.1016/0024-3205(93)90081-d. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther. 2011;9:433–449. doi: 10.1586/erc.11.34. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- Cunningham RT, Morrow JI, Johnston CF, Buchanan KD. Serum neurone-specific enolase concentrations in patients with neurological disorders. Clin Chim Acta. 1994;230:117–124. doi: 10.1016/0009-8981(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJ, Rinkel GJ. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–819. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debdi M, Seylaz J, Sercombe R. Early changes in rabbit cerebral artery reactivity after subarachnoid hemorrhage. Stroke. 1992;23:1154–1162. doi: 10.1161/01.str.23.8.1154. [DOI] [PubMed] [Google Scholar]
- Debdi M, Seylaz J, Sercombe R. Increased influence of calcium and nicardipine on rabbit basilar artery reactivity after brief subarachnoid hemorrhage. J Cardiovasc Pharmacol. 1993;21:754–759. doi: 10.1097/00005344-199305000-00010. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Microvascular responses to cerebral ischemia/inflammation. Ann N Y Acad Sci. 1997;823:132–147. doi: 10.1111/j.1749-6632.1997.tb48386.x. [DOI] [PubMed] [Google Scholar]
- Delgado-Zygmunt TJ, Arbab MA, Shiokawa Y, Svendgaard NA. A primate model for acute and late cerebral vasospasm: angiographic findings. Acta Neurochir. 1992;118:130–136. doi: 10.1007/BF01401298. [DOI] [PubMed] [Google Scholar]
- Demirgil BT, Tugcu B, Postalci L, Guclu G, Dalgic A, Oral Z. Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Minim Invasive Neurosurg. 2003;46:344–348. doi: 10.1055/s-2003-812500. [DOI] [PubMed] [Google Scholar]
- Denton IC, Robertson JT, Dugdale M. An assessment of early platelet activity in experimental subarachnoid hemorrhage and middle cerebral artery thrombosis in the cat. Stroke. 1971;2:268–272. doi: 10.1161/01.str.2.3.268. [DOI] [PubMed] [Google Scholar]
- Deshaies EM, Boulos AS, Drazin D, Popp AJ. Evidence-based pharmacotherapy for cerebral vasospasm. Neurol Res. 2009;31:615–620. doi: 10.1179/174313209X382377. [DOI] [PubMed] [Google Scholar]
- Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hardemark HG, Rodichok L. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- Diringer MN. Management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2009;37:432–440. doi: 10.1097/CCM.0b013e318195865a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doczi T. The pathogenetic and prognostic significance of blood-brain barrier damage at the acute stage of aneurysmal subarachnoid haemorrhage. Clinical and experimental studies. Acta Neurochir (Wien) 1985;77:110–132. doi: 10.1007/BF01476215. [DOI] [PubMed] [Google Scholar]
- Doczi T, Bende J, Huszka E, Kiss J. Syndrome of inappropriate secretion of antidiuretic hormone after subarachnoid hemorrhage. Neurosurgery. 1981;9:394–397. doi: 10.1227/00006123-198110000-00008. [DOI] [PubMed] [Google Scholar]
- Doczi T, Joo F, Adam G, Bozoky B, Szerdahelyi P. Blood-brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery. 1986a;18:733–739. doi: 10.1227/00006123-198606000-00010. [DOI] [PubMed] [Google Scholar]
- Doczi T, Joo F, Sonkodi S, Adam G. Increased vulnerability of the blood-brain barrier to experimental subarachnoid hemorrhage in spontaneously hypertensive rats. Stroke. 1986b;17:498–501. doi: 10.1161/01.str.17.3.498. [DOI] [PubMed] [Google Scholar]
- Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763–769. doi: 10.1227/01.neu.0000053222.74852.2d. discussion 769-771. [DOI] [PubMed] [Google Scholar]
- Dorhout Mees SM, Bertens D, van der Worp HB, Rinkel GJ, van den Bergh WM. Magnesium and headache after aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2010;81:490–493. doi: 10.1136/jnnp.2009.181404. [DOI] [PubMed] [Google Scholar]
- Dorsch N, Branston NM, Symon L, Jakubowski J. Intracranial pressure changes following primate subarachnoid haemorrhage. Neurol Res. 1989;11:201–204. doi: 10.1080/01616412.1989.11739893. [DOI] [PubMed] [Google Scholar]
- Dorsch NW. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care. 2002;8:128–133. doi: 10.1097/00075198-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Ebert N, Priller J, Megow D, Lindauer U, Klee R, Reuter U, Imai Y, Einhaupl KM, Victorov I, Dirnagl U. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage? J Neurosurg. 2000;93:658–666. doi: 10.3171/jns.2000.93.4.0658. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Windmuller O, Petzold G, Lindauer U, Einhaupl KM, Dirnagl U. Ischemia triggered by red blood cell products in the subarachnoid space is inhibited by nimodipine administration or moderate volume expansion/hemodilution in rats. Neurosurgery. 2002;51:1457–1465. discussion 1465-1457. [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Dumont AS, Dumont RJ, Chow MM, Lin CL, Calisaneller T, Ley KF, Kassell NF, Lee KS. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–133. doi: 10.1227/01.neu.0000068863.37133.9e. discussion 133-125. [DOI] [PubMed] [Google Scholar]
- Dupont SA, Wijdicks EF, Manno EM, Lanzino G, Rabinstein AA. Prediction of angiographic vasospasm after aneurysmal subarachnoid hemorrhage: value of the Hijdra sum scoring system. Neurocrit Care. 2009;11:172–176. doi: 10.1007/s12028-009-9247-3. [DOI] [PubMed] [Google Scholar]
- Durmaz R, Ozkara E, Kanbak G, Arslan O, Dokumacioğlu A, Kartkaya K, Atasoy M. Nitric oxide level and adenosine deaminase activity in cerebrospinal fluid of patients with subarachnoid hemorrhage. Turkish neurosurgery. 2008;18:157–164. [PubMed] [Google Scholar]
- Ebel H, Rust DS, Leschinger A, Ehresmann N, Kranz A, Hoffmann O, Boker DK. Vasomotion, regional cerebral blood flow and intracranial pressure after induced subarachnoid haemorrhage in rats. Zentralbl Neurochir. 1996;57:150–155. [PubMed] [Google Scholar]
- Echlin F. Experimental vasospasm, acute and chronic, due to blood in the subarachnoid space. J Neurosurg. 1971;35:646–656. doi: 10.3171/jns.1971.35.6.0646. [DOI] [PubMed] [Google Scholar]
- Ecker A, Riemenschneider PA. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951;8:660–667. doi: 10.3171/jns.1951.8.6.0660. [DOI] [PubMed] [Google Scholar]
- Eddleman CS, Hurley MC, Naidech AM, Batjer HH, Bendok BR. Endovascular options in the treatment of delayed ischemic neurological deficits due to cerebral vasospasm. Neurosurg Focus. 2009;26:E6. doi: 10.3171/2008.11.FOCUS08278. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Byrne JV, Griffith TM. The effect of chronic subarachnoid hemorrhage on basal endothelium-derived relaxing factor activity in intrathecal cerebral arteries. J Neurosurg. 1992;76:830–837. doi: 10.3171/jns.1992.76.5.0830. [DOI] [PubMed] [Google Scholar]
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- Enblad P, Valtysson J, Andersson J, Lilja A, Valind S, Antoni G, Langstrom B, Hillered L, Persson L. Simultaneous intracerebral microdialysis and positron emission tomography in the detection of ischemia in patients with subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1996;16:637–644. doi: 10.1097/00004647-199607000-00014. [DOI] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cereb Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiner EA, Leikis R, Ferch RD, MacFarlane MR, Bonkowski JA, Frampton CM, Richards AM. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002;56:629–635. doi: 10.1046/j.1365-2265.2002.01285.x. [DOI] [PubMed] [Google Scholar]
- Espinosa F, Weir B, Shnitka T, Overton T, Boisvert D. A randomized placebo-controlled double-blind trial of nimodipine after SAH in monkeys. Part 2: Pathological findings. J Neurosurg. 1984;60:1176–1185. doi: 10.3171/jns.1984.60.6.1176. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schutt S, Fritzinger M, Horn P, Vajkoczy P, Kreisel S, Brunner J, Schmiedek P, Hennerici M. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001;70:534–537. doi: 10.1136/jnnp.70.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Patron C, Martinez-Cuesta MA, Salas E, Sawicki G, Wozniak M, Radomski MW, Davidge ST. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb Haemost. 1999;82:1730–1735. [PubMed] [Google Scholar]
- Fiebach JB, Schellinger PD, Geletneky K, Wilde P, Meyer M, Hacke W, Sartor K. MRI in acute subarachnoid haemorrhage; findings with a standardised stroke protocol. Neuroradiology. 2004;46:44–48. doi: 10.1007/s00234-003-1132-8. [DOI] [PubMed] [Google Scholar]
- Fisher CM. Clinical syndromes in cerebral thrombosis, hypertensive hemorrhage, and ruptured saccular aneurysm. Clin Neurosurg. 1975;22:117–147. doi: 10.1093/neurosurgery/22.cn_suppl_1.117. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Fountas KN, Tasiou A, Kapsalaki EZ, Paterakis KN, Grigorian AA, Lee GP, Robinson JS., Jr. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus. 2009;26:E22. doi: 10.3171/2009.2.FOCUS08311. [DOI] [PubMed] [Google Scholar]
- Frederickson RG, Low FN. Blood vessels and tissue space associated with the brain of the rat. Am J Anat. 1969;125:123–145. doi: 10.1002/aja.1001250202. [DOI] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Muller A, Bi W, Peerschke EI, Sehba FA. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J Neuroinflammation. 2011;8:103. doi: 10.1186/1742-2094-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Muller A, Sehba FA. Escape of intraluminal platelets into brain parenchyma after subarachnoid hemorrhage. Neuroscience. 2010a;165:968–975. doi: 10.1016/j.neuroscience.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Muller A, Sehba FA. Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res. 2010b;1354:179–187. doi: 10.1016/j.brainres.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Sehba FA. Cell death starts early after subarachnoid hemorrhage. Neurosci Lett. doi: 10.1016/j.neulet.2012.01.036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- Frykholm P, Andersson JL, Langstrom B, Persson L, Enblad P. Haemodynamic and metabolic disturbances in the acute stage of subarachnoid haemorrhage demonstrated by PET. Acta Neurol Scand. 2004;109:25–32. doi: 10.1034/j.1600-0404.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg. 1996;84:35–42. doi: 10.3171/jns.1996.84.1.0035. [DOI] [PubMed] [Google Scholar]
- Fujikawa H, Tani E, Yamaura I, Ozaki I, Miyaji K, Sato M, Takahashi K, Imajoh-Ohmi S. Activation of protein kinases in canine basilar artery in vasospasm. J Cereb Blood Flow Metab. 1999;19:44–52. doi: 10.1097/00004647-199901000-00005. [DOI] [PubMed] [Google Scholar]
- Fukami MH, Holmsen H, Kowalska MA, Niewiarowski S. Platelet Secretion. In: Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Hemostatis and thrombosis basic principles and clinical practice. Lippincott Williams & Wilkins; Phialdelphia, PA, USA: 2001. pp. 562–573. [Google Scholar]
- Fukuhara T, Douville CM, Eliott JP, Newell DW, Winn HR. Relationship between intracranial pressure and the development of vasospasm after aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 1998;38:710–715. doi: 10.2176/nmc.38.710. discussion 716-717. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Cafe C, Rodriguez y Baena R, Tancioni F, Torri C, Tartara F, Marzatico F. Superoxide dismutase activity in cisternal cerebrospinal fluid after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1997;139:1033–1037. doi: 10.1007/BF01411556. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Marzatico F, Renault B, Fulle I, Lombardi D, Ferlenga P, Rodriguez y Baena R. High-dose methylprednisolone and ‘ex vivo’ release of eicosanoids after experimental subarachnoid haemorrhage. Neurol Res. 1990a;12:111–116. doi: 10.1080/01616412.1990.11739928. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Marzatico F, Rodriguez y Baena R, Pacchiarini L, Vigano T, Grignani G, Crivellari MT, Benzi G. Arachidonic acid metabolism and pathophysiologic aspects of subarachnoid hemorrhage in rats. Stroke. 1990b;21:328–332. doi: 10.1161/01.str.21.2.328. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Pasqualin A, Baena R, Borasio E, Marzatico F. Oxidative stress in the human brain after subarachnoid hemorrhage. J Neurosurg. 1998;89:748–754. doi: 10.3171/jns.1998.89.5.0748. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Rodriguez y Baena R, Quaglini S, Bellazzi R, Cafe C, Torri C, Marzatico F. Experimental subarachnoid hemorrhage: events related to anti-oxidant enzymatic systems and eicosanoid peroxide enhancement. Neurochem Res. 1994;19:839–844. doi: 10.1007/BF00967453. [DOI] [PubMed] [Google Scholar]
- Gallerani M, Portaluppi F, Maida G, Chieregato A, Calzolari F, Trapella G, Manfredini R. Circadian and circannual rhythmicity in the occurrence of subarachnoid hemorrhage. Stroke. 1996;27:1793–1797. doi: 10.1161/01.str.27.10.1793. [DOI] [PubMed] [Google Scholar]
- Gambardella G, De Blasi F, Caruso G, Zema A, Turiano F, Collufio D. Intracranial pressure, cerebral perfusion pressure, and SPECT in the management of patients with SAH Hunt and Hess grades I-II. Acta Neurochir Suppl. 1998;71:215–218. doi: 10.1007/978-3-7091-6475-4_62. [DOI] [PubMed] [Google Scholar]
- Germano A, Caffo M, Angileri FF, Arcadi F, Newcomb-Fernandez J, Caruso G, Meli F, Pineda JA, Lewis SB, Wang KK, Bramanti P, Costa C, Hayes RL. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–744. doi: 10.1089/neu.2006.0181. [DOI] [PubMed] [Google Scholar]
- Germano A, Costa C, DeFord SM, Angileri FF, Arcadi F, Pike BR, Bramanti P, Bausano B, Zhao X, Day AL, Anderson DK, Hayes RL. Systemic administration of a calpain inhibitor reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2002;19:887–896. doi: 10.1089/08977150260190474. [DOI] [PubMed] [Google Scholar]
- Germano A, d’Avella D, Cicciarello R, Hayes RL, Tomasello F. Blood-brain barrier permeability changes after experimental subarachnoid hemorrhage. Neurosurgery. 1992;30:882–886. doi: 10.1227/00006123-199206000-00011. [DOI] [PubMed] [Google Scholar]
- Germano A, d’Avella D, Imperatore C, Caruso G, Tomasello F. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir. 2000;142:575–580. doi: 10.1007/s007010050472. [DOI] [PubMed] [Google Scholar]
- Germano AF, Dixon CE, d’Avella D, Hayes RL, Tomasello F. Behavioral deficits following experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 1994;11:345–353. doi: 10.1089/neu.1994.11.345. [DOI] [PubMed] [Google Scholar]
- Gewirtz RJ, Dhillon HS, Goes SE, DeAtley SM, Scheff SW. Lactate and free fatty acids after subarachnoid hemorrhage. Brain Res. 1999;840:84–91. doi: 10.1016/s0006-8993(99)01752-7. [DOI] [PubMed] [Google Scholar]
- Gomis P, Graftieaux JP, Sercombe R, Hettler D, Scherpereel B, Rousseaux P. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2010;112:681–688. doi: 10.3171/2009.4.JNS081377. [DOI] [PubMed] [Google Scholar]
- Graetz D, Nagel A, Schlenk F, Sakowitz O, Vajkoczy P, Sarrafzadeh A. High ICP as trigger of proinflammatory IL-6 cytokine activation in aneurysmal subarachnoid hemorrhage. Neurol Res. 2010;32:728–735. doi: 10.1179/016164109X12464612122650. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR, Torner J, Adams HP, Jr., Kassell NF. Factors associated with hydrocephalus after subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Arch Neurol. 1989;46:744–752. doi: 10.1001/archneur.1989.00520430038014. [DOI] [PubMed] [Google Scholar]
- Grobelny BT, Ducruet AF, DeRosa PA, Kotchetkov IS, Zacharia BE, Hickman ZL, Fernandez L, Narula R, Claassen J, Lee K, Badjatia N, Mayer SA, Connolly ES., Jr. Gain-of-function polymorphisms of cystathionine beta-synthase and delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2011;115:101–107. doi: 10.3171/2011.2.JNS101414. [DOI] [PubMed] [Google Scholar]
- Grosset DG, Straiton J, McDonald I, Bullock R. Angiographic and Doppler diagnosis of cerebral artery vasospasm following subarachnoid haemorrhage. Br J Neurosurg. 1993;7:291–298. doi: 10.3109/02688699309023812. [DOI] [PubMed] [Google Scholar]
- Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–661. doi: 10.1227/00006123-198804000-00006. [DOI] [PubMed] [Google Scholar]
- Gruber A, Dietrich W, Czech T, Richling B. Recurrent aneurysmal subarachnoid haemorrhage: bleeding pattern and incidence of posthaemorrhagic ischaemic infarction. Br J Neurosurg. 1997;11:121–126. doi: 10.1080/02688699746465. [DOI] [PubMed] [Google Scholar]
- Gruber A, Roessler K, Georgopoulos A, Missbichler A, Bonelli R, Richling B. Evaluation of big endothelin-1 concentrations in serum and ventricular cerebrospinal fluid after early surgical compared with nonsurgical management of ruptured intracranial aneurysms. Neurosurg Focus. 2000a;8:e6. doi: 10.3171/foc.2000.8.5.6. [DOI] [PubMed] [Google Scholar]
- Gruber A, Rossler K, Graninger W, Donner A, Illievich MU, Czech T. Ventricular cerebrospinal fluid and serum concentrations of sTNFR-I, IL-1ra, and IL-6 after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2000b;12:297–306. doi: 10.1097/00008506-200010000-00001. [DOI] [PubMed] [Google Scholar]
- Gules I, Satoh M, Clower BR, Nanda A, Zhang JH. Comparison of three rat models of cerebral vasospasm. Am J Physiol Heart Circ Physiol. 2002;283:H2551–2559. doi: 10.1152/ajpheart.00616.2002. [DOI] [PubMed] [Google Scholar]
- Gules I, Satoh M, Nanda A, Zhang JH. Apoptosis, blood-brain barrier, and subarachnoid hemorrhage. Acta Neurochir Suppl. 2003;86:483–487. doi: 10.1007/978-3-7091-0651-8_99. [DOI] [PubMed] [Google Scholar]
- Guo Z, Sun X, He Z, Jiang Y, Zhang X, Zhang JH. Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol Res. 2010;32:715–720. doi: 10.1179/016164109X12478302362491. [DOI] [PubMed] [Google Scholar]
- Hackam DG. Translating animal research into clinical benefit. BMJ. 2007;334:163–164. doi: 10.1136/bmj.39104.362951.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeishi H, Suzuki A, Yasui N, Hatazawa J, Shimosegawa E. Diffusion-weighted magnetic resonance imaging in patients with subarachnoid hemorrhage. Neurosurgery. 2002;50:741–747. doi: 10.1097/00006123-200204000-00010. discussion 747-748. [DOI] [PubMed] [Google Scholar]
- Haining JL, Clower BR, Honma Y, Smith RR. Accumulation of intimal platelets in cerebral arteries following experimental subarachnoid hemorrhage in cats. Stroke. 1988;19:898–902. doi: 10.1161/01.str.19.7.898. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr., Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205–214. doi: 10.1161/01.str.23.2.205. [DOI] [PubMed] [Google Scholar]
- Hall ED, Travis MA. Effects of the nonglucocorticoid 21-aminosteroid U74006F on acute cerebral hypoperfusion following experimental subarachnoid hemorrhage. Exp Neurol. 1988;102:244–248. doi: 10.1016/0014-4886(88)90100-8. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- Handa Y, Kubota T, Kaneko M, Tsuchida A, Kobayashi H, Kawano H. Expression of intercellular adhesion molecule 1 (ICAM-1) on the cerebral artery following subarachnoid haemorrhage in rats. Acta Neurochir (Wien) 1995;132:92–97. doi: 10.1007/BF01404854. [DOI] [PubMed] [Google Scholar]
- Handa Y, Weir BK, Nosko M, Mosewich R, Tsuji T, Grace M. The effect of timing of clot removal on chronic vasospasm in a primate model. J Neurosurg. 1987;67:558–564. doi: 10.3171/jns.1987.67.4.0558. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J, Hoel NL, Zhou M, Xu CB, Svendgaard NA, Edvinsson L. Subarachnoid hemorrhage enhances endothelin receptor expression and function in rat cerebral arteries. Neurosurgery. 2003;52:1188–1194. [PubMed] [Google Scholar]
- Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Ann Neurol. 1990;27:106–108. doi: 10.1002/ana.410270118. [DOI] [PubMed] [Google Scholar]
- Hatake K, Wakabayashi I, Kakishita E, Hishida S. Impairment of endothelium-dependent relaxation in human basilar artery after subarachnoid hemorrhage. Stroke. 1992;23:1111–1116. doi: 10.1161/01.str.23.8.1111. discussion 1116-1117. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Suzuki A, Hatazawa J, Hadeishi H, Shirane R, Tominaga T, Yasui N. Post-operative changes of cerebral circulation and metabolism in the acute stage of low-grade aneurysmal subarachnoid hemorrhage. Neurol Res. 2008;30:678–683. doi: 10.1179/174313208X291676. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Suzuki A, Hatazawa J, Kanno I, Shirane R, Yoshimoto T, Yasui N. Cerebral circulation and metabolism in the acute stage of subarachnoid hemorrhage. J Neurosurg. 2000;93:1014–1018. doi: 10.3171/jns.2000.93.6.1014. [DOI] [PubMed] [Google Scholar]
- Heilbrun MP, Olesen J, Lassen NA. Regional cerebral blood flow studies in subarachnoid hemorrhage. J Neurosurg. 1972;37:36–44. doi: 10.3171/jns.1972.37.1.0036. [DOI] [PubMed] [Google Scholar]
- Hendryk S, Jarzab B, Josko J. Increase of the IL-1 beta and IL-6 levels in CSF in patients with vasospasm following aneurysmal SAH. Neuro Endocrinol Lett. 2004;25:141–147. [PubMed] [Google Scholar]
- Heuer GG, Smith MJ, Elliott JP, Winn HR, LeRoux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:408–416. doi: 10.3171/jns.2004.101.3.0408. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Hattori H, Hattori R, Furusho K. Increased neurons containing neuronal nitric oxide synthase in the brain of a hypoxic-ischemic neonatal rat model. Brain Dev. 1996;18:369–375. doi: 10.1016/0387-7604(96)00019-8. [DOI] [PubMed] [Google Scholar]
- Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- Hillman J, von Essen C, Leszniewski W, Johansson I. Significance of “ultra-early” rebleeding in subarachnoid hemorrhage. J Neurosurg. 1988;68:901–907. doi: 10.3171/jns.1988.68.6.0901. [DOI] [PubMed] [Google Scholar]
- Hirashima Y, Hamada H, Kurimoto M, Origasa H, Endo S. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;102:882–887. doi: 10.3171/jns.2005.102.5.0882. [DOI] [PubMed] [Google Scholar]
- Hoelper BM, Hofmann E, Sporleder R, Soldner F, Behr R. Transluminal balloon angioplasty improves brain tissue oxygenation and metabolism in severe vasospasm after aneurysmal subarachnoid hemorrhage: case report. Neurosurgery. 2003;52:970–974. doi: 10.1227/01.neu.0000053033.98317.a3. discussion 974-976. [DOI] [PubMed] [Google Scholar]
- Holmin S, Soderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. discussion 298-299. [DOI] [PubMed] [Google Scholar]
- Hongo K, Kassell NF, Nakagomi T, Sasaki T, Tsukahara T, Ogawa H, Vollmer DG, Lehman RM. Subarachnoid hemorrhage inhibition of endothelium-derived relaxing factor in rabbit basilar artery. J Neurosurg. 1988;69:247–253. doi: 10.3171/jns.1988.69.2.0247. [DOI] [PubMed] [Google Scholar]
- Honma Y, Clower BR, Haining JL, Smith RR. Comparison of intimal platelet accumulation in cerebral arteries in two experimental models of subarachnoid hemorrhage. Neurosurgery. 1989;24:487–490. doi: 10.1227/00006123-198904000-00001. [DOI] [PubMed] [Google Scholar]
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- Hsieh YP, Lin CL, Shiue AL, Yin H, Morrow JD, Hsu JC, Hsieh TC, Wei HJ, Yen HC. Correlation of F4-neuroprostanes levels in cerebrospinal fluid with outcome of aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2009;47:814–824. doi: 10.1016/j.freeradbiomed.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Huang CY, Chan FL, Yu YL, Woo E, Chin D. Cerebrovascular disease in Hong Kong Chinese. Stroke. 1990;21:230–235. doi: 10.1161/01.str.21.2.230. [DOI] [PubMed] [Google Scholar]
- Huang J, van Gelder JM. The probability of sudden death from rupture of intracranial aneurysms: a meta-analysis. Neurosurgery. 2002;51:1101–1105. doi: 10.1097/00006123-200211000-00001. discussion 1105-1107. [DOI] [PubMed] [Google Scholar]
- Hubschmann OR. The role of calcium in parenchymal cell injury in subarachnoid haemorrhage. Neurol Res. 1987;9:265–269. doi: 10.1080/01616412.1987.11739806. [DOI] [PubMed] [Google Scholar]
- Hubschmann OR, Kornhauser D. Cortical cellular response in acute subarachnoid hemorrhage. J Neurosurg. 1980;52:456–462. doi: 10.3171/jns.1980.52.4.0456. [DOI] [PubMed] [Google Scholar]
- Hubschmann OR, Kornhauser D. Effect of subarachnoid hemorrhage on the extracellular microenvironment. J Neurosurg. 1982;56:216–221. doi: 10.3171/jns.1982.56.2.0216. [DOI] [PubMed] [Google Scholar]
- Hughes MA, Grover PJ, Butler CR, Elwell VA, Mendoza ND. A 5-year retrospective study assessing the association between seasonal and meteorological change and incidence of aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 2010;24:396–400. doi: 10.3109/02688697.2010.499154. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O’Connell MT, Al-Rawi PG, Kett-White CR, Gupta AK, Maskell LB, Pickard JD, Kirkpatrick PJ. Increases in GABA concentrations during cerebral ischaemia: a microdialysis study of extracellular amino acids. J Neurol Neurosurg Psychiatry. 2002;72:99–105. doi: 10.1136/jnnp.72.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter BO, Gilsbach JM, Kreitschmann I. Quality of life and cognitive deficits after subarachnoid haemorrhage. Br J Neurosurg. 1995;9:465–475. doi: 10.1080/02688699550041106. [DOI] [PubMed] [Google Scholar]
- Hutter BO, Kreitschmann-Andermahr I, Gilsbach JM. Health-related quality of life after aneurysmal subarachnoid hemorrhage: impacts of bleeding severity, computerized tomography findings, surgery, vasospasm, and neurological grade. J Neurosurg. 2001;94:241–251. doi: 10.3171/jns.2001.94.2.0241. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Imperatore C, Germano A, d’Avella D, Tomasello F, Costa G. Effects of the radical scavenger AVS on behavioral and BBB changes after experimental subarachnoid hemorrhage. Life Sci. 2000;66:779–790. doi: 10.1016/s0024-3205(99)00651-7. [DOI] [PubMed] [Google Scholar]
- Inagawa T. What are the actual incidence and mortality rates of subarachnoid hemorrhage? Surg Neurol. 1997;47:47–52. doi: 10.1016/s0090-3019(96)00370-9. discussion 52-43. [DOI] [PubMed] [Google Scholar]
- Inagawa T, Kamiya K, Ogasawara H, Yano T. Rebleeding of ruptured intracranial aneurysms in the acute stage. Surg Neurol. 1987;28:93–99. doi: 10.1016/0090-3019(87)90079-6. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Murakami K, Link T, Zvarova K, Tranmer BI, Morielli AD, Wellman GC. Acute and chronic effects of oxyhemoglobin on voltage-dependent ion channels in cerebral arteries. Acta Neurochir Suppl. 2008;104:99–102. doi: 10.1007/978-3-211-75718-5_19. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kusaka G, Yamaguchi N, Sekizuka E, Nakadate H, Minamitani H, Shinoda S, Watanabe E. Platelet and leukocyte adhesion in the microvasculature at the cerebral surface immediately after subarachnoid hemorrhage. Neurosurgery. 2009;64:546–553. doi: 10.1227/01.NEU.0000337579.05110.F4. discussion 553-544. [DOI] [PubMed] [Google Scholar]
- Isotani E, Suzuki R, Tomita K, Hokari M, Monma S, Marumo F, Hirakawa K. Alterations in plasma concentrations of natriuretic peptides and antidiuretic hormone after subarachnoid hemorrhage. Stroke. 1994;25:2198–2203. doi: 10.1161/01.str.25.11.2198. [DOI] [PubMed] [Google Scholar]
- Iuliano BA, Pluta RM, Jung C, Oldfield EH. Endothelial dysfunction in a primate model of cerebral vasospasm. J Neurosurg. 2004;100:287–294. doi: 10.3171/jns.2004.100.2.0287. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Sugawara T, Zhang J, Jacobson P, Obenaus A. Magnetic resonance imaging detects and predicts early brain injury after subarachnoid hemorrhage in a canine experimental model. J Neurotrauma. 2008;25:1099–1106. doi: 10.1089/neu.2008.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger M, Soehle M, Meixensberger J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 2003;74:513–515. doi: 10.1136/jnnp.74.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen M. Role of initial brain ischemia in subarachnoid hemorrhage following aneurysm rupture. A pathophysiological survey. Acta Neurol Scand Suppl. 1992;141:1–33. [PubMed] [Google Scholar]
- Jakobsen M, Enevoldsen E, Bjerre P. Cerebral blood flow and metabolism following subarachnoid haemorrhage: cerebral oxygen uptake and global blood flow during the acute period in patients with SAH. Acta Neurol Scand. 1990;82:174–182. doi: 10.1111/j.1600-0404.1990.tb04485.x. [DOI] [PubMed] [Google Scholar]
- Jakubowski J, Bell BA, Symon L, Zawirski MB, Francis DM. A primate model of subarachnoid hemorrhage: change in regional cerebral blood flow, autoregulation carbon dioxide reactivity, and central conduction time. Stroke. 1982;13:601–611. doi: 10.1161/01.str.13.5.601. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Berenstein V, Li X, Mayer J, Silane M, Shin YS, Niimi Y, Kilic T, Gunel M, Berenstein A. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery. 2005;57:558–564. doi: 10.1227/01.neu.0000170439.89041.d6. discussion 558-564. [DOI] [PubMed] [Google Scholar]
- Jedrzejowska-Szypulka H, Larysz-Brysz M, Kukla M, Snietura M, Lewin-Kowalik J. Neutralization of interleukin-1beta reduces vasospasm and alters cerebral blood vessel density following experimental subarachnoid hemorrhage in rats. Curr Neurovasc Res. 2009;6:95–103. doi: 10.2174/156720209788185669. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–1418. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- Josko J, Hendryk S, Jedrzejowska-Szypula H, Gwozdz B, Herman ZS, Gawlik R. Influence endothelin ETA receptor antagonist--BQ-123--on changes of endothelin-1 level in plasma of rats with acute vasospasm following subarachnoid hemorrhage. J Physiol Pharmacol. 1998;49:367–375. [PubMed] [Google Scholar]
- Josko J, Hendryk S, Jedrzejowska-Szypulka H, Slowinski J, Gwozdz B, Lange D, Snietura M, Zwirska-Korczala K, Jochem J. Cerebral angiogenesis after subarachnoid hemorrhage (SAH) and endothelin receptor blockage with BQ-123 antagonist in rats. J Physiol Pharmacol. 2001;52:237–248. [PubMed] [Google Scholar]
- Jung CS, Iuliano BA, Harvey-White J, Espey MG, Oldfield EH, Pluta RM. Association between cerebrospinal fluid levels of asymmetric dimethyl-L-arginine, an endogenous inhibitor of endothelial nitric oxide synthase, and cerebral vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 2004;101:836–842. doi: 10.3171/jns.2004.101.5.0836. [DOI] [PubMed] [Google Scholar]
- Jung CS, Oldfield EH, Harvey-White J, Espey MG, Zimmermann M, Seifert V, Pluta RM. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:945–950. doi: 10.3171/JNS-07/11/0945. [DOI] [PubMed] [Google Scholar]
- Kacira T, Kemerdere R, Atukeren P, Hanimoglu H, Sanus GZ, Kucur M, Tanriverdi T, Gumustas K, Kaynar MY. Detection of caspase-3, neuron specific enolase, and high-sensitivity C-reactive protein levels in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:674–679. doi: 10.1227/01.NEU.0000255394.77538.BB. discussion 679-680. [DOI] [PubMed] [Google Scholar]
- Kader A, Krauss WE, Onesti ST, Elliott JP, Solomon RA. Chronic cerebral blood flow changes following experimental subarachnoid hemorrhage in rats. Stroke. 1990;21:577–581. doi: 10.1161/01.str.21.4.577. [DOI] [PubMed] [Google Scholar]
- Kajita Y, Suzuki Y, Oyama H, Tanazawa T, Takayasu M, Shibuya M, Sugita K. Combined effect of L-arginine and superoxide dismutase on the spastic basilar artery after subarachnoid hemorrhage in dogs. J Neurosurg. 1994;80:476–483. doi: 10.3171/jns.1994.80.3.0476. [DOI] [PubMed] [Google Scholar]
- Kamezaki T, Yanaka K, Nagase S, Fujita K, Kato N, Nose T. Increased levels of lipid peroxides as predictive of symptomatic vasospasm and poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:1302–1305. doi: 10.3171/jns.2002.97.6.1302. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Kuyama H, Symon L. An experimental study of the acute stage of subarachnoid hemorrhage. J Neurosurg. 1983;59:917–924. doi: 10.3171/jns.1983.59.6.0917. [DOI] [PubMed] [Google Scholar]
- Kao L, Al-Lawati Z, Vavao J, Steinberg GK, Katznelson L. Prevalence and clinical demographics of cerebral salt wasting in patients with aneurysmal subarachnoid hemorrhage. Pituitary. 2009;12:347–351. doi: 10.1007/s11102-009-0188-9. [DOI] [PubMed] [Google Scholar]
- Kapp J, Mahaley MS, Jr., Odom GL. Cerebral arterial spasm. 2. Experimental evaluation of mechanical and humoral factors in pathogenesis. J Neurosurg. 1968;29:339–349. doi: 10.3171/jns.1968.29.4.0339. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Haley EC, Jr., Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. see comments. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Torner JC. The International Cooperative Study on Timing of Aneurysm Surgery--an update. Stroke. 1984;15:566–570. doi: 10.1161/01.str.15.3.566. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Weir BK, White DM, Stefansson K. Mechanism of oxyhemoglobin-induced release of endothelin-1 from cultured vascular endothelial cells and smooth-muscle cells. J Neurosurg. 1993;79:892–898. doi: 10.3171/jns.1993.79.6.0892. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Suzuki A, Hadeishi H, Yasui N, Hatazawa J. Cerebral blood flow and oxygen metabolism during mild hypothermia in patients with subarachnoid haemorrhage. Acta Neurochir (Wien) 2000;142:1117–1121. doi: 10.1007/s007010070039. discussion 1121-1112. [DOI] [PubMed] [Google Scholar]
- Kaynar MY, Tanriverdi T, Kafadar AM, Kacira T, Uzun H, Aydin S, Gumustas K, Dirican A, Kuday C. Detection of soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:1030–1036. doi: 10.3171/jns.2004.101.6.1030. [DOI] [PubMed] [Google Scholar]
- Kerner A, Schlenk F, Sakowitz O, Haux D, Sarrafzadeh A. Impact of hyperglycemia on neurological deficits and extracellular glucose levels in aneurysmal subarachnoid hemorrhage patients. Neurol Res. 2007;29:647–653. doi: 10.1179/016164107X248983. [DOI] [PubMed] [Google Scholar]
- Khajavi K, Ayzman I, Shearer D, Jones SC, Levy JH, Prayson RA, Skibinski CI, Hahn JF, Chyatte D. Prevention of chronic cerebral vasospasm in dogs with milrinone. Neurosurgery. 1997;40:354–362. doi: 10.1097/00006123-199702000-00025. discussion 362-353. [DOI] [PubMed] [Google Scholar]
- Khaldi A, Zauner A, Reinert M, Woodward JJ, Bullock MR. Measurement of nitric oxide and brain tissue oxygen tension in patients after severe subarachnoid hemorrhage. Neurosurgery. 2001;49:33–38. doi: 10.1097/00006123-200107000-00005. discussion 38-40. [DOI] [PubMed] [Google Scholar]
- Kim P, Yaksh TL, Romero SD, Sundt TM., Jr. Production of uric acid in cerebrospinal fluid after subarachnoid hemorrhage in dogs: investigation of the possible role of xanthine oxidase in chronic vasospasm. Neurosurgery. 1987;21:39–44. doi: 10.1227/00006123-198707000-00008. [DOI] [PubMed] [Google Scholar]
- Kitsuta Y, Suzuki N, Sugiyama M, Yamamoto I. Changes in level of consciousness and association with hyperglycemia as tool for predicting and preventing re-bleeding after spontaneous subarachnoid hemorrhage. Prehosp Disaster Med. 2006;21:190–195. doi: 10.1017/s1049023x00003666. [DOI] [PubMed] [Google Scholar]
- Ko NU, Rajendran P, Kim H, Rutkowski M, Pawlikowska L, Kwok PY, Higashida RT, Lawton MT, Smith WS, Zaroff JG, Young WL. Endothelial nitric oxide synthase polymorphism (−786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:1103–1108. doi: 10.1161/STROKEAHA.107.496596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Hayashi M, Kobayashi S, Kabuto M, Handa Y, Kawano H, Ide H. Cerebral vasospasm and vasoconstriction caused by endothelin. Neurosurgery. 1991;28:673–678. doi: 10.1097/00006123-199105000-00006. discussion 678-679. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ide H, Ishii H, Kabuto M, Handa Y, Kubota T. Endothelin-1 levels in plasma and cerebrospinal fluidfollowing subarachnoid haemorrhage. J Clin Neurosci. 1995;2:252–256. doi: 10.1016/s0967-5868(95)80011-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ishii R, Roike T. Cerebral blood flow and metabolism in patients with ruptured aneurysms. Acta Neurol Scand Suppl. 1979;60:492–493. [Google Scholar]
- Kohno K, Sakaki S, Ohue S, Kumon Y, Matsuoka K. Intracellular calcium levels in canine basilar artery smooth muscle following experimental subarachnoid hemorrhage: an electron microscopic cytochemical study. Acta Neuropathol. 1991;81:664–669. doi: 10.1007/BF00296377. [DOI] [PubMed] [Google Scholar]
- Kokubo Y, Chowdhury AH, Date C, Yokoyama T, Sobue H, Tanaka H. Age-dependent association of apolipoprotein E genotypes with stroke subtypes in a Japanese rural population. Stroke. 2000;31:1299–1306. doi: 10.1161/01.str.31.6.1299. [DOI] [PubMed] [Google Scholar]
- Kongable GL, Lanzino G, Germanson TP, Truskowski LL, Alves WM, Torner JC, Kassell NF. Gender-related differences in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1996;84:43–48. doi: 10.3171/jns.1996.84.1.0043. [DOI] [PubMed] [Google Scholar]
- Kosteljanetz M. CSF dynamics in patients with subarachnoid and/or intraventricular hemorrhage. J Neurosurg. 1984;60:940–946. doi: 10.3171/jns.1984.60.5.0940. [DOI] [PubMed] [Google Scholar]
- Kramer A, Fletcher J. Do endothelin-receptor antagonists prevent delayed neurological deficits and poor outcomes after aneurysmal subarachnoid hemorrhage?: a meta-analysis. Stroke. 2009;40:3403–3406. doi: 10.1161/STROKEAHA.109.560243. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Ogasawara K, Kakino S, Kashimura H, Tomitsuka N, Sugawara A, Ogawa A. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008;69:592–596. doi: 10.1016/j.surneu.2008.02.014. discussion 596. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Tanabe H, Arai M, Ohta T. Measurement of serum neuron-specific enolase levels after subarachnoid hemorrhage and intracerebral hemorrhage. No Shinkei Geka. 1994;22:531–535. [PubMed] [Google Scholar]
- Kuyama H, Ladds A, Branston NM, Nitta M, Symon L. An experimental study of acute subarachnoid haemorrhage in baboons: changes in cerebral blood volume, blood flow, electrical activity and water content. J Neurol Neurosurg Psychiatry. 1984;47:354–364. doi: 10.1136/jnnp.47.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JM, Smielewski P, Czosnyka M, Pickard JD, Kirkpatrick PJ. Predicting delayed ischemic deficits after aneurysmal subarachnoid hemorrhage using a transient hyperemic response test of cerebral autoregulation. Neurosurgery. 2000;47:819–825. doi: 10.1097/00006123-200010000-00004. discussions 825-816. [DOI] [PubMed] [Google Scholar]
- Lambert G, Naredi S, Eden E, Rydenhag B, Friberg P. Monoamine metabolism and sympathetic nervous activation following subarachnoid haemorrhage: influence of gender and hydrocephalus. Brain Res Bull. 2002;58:77–82. doi: 10.1016/s0361-9230(02)00762-1. [DOI] [PubMed] [Google Scholar]
- Landau B, Ransohoff J. Prolonged cerebral vasospasm in experimental subarachnoid hemorrhage. Neurology. 1968;18:1056–1065. doi: 10.1212/wnl.18.11.1056. [DOI] [PubMed] [Google Scholar]
- Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001;29:158–163. doi: 10.1097/00003246-200101000-00031. [DOI] [PubMed] [Google Scholar]
- Lanterna LA, Rigoldi M, Tredici G, Biroli F, Cesana C, Gaini SM, Dalpra L. APOE influences vasospasm and cognition of noncomatose patients with subarachnoid hemorrhage. Neurology. 2005;64:1238–1244. doi: 10.1212/01.WNL.0000156523.77347.B4. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR. Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg. 1996;85:39–49. doi: 10.3171/jns.1996.85.1.0039. [DOI] [PubMed] [Google Scholar]
- Le Roux PD, Winn HR. Management of the ruptured aneurysm. Neurosurg Clin N Am. 1998;9:525–540. [PubMed] [Google Scholar]
- Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G. Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res. 2009a;1287:126–135. doi: 10.1016/j.brainres.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Lee JY, Sagher O, Keep R, Hua Y, Xi G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009b;65:331–343. doi: 10.1227/01.NEU.0000345649.78556.26. discussion 343. [DOI] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hardemark HG, Wasiewski WW. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Leist M, Nicotera P. Apoptosis, excitotoxicity, and neuropathology. Exp Cell Res. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- Leung CH, Poon WS, Yu LM, Wong GK, Ng HK. Apolipoprotein e genotype and outcome in aneurysmal subarachnoid hemorrhage. Stroke. 2002;33:548–552. doi: 10.1161/hs0202.102326. [DOI] [PubMed] [Google Scholar]
- Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- Levitt P, Wilson WP, Wilkins RH. The effects of subarachnoid blood on the electrocorticogram of the cat. J Neurosurg. 1971;35:185–191. doi: 10.3171/jns.1971.35.2.0185. [DOI] [PubMed] [Google Scholar]
- Lin CL, Dumont AS, Calisaneller T, Kwan AL, Hwong SL, Lee KS. Monoclonal antibody against E selectin attenuates subarachnoid hemorrhage-induced cerebral vasospasm. Surg Neurol. 2005;64:201–205. doi: 10.1016/j.surneu.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Lin CL, Hsu YT, Lin TK, Morrow JD, Hsu JC, Hsu YH, Hsieh TC, Tsay PK, Yen HC. Increased levels of F2-isoprostanes following aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2006;40:1466–1473. doi: 10.1016/j.freeradbiomed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Liu TH, Xu JA, Marshall PA, Thompson JK, Parks DA, Freeman BA, Hsu CY, Beckman JS. Role of xanthine dehydrogenase and oxidase in focal cerebral ischemic injury to rat. Am J Physiol. 1991;261:H2051–2057. doi: 10.1152/ajpheart.1991.261.6.H2051. [DOI] [PubMed] [Google Scholar]
- Liu S, Tang J, Ostrowski RP, Titova E, Monroe C, Chen W, Lo W, Martin R, Zhang JH. Oxidative stress after subarachnoid hemorrhage in gp91phox knockout mice. Can J Neurol Sci. 2007;34:356–361. doi: 10.1017/s031716710000682x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- Losiniecki A, Zuccarello M. Subarachnoid hemorrhage: effect on cerebral blood flow and cerebral metabolism. Front Biosci. 2008;13:1845–1856. doi: 10.2741/2804. [DOI] [PubMed] [Google Scholar]
- Mabe H, Suzuki S, Mase M, Umemura A, Nagai H. Serum neuron-specific enolase levels after subarachnoid hemorrhage. Surg Neurol. 1991;36:170–174. doi: 10.1016/0090-3019(91)90108-l. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Marr A, Roux S, Kassell N. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Mocco J, Hoh DJ, Huang J, Choudhri TF, Kreiter KT, Lozier A, Opperman M, Poisik A, Yorgason J, Solomon RA, Mayer SA, Connolly ES. Outcome prediction with serum intercellular adhesion molecule-1 levels after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;96:71–75. doi: 10.3171/jns.2002.96.1.0071. [DOI] [PubMed] [Google Scholar]
- Macrae I, Robinson M, McAuley M, Reid J, McCulloch J. Effects of intracisternal endothelin-1 injection on blood flow to the lower brain stem. Eur J Pharmacol. 1991;203:85–91. doi: 10.1016/0014-2999(91)90794-q. [DOI] [PubMed] [Google Scholar]
- Marbacher S, Sherif C, Neuschmelting V, Schlappi JA, Takala J, Jakob SM, Fandino J. Extra-intracranial blood shunt mimicking aneurysm rupture: intracranial-pressure-controlled rabbit subarachnoid hemorrhage model. J Neurosci Methods. 2010;191:227–233. doi: 10.1016/j.jneumeth.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992;262:C854–861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- Marzatico F, Gaetani P, Cafe C, Spanu G, Rodriguez y Baena R. Antioxidant enzymatic activities after experimental subarachnoid hemorrhage in rats. Acta Neurol Scand. 1993;87:62–66. doi: 10.1111/j.1600-0404.1993.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Marzatico F, Gaetani P, Tartara F, Bertorelli L, Feletti F, Adinolfi D, Tancioni F, Rodriguez y Baena R. Antioxidant status and alpha1-antiproteinase activity in subarachnoid hemorrhage patients. Life Sci. 1998;63:821–826. doi: 10.1016/s0024-3205(98)00338-5. [DOI] [PubMed] [Google Scholar]
- Mathiesen T, Edner G, Ulfarsson E, Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. J Neurosurg. 1997;87:215–220. doi: 10.3171/jns.1997.87.2.0215. [DOI] [PubMed] [Google Scholar]
- Matz PG, Copin JC, Chan PH. Cell death after exposure to subarachnoid hemolysate correlates inversely with expression of CuZn-superoxide dismutase. Stroke. 2000;31:2450–2459. doi: 10.1161/01.str.31.10.2450. [DOI] [PubMed] [Google Scholar]
- Matz PG, Fujimura M, Lewen A, Morita-Fujimura Y, Chan PH. Increased cytochrome c-mediated DNA fragmentation and cell death in manganese-superoxide dismutase-deficient mice after exposure to subarachnoid hemolysate. Stroke. 2001;32:506–515. doi: 10.1161/01.str.32.2.506. [DOI] [PubMed] [Google Scholar]
- McCulloch J. Excitatory amino acid antagonists and their potential for the treatment of ischaemic brain damage in man. Br J Clin Pharmacol. 1992;34:106–114. doi: 10.1111/j.1365-2125.1992.tb04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating EG, Andrews PJ. Cytokines and adhesion molecules in acute brain injury. Br J Anaesth. 1998;80:77–84. doi: 10.1093/bja/80.1.77. [DOI] [PubMed] [Google Scholar]
- McLean RM. Magnesium and its therapeutic uses: a review. Am J Med. 1994;96:63–76. doi: 10.1016/0002-9343(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Medele RJ, Stummer W, Reulen HJ, Steiger HJ. Evidence for peroxidative damage by nitric oxide in experimental chronic cerebral vasospasm. Neurol Res. 1996;18:277–280. doi: 10.1080/01616412.1996.11740420. [DOI] [PubMed] [Google Scholar]
- Meguro T, Chen B, Lancon J, Zhang JH. Oxyhemoglobin induces caspase-mediated cell death in cerebral endothelial cells. J Neurochem. 2001a;77:1128–1135. doi: 10.1046/j.1471-4159.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- Meguro T, Clower BR, Carpenter R, Parent AD, Zhang JH. Improved rat model for cerebral vasospasm studies. Neurol Res. 2001b;23:761–766. doi: 10.1179/016164101101199144. [DOI] [PubMed] [Google Scholar]
- Meguro T, Klett CP, Chen B, Parent AD, Zhang JH. Role of calcium channels in oxyhemoglobin-induced apoptosis in endothelial cells. J Neurosurg. 2000;93:640–646. doi: 10.3171/jns.2000.93.4.0640. [DOI] [PubMed] [Google Scholar]
- Megyesi JF, Findlay JM, Vollrath B, Cook DA, Chen MH. In vivo angioplasty prevents the development of vasospasm in canine carotid arteries. Pharmacological and morphological analyses. Stroke. 1997;28:1216–1224. doi: 10.1161/01.str.28.6.1216. [DOI] [PubMed] [Google Scholar]
- Mehta V, Holness RO, Connolly K, Walling S, Hall R. Acute hydrocephalus following aneurysmal subarachnoid hemorrhage. Can J Neurol Sci. 1996;23:40–45. doi: 10.1017/s0317167100039160. [DOI] [PubMed] [Google Scholar]
- Memon ZI, Altura BT, Benjamin JL, Cracco RQ, Altura BM. Predictive value of serum ionized but not total magnesium levels in head injuries. Scand J Clin Lab Invest. 1995;55:671–677. doi: 10.3109/00365519509075397. [DOI] [PubMed] [Google Scholar]
- Milhorat TH. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1987;20:15–20. doi: 10.1227/00006123-198701000-00004. [DOI] [PubMed] [Google Scholar]
- Miller AA, Budzyn K, Sobey CG. Vascular dysfunction in cerebrovascular disease: mechanisms and therapeutic intervention. Clin Sci (Lond) 2010;119:1–17. doi: 10.1042/CS20090649. [DOI] [PubMed] [Google Scholar]
- Minato H, Honda Y, Masuda Y, Fujitani B, Hosoki K. Prevention by the new Ca2+ channel antagonist, AJ-3941, of loss of endothelium-dependent relaxation after subarachnoid hemorrhage in rats. Eur J Pharmacol. 1996;315:297–303. doi: 10.1016/s0014-2999(96)00632-2. [DOI] [PubMed] [Google Scholar]
- Miranda P, Lagares A, Alen J, Perez-Nunez A, Arrese I, Lobato RD. Early transcranial Doppler after subarachnoid hemorrhage: clinical and radiological correlations. Surg Neurol. 2006;65:247–252. doi: 10.1016/j.surneu.2005.06.042. discussion 252. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- Miura K. Changes in Mg++ concentration of CSF after subarachnoid hemorrhage and Mg++--effects on the contractions of bovine cerebral artery. No Shinkei Geka. 1988;16:1251–1259. [PubMed] [Google Scholar]
- Mocco J, Mack WJ, Kim GH, Lozier AP, Laufer I, Kreiter KT, Sciacca RR, Solomon RA, Mayer SA, Connolly ES., Jr. Rise in serum soluble intercellular adhesion molecule-1 levels with vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:537–541. doi: 10.3171/jns.2002.97.3.0537. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Skelly JP, Batra V, editors. Toxicokinetics and New Drug Development. Pergamon Press; New York: 1989. pp. 42–96. [Google Scholar]
- Muroi C, Yonekawa Y, Khan N, Rousson V, Keller E. Seasonal variations in hospital admissions due to aneurysmal subarachnoid haemorrhage in the state of Zurich, Switzerland. Acta Neurochir (Wien) 2004;146:659–665. doi: 10.1007/s00701-004-0278-4. [DOI] [PubMed] [Google Scholar]
- Nadler JL, Goodson S, Rude RK. Evidence that prostacyclin mediates the vascular action of magnesium in humans. Hypertension. 1987;9:379–383. doi: 10.1161/01.hyp.9.4.379. [DOI] [PubMed] [Google Scholar]
- Nagel A, Graetz D, Schink T, Frieler K, Sakowitz O, Vajkoczy P, Sarrafzadeh A. Relevance of intracranial hypertension for cerebral metabolism in aneurysmal subarachnoid hemorrhage. Clinical article. J Neurosurg. 2009a;111:94–101. doi: 10.3171/2009.1.JNS08587. [DOI] [PubMed] [Google Scholar]
- Nagel A, Graetz D, Vajkoczy P, Sarrafzadeh AS. Decompressive Craniectomy in Aneurysmal Subarachnoid Hemorrhage: Relation to Cerebral Perfusion Pressure and Metabolism. Neurocrit Care. 2009b doi: 10.1007/s12028-009-9269-x. [DOI] [PubMed] [Google Scholar]
- Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, Commichau C, Connolly ES, Mayer SA. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410–416. doi: 10.1001/archneur.62.3.410. [DOI] [PubMed] [Google Scholar]
- Nakagomi T, Kassell NF, Sasaki T, Fujiwara S, Lehman RM, Johshita H, Nazar GB, Torner JC. Effect of subarachnoid hemorrhage on endothelium-dependent vasodilation. J Neurosurg. 1987;66:915–923. doi: 10.3171/jns.1987.66.6.0915. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Okuchi K, Matsuyama T, Fukushima H, Seki T, Konobu T, Nishio K. Clinical significance of elevated natriuretic peptide levels and cardiopulmonary parameters after subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2009;49:185–191. doi: 10.2176/nmc.49.185. discussion 191-182. [DOI] [PubMed] [Google Scholar]
- Nau R, Haase S, Bunkowski S, Bruck W. Neuronal apoptosis in the denate gyrus in humans with subarachnoid hemorrage and cerebral hypoxia. Brain Pathol. 2002;12:329–336. doi: 10.1111/j.1750-3639.2002.tb00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naval NS, Stevens RD, Mirski MA, Bhardwaj A. Controversies in the management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2006;34:511–524. doi: 10.1097/01.ccm.0000198331.45998.85. [DOI] [PubMed] [Google Scholar]
- Neil-Dwyer G, Cruickshank J. The blood leucocyte count and its prognostic significance in subarachnoid haemorrhage. Brain. 1974;97:79–86. doi: 10.1093/brain/97.1.79. [DOI] [PubMed] [Google Scholar]
- Ng WH, Moochhala S, Yeo TT, Ong PL, Ng PY. Nitric oxide and subarachnoid hemorrhage: elevated level in cerebrospinal fluid and their implications. Neurosurgery. 2001;49:622–626. doi: 10.1097/00006123-200109000-00016. discussion 626-627. [DOI] [PubMed] [Google Scholar]
- Nieuwkamp DJ, de Gans K, Algra A, Albrecht KW, Boomstra S, Brouwers PJ, Groen RJ, Metzemaekers JD, Nijssen PC, Roos YB, Tulleken CA, Vandertop WP, van Gijn J, Vos PE, Rinkel GJ. Timing of aneurysm surgery in subarachnoid haemorrhage--an observational study in The Netherlands. Acta Neurochir (Wien) 2005;147:815–821. doi: 10.1007/s00701-005-0536-0. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Saveland H, Boris-Moller F, Brandt L, Wieloch T. Increased levels of glutamate in patients with subarachnoid haemorrhage as measured by intracerebral microdialysis. Acta Neurochir Suppl. 1996;67:45–47. doi: 10.1007/978-3-7091-6894-3_10. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Bodock MJ, Koroshetz WJ, Topcuoglu MA, Carter BS, Ogilvy CS, Pryor JC, Buonanno FS. High-dose bosentan in the prevention and treatment of subarachnoid hemorrhage-induced cerebral vasospasm: an open-label feasibility study. Neurocrit Care. 2007;7:194–202. doi: 10.1007/s12028-007-0070-4. [DOI] [PubMed] [Google Scholar]
- Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysm. J Neurosurg. 1973;39:226–234. doi: 10.3171/jns.1973.39.2.0226. [DOI] [PubMed] [Google Scholar]
- Nornes H. Cerebral arterial flow dynamics during aneurysm haemorrhage. Acta Neurochir. 1978;41:39–48. doi: 10.1007/BF01809135. [DOI] [PubMed] [Google Scholar]
- Nornes H, Magnaes B. Intracranial pressure in patients with ruptured saccular aneurysm. J Neurosurg. 1972;36:537–547. doi: 10.3171/jns.1972.36.5.0537. [DOI] [PubMed] [Google Scholar]
- Nosko M, Weir BK, Lunt A, Grace M, Allen P, Mielke B. Effect of clot removal at 24 hours on chronic vasospasm after SAH in the primate model. J Neurosurg. 1987;66:416–422. doi: 10.3171/jns.1987.66.3.0416. [DOI] [PubMed] [Google Scholar]
- Oertel M, Schumacher U, McArthur DL, Kastner S, Boker DK. S-100B and NSE: markers of initial impact of subarachnoid haemorrhage and their relation to vasospasm and outcome. J Clin Neurosci. 2006;13:834–840. doi: 10.1016/j.jocn.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Ohkuma H, Itoh K, Shibata S, Suzuki S. Morphological changes of intraparenchymal arterioles after experimental subarachnoid hemorrhage in dogs. Neurosurgery. 1997;41:230–235. doi: 10.1097/00006123-199707000-00036. discussion 235-236. [DOI] [PubMed] [Google Scholar]
- Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001;32:1176–1180. doi: 10.1161/01.str.32.5.1176. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- Ono S, Date I, Nakajima M, Onoda K, Ogihara K, Shiota T, Asari S, Ninomiya Y, Yabuno N, Ohmoto T. Three-dimensional analysis of vasospastic major cerebral arteries in rats with the corrosion cast technique. Stroke. 1997;28:1631–1637. doi: 10.1161/01.str.28.8.1631. discussion 1638. [DOI] [PubMed] [Google Scholar]
- Ono S, Date I, Onoda K, Ohmoto T. Time course of the diameter of the major cerebral arteries after subarachnoid hemorrhage using corrosion cast technique. Neurol Res. 2003;25:383–389. doi: 10.1179/016164103101201535. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- Owens J, Wyper DJ, Patterson J, Brown DR, Elliott AT, Teasdale GM, McCulloch J. First SPET images of glutamate (NMDA) receptor activation in vivo in cerebral ischaemia. Nucl Med Commun. 1997;18:149–158. doi: 10.1097/00006231-199702000-00010. [DOI] [PubMed] [Google Scholar]
- Palmer GC, Cregan EF, Borrelli AR, Willett F. Neuroprotective properties of the uncompetitive NMDA receptor antagonist remacemide hydrochloride. Ann N Y Acad Sci. 1995;765:236–247. doi: 10.1111/j.1749-6632.1995.tb16580.x. discussion 248. [DOI] [PubMed] [Google Scholar]
- Papadopoulos SM, Gilbert LL, Webb RC, D’Amato CJ. Characterization of contractile responses to endothelin in human cerebral arteries: implications for cerebral vasospasm. Neurosurgery. 1990;26:810–815. doi: 10.1097/00006123-199005000-00013. [DOI] [PubMed] [Google Scholar]
- Park KW, Metais C, Dai HB, Comunale ME, Sellke FW. Anesth Analg. 2001. Microvascular endothelial dysfunction and its mechanism in a rat model of subarachnoid hemorrhage; pp. 990–996. [DOI] [PubMed] [Google Scholar]
- Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Pepper EM, Bateman GA, Wang Y, Levi CR. Identification of the penumbra and infarct core on hyperacute noncontrast and perfusion CT. Neurology. 2007;68:730–736. doi: 10.1212/01.wnl.0000256366.86353.ff. [DOI] [PubMed] [Google Scholar]
- Paul R, Koedel U, Winkler F, Kieseier BC, Fontana A, Kopf M, Hartung HP, Pfister HW. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain. 2003;126:1873–1882. doi: 10.1093/brain/awg171. [DOI] [PubMed] [Google Scholar]
- Peltonen S, Juvela S, Kaste M, Lassila R. Hemostasis and fibrinolysis activation after subarachnoid hemorrhage. J Neurosurg. 1997;87:207–214. doi: 10.3171/jns.1997.87.2.0207. [DOI] [PubMed] [Google Scholar]
- Pennings FA, Bouma GJ, Ince C. Direct observation of the human cerebral microcirculation during aneurysm surgery reveals increased arteriolar contractility. Stroke. 2004;35:1284–1288. doi: 10.1161/01.STR.0000126039.91400.cb. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AR, Sanchez-Pena P, Biondi A, Sourour N, Boch AL, Colonne C, Lejean L, Abdennour L, Puybasset L. Predictors of 1-year outcome after coiling for poor-grade subarachnoid aneurysmal hemorrhage. Neurocrit Care. 2007;7:18–26. doi: 10.1007/s12028-007-0053-5. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Kwun BD, Hackett JD, Zervas NT. The role of inflammation in experimental cerebral vasospasm. J Neurosurg. 1990a;72:767–774. doi: 10.3171/jns.1990.72.5.0767. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Roussos L, Kwun BD, Hackett JD, Owen CJ, Zervas NT. Evidence of the role of hemolysis in experimental cerebral vasospasm. J Neurosurg. 1990b;72:775–781. doi: 10.3171/jns.1990.72.5.0775. [DOI] [PubMed] [Google Scholar]
- Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, Disney LB, Khan MI, Grace M, Holness RO, et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–517. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- Petzold A, Rejdak K, Belli A, Sen J, Keir G, Kitchen N, Smith M, Thompson EJ. Axonal pathology in subarachnoid and intracerebral hemorrhage. J Neurotrauma. 2005a;22:407–414. doi: 10.1089/neu.2005.22.407. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Einhaupl KM, Dirnagl U, Dreier JP. Ischemia triggered by spreading neuronal activation is induced by endothelin-1 and hemoglobin in the subarachnoid space. Ann Neurol. 2003;54:591–598. doi: 10.1002/ana.10723. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Haack S, von Bohlen Und Halbach O, Priller J, Lehmann TN, Heinemann U, Dirnagl U, Dreier JP. Nitric oxide modulates spreading depolarization threshold in the human and rodent cortex. Stroke. 2008;39:1292–1299. doi: 10.1161/STROKEAHA.107.500710. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Windmuller O, Haack S, Major S, Buchheim K, Megow D, Gabriel S, Lehmann TN, Drenckhahn C, Peters O, Meierkord H, Heinemann U, Dirnagl U, Dreier JP. Increased extracellular K+ concentration reduces the efficacy of N-methyl-D-aspartate receptor antagonists to block spreading depression-like depolarizations and spreading ischemia. Stroke. 2005b;36:1270–1277. doi: 10.1161/01.STR.0000166023.51307.e0. [DOI] [PubMed] [Google Scholar]
- Philippon J, Grob R, Dagreou F, Guggiari M, Rivierez M, Viars P. Prevention of vasospasm in subarachnoid haemorrhage. A controlled study with nimodipine. Acta Neurochir (Wien) 1986;82:110–114. doi: 10.1007/BF01456369. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. discussion 332. [DOI] [PubMed] [Google Scholar]
- Pickard JD, Murray GD, Illingworth R, Shaw MD, Teasdale GM, Foy PM, Humphrey PR, Lang DA, Nelson R, Richards P, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid hemorrhage. BMJ. 1989;298:636–643. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard JD, Walker V, Perry S, Smythe PJ, Eastwood S, Hunt R. Arterial eicosanoid production following chronic exposure to a periarterial haematoma. J Neurol Neurosurg Psychiatry. 1984;47:661–667. doi: 10.1136/jnnp.47.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepgras A, Elste V, Frietsch T, Schmiedek P, Reith W, Schilling L. Effect of moderate hypothermia on experimental severe subarachnoid hemorrhage, as evaluated by apparent diffusion coefficient changes. Neurosurgery. 2001;48:1128–1134. doi: 10.1097/00006123-200105000-00033. discussion 1134-1125. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Boock RJ, Afshar JK, Clouse K, Bacic M, Ehrenreich H, Oldfield EH. Source and cause of endothelin-1 release into cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 1997a;87:287–293. doi: 10.3171/jns.1997.87.2.0287. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. Jama. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Oldfield EH, Boock RJ. Reversal and prevention of cerebral vasospasm by intracarotid infusions of nitric oxide donors in a primate model of subarachnoid hemorrhage. J Neurosurg. 1997b;87:746–751. doi: 10.3171/jns.1997.87.5.0746. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Frei B, Rordorf G, Ogilvy CS, Koroshetz WJ, Beal MF. Increased levels of plasma cholesteryl ester hydroperoxides in patients with subarachnoid hemorrhage. Free Radic Biol Med. 1997;23:762–767. doi: 10.1016/s0891-5849(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol. 2005;25:435–444. doi: 10.1055/s-2005-923537. [DOI] [PubMed] [Google Scholar]
- Prunell GF, Mathiesen T, Diemer NH, Svendgaard NA. Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery. 2003;52:165–175. doi: 10.1097/00006123-200301000-00022. discussion 175-166. [DOI] [PubMed] [Google Scholar]
- Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Delayed cell death related to acute cerebral blood flow changes following subarachnoid hemorrhage in the rat brain. J Neurosurg. 2005;102:1046–1054. doi: 10.3171/jns.2005.102.6.1046. [DOI] [PubMed] [Google Scholar]
- Pyne-Geithman GJ, Caudell DN, Prakash P, Clark JF. Glutathione peroxidase and subarachnoid hemorrhage: implications for the role of oxidative stress in cerebral vasospasm. Neurol Res. 2009;31:195–199. doi: 10.1179/174313209X393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne GJ, Cadoux-Hudson TA, Clark JF. Magnesium protection against in vitro cerebral vasospasm after subarachnoid haemorrhage. Br J Neurosurg. 2001;15:409–415. doi: 10.1080/02688690120082413. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–1866. doi: 10.1161/01.STR.0000133132.76983.8e. [DOI] [PubMed] [Google Scholar]
- Ram Z, Sahar A, Hadani M. Vasospasm due to massive subarachnoid haemorrhage--a rat model. Acta Neurochir. 1991;110:181–184. doi: 10.1007/BF01400688. [DOI] [PubMed] [Google Scholar]
- Rasmussen G, Hauerberg J, Waldemar G, Gjerris F, Juhler M. Cerebral blood flow autoregulation in experimental subarachnoid haemorrhage in rat. Acta Neurochir. 1992;119:128–133. doi: 10.1007/BF01541796. [DOI] [PubMed] [Google Scholar]
- Ratsep T, Asser T. Cerebral hemodynamic impairment after aneurysmal subarachnoid hemorrhage as evaluated using transcranial doppler ultrasonography: relationship to delayed cerebral ischemia and clinical outcome. J Neurosurg. 2001;95:393–401. doi: 10.3171/jns.2001.95.3.0393. [DOI] [PubMed] [Google Scholar]
- Reed GL. Platelet secretion. In: Michelson AD, editor. Platelets. Academic press; San Diego, California, USA: 2002. pp. 181–195. [Google Scholar]
- Reijneveld JC, Wermer M, Boonman Z, van Gijn J, Rinkel GJ. Acute confusional state as presenting feature in aneurysmal subarachnoid hemorrhage: frequency and characteristics. J Neurol. 2000;247:112–116. doi: 10.1007/pl00007791. [DOI] [PubMed] [Google Scholar]
- Rojas S, Martin A, Justicia C, Falcon C, Bargallo N, Chamorro A, Planas AM. Modest MRI signal intensity changes precede delayed cortical necrosis after transient focal ischemia in the rat. Stroke. 2006;37:1525–1532. doi: 10.1161/01.STR.0000221713.06148.16. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res. 1992;576:203–207. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. Platelet adhesion and aggregation without endothelial denudation or exposure of basal lamina and/or collagen. J Vasc Res. 1997;34:409–417. doi: 10.1159/000159251. [DOI] [PubMed] [Google Scholar]
- Rothberg C, Weir B, Overton T, Grace M. Responses to experimental subarachnoid hemorrhage in the spontaneously breathing primate. J Neurosurg. 1980;52:302–308. doi: 10.3171/jns.1980.52.3.0302. [DOI] [PubMed] [Google Scholar]
- Rothlein R. Overview of leukocyte adhesion. Neurology. 1997;49:S3–4. doi: 10.1212/wnl.49.5_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A. Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2006;18:68–72. doi: 10.1097/01.ana.0000181693.30750.af. [DOI] [PubMed] [Google Scholar]
- Rothoerl RD, Ringel F. Molecular mechanisms of cerebral vasospasm following aneurysmal SAH. Neurol Res. 2007;29:636–642. doi: 10.1179/016164107X240224. [DOI] [PubMed] [Google Scholar]
- Said S, Rosenblum WI, Povlishock JT, Nelson GH. Correlations between morphological changes in platelet aggregates and underlying endothelial damage in cerebral microcirculation of mice. Stroke. 1993;24:1968–1976. doi: 10.1161/01.str.24.12.1968. [DOI] [PubMed] [Google Scholar]
- Sakaki S, Ohue S, Kohno K, Takeda S. Impairement of vascular reactivity and changes in intracellular calcium and calmodulin levels of smooth muscle cells in canine basilar arteries after subarachnoid hemorrhage. Neurosurgery. 1989;25:753–761. doi: 10.1097/00006123-198911000-00010. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Hillered L, Enblad P, Engstrom E. Microdialysis patterns in subarachnoid hemorrhage patients with focus on ischemic events and brain interstitial glutamine levels. Acta Neurochir (Wien) 2009a;151:437–446. doi: 10.1007/s00701-009-0265-x. discussion 446. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Hillered L, Zetterling M, Enblad P, Hesselager G, Ryttlefors M, Kumlien E, Lewen A, Marklund N, Nilsson P, Salci K, Ronne-Engstrom E. Cerebral glutamine and glutamate levels in relation to compromised energy metabolism: a microdialysis study in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2007;27:1309–1317. doi: 10.1038/sj.jcbfm.9600433. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Howells T, Kumlien E, Enblad P, Hillered L, Ronne-Engstrom E. Relationship between intracranial hemodynamics and microdialysis markers of energy metabolism and glutamate-glutamine turnover in patients with subarachnoid hemorrhage. Clinical article. J Neurosurg. 2009b;111:910–915. doi: 10.3171/2008.8.JNS0889. [DOI] [PubMed] [Google Scholar]
- Sano K. Acute ischaemic and delayed ischaemic neurological deficits as the causes of bad grading in aneurysmal subarachnoid haemorrhage. Neurol Res. 1994;16:35–39. doi: 10.1080/01616412.1994.11740189. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A, Haux D, Sakowitz O, Benndorf G, Herzog H, Kuechler I, Unterberg A. Acute focal neurological deficits in aneurysmal subarachnoid hemorrhage: relation of clinical course, CT findings, and metabolite abnormalities monitored with bedside microdialysis. Stroke. 2003;34:1382–1388. doi: 10.1161/01.STR.0000074036.97859.02. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Sakowitz OW, Kiening KL, Benndorf G, Lanksch WR, Unterberg AW. Bedside microdialysis: a tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit Care Med. 2002;30:1062–1070. doi: 10.1097/00003246-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Thomale UW, Haux D, Unterberg AW. Cerebral metabolism and intracranial hypertension in high grade aneurysmal subarachnoid haemorrhage patients. Acta Neurochir Suppl. 2005;95:89–92. doi: 10.1007/3-211-32318-x_19. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Unterberg AW, Lanksch WR. Bedside-microdialysis for early detection of vasospasm after subarachnoid hemorrhage. Case report and review of the literature. Zentralbl Neurochir. 1998;59:269–273. [PubMed] [Google Scholar]
- Satoh M, Date I, Nakajima M, Takahashi K, Iseda K, Tamiya T, Ohmoto T, Ninomiya Y, Asari S. Inhibition of poly(ADP-ribose) polymerase attenuates cerebral vasospasm after subarachnoid hemorrhage in rabbits. Stroke. 2001;32:225–231. doi: 10.1161/01.str.32.1.225. [DOI] [PubMed] [Google Scholar]
- Saveland H, Nilsson OG, Boris-Moller F, Wieloch T, Brandt L. Intracerebral microdialysis of glutamate and aspartate in two vascular territories after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1996;38:12–19. doi: 10.1007/BF01921273. discussion 19-20. [DOI] [PubMed] [Google Scholar]
- Sayama T, Suzuki S, Fukui M. Role of inducible nitric oxide synthase in the cerebral vasospasm after subarachnoid hemorrhage in rats. Neurol Res. 1999;21:293–298. doi: 10.1080/01616412.1999.11740934. [DOI] [PubMed] [Google Scholar]
- Schatlo B, Glasker S, Zauner A, Thompson GB, Oldfield EH, Pluta RM. Correlation of end-tidal CO2 with transcranial Doppler flow velocity is decreased during chemoregulation in delayed cerebral vasospasm after subarachnoid haemorrhage--results of a pilot study. Acta Neurochir Suppl. 2008;104:249–250. doi: 10.1007/978-3-211-75718-5_49. [DOI] [PubMed] [Google Scholar]
- Schmieder K, Moller F, Engelhardt M, Scholz M, Schregel W, Christmann A, Harders A. Dynamic cerebral autoregulation in patients with ruptured and unruptured aneurysms after induction of general anesthesia. Zentralbl Neurochir. 2006;67:81–87. doi: 10.1055/s-2006-933374. [DOI] [PubMed] [Google Scholar]
- Scholler K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, Hamann GF, Schmid-Elsaesser R, Zausinger S. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Schubert GA, Poli S, Mendelowitsch A, Schilling L, Thome C. Hypothermia reduces early hypoperfusion and metabolic alterations during the acute phase of massive subarachnoid hemorrhage: a laser-Doppler-flowmetry and microdialysis study in rats. J Neurotrauma. 2008a;25:539–548. doi: 10.1089/neu.2007.0500. [DOI] [PubMed] [Google Scholar]
- Schubert GA, Poli S, Schilling L, Heiland S, Thome C. Hypothermia reduces cytotoxic edema and metabolic alterations during the acute phase of massive SAH: a diffusion-weighted imaging and spectroscopy study in rats. J Neurotrauma. 2008b;25:841–852. doi: 10.1089/neu.2007.0443. [DOI] [PubMed] [Google Scholar]
- Schulz MK, Wang LP, Tange M, Bjerre P. Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;93:808–814. doi: 10.3171/jns.2000.93.5.0808. [DOI] [PubMed] [Google Scholar]
- Schwartz AY, Masago A, Sehba FA, Bederson JB. Experimental models of subarachnoid hemorrhage in the rat: A refinement of the endovascular filament model. J Neurosci Methods. 2000a;96:161–167. doi: 10.1016/s0165-0270(00)00156-4. [DOI] [PubMed] [Google Scholar]
- Schwartz AY, Sehba FA, Bederson JB. Decreased nitric oxide availability contributes to acute cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2000b;47:208–214. doi: 10.1097/00006123-200007000-00042. discussion 214-205. [DOI] [PubMed] [Google Scholar]
- Sehba F, Bederson JB. Rodent models of Hemorrhagic stroke. In: Tatlisumak T, Fisher MJ, editors. Handbook of experimental neurology : Methods & techniques in animal research. Cambridge University Press; 2006a. in press. [Google Scholar]
- Sehba FA, Bederson JB. Mechanisms of Acute Brain injury after Subarachnoid Hemorrhage. Neurol Res. 2006b;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Chereshnev I, Maayani S, Friedrich V, Jr., Bederson JB. Nitric Oxide synthase in acute alteration of Nitric Oxide levels after subarachnoid hemorrhage. Neurosurgery. 2004a;55:671–677. doi: 10.1227/01.neu.0000134557.82423.b2. discussion 677-678. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Ding WH, Chereshnev I, Bederson JB. Effects of S-nitrosoglutathione on acute vasoconstriction and glutamate release after subarachnoid hemorrhage. Stroke. 1999;30:1955–1961. doi: 10.1161/01.str.30.9.1955. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Flores R, Muller A, Friedrich V, Bederson JB. *Early decrease in cerebral endothelial nitric oxide synthase occurs after Subarachnoid Hemorrhage; Annual Stroke conference; 2007a.p. 527. [Google Scholar]
- Sehba FA, Flores R, Muller A, Friedrich V, Chen JF, Britz GW, Winn HR, Bederson JB. Adenosine A(2A) receptors in early ischemic vascular injury after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg. 2010;113:826–834. doi: 10.3171/2009.9.JNS09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehba FA, Flores R, Ono K, Suzuki H, Sawada MS, Kanzawa T. *Expression of Cytokines and Neurotrophins after acute Subarachnoid Hemorrhage; Annual Stroke conference; 2008; Session: 126. [Google Scholar]
- Sehba FA, Friedrich V. Cerebral Microvasculature is an Early Target of Subarachnoid Hemorrhage. Acta Neurochir (Wien) supp. doi: 10.1007/978-3-7091-1192-5_37. submitted. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Makonnen G, Friedrich V, Bederson JB. Acute cerebral vascular injury occurs after subarachnoid hemorrhage and can be prevented by administration of a Nitric Oxide donor. J Neurosurg. 2007b;106:321–329. doi: 10.3171/jns.2007.106.2.321. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Mostafa G, Knopman J, Friedrich V, Jr., Bederson JB. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J Neurosurg. 2004b;101:633–640. doi: 10.3171/jns.2004.101.4.0633. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Mustafa G, Friedrich V, Bederson JB. Acute microvascular platelet aggregation after Subarachnoid hemorrhage. J Neurosurg. 2005;102:1094–1100. doi: 10.3171/jns.2005.102.6.1094. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Pluta RM, Zhang JH. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43:27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehba FA, Schwartz AY, Chereshnev I, Bederson JB. Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2000;20:604–611. doi: 10.1097/00004647-200003000-00018. [DOI] [PubMed] [Google Scholar]
- Sercombe R, Dinh YR, Gomis P. Cerebrovascular inflammation following subarachnoid hemorrhage. Jpn J Pharmacol. 2002;88:227–249. doi: 10.1254/jjp.88.227. [DOI] [PubMed] [Google Scholar]
- Sermet A, Tasdemir N, Deniz B, Atmaca M. Time-dependent changes in superoxide dismutase, catalase, xanthine dehydrogenase and oxidase activities in focal cerebral ischaemia. Cytobios. 2000;102:157–172. [PubMed] [Google Scholar]
- Shaw MD, Vermeulen M, Murray GD, Pickard JD, Bell BA, Teasdale GM. Efficacy and safety of the endothelin, receptor antagonist TAK-044 in treating subarachnoid hemorrhage: a report by the Steering Committee on behalf of the UK/Netherlands/Eire TAK-044 Subarachnoid Haemorrhage Study Group. J Neurosurg. 2000;93:992–997. doi: 10.3171/jns.2000.93.6.0992. [DOI] [PubMed] [Google Scholar]
- Sherlock M, O’Sullivan E, Agha A, Behan LA, Rawluk D, Brennan P, Tormey W, Thompson CJ. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2006;64:250–254. doi: 10.1111/j.1365-2265.2006.02432.x. [DOI] [PubMed] [Google Scholar]
- Shimoda M, Takeuchi M, Tominaga J, Oda S, Kumasaka A, Tsugane R. Asymptomatic versus symptomatic infarcts from vasospasm in patients with subarachnoid hemorrhage: serial magnetic resonance imaging. Neurosurgery. 2001;49:1341–1348. doi: 10.1097/00006123-200112000-00010. discussion 1348-1350. [DOI] [PubMed] [Google Scholar]
- Shin HK, Lee JH, Kim CD, Kim YK, Hong JY, Hong KW. Prevention of impairment of cerebral blood flow autoregulation during acute stage of subarachnoid hemorrhage by gene transfer of Cu/Zn SOD-1 to cerebral vessels. J Cereb Blood Flow Metab. 2003;23:111–120. doi: 10.1097/01.WCB.0000036561.60552.63. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Silasi G, Colbourne F. Long-term assessment of motor and cognitive behaviours in the intraluminal perforation model of subarachnoid hemorrhage in rats. Behav Brain Res. 2009;198:380–387. doi: 10.1016/j.bbr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Simeone FA, Ryan KG, Cotter JR. Prolonged experimental cerebral vasospasm. J Neurosurg. 1968;29:357–366. doi: 10.3171/jns.1968.29.4.0357. [DOI] [PubMed] [Google Scholar]
- Skjoth-Rasmussen J, Schulz M, Kristensen SR, Bjerre P. Delayed neurological deficits detected by an ischemic pattern in the extracellular cerebral metabolites in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;100:8–15. doi: 10.3171/jns.2004.100.1.0008. [DOI] [PubMed] [Google Scholar]
- Smith SL, Larson PG, Hall ED. A comparison of the effects of tirilazad on subarachnoid hemorrhage-induced blood-brain barrier permeability in male and female rats. J Stroke Cerebrovasc Dis. 1997;6:389–393. doi: 10.1016/s1052-3057(97)80039-0. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM. Subarachnoid haemorrhage: what happens to the cerebral arteries? Clin Exp Pharmacol Physiol. 1998;25:867–876. doi: 10.1111/j.1440-1681.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Soehle M, Chatfield DA, Czosnyka M, Kirkpatrick PJ. Predictive value of initial clinical status, intracranial pressure and transcranial Doppler pulsatility after subarachnoid haemorrhage. Acta Neurochir (Wien) 2007;149:575–583. doi: 10.1007/s00701-007-1149-6. [DOI] [PubMed] [Google Scholar]
- Solomon RA, Antunes JL, Chen RY, Bland L, Chien S. Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke. 1985;16:58–64. doi: 10.1161/01.str.16.1.58. [DOI] [PubMed] [Google Scholar]
- Solomon RA, Fink ME, Lennihan L. Early aneurysm surgery and prophylactic hypervolemic hypertensive therapy for the treatment of aneurysmal subarachnoid hemorrhage. Neurosurgery. 1988;23:699–704. doi: 10.1227/00006123-198812000-00002. [DOI] [PubMed] [Google Scholar]
- Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, Zhang JH. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Starke RM, Kim GH, Komotar RJ, Hickman ZL, Black EM, Rosales MB, Kellner CP, Hahn DK, Otten ML, Edwards J, Wang T, Russo JJ, Mayer SA, Connolly ES., Jr. Endothelial nitric oxide synthase gene single-nucleotide polymorphism predicts cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008;28:1204–1211. doi: 10.1038/jcbfm.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke RM, Komotar RJ, Kim GH, Kellner CP, Otten ML, Hahn DK, Michael Schmidt J, Sciacca RR, Mayer SA, Sander Connolly E. Evaluation of a revised Glasgow Coma Score scale in predicting long-term outcome of poor grade aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. 2009;16:894–899. doi: 10.1016/j.jocn.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Staub F, Graf R, Gabel P, Kochling M, Klug N, Heiss WD. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery. 2000;47:1106–1115. doi: 10.1097/00006123-200011000-00016. discussion 1115-1106. [DOI] [PubMed] [Google Scholar]
- Stein SC, Browne KD, Chen XH, Smith DH, Graham DI. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery. 2006a;59:781–787. doi: 10.1227/01.NEU.0000227519.27569.45. discussion 787-788. [DOI] [PubMed] [Google Scholar]
- Stein SC, Levine JM, Nagpal S, LeRoux PD. Vasospasm as the sole cause of cerebral ischemia: how strong is the evidence? Neurosurg Focus. 2006b;21:E2. doi: 10.3171/foc.2006.21.3.2. [DOI] [PubMed] [Google Scholar]
- Steiner L, Lofgren J, Zwetnow NN. Characteristics and limits of tolerance in repeated subarachnoid hemorrhage in dogs. Acta Neurol Scand. 1975;52:241–267. doi: 10.1111/j.1600-0404.1975.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Stoltenberg-Didinger G, Schwartz K. Brain Lesions Secondary to Subarachnoid Hemorrhage due to ruptured aneurysms. In: Cervos-Navarro J, Ferst R, editors. Stroke and Microcirculation. Raven Press; New York: 1987. pp. 471–480. [Google Scholar]
- Stoodley M, MacDonald RL, Weir B, Marton LS, Johns L, Du Zhang Z, Kowalczuk A. Subarachnoid hemorrhage as a cause of an adaptive response in cerebral arteries. J Neurosurg. 2000;93:463–470. doi: 10.3171/jns.2000.93.3.0463. [DOI] [PubMed] [Google Scholar]
- Suarez-Rivera O. Acute hydrocephalus after subarachnoid hemorrhage. Surg Neurol. 1998;49:563–565. doi: 10.1016/s0090-3019(97)00342-x. [DOI] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP, International Stroke Incidence Collaboration Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, Narasimhan P, Maier CM, Chan PH. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T, Fujishima M, Omae T. Lactate and pyruvate concentrations, and acid-base balance of cerebrospinal fluid in experimentally induced intracerebral and subarachnoid hemorrhage in dogs. Stroke. 1975;6:715–719. doi: 10.1161/01.str.6.6.715. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kajita Y, Oyama H, Tanazawa T, Takayasu M, Shibuya M, Sugita K. Dysfunction of nitric oxide in the spastic basilar arteries after subarachnoid hemorrhage. J Auton Nerv Syst. 1994;49(Suppl):S83–87. doi: 10.1016/0165-1838(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Symon L. Disordered cerebro-vascular physiology in aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1978;41:7–22. doi: 10.1007/BF01809133. [DOI] [PubMed] [Google Scholar]
- Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Tada T, Kitazawa K, Tanaka Y, Hongo K, Kameko M, Uemura KI. Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res. 2001;23:724–730. doi: 10.1179/016164101101199243. [DOI] [PubMed] [Google Scholar]
- Tanriverdi T, Sanus GZ, Ulu MO, Tureci E, Uzun H, Aydin S, Kaynar MY. Serum and cerebrospinal fluid concentrations of E-selectin in patients with aneurysmal subarachnoid hemorrhage. Braz J Med Biol Res. 2005;38:1703–1710. doi: 10.1590/s0100-879x2005001100020. [DOI] [PubMed] [Google Scholar]
- Tettenborn D, Dycka J. Prevention and treatment of delayed ischemic dysfunction in patients with aneurysmal subarachnoid hemorrhage. Stroke. 1990;21:IV85–89. [PubMed] [Google Scholar]
- Thal SC, Mebmer K, Schmid-Elsaesser R, Zausinger S. Neurological impairment in rats after subarachnoid hemorrhage--a comparison of functional tests. J Neurol Sci. 2008;268:150–159. doi: 10.1016/j.jns.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Thal SC, Sporer S, Klopotowski M, Thal SE, Woitzik J, Schmid-Elsaesser R, Plesnila N, Zausinger S, Laboratory investigation Brain edema formation and neurological impairment after subarachnoid hemorrhage in rats. J Neurosurg. 2009;111:988–994. doi: 10.3171/2009.3.JNS08412. [DOI] [PubMed] [Google Scholar]
- Tomassoni D, Lanari A, Silvestrelli G, Traini E, Amenta F. Nimodipine and its use in cerebrovascular disease: evidence from recent preclinical and controlled clinical studies. Clin Exp Hypertens. 2008;30:744–766. doi: 10.1080/10641960802580232. [DOI] [PubMed] [Google Scholar]
- Tomida M, Muraki M, Uemura K, Yamasaki K. Plasma concentrations of brain natriuretic peptide in patients with subarachnoid hemorrhage. Stroke. 1998;29:1584–1587. doi: 10.1161/01.str.29.8.1584. [DOI] [PubMed] [Google Scholar]
- Travis MA, Hall ED. The effects of chronic two-fold dietary vitamin E supplementation on subarachnoid hemorrhage-induced brain hypoperfusion. Brain Res. 1987;418:366–370. doi: 10.1016/0006-8993(87)90105-3. [DOI] [PubMed] [Google Scholar]
- Trojanowski T. Experimental subarachnoid haemorrhage. Part I. a new approach to subarachnoid blood injection in cats. Acta Neurochir. 1982a;62:171–175. doi: 10.1007/BF01403621. [DOI] [PubMed] [Google Scholar]
- Trojanowski T. Experimental subarachnoid haemorrhage. Part II: extravasation volume and dynamics of subarachnoid arterial bleeding in cats. Acta Neurochir. 1982b;64:103–108. doi: 10.1007/BF01405623. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Cook DA, Weir BK, Handa Y. Effect of clot removal on cerebrovascular contraction after subarachnoid hemorrhage in the monkey: pharmacological study. Heart Vessels. 1996;11:69–79. doi: 10.1007/BF01744506. [DOI] [PubMed] [Google Scholar]
- Uhl E, Lehmberg J, Steiger HJ, Messmer K. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery. 2003;52:1307–1315. doi: 10.1227/01.neu.0000065154.04824.9e. discussion 1315-1307. [DOI] [PubMed] [Google Scholar]
- Umansky F, Kaspi T, Shalit MN. Regional cerebral blood flow in the acute stage of experimentally induced subarachnoid hemorrhage. J Neurosurg. 1983;58:210–216. doi: 10.3171/jns.1983.58.2.0210. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Meyer B, Weidauer S, Raabe A, Thome C, Ringel F, Breu V, Schmiedek P. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- Vale FL, Bradley EL, Fisher WS., 3rd The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997;86:462–466. doi: 10.3171/jns.1997.86.3.0462. [DOI] [PubMed] [Google Scholar]
- van Asch CJ, van der Schaaf IC, Rinkel GJ. Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010;31:67–70. doi: 10.3174/ajnr.A1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh WM, Algra A, van der Sprenkel JW, Tulleken CA, Rinkel GJ. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:276–281. doi: 10.1227/01.neu.0000043984.42487.0e. discussion 281-272. [DOI] [PubMed] [Google Scholar]
- van den Bergh WM, Dijkhuizen RM, Rinkel GJ. Potentials of magnesium treatment in subarachnoid haemorrhage. Magnes Res. 2004;17:301–313. [PubMed] [Google Scholar]
- van den Bergh WM, Zuur JK, Kamerling NA, van Asseldonk JT, Rinkel GJ, Tulleken CA, Nicolay K. Role of magnesium in the reduction of ischemic depolarization and lesion volume after experimental subarachnoid hemorrhage. J Neurosurg. 2002;97:416–422. doi: 10.3171/jns.2002.97.2.0416. [DOI] [PubMed] [Google Scholar]
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- Vecchione C, Frati A, Di Pardo A, Cifelli G, Carnevale D, Gentile MT, Carangi R, Landolfi A, Carullo P, Bettarini U, Antenucci G, Mascio G, Busceti CL, Notte A, Maffei A, Cantore GP, Lembo G. Tumor necrosis factor-alpha mediates hemolysis-induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension. 2009;54:150–156. doi: 10.1161/HYPERTENSIONAHA.108.128124. [DOI] [PubMed] [Google Scholar]
- Veelken JA, Laing RJ, Jakubowski J. The Sheffield model of subarachnoid hemorrhage in rats. Stroke. 1995;26:1279–1283. doi: 10.1161/01.str.26.7.1279. discussion 1284. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD. Effect of Endothelin-Receptor Antagonists on Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage Remains Unclear. Stroke. 2009;40:e714. doi: 10.1161/STROKEAHA.109.565887. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- Vieira AC, Azevedo-Filho HR, Quinino S, Ponte de Souza ML, Camara D, Jr., Leitao L, Andrade G. Language, memory, and verbal fluency changes in patients with aneurysmal subarachnoid hemorrhage: results of a preoperative investigation. World Neurosurg. 2011;75:653–659. doi: 10.1016/j.wneu.2010.10.044. discussion 596-657. [DOI] [PubMed] [Google Scholar]
- Vikman P, Beg S, Khurana TS, Hansen-Schwartz J, Edvinsson L. Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J Neurosurg. 2006;105:438–444. doi: 10.3171/jns.2006.105.3.438. [DOI] [PubMed] [Google Scholar]
- Voldby B. Pathophysiology of subarachnoid haemorrhage. Experimental and clinical data. Acta Neurochir Suppl (Wien) 1988;45:1–6. [PubMed] [Google Scholar]
- Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62:59–67. doi: 10.3171/jns.1985.62.1.0059. [DOI] [PubMed] [Google Scholar]
- von Holst H, Mathiesen T. Electrolyte concentrations in serum and CSF following subarachnoid haemorrhage. Br J Neurosurg. 1990;4:123–126. doi: 10.3109/02688699008992710. [DOI] [PubMed] [Google Scholar]
- Holst H, Sollevi A. Increased concentration of hypoxanthine in human central cerebrospinal fluid after subarachnoid haemorrhage. Acta Neurochir (Wien) 1985;77:52–59. doi: 10.1007/BF01402306. [DOI] [PubMed] [Google Scholar]
- Wanebo JE, Arthur AS, Louis HG, West K, Kassell NF, Lee KS, Helm GA. Systemic administration of the endothelin-A receptor antagonist TBC 11251 attenuates cerebral vasospasm after experimental subarachnoid hemorrhage: dose study and review of endothelin-based therapies in the literature on cerebral vasospasm. Neurosurgery. 1998;43:1409–1417. discussion 1417-1408. [PubMed] [Google Scholar]
- Wang J, Ohta S, Sakaki S, Araki N, Matsuda S, Sakanaka M. Changes in Ca(++)-ATPase activity in smooth-muscle cell membranes of the canine basilar artery with experimental subarachnoid hemorrhage. J Neurosurg. 1994;80:269–275. doi: 10.3171/jns.1994.80.2.0269. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu C, Zhang G, Lu Y. Changes of endothelin during cerebral vasospasm after experimental subarachnoid hemorrhage. Chin Med J (Engl) 1995;108:586–590. [PubMed] [Google Scholar]
- Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–623. doi: 10.1097/01.ccm.0000201903.46435.35. quiz 624. [DOI] [PubMed] [Google Scholar]
- Watkins LD. Nitric oxide and cerebral blood flow: an update. Cerebrovasc Brain Metab Rev. 1995;7:324–337. [PubMed] [Google Scholar]
- Weidauer S, Vatter H, Beck J, Raabe A, Lanfermann H, Seifert V, Zanella F. Focal laminar cortical infarcts following aneurysmal subarachnoid haemorrhage. Neuroradiology. 2008;50:1–8. doi: 10.1007/s00234-007-0294-1. [DOI] [PubMed] [Google Scholar]
- Weir B, Erasmo R, Miller J, McIntyre J, Secord D, Mielke B. Vasospasm in response to repeated subarachnoid hemorrhages in the monkey. J Neurosurg. 1970;33:395–406. doi: 10.3171/jns.1970.33.4.0395. [DOI] [PubMed] [Google Scholar]
- Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48:173–178. doi: 10.3171/jns.1978.48.2.0173. [DOI] [PubMed] [Google Scholar]
- Westermaier T, Jauss A, Eriskat J, Kunze E, Roosen K. Acute vasoconstriction: decrease and recovery of cerebral blood flow after various intensities of experimental subarachnoid hemorrhage in rats. J Neurosurg. 2009;110:996–1002. doi: 10.3171/2008.8.JNS08591. [DOI] [PubMed] [Google Scholar]
- Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol. 1985;18:211–216. doi: 10.1002/ana.410180208. [DOI] [PubMed] [Google Scholar]
- Wilkins RH, Alexander JA, Odom GL. Intracranial arterial spasm: a clinical analysis. J Neurosurg. 1968;29:121–134. doi: 10.3171/jns.1968.29.2.0121. [DOI] [PubMed] [Google Scholar]
- Windmuller O, Lindauer U, Foddis M, Einhaupl KM, Dirnagl U, Heinemann U, Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain. 2005;128:2042–2051. doi: 10.1093/brain/awh545. [DOI] [PubMed] [Google Scholar]
- Wirth FP. Surgical treatment of incidental intracranial aneurysms. Clin Neurosurg. 1986;33:125–135. [PubMed] [Google Scholar]
- Wong GK, Chan MT, Poon WS, Boet R, Gin T. Neurol Res. 2006. Magnesium therapy within 48 hours of an aneurysmal subarachnoid hemorrhage: neuro-panacea; pp. 431–435. [DOI] [PubMed] [Google Scholar]
- Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010a;41:921–926. doi: 10.1161/STROKEAHA.109.571125. [DOI] [PubMed] [Google Scholar]
- Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC. Plasma magnesium concentrations and clinical outcomes in aneurysmal subarachnoid hemorrhage patients: post hoc analysis of intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage trial. Stroke. 2010b;41:1841–1844. doi: 10.1161/STROKEAHA.110.585232. [DOI] [PubMed] [Google Scholar]
- Woszczyk A, Deinsberger W, Boker DK. Nitric oxide metabolites in cisternal CSF correlate with cerebral vasospasm in patients with a subarachnoid haemorrhage. Acta Neurochir (Wien) 2003;145:257–264. doi: 10.1007/s00701-003-0004-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Nishizawa S, Yokoyama T, Ryu H, Uemura K. Subarachnoid hemorrhage impairs cerebral blood flow response to nitric oxide but not to cyclic GMP in large cerebral arteries. Brain Res. 1997;757:1–9. doi: 10.1016/s0006-8993(96)01433-3. [DOI] [PubMed] [Google Scholar]
- Yamaura I, Tani E, Saido TC, Suzuki K, Minami N, Maeda Y. Calpain-calpastatin system of canine basilar artery in vasospasm. J Neurosurg. 1993;79:537–543. doi: 10.3171/jns.1993.79.4.0537. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM. Mg(2+)-induced endothelium-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol. 2000;278:R628–639. doi: 10.1152/ajpregu.2000.278.3.R628. [DOI] [PubMed] [Google Scholar]
- Yatsushige H, Calvert JW, Cahill J, Zhang JH. Limited role of inducible nitric oxide synthase in blood-brain barrier function after experimental subarachnoid hemorrhage. J Neurotrauma. 2006;23:1874–1882. doi: 10.1089/neu.2006.23.1874. [DOI] [PubMed] [Google Scholar]
- Yatsushige H, Ostrowski RP, Tsubokawa T, Colohan A, Zhang JH. Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J Neurosci Res. 2007;85:1436–1448. doi: 10.1002/jnr.21281. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y, Kim P, Sasaki T, Takakura K. Temporal profile and significance of metabolic failure and trophic changes in the canine cerebral arteries during chronic vasospasm after subarachnoid hemorrhage. J Neurosurg. 1993;78:807–812. doi: 10.3171/jns.1993.78.5.0807. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang L, Liu M, Wu B. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010;2:CD006778. doi: 10.1002/14651858.CD006778.pub2. [DOI] [PubMed] [Google Scholar]
- Zhang ZD, Yamini B, Komuro T, Ono S, Johns L, Marton LS, Weir B, Macdonald RL. Vasospasm in monkeys resolves because of loss of and encasement of subarachnoid blood clot. Stroke. 2001;32:1868–1874. doi: 10.1161/01.str.32.8.1868. [DOI] [PubMed] [Google Scholar]
- Zhou C, Yamaguchi M, Kusaka G, Schonholz C, Nanda A, Zhang JH. J Cereb Blood Flow Metab. 2004. Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage; pp. 419–431. [DOI] [PubMed] [Google Scholar]
- Zubkov AY, Aoki K, Parent AD, Zhang JH. Preliminary study of the effects of caspase inhibitors on vasospasm in dog penetrating arteries. Life Sci. 2002a;70:3007–3018. doi: 10.1016/s0024-3205(02)01550-3. [DOI] [PubMed] [Google Scholar]
- Zubkov AY, Tibbs RE, Aoki K, Zhang JH. Morphological changes of cerebral penetrating arteries in a canine double hemorrhage model. Surg Neurol. 2000;54:212–219. doi: 10.1016/s0090-3019(00)00305-0. discussion 219-220. [DOI] [PubMed] [Google Scholar]
- Zubkov AY, Tibbs RE, Clower B, Ogihara K, Aoki K, Zhang JH. Morphological changes of cerebral arteries in a canine double hemorrhage model. Neurosci Lett. 2002b;326:137–141. doi: 10.1016/s0304-3940(02)00188-x. [DOI] [PubMed] [Google Scholar]
- Zuccarello M, Lewis AI, Upputuri S, Farmer JB, Anderson DK. Effect of remacemide hydrochloride on subarachnoid hemorrhage-induced vasospasm in rabbits. J Neurotrauma. 1994;11:691–698. doi: 10.1089/neu.1994.11.691. [DOI] [PubMed] [Google Scholar]