Abstract
Genomic and pharmacologic data have suggested the involvement of the α3β4 subtype of nicotinic acetylcholine receptors (nAChRs) in drug seeking to nicotine and other drugs of abuse. In order to better examine this receptor subtype, we have identified and characterized the first high affinity and selective α3β4 nAChR antagonist, AT-1001, both in vitro and in vivo. This is the first reported compound with a Ki below 10 nM at α3β4 nAChR and >90-fold selectivity over the other major subtypes, the α4β2 and α7 nAChR. AT-1001 competes with epibatidine, allowing for [3H]epibatidine binding to be used for structure-activity studies, however, both receptor binding and ligand-induced Ca2+ flux are not strictly competitive because increasing ligand concentration produces an apparent decrease in receptor number and maximal Ca2+ fluorescence. AT-1001 also potently and reversibly blocks epibatidine-induced inward currents in HEK cells transfected with α3β4 nAChR. Importantly, AT-1001 potently and dose-dependently blocks nicotine self-administration in rats, without affecting food responding. When tested in a nucleus accumbens (NAcs) synaptosomal preparation, AT-1001 inhibits nicotine-induced [3H]dopamine release poorly and at significantly higher concentrations compared with mecamylamine and conotoxin MII. These results suggest that its inhibition of nicotine self-administration in rats is not directly due to a decrease in dopamine release from the NAc, and most likely involves an indirect pathway requiring α3β4 nAChR. In conclusion, our studies provide further evidence for the involvement of α3β4 nAChR in nicotine self-administration. These findings suggest the utility of this receptor as a target for smoking cessation medications, and highlight the potential of AT-1001 and congeners as clinically useful compounds.
Keywords: nicotine, self-administration, α3β4 nicotinic acetylcholine receptor antagonist, AT-1001
INTRODUCTION
Neuronal nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels in the same family as the GABA, glycine, and 5-HT3 receptors and mediate cation flux (Gotti et al, 1997; McGehee et al, 1995a). In mammalian neural tissue, eight α-subunits and three β-subunits have been identified. The receptor is usually made up of a combination of subunit types (McGehee and Role, 1995b), although there are also fully functional homomeric receptor proteins, particularly the prominent α7 nAChR. The α4β2 and the α7 receptors are by far the most prevalent in the CNS (Perry et al, 2002; Xiao and Kellar, 2004).
Nicotine addiction, like addiction to other psychomotor stimulants, is thought to be due to the activation of the dopaminergic mesocorticolimbic pathway (Picciotto, 1998; Stolerman and Shoaib, 1991). The nicotinic receptor subtype most prevalent in this region is the α4β2 nAChR, although there is a significant level of the α6 subunit (Perry et al, 2002, 2007). Furthermore, mRNA from multiple subunits is known to exist in small brain regions and even in single cells (Klink et al, 2001). Significant evidence suggests that the α4β2 receptor is involved in nicotine dependence. In particular, β2-knockout mice do not self-administer nicotine (Epping-Jordan et al, 1999; Picciotto et al, 1998), the somewhat selective α4β2 antagonist DHβE blocks nicotine self-administration (Watkins et al, 1999), and the α4β2 partial agonist varenicline is clinically used as a smoking cessation medication. More recently, the importance of the α6 subunit to nicotine self-administration has also been demonstrated (Pons et al, 2008).
After α4β2 and α7 nAChR, the next most prominent receptor subtype is the α3β4 nAChR. The α3β4 receptor, which predominates in sensory and autonomic ganglia and in the adrenal gland, is sometimes referred to as the ‘ganglionic nAChR' (Mandelzys et al, 1994; Poth et al, 1997). In the CNS, the mesolimbic dopamine pathway contains some α3β4 receptors; however, the majority of this receptor subtype resides in the medial habenula (MHb), interpeduncular nucleus (IPN), and pineal gland (Perry et al, 2002; Quick et al, 1999). Interestingly, the MHb and IPN have recently been implicated in nicotine self-administration and reward along with the α3, α5, and/or β4 nAChR subunits. In α5 null mice, there is an increase in nicotine conditioned place preference (CPP) and nicotine self-administration at higher nicotine doses, indicating that nAChRs containing this subunit may be involved in limiting the reward mediated by higher nicotine doses (Fowler et al, 2011; Jackson et al, 2010). Genome-wide association studies (GWAS) have implicated variants in the α3-α5-β4 gene cluster on chromosome 15 to be associated with an increased risk of whether a smoker becomes nicotine dependent, and to smoking a greater number of cigarettes per day (Berrettini et al, 2008; Saccone et al, 2008). These GWAS studies, therefore, also suggest a possible involvement of the α3β4 nAChR subtype in tobacco dependence.
Another line of evidence also implicates the α3β4 nAChR subtype and the MHb in self-administration of not only nicotine, but also a variety of abused drugs. Glick et al have identified a compound, 18-methoxycoronaridine (18-MC), which inhibits the reward induced by a large number of abused drugs (Maisonneuve and Glick, 2003). 18-MC is a derivative of the alkaloid ibogaine, a compound that has considerable anecdotal evidence for utility as a drug abuse medication, but no demonstration of clinical efficacy. 18-MC reduces methamphetamine, nicotine, and morphine self-administration in rats (Glick et al, 2000a, 2006). It also attenuates alcohol consumption and blocks acquisition of cocaine place preference (McCallum and Glick, 2009; Rezvani et al, 1997). 18-MC apparently mediates these actions through inhibition of α3β4 nAChR (Glick et al, 2011; Maisonneuve and Glick, 2003). However, although 18-MC may be more selective than ibogaine, which binds to a large number of receptors, 18-MC is neither a high affinity nor a selective α3β4 nAChR antagonist. To better demonstrate the involvement of the α3β4 nAChR in drug abuse, we have synthesized a series of high affinity and selective α3β4 nAChR antagonists. Here, we demonstrate that AT-1001 from this series, has nanomolar affinity for α3β4, low affinity at α4β2 and α7 nAChR, and acts as a non-competitive antagonist at α3β4 nAChR in vitro. Furthermore, AT-1001 potently blocks nicotine self-administration in rats.
MATERIALS AND METHODS
Drugs
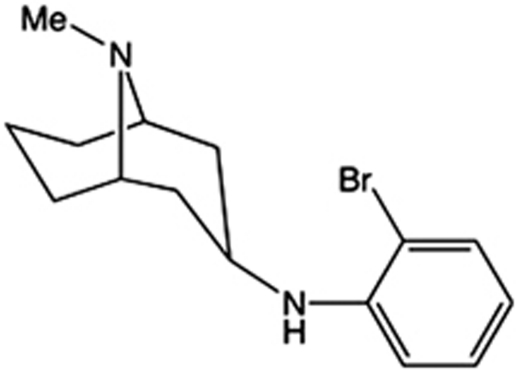
AT-1001 (N-(2-bromophenyl)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine) (Figure 1), was synthesized by methods to be published elsewhere. Nicotine tartrate was purchased from Sigma (St Louis, MO), dissolved in saline and adjusted to neutral pH (7.2–7.4), with doses reported as free base. Mecamylamine HCl (Sigma) was dissolved in saline with doses reported as salt.
Figure 1.
Chemical structure of AT-1001.
In vitro Characterization
Cell culture
KXα3β4R2 and KXα4β2R2 cells (HEK cells transfected with rat α3β4 and α4β2 respectively, obtained from Dr Kenneth Kellar, Georgetown University) were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 0.5% penicillin/streptomycin, 0.4 mg/ml of geneticin, and were maintained in an atmosphere of 7.5% CO2 at 37 °C. For binding assays, cells were passaged on 150-mm dishes and harvested when confluent. For functional assays, the cells were seeded into 96-well collagen-coated plates (Becton Dickinson Biocoat) at a density of approximately 50 000 cells per well. Cells seeded at this density grow into a confluent monolayer in 24 to 30 h.
Binding assays
Binding to α3β4 and α4β2 nAChR was conducted, as described previously (Zaveri et al, 2010). Briefly, cells were harvested by scraping the plates with a rubber policeman, suspended in Tris buffer, homogenized in a Polytron homogenizer, and washed twice by centrifugation at 20 000 × g for 20 min. For binding, the cell membranes were incubated with test compounds at concentrations ranging from 10−5 to 10−10 M in the presence of 0.3 nM of [3H]epibatidine. After a 2-h incubation at room temperature, samples were filtered through glass fiber filters by using a Tomtec cell harvester, and then counted. Nonspecific binding was determined with 0.1 μM of the unlabeled epibatidine. IC50 values and Hill coefficients were determined using the program Graphpad/PRISM. Ki values were calculated using the Cheng Prusoff transformation (Cheng and Prusoff, 1973). Binding to α7 nAChR was conducted with 1.0 nM [125I]-α-Bungarotoxin binding to rat brain membranes (Francis et al, 2001). Filtration and data analysis were conducted as described for [3H]epibatidine binding.
Ca2+ fluorescence assays
Ca2+ uptake was determined using FLIPR, as described previously (Zaveri et al, 2010). For dye loading, the no-wash dye (Molecular Devices Calcium Assay Kit, R8033) was reconstituted in assay buffer (Hank's Balanced Salt Solution with 20 mM of HEPES, pH 7.4) containing 2.5 μM of probenecid (Sigma). For antagonist assays, test compounds were diluted in assay buffer containing 100 nM of epibatidine. For calcium measurements, the FLIPR was equipped with a 5-W argon-ion laser source with an excitation wavelength of 488 nM, and a 510–570 nM bandpass emission filter was used for detection. For the assay, the FLIPR was set up to make fluorescence readings every 1 s for the first minute, and every 6 s for the next 2 min. The compounds were added at 10 s into the fluorescence readings. The change in fluorescence represents the maximum response, minus the minimum response for each well. Graphpad/PRISM was used to determine the EC50s and IC50s for the compounds by plotting the changes in fluorescence vs the logs of the compound concentrations.
Patch clamp recordings
Stably transfected HEK293 cells expressing α3β4 nAChR were plated on poly--lysine-coated coverslips and grown in 35 mm dishes for 1–2 days before patch clamp recordings. Voltage-clamp recordings were made in the whole-cell configuration using an Axopatch 700A patch-clamp amplifier (Axon Instruments, Unit City, CA). Thin-walled borosilicate glass microelectrodes (TW150F, World Precision Instruments, Sarasota, FL) with resistances of 3–5 MΩ when filled with an internal solution containing (in mM): 135 CsCl, 10 CsF, 10 HEPES (N-[2-hydroxyethyl] piperazine-NV-[2-ethanesulfonic acid]), 5 EGTA (ethylene glycol-bis[h-aminoethyl ether]-N,N,NV,NV-tetraacetic acid), 1 MgCl2, 0.5 CaCl2, pH 7.2. Whole-cell capacitance and series resistance were recorded and automatically compensated using the circuitry of the amplifier and PClamp 9.0 software. Cells were continuously superfused with extracellular solution containing (in mM): 150 NaCl, 3 KCl, 5 HEPES, 1 MgCl2, 1.8 CaCl2, 10 glucose, pH 7.3–7.4. Control, agonist, and drug solutions were applied to individual cells by a local, rapid perfusion driven by gravity for 500 ms or 2 s, each time with a minimum 30-s interval between applications.
Dopamine release experiments
The effect of nicotine and other compounds on release of [3H]dopamine was determined on synaptosomes prepared from rat nucleus accumbens (NAc). Synaptosomes were produced by a modification of the method of Quik et al (2003). Briefly, the NAc was dissected from freshly harvested Sprague–Dawley rat brain, and placed into ice cold Hybernate A medium. Dissected NAc was homogenized in 8.0 ml of 0.32 M sucrose, buffered to pH 7.35 with 5 mM HEPES. A crude synaptosome preparation was obtained by centrifugation at 12 000 × g for 20 min. To initialize uptake, a single pellet was drained and resuspended into 800 μl of uptake buffer (128 mM NaCl, 2.4 mM KCl, 1.2 mM KH2PO4, 25 mM HEPES, pH 7.5, 10 mM glucose, 3.2 mM CaCl2, 1.2 mM MgSO4, 1 mM ascorbic acid, 10 μM pargyline, pH 7.5). The solution was then immediately placed in a 37 °C water bath for 10 min. Test compounds were also added at this point. Next, [3H]dopamine was added to a final concentration of 125 nM and synaptosomes were incubated another 5 min at 37 °C. Triplicate samples of 80 μl each were then applied to the perfusion chambers. From this point on, washes and release were carried out in perfusion buffer (uptake buffer containing 0.1% BSA and 10 μM nomifensin). Unbound test compounds and any uninternalized [3H]-DA were removed with this wash. Perfusion was carried out with a flow rate of 1 ml/min, and the first 20 ml were typically discarded. Finally, 12 × 15 drop (∼20 s) fractions were collected. At the fourth fraction, 10 μM nicotine, or nicotine plus readministered test compound were passed through the chamber. Subsequent fractions were collected by rinsing in perfusion buffer and subsequently counted in a liquid scintillation counter.
Self-Administration Experiments
Food training
Male Sprague–Dawley rats (age P81, Charles River Labs, Hollister, CA; group housed two per cage) were trained to lever-press to earn a food pellet (45 mg rodent purified diet; Bio-Serv, Frenchtown, NJ) in an operant testing chamber (Med Associates, St Albans, VT). The chamber contained two levers; reinforced (R), responses at which led to reward delivery, non-reinforced (NR), responses at which were recorded but had no consequence. On earning a reward, a cue light above the R lever was illuminated and the house light extinguished for the entire duration of the timeout period. Food training began with a fixed-ratio 1, 1-s timeout (FR1TO1) protocol, followed by FR1TO10, FR2TO20, and FR5TO20. Rats progressed to the next ratio after earning at least 50 pellets during the 30-min session. Throughout the food training protocol, animals were food restricted to maintain approximately 85% of their free-feeding body weight.
Surgery
After completing food training, rats were anesthetized with equithesin (0.0035 ml/g body weight), and implanted with an indwelling catheter into the right external jugular vein using previously published procedures (Belluzzi et al, 2005). Catheters were flushed daily with heparinized saline solution (0.6 ml of 1000 units/ml heparin in 30 ml saline) to maintain patency. Patency was verified by infusing propofol (0.1 ml, Abbott Laboratories, Chicago, IL) to test for rapid anesthesia.
Nicotine self-administration
Following 2 days of recovery, animals were maintained at 90–95% of free-feeding weight and allowed to self-administer nicotine (30 μg/kg/infusion, 100 μl i.v. over 5.6 s) in the same chambers as used for food training, during daily 1-h sessions. Rats self-administered nicotine under the FR5TO20 schedule for a minimum of 10 days until reaching stable responding (R±20% of the mean over 3 days; R⩾6; R⩾2*NR). Following stable responding, AT-1001 (0, 0.75, 1.5, or 3 mg/kg, s.c., 10 min before testing n=6 per group) was administered to all animals in a within-subjects latin-square design, where all animals received all test doses in randomized order. Animals returned to baseline responding for a minimum of 3 days, or until reaching stable responding, before testing the next dose. Following the last test dose, we examined the effects of mecamylamine (3 mg/kg, s.c., 15 min before testing) on nicotine self-administration. Animals were allowed to self-administer nicotine for an additional day to confirm that responding returned to baseline.
Food self-administration
To assess any nonspecific effects of AT-1001 treatment a separate group of animals was trained to respond for food pellets. The same testing procedures were used as described above except these animals were given a 3-day rest period rather than catheter implantation surgery and allowed to respond for food pellets during the 1-h test sessions rather than nicotine. They underwent the same drug treatments as above.
All procedures were conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Animals (2002) and were approved by the Institutional Animal Care and Use Committees.
Data analysis
The effect of AT-1001 on the number of responses was analyzed by repeated-measures ANOVA, with drug dose as the within-subjects factor. Significant main effects were further analyzed by paired t-tests, Bonferroni corrected for multiple comparisons. AT-1001 effects on nicotine self-administration and food-maintained responding were analyzed separately.
RESULTS
Receptor Binding and Ca2+ Fluorescence Assays
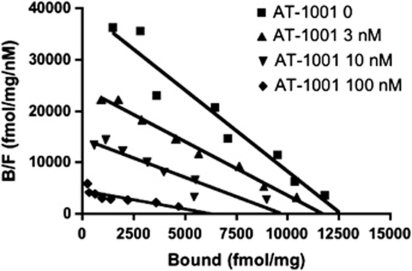
AT-1001 binds to α3β4 nAChRs in membranes of HEK cells transfected with this receptor type. It has high affinity and selectivity for the α3β4 nAChR, with Ki values of 2.4, 476, and 221 nM at α3β4, α4β2, and α7 nAChR, respectively (Table 1). The affinity of AT-1001 is less than that of epibatidine, but is far higher than the other agonists nicotine and cytisine. The nAChR antagonists mecamylamine and 18-MC did not compete with [3H]epibatidine binding. As seen in Figure 2, increasing concentrations of AT-1001 did not induce a parallel shift in the Scatchard Plot derived from a [3H]epibatidine saturation experiment. Rather, AT-1001 induced a decrease in Bmax as well as an increase in Kd. Similar data are shown with the direct binding saturation curves in Supplementary Figure S1. This demonstrates that AT-1001 is not strictly a competitive antagonist of epibatidine binding to α3β4 nAChR. Nevertheless, it competes with [3H]epibatidine, unlike mecamylamine and 18-MC.
Table 1. Binding Affinity Ki (nM) of AT-1001, Compared with Other Prototypical Agonists at α3β4, α4β2, and α7 Receptors.
| Compound |
Ki (nM) |
||
|---|---|---|---|
| α3β4 | α4β2 | α7 | |
| Epibatidine | 0.15±0.05 | 0.06±0.0 | 4.16±0.47 |
| Nicotine | 480.69±59.38 | 11.13±1.11 | |
| Cytisine | 202.89±18.85 | 1.53±0.20 | |
| AT-1001 | 2.64±0.24 | 476.52±98.75 | 221.72±9.88 |
| SR16584 | 507.86±162.39 | >10K | >10K |
| 18-MC | >10K | >10K | |
Data show mean±SD for at least two experiments conducted in triplicate.
Figure 2.
Inhibition of [3H]epibatidine binding to α3β4-containing HEK cell membranes by AT-1001. Scatchard analysis was plotted from saturation binding to HEK cell membranes, using [3H]epibatidine concentrations ranging from 0.04 to 3.2 nM. Saturation experiments were conducted in the presence of 0 (▪) 3 nM (▴), 10 nM (▾), and 100 nM (⧫) AT-1001. Data are from a single experiment repeated twice with similar results. Kd and Bmax values were: 0.32 nM, 12 620 fmol/mg protein; 0.48 nM, 11 700 fmol/mg protein; 0.66 nM, 9723 fmol/mg protein; and 1.40 nM, 6320 fmol/mg protein for Scatchard analysis in the presence of 0, 3, 10, and 100 nM AT-1001, respectively. Therefore, increasing concentrations of AT-1001 show a decrease in Bmax and increase in Kd, suggesting non-competitive, or mixed inhibition of binding. As inhibition was not strictly competitive, a pA2 value could not be determined.
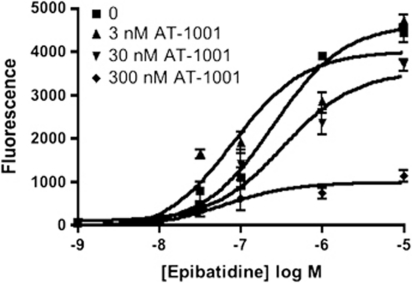
Efficacy at α3β4 nAChR was determined by measuring Ca2+ flux in HEK cells transfected with α3β4 nAChR (Table 2). AT-1001 has no agonist activity of its own but potently inhibits an epibatidine-induced increase in intracellular Ca2+-induced fluorescence. Epibatidine treatment produced a 1000-fold increase in intracellular Ca2+-induced fluorescence, and this was inhibited by several nicotinic antagonists (see Figure 3 and Table 2). AT-1001 (IC50 35.2±8.1 nM) was more than twice as potent as mecamylamine and nearly 30 times more potent than 18-MC. Once again, inhibition was non-competitive, as AT-1001 induced a decrease in maximal Ca2+ flux rather than a parallel shift to the right of the epibatidine dose response curve (Figure 3). Unlike with [3H]epibatidine binding, both mecamylamine and 18-MC were able to inhibit epibatidine-induced Ca2+ flux in these cells, indicating that both of these compounds are also α3β4 nAChR antagonists. However, neither of these compounds was nearly as potent as AT-1001.
Table 2. Effect of nAChR Agonists and Antagonists on Ca2+-Induced Fluorescence in α3β4-Containing HEK Cells.
| Compound | α3β4 |
|---|---|
| EC50 (nM)±SEM | |
| Epibatidine | 43.5±12 |
| IC50 (nM)±SEM | |
| AT-1001 | 35.2±8.1 |
| 18-MC | 1058±530 |
| Mecamylamine | 83.7±13.3 |
| DHβE | >10 000 |
Data show mean±SD for at least two experiments conducted in quadruplicate.
Figure 3.
Inhibition of epibatidine-induced Ca2+ fluorescence by AT-1001 in HEK cells containing α3β4 nAChR. Ca2+ fluorescence induced by increasing concentrations of epibatidine was measured as described in Materials and methods section. Epibatidine dose-response experiments conducted in the presence of 0 (▪), 3 nM (▴), 30 nM (▾), and 300 nM (♦) of AT1001 induced a rightward shift in EC50 and progressive decrease in maximal effect suggesting a non-competitive mode of inhibition. Data shown are mean±SD from a single experiment conducted in quadruplicate that was repeated with similar results.
Assessment of Off-Target Activity of AT-1001
AT-1001 has been evaluated against 48 different receptor and ion channel targets in the NIMH Psychoactive Drug Screening Program (Supplementary Table T1). The only receptors for which AT-1001 had affinity below a Ki of 1 μM, are the 5HT3 (Ki 600 nM), histamine H1 (Ki 201 nM), muscarinic M4 (Ki 661 nM) and M5 (Ki 325 nM), SERT (271 nM) sigma2 (Ki 418 nM), and sigma1 (Ki 84 nM). Given the high binding affinity of AT-1001 for its intended target, AT-1001 demonstrates a selectivity of >100-fold for everything except other β4-containing nAChRs and sigma1, and still maintains nearly 50-fold selectivity over these binding sites.
Whole-Cell Voltage Clamp Experiments
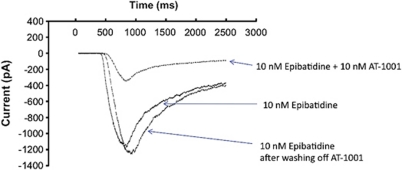
Inward currents were induced in HEK cells transfected with α3β4 nAChR by either nicotine or epibatidine in a concentration-dependent manner (Supplementary Figure S2). Epibatidine had an EC50 of approximately 150 nM. AT-1001 potently inhibits the epibatidine-induced current in HEK cells. As shown in Figure 4, inward current, induced by 10 nM epibatidine, was inhibited 66±15% by the inclusion of 10 nM AT-1001. The inhibition induced by a 2-s application of AT-1001 was fully reversed by a minimum of a 30-s perfusion of control solution, indicating that the inhibition by AT-1001 at the α3β4 nAChR-gated channel is fully reversible.
Figure 4.
Patch clamp recording in α3β4-containing HEK cells. Sample trace of the blocking effect of 10 nM AT-1001 against 10 nM Epibatidine activation (66% block, n=3). Whole-cell voltage-clamp recordings were made using Axon 700A and PClamp 8.2. Experiments were conducted within 24–72 h after replating the stable cells. Cells were clamped at –60 mV and drugs were delivered via local rapid perfusion for 500 ms, as described in Materials and methods section.
Effect on [3H]dopamine Release In Vitro
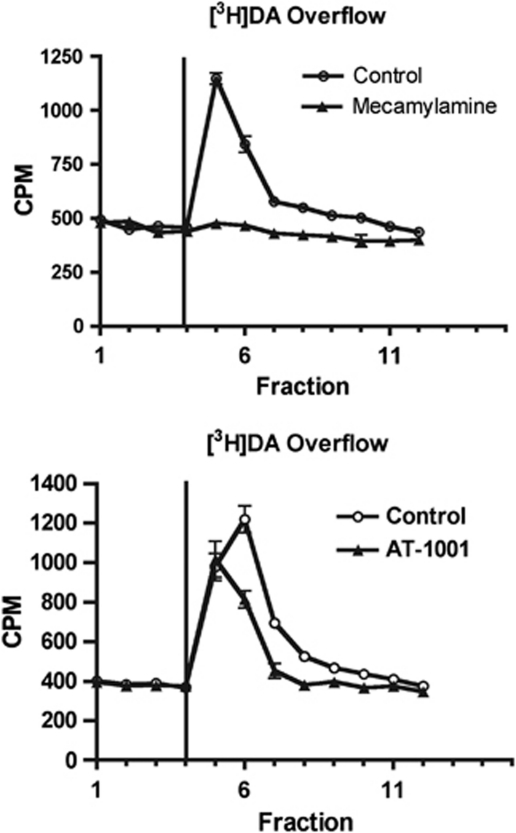
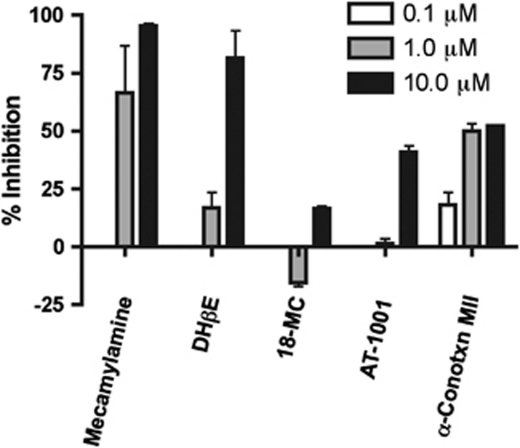
If α3β4 nAChRs have an effect on reward, they may be expected to inhibit release of dopamine from a striatal slice or synaptosome preparation. To test this hypothesis, we examined whether AT-1001 could inhibit nicotine-induced release of [3H]dopamine from NAc synaptosomes. In this preparation, synaptosomes were perfused with 10 μM nicotine, inducing a 3–4-fold increase in release of previously accumulated [3H]dopamine (Figure 5). This release was inhibited at 1 and 10 μM by the non-selective nAChR antagonist mecamylamine and the α4β2-selective antagonist DHβE, which has 100-fold higher affinity for α4β2 than α3β4 nAChRs (Chavez-Noriega et al, 1997). The selective α3β4 antagonist AT-1001 had a partial effect (40% inhibition) on [3H]dopamine release only at the high dose of 10 μM, while 18-MC had a smaller effect, inhibiting [3H]dopamine release only approximately 15% at 10 μM. Consistent with other studies, the α6-selective antagonist conotoxin MII inhibited approximately 50% at both 1 and 10 μM, indicating roughly half of the receptors contain the α6 subunit (Figure 6) (Grady et al, 2002).
Figure 5.
Inhibition of nicotine-induced [3H]DA release from nucleus accumbens synaptosomes. Synaptosomes were perfused with buffer and then 10 μM nicotine was added as indicated by the vertical line at fraction 4. Nicotine-induced [3H]DA release was inhibited completely by 10 μM mecamylamine (a) and to a much lesser extent by 10 μM AT-1001 (b). Data shown are mean±SD from a single experiment conducted in triplicate that was repeated with similar results.
Figure 6.
Inhibition of nicotine-induced [3H]DA release by nAChR antagonists. Data shown represents mean inhibition±SD from three experiments conducted in triplicate. Mecamylamine and conotoxin MII significantly inhibited [3H]DA at both 1 and 10 μM while DHβE and AT-1001 inhibited release only at 10 μM.
Effect of AT-1001 on Nicotine Self-Administration in Rats
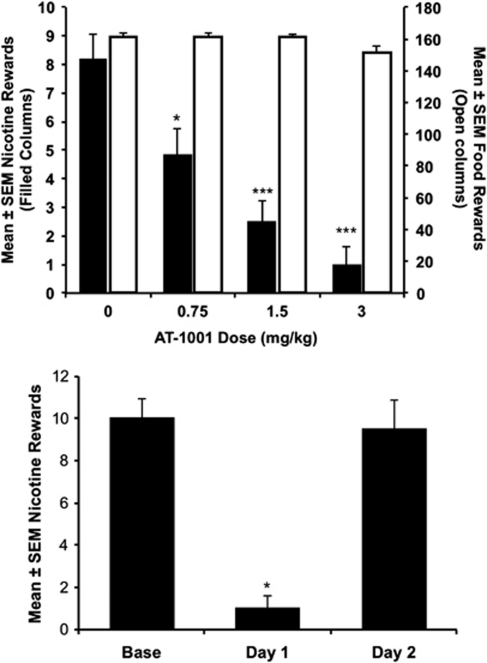
AT-1001 dose-dependently decreased responding for nicotine, but not food pellets (Figure 7a). In the nicotine group, there was a significant main effect of drug dose (F3, 15=46.685; p<0.001). Further testing revealed that all AT-1001 doses significantly reduced responding compared with vehicle treatment. In contrast, vehicle administration did not produce a significant change from baseline responding. Although there was a significant main effect of AT-1001 treatment in the food-responding group (F3, 15=7.582; p=0.003), there were no significant differences in post-hoc tests (p=0.054 corrected, AT-1001, 3 mg/kg vs vehicle).
Figure 7.
AT-1001 reduces nicotine self-administration in rats. (a) Acute effects of AT-1001 treatment. Each data point is the mean±SEM number of rewards earned in the 1-h session following AT-1001 treatment. Filled bars represent animals responding for nicotine (30 μg/kg/inj, left axis), solid bars are animals responding for food pellets (45 mg pellets, right axis). AT-1001 doses (mg/kg, s.c.) are indicated on the x axis. *p<0.05 vs vehicle, *** p<0.001 vs vehicle. (b) AT-1001 (3 mg/kg) does not have long-lasting effects. Base is the number of reinforced responses on the day before AT-1001 treatment. Day 1 is the number of reinforced responses on the test day of AT-1001 (3 mg/kg) treatment, while day 2 is the day following the testday. *p<0.05 vs base and day 2. n=6 per group.
As 18-MC treatment has long-lasting effects (Glick et al, 2000a), we compared responding the day before testing with the 3 mg/kg dose of AT-1001, the day of testing, and the day after testing (Figure 7b). There was a significant main effect of day (F2, 10=40.043; p<0.001), with responding on the test day significantly lower than either other day (p<0.001). Animals responded the day after treatment at baseline levels, indicating that AT-1001 did not have long-lasting effects. Finally, we tested whether the nonspecific nAChR antagonist mecamylamine, which reduces nicotine self-administration in this paradigm (Corrigall and Coen, 1989), was able to reduce responding to a similar extent as AT-1001. Consistent with past reports, mecamylamine (3 mg/kg; s.c.) significantly reduced responding for nicotine (p=0.011). However, AT-1001 (3 mg/kg) reduced responding significantly more than did mecamylamine (90.5%±7.1 vs 48%±8.9; p<0.05) (Supplementary Figure S3).
DISCUSSION
Considerable research has been conducted to determine the nAChR subunits involved in drug abuse and nicotine abuse in particular. The α4β2 nAChR is the most prevalent in brain tissue and it was demonstrated relatively early on that both the α4 and β2 subunits have a role in nicotine-seeking activity. β2 nAChR knockout mice do not self-administer nicotine, an activity that can be restored by viral vector-induced replacement of this subunit into the VTA (Maskos et al, 2005; Picciotto et al, 1998; Pons et al, 2008). Complementary experiments demonstrated that selective activation of a mutated α4 subunit that responds to lower nicotine concentrations, by low doses of nicotine that do not activate wild-type nAChR, is sufficient for nicotine-induced reward, as determined by CPP (Tapper et al, 2004). In addition, the clinically used medication for smoking cessation, varenicline, is a potent α4β2 nAChR partial agonist that decreases nicotine-induced increase in NAc dopamine, and attenuates nicotine self-administration (Rollema et al, 2007).
The α6 subunit has been demonstrated to be equally important for nicotine self-administration. Deletion of the α6 subunit blocks nicotine self-administration in mice, which can be restored by replacement of the α6 subunit into the VTA (Pons et al, 2008). In addition, inhibition of α6-containing nAChR with α-conotoxin MII (H9A;L15A) blocks both nicotine CPP and nicotine withdrawal-associated CPA and anxiety (Jackson et al, 2009; McIntosh et al, 2004). In fact, both α4 and α6 receptors are required in the VTA to induce the rewarding effects of nicotine, suggesting that the α4α6β2 receptor might be the most important player in mediating nicotine abuse (Pons et al, 2008).
More recently, the importance of the α5 subunit to nicotine addiction has been demonstrated. However, deletion of the α5 subunit modulates nicotine self-administration and reward in the opposite direction to the deletion of β2 or α6. Nicotine demonstrates an inverted U shaped dose response curve for self-administration and CPP, with higher doses being less rewarding. Deleting the α5 subunit appears to block the aversive properties of higher nicotine doses, thereby inhibiting the falling arm of the dose response curve and increasing both self-administration and CPP at the higher nicotine doses (Fowler et al, 2011; Jackson et al, 2010). These results are particularly interesting because several studies have identified the α5-α3-β4 nicotinic receptor gene cluster found on chromosome 15 as associated with increased risk for tobacco dependence. Variants in this gene cluster lead to an increased likelihood of a smoker becoming dependent and to smoking a greater number of cigarettes per day (Berrettini et al, 2008; Caporaso et al, 2009; Saccone et al, 2008; Thorgeirsson et al, 2008). A variant (rs16969968) leading to a D398N change in amino acid sequence of the α5 subunit may be particularly important in this regard as the N variant of the receptor appears to less efficiently induce Ca2+ flux than the more prevalent D-containing receptor in HEK cells containing α4α5β2 receptors transfected with the different receptor variants (Bierut et al, 2008).
Although the α5 subunit of the nAChR can be found in the midbrain reward center, one location proven to be crucial for its effect on nicotine self-administration is the MHb to IPN pathway. The MHb sends projections through the fasciculus retroflexus to the IPN. Fowler et al. demonstrated that reintroduction of the α5 subunit via a viral vector into the IPN restores self-administration to w.t. levels (Fowler et al, 2011). Inactivation of the MHb or IPN by lidocaine microinjection produces self-administration levels similar to the α5 KO animals. The MHb and IPN have a very high level of nAChRs. Furthermore, a large portion of these receptors represent the α3β4 nAChR subtype and these regions, along with the pineal gland, are the only brain regions with a high density of this subtype (Perry et al, 2002). However, most α3β4 subunits in the MHb-IPN are not complexed with α5, and the α3β4 nAChR controls ACh release in the fasciculus retroflexus (Grady et al, 2009).
In addition to the α4, α5, α6, and β2 containing receptors, the α3β4 nAChR has also been demonstrated to be involved in drug-seeking behavior, not only of nicotine but a variety of other drugs of abuse as well. As one example, deletion of the β4 subunit eliminated nicotine withdrawal symptoms (Salas et al, 2004). Glick et al (2000a, 2006) have demonstrated repeatedly that the ibogaine analog 18-MC is able to attenuate self-administration of a variety of abused drugs, including nicotine, morphine, cocaine, methamphetamine, and alcohol (Rezvani et al, 1997). It also blocked the acquisition of cocaine CPP, but surprisingly potentiated cocaine prime-induced reinstatement (McCallum and Glick, 2009). Unlike ibogaine, 18-MC appears to be a somewhat selective, though not particularly potent, α3β4 nAChR antagonist (Glick et al, 2000b).
Our studies were undertaken to identify new selective α3β4 nAChR antagonists and determine if this receptor is a viable target for treating tobacco dependence. AT-1001 is the first reported α3β4 antagonist with nearly 100-fold selectivity over α4β2 and α7 nAChRs. It has very high binding affinity (Ki <10 nM) for the α3β4 nAChR, as determined by inhibition of [3H]epibatidine binding, and has about a 200-fold higher affinity for α3β4 nAChR than our original lead compound SR16584 (Zaveri et al, 2010). AT-1001 is not a congener of 18-MC and clearly binds differently to the α3β4 nAChR because 18-MC does not block [3H]epibatidine binding but in fact binds to the mecamylamine site in the central lumen of the receptor (Arias et al, 2010). Although AT-1001 displaces [3H]epibatidine, Scatchard analysis indicates at least partial non-competitive inhibition of [3H]epibatidine binding. Both AT-1001 and 18-MC inhibit α3β4 nAChR activity, with the ability to block epibatidine-induced Ca2+ flux in transfected HEK cells. However, AT-1001 is approximately 30-fold more potent than 18-MC as an inhibitor of Ca2+ flux. AT-1001 is also more potent than mecamylamine in inhibiting epibatidine-induced Ca2+ flux through α3β4 nAChR.
Consistent with past reports implicating the α3β4 nAChR subtype in drug-seeking behavior, AT-1001 potently blocked self-administration of nicotine in rats. In this model of addiction, AT-1001 (0, 0.75, 1.5, and 3.0 mg/kg) produced a dose-dependent inhibition of nicotine self-administration, almost completely blocking nicotine responding at 3 mg/kg. This ability to block nicotine self-administration was rapidly reversible, with nicotine responding returning to pre-drug levels by 24 h. In contrast, AT-1001 did not affect food-seeking behavior, suggesting that the drug acts specifically on nicotine-induced rather than natural reward processes. In addition, AT-1001 did not show any reward of its own up to 10 mg/kg in mice using the CPP paradigm (Supplementary Figure S4). We have not yet examined this compound for ganglionic blockade-induced hypotension or constipation. Based on previous studies by Glick et al (2006) and Fowler et al (2011), and the discrete localization of α3β4 nAChR (Perry et al, 2002), we believe that AT-1001 is acting at the level of the MHb and IPN, supporting the hypothesis that both these brain regions and the α3β4 nAChR subtype are involved in nicotine self-administration. In addition, the fact that some animals at the highest dose did not self-administer any nicotine suggests that AT-1001 is affecting drive for the drug reward rather than antagonizing nicotine's rewarding effects. This is also consistent with studies by Glick et al (2000a, 2006) demonstrating a reward-attenuating effect of 18-MC for more than just nicotine (McCallum and Glick, 2009; Rezvani et al, 1997). On the other hand, varenicline, DHβE, and mecamylamine, which probably inhibit nicotine reward directly at the level of the NAc/VTA, reduce rather than eliminate, responding for nicotine (Corrigall and Coen, 1989; Glick et al, 2011; Rollema et al, 2007; Watkins et al, 1999).
Since AT-1001 potently blocked nicotine self-administration, we investigated whether AT-1001 had any effect on DA release in the NAc. AT-1001 was tested for its ability to block nicotine-induced [3H]DA release from synaptosomes produced from tissue taken from rat NAc. In this system, mecamylamine was the most potent compound, and completely blocked [3H]DA release when used at 10 μM. AT-1001, which is selective for the α3β4 and far more potent than mecamylamine for inhibition of Ca2+ flux in HEK cells transfected with the α3β4 nAChR, produces only partial inhibition of [3H]DA release at 10 μM, a far higher concentration than at which it inhibits Ca2+ flux. nAChR in the striatum are mostly α4β2, α4α5β2, and α4α6β2 nAChR (Gotti et al, 2010). Nicotine-induced [3H]DA release from this brain region is attenuated by the antagonist DHβE, which has 100-fold higher affinity for α4β2 than α3β4, and partially inhibited by the α6-selective antagonist conotoxin MII. This is consistent with literature values and suggests that a subset of nAChR receptors in this brain region contain the α6 subunit (Grady et al, 2002). Bivalent anionic compounds, such as N,N-alkane-diylbis-3-picolinium, and N,N-decane-1,10-diyl-bis-3-picolinium diiodide (bPiDI), which inhibit α4β2 and α6-containing nAChR have also been demonstrated to block DA release from a striatal brain slice preparation (Dwoskin et al, 2008; Wooters et al, 2011). The poor potency of inhibition of DA release by AT-1001 probably reflects inhibition of the α4β2 subtype at this high concentration. These results indicate that the inhibition of nicotine self-administration by AT-1001 is not a consequence of a decrease in striatal (NAc) DA levels by acting directly at this brain region, as is the case with the α6β2-selective antagonist, bPiDI (Wooters et al, 2011), and suggest that the role of the α3β4 nAChR receptor subtype in decreasing nicotine self-administration still needs further investigation. Although the α3β4 nAChR has a relatively low affinity for nicotine, we believe that serum nicotine levels are sufficiently high to activate this receptor in the self-administration assay. We have previously demonstrated that two intravenous infusions of nicotine at the dose used in this study yield blood levels of 1.85 μM that remain high over a 30-min period (Cao et al, 2007). Thus, the potent effect of AT-1001 on nicotine self-administration is a good starting point for the demonstration of the role of this important nAChR subtype, as a target for smoking cessation medications.
The mechanism by which α3β4 nAChR antagonism reduces nicotine self-administration is unclear. The fact that α3β4 nAChR antagonism reduces nicotine self-administration strongly suggests that, in some way, α3β4 receptor activation, probably originating in the MHb and/or IPN, promotes self-administration. Nevertheless, studies by Fowler et al (2011) clearly demonstrate that nAChRs containing α5 subunits, in these same brain regions, seem to limit self-administration. Potentially, the α5 subunits are on α4β2 nAChRs and therefore α3β4 and α4α5β2 may work against each other with respect to nicotine's rewarding properties. At this time, the relative levels of α3β4 and α4β2 nAChR in the MHb and IPN are somewhat difficult to determine and still in question (Gotti et al, 2010; Perry et al, 2002). Furthermore, the pathway by which the IPN projects to the VTA or NAc is not well understood. Resolving these issues may require new tools for identifying and labeling receptors. The family of compounds represented by AT-1001 may provide some of these tools. In addition to its potential as a drug abuse medication, AT-1001 and other compounds in this family will certainly have significant research benefits. We have recently synthesized an 125I-analog of AT-1001. Preliminary studies suggest that this radiolabeled ligand will be useful for radioligand binding and in vitro autoradiography to further characterize the localization of this receptor subtype in brain.
In conclusion, we have characterized the first high affinity and selective α3β4 nAChR antagonist, AT-1001. This compound has nanomolar affinity at the α3β4 nAChR, and nearly 100-fold selectivity vs α4β2 and α7 nAChR. AT-1001 binds in a non-competitive manner to the α3β4 nAChR, but unlike channel blockers such as mecamylamine, it competes for the [3H]epibatidine binding site, facilitating structure-activity studies. In addition to its selectivity, AT-1001 is much more potent than either 18-MC or the classical nicotinic antagonist mecamylamine in inhibiting receptor activation in vitro. Finally, when administered systemically, AT-1001 dose-dependently blocks nicotine self-administration in rats without affecting food responding. This compound shows great potential as a novel pharmacotherapy for smoking cessation and validates the α3β4 nAChR as a target for tobacco dependence medications.
Acknowledgments
Determination of binding affinity of AT-1001 at a panel of various receptors and ion channels was generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. This research was supported by grants from the National Institute on Drug Abuse DA020811 (LT) and the UC Tobacco Related Diseases Research Program 18XT-0085 (FL).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Preliminary reports of these data were presented at the annual meetings of the Society for Neuroscience.
Supplementary Material
References
- Arias HR, Rosenberg A, Feuerbach D, Targowska-Duda KM, Maciejewski R, Jozwiak K, et al. Interaction of 18-methoxycoronaridine with nicotinic acetylcholine receptors in different conformational states. Biochim Biophys Acta. 2010;1798:1153–1163. doi: 10.1016/j.bbamem.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Wooters TE, Sumithran SP, Siripurapu KB, Joyce BM, Lockman PR, et al. N,N'-alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity. J Pharmacol Exp Ther. 2008;326:563–576. doi: 10.1124/jpet.108.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Cheng EY, Weiland GA, Oswald RE. Specific activation of the alpha 7 nicotinic acetylcholine receptor by a quaternary analog of cocaine. Mol Pharmacol. 2001;60:71–79. doi: 10.1124/mol.60.1.71. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000a;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann NY Acad Sci. 2000b;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur J Pharmacol. 2006;537 (1–3:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;599:91–95. doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, et al. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, Pie B, Deneris ES, Cooper E. The developmental increase in ACh current densities on rat sympathetic neurons correlates with changes in nicotinic ACh receptor alpha-subunit gene expression and occurs independent of innervation. J Neurosci. 1994;14:2357–2364. doi: 10.1523/JNEUROSCI.14-04-02357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Glick SD. 18-Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett. 2009;458:57–59. doi: 10.1016/j.neulet.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995a;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995b;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, et al. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates alpha6- and beta3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend. 1998;51:165–172. doi: 10.1016/s0376-8716(98)00074-x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurons from rat intracardiac ganglia. J Neurosci. 1997;17:586–596. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, et al. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–1179. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, et al. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Saccone NL, Swan GE, Madden PA, Goate AM, Rice JP, et al. Systematic biological prioritization after a genome-wide association study: an application to nicotine dependence. Bioinformatics. 2008;24:1805–1811. doi: 10.1093/bioinformatics/btn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12:467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang Z, et al. bPiDI: a novel selective alpha6beta2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol. 2011;163:346–357. doi: 10.1111/j.1476-5381.2011.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. Novel alpha3beta4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. J Med Chem. 2010;53:8187–8191. doi: 10.1021/jm1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.