Abstract
OBJECTIVES
Neurological injury after global brain ischaemia (i.e. sudden death) remains problematic, despite improving cardiac survival. Unfortunately, sudden death models introduce unwanted variables for studying the brain because of multiple organ injury. To circumvent this, a new minimally invasive large animal model of isolated global brain ischaemia, together with baseline perfusion studies is described.
METHODS
The model employs neck and small (3–4 inches) supra-sternal incisions to block inflow from carotid and vertebral arteries for 30 min of normothermic ischaemia. Neurological changes after 24 h in six pigs was compared with six Sham pigs assessing neurological deficit score (NDS, 0 = normal, 500 = brain death), brain oedema and cerebral infarction by 2,3,5-triphenyltetrazolium chloride (TTC) stain. Six other pigs had baseline perfusion characteristics in this new model evaluated at carotid flows of 750, 550 and 450 cc/min, with cerebral perfusion pressure, cerebral oximeter saturation [IN Vivo Optical Spectroscopy (INVOS)] and transcranial O2 uptake measurements.
RESULTS
The model never altered cardiac or pulmonary function, and six Sham pigs had normal (NDS = 0) neurological recovery without brain injury. Conversely, 24 h analysis showed that 30 min of global normothermic brain ischaemia caused multiple post-reperfusion seizures (P < 0.001 versus Sham), raised NDS (231 ± 16; P < 0.001 versus Sham) in four of six survivors and caused marked post-brain oedema (P < 0.001 versus Sham) and extensive cerebral infarctions (TTC stain; P < 0.001 versus Sham). Baseline perfusion showed 750 cc/min flow rate produced normal INVOS levels and O2 consumption at mean 90–100 mmHg carotid pressure. Carotid pressure and INVOS fell at mid- and low-flow rates. Although INVOS did not change, 450 cc/min flow lowered global O2 consumption, which further decreased after transient ischaemia (30 s) and 5 min of reperfusion.
CONCLUSIONS
This new isolated global brain model consistently caused anatomic, biochemical and functional neurological damage in pigs after 30 min of ischaemia. Flows of 750 cc/min maintained normal mean systemic arterial (90–100 mmHg) pressure, INVOS levels and O2 consumption. Cerebral pressure and INVOS fell in mid- and low-flow studies. A disparity existed between INVOS oxygen saturation and global O2 consumption at lower flow rates of 450 cc/min following transient ischaemia, indicating that surface oxygen saturation measurement does not reflect global brain O2 consumption.
Keywords: Brain death, Brain ischaemia, Reperfusion injury, Sudden death, New model, Global brain ischaemic model
INTRODUCTION
Sudden death from cardiac arrest (CA) [1, 2] occurs in ∼450 000 US citizens/year and reflects an unresolved health care problem. Survival to hospital discharge following a ‘witnessed arrest’ is only 5–15%, and up to 70% of survivors suffer neurological damage that impacts on long-term survival [1, 2]. Although survival improves to 30% by employing emergency cardiopulmonary bypass (CPB) [1, 2], devastating neurological damage persists. Furthermore, an ‘unwitnessed arrest’ [delayed cardiopulmonary resuscitation (CPR)] is almost universally fatal, with severe brain damage in the rare survivor [2]. Consequently, brain injury remains the basic investigative challenge in all CA patients.
Ischaemic/reperfusion injury is the mechanism of brain damage. Ischaemia causes progressive changes in cellular architecture and physiology, which may ultimately cause cell destruction [3, 4], and simultaneously introduces a susceptibility to damage that may either be accelerated by reperfusion with normal blood or reversed by controlling the conditions and composition of the reperfusate (controlled reperfusion), as done in other organs [2, 4]. Currently controlled reperfusate's capacity to reverse brain neurological damage is uncertain. However, complete cardiac and neurological functional recovery was recently reported following employment of CPB with a modified prime to deliver controlled reperfusion after 15 min of CA (sudden death) without CPR [5]. Nevertheless, this investigation did not reflect clinical practice, because at least 10–15 more minutes of ischaemia (total 30 min) is required to insert peripheral cannulation for emergency bypass after the CA [1, 2].
Whole body sudden death models have limitations in evaluating controlled brain reperfusion that include: (i) release of inflammatory mediators from remote ischaemic regions (heart, lung, liver etc.) that may affect the brain [2, 4, 6, 7], (ii) generalized inflammation secondary to CPB and (iii) inability to completely control the CBP prime after the patient's blood admixture [2, 4, 8, 9]. Conversely, an isolated ‘brain ischaemia model’ circumvents these problems, and simultaneously parallels prior heart and lung controlled reperfusion studies [2, 4].
The pig model was selected because it is more similar to humans [10, 11], offsets cannulation problems in smaller species and allows the same perfusion systems used for controlled reperfusion in other organs [2, 4, 11]. This study will first evaluate the ability to develop a new isolated model of global brain ischaemia (simulating sudden death) employing a minimally surgically invasive method. Following its described success, the second component defines its baseline perfusion characteristics and evaluates its underlying carotid and vertebral perfusion contributions.
MATERIALS AND METHODS
The study was approved by the Institutional Animal Research Committee of the University of California at Los Angeles. All animals received care in compliance with the 1996 NRC Guide for the Care and Use of Laboratory Animals.
Twenty-five Yorkshire-Duroc pigs (47.2 ± 1.4 kg) were premedicated with 15 mg/kg ketamine and 0.5 mg/kg diazepam intramuscularly, and received neuromuscular blockade with pancuronium (0.2 mg/kg) during surgical dissection. After endotracheal intubation, anaesthesia was achieved with inhaled isoflurane (1.0–3.0%) and 12–14 breaths/min were provided by a volume-controlled ventilator (Servo 900C, Siemens-Elema, Sweden) at 10–15 ml/kg tidal volume, 4 mmHg positive end-expiratory pressure, with setting adjustment to normalize pH (7.37–7.45) and oxygen (PaO2 = 80–200 mmHg) and carbon dioxide tension (PaCO2 = 35–45 mmHg). Anaesthesia was supplemented with fentanyl (1 mg/kg) every hour as needed.
Electrocardiography monitored heart rate and rhythm, and left internal thoracic artery intubation monitored arterial pressure and allowed blood gas samples. Pulmonary artery pressure and cardiac output were determined with a balloon-tipped pulmonary artery catheter (Model 132F5, Baxter Healthcare, Irvine, CA, USA) via the right external jugular vein. Sterile incisions exposed vessels used for cerebral ischaemia (see below), Cefazolin (Ancef 30 mg/kg, GlaxoSmithKline, Philadelphia, PA, USA) was given perioperativly every 8 h, and saline infused intravenously at 5–10 ml/kg/h. Body temperature was kept at 37°C using a heating pad and lamp as needed.
Euthanasia was accomplished using pentobarbital and potassium chloride at the completion of each study.
Isolated global brain ischaemia
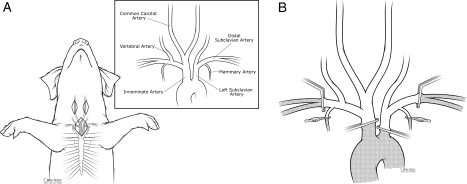
Figure 1 shows the new pig model of isolated global brain ischaemia employing neck incisions (carotid arteries) and a small (3–4 inches) supra-sternal incision to occlude the innominate, proximal left subclavian and both the internal mammary and distal subclavian arteries (Fig. 1A). The upper part of the manubrium was ‘partially’ split as needed to allow better access to these major vessels.
Figure 1:
(A) Surgical incisions used in this model and the underling arterial anatomy of the pig. (B) Method of vascular occlusion to create complete (global) brain ischaemia.
Development of global cerebral ischaemic model
Experimental protocol
The first step in the new model was to determine the injury extent in eight pigs undergoing 30 min of complete (global) 37°C brain ischaemia, in comparison with six control (Sham) pigs undergoing the dissection without brain ischaemia.
Method of inflow occlusion
Carotid artery inflow was occluded by clamping the pig's innominate artery containing left and right carotid and right subclavian artery branches. Vertebral artery inflow was prevented by clamping the left subclavian just distal to the aortic arch and both internal mammary arteries and both distal subclavian arteries just beyond their mammary arteries (Fig. 1B).
Sudden cerebral ischaemia caused a catecholamine surge producing severe hypertension and tachycardia requiring temporary nitroprusside and esmolol infusions to maintain normal haemodynamic stability, titrating them as needed. With ischaemia, each pig also showed a markedly reduced IN Vivo Optical Spectroscopy (INVOS) cerebral oximeter level, dilated non-reactive pupils and carotid pressure <12 mmHg. Reperfusion was accomplished by removing all vascular clamps.
Postoperative care
One hour after reperfusion, the mediastinum was drained by a 20 French drainage tube, a single IV cannula was retained and wounds closed. Extubation was done after spontaneous breathing occurred with normal PaO2 and PaCO2 levels. Pigs were monitored for 24 h unless they died prematurely. The incidence and duration of postoperative seizures were recorded and IV Midazolam (0.1 mg/kg) delivered if prolonged (>few minutes).
Haemodynamic data
Cardiac output was determined before and during ischaemia and following reperfusion by triplicate injections of 4°C cold saline solution (thermodilution technique) and cardiac index, and left ventricular stroke work index calculated using standard equations.
Biochemical data
Arterial and internal jugular blood samples at baseline and during reperfusion were immediately centrifuged (3000 rpm) for 10 min and stored at −20°C until biochemical analysis to evaluate oxidant mediated-lipid peroxidation. Conjugated diene levels were determined spectrophotometrically after chloroform–methanol 2:1 (vol/vol) extraction, and expressed as absorbance at a wave length of 240 nm/0.5 ml plasma.
Neurological deficit score
Neurological behaviour was independently assessed by two laboratory team members at 4 and 24 h after reperfusion using a species-specific behaviour scale [1, 5, 8, 9]. Neurological deficit score (NDS) evaluates five general neurological examination components with a maximal score of 100 in each category: total score of 0 = normal and 500 indicates brain death. The five components are as follows: (i) central nerve function (0–100 points): pupil size (0–10), eye position (0–10), light, lid and corneal reflex (each 0–10), ciliospinal, occulocephalic reflex (each 0–10), auditory, gag reflex (each 0–10), carinal reflex (0–10), (ii) respiration (0–100 points): normal (0), hyperventilation (25), abnormal (50), absent (100), (iii) motor sensory function (0–100 points): stretch reflex (0–25), motor response to pain (0–25), positioning (0–25), muscle tonus (0–25), (iv) level of consciousness (0–100 points): normal (0), cloudy (30), delirium (45), stupor (60), coma (100) and (v) behaviour (0–100 points): drinking, chewing, sitting, standing (each 0–15), walking (0–40). Numbers in parentheses indicate scores for each parameter. A mean value was recorded if different scores were reported by each team member.
Tissue oedema and analysis of infarction
Following euthanasia, the brain was excised, weighted and placed in the freezer for 10 min. Coronal samples 3–5 mm thick were sliced from the frontal lobe, thalamus and hippocampus and sagittal samples sliced from the posterior brain stem and cerebellum. Tissue oedema (wet to dry weight) was calculated from the frontal cortex, hippocampus and cerebellum by weighing a small piece of tissue immediately and then reweighing it following 24 h in an oven at 80°C for 24 h. Tissue oedema equals the percentage of tissue water = (Wet weight−Dry weight/wet weight) × 100. Brain infarction was determined by placing the remaining cut brain samples in 1% TTC (2,3,5-triphenyltetrazolium chloride) solution at 37°C for 20–30 min.
Baseline perfusion and flow studies
The ‘second step’ was the analysis in 11 pigs of normal baseline perfusion characteristics and inflow contributions from carotid and vertebral vessels. This sequence in normal brains was developed because pilot studies, to be described subsequently [12], defined severe limitations to the use of controlled reperfusion at a flow of 350 ml/min and perfusion pressure ∼30 mmHg, a reflow method patterned on the prior success of low pressure reperfusion in heart and lung studies [2, 4, 13, 14].
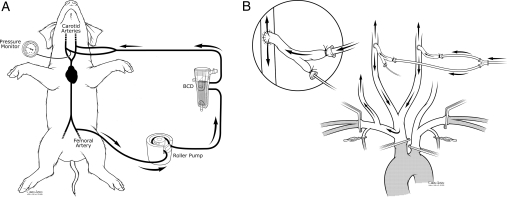
These studies employed the same new isolated brain model of inflow occlusion, but added perfusion into both carotid arteries via arterial grafts that were harvested from donor pigs. Inflow carotid pressure and blood sampling was via a catheter placed within a branch of carotid graft. After heparinization (12 000 units), a Quest MPS system (Quest Medical, Allen, TX, USA) drained whole blood from the right femoral artery via a 14 Fr femoral cannula and delivered flow into both carotid arteries via 10 Fr cannulas. (Fig. 2A and B).
Figure 2:
(A) Cerebral perfusion system and (B) model of isolate brain perfusion.
The following variables were measured.
Cerebral perfusion pressure (CPP): carotid arterial and internal jugular venous pressures were recorded continuously and CPP was defined as the difference between arterial and venous mean pressure at fixed perfusion flow rate.
Cerebral oxygen saturation: an INVOS cerebral oximeter system monitored oxygen saturation using near infrared light to measure oxygen saturation in the cerebral cortex under the sensor [15, 16].
- Cerebral oxygen uptake: transcranial O2 uptake was determined by the difference between carotid arterial and jugular venous oxygen content after determining haemoglobin concentration, percent saturation and dissolved oxygen by the following standard formulas:
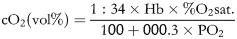
where cO2 is the oxygen content, Hb is the haemoglobin, sat. is the saturation, CMRO2, is the transcranial O2 consumption, cAO2 is the arterial oxygen content, cSjO2 is the internal jugular venous blood oxygen content and CBF is the cerebral blood flow.
Flow studies
Ischaemia was avoided by clamping all cerebral inflow and thereby simultaneously delivering a flow rate to maintain a normal mean systemic pressure of 90–100 mmHg. A flow of 750 cc/min gave a normal pressure in the first two pigs and so was initially used in every experiment. Subsequently, six pigs were evaluated to examine flow variation and the response to transient 30 s ischaemia at flow rates of 750 cc/min (high flow), 550 cc/min (mid flow) and finally 450 cc/min (low flow). The low 450 cc/min flow rate was selected because of limitations of 350 cc/min in previous pilot studies [12].
Transient 30 s ischaemia was employed to determine the brain's normal response at each flow rate. Five minutes after perfusion at each flow, pump flow was discontinued for 30 s and resumed at that flow rate while cerebral perfusion pressure, oxygen saturation and transcranial cerebral oxygen uptake were monitored. The next stage (flow rate) was then studied 5 min later. During the initial 5 min following transient ischaemia, baseline measurements of cerebral perfusion pressure and cerebral oxygen saturation were repeated at 10, 20, 30 s, and 1, 2, 5 min intervals and transcranial O2 uptake was determined at baseline and 30 s and 5 min after ischaemia from carotid artery and high jugular vein sample sites.
The completeness of cerebral isolation was verified at the end of each experiment by stopping pump flow while occluding the inflow vessels and demonstrating the catecholamine surge, decrease in cerebral oxygen saturation to 15% (its minimum expression), carotid artery pressure <12 mmHg and bilateral pupil dilation. The position of the internal jugular venous catheter at the internal jugular vein bulb was confirmed post-mortem.
Perfusion distribution studies
‘The third step’ determined vascular flow to individual vessels (Fig. 2B) in five pigs. Flow during isolated brain perfusion was fixed at a pressure of 90–100 mmHg. Cerebral pressure and INVOS was then measured after 15–30 s occlusion of the following vessels; (i) proximal right and left carotid arteries separately and together, (ii) distal right and left carotid arteries separately and together and (iii) the right vertebral artery. Proximal and distal carotid artery occlusion refers to closure occurring before or after the site of carotid inflow vascular anastomosis. Baseline flow was resumed for 5 min prior to each transient occlusion. Perfusion flow was adjusted to keep the pressure 90–100 mmHg if the mean pressure changed by more than 30 mmHg with occlusion.
Statistical analysis
Data were expressed as mean ± SEM. Two-way analysis of variance with Fisher's least significant difference procedure for post hoc repeated measurements was used to analyse intra- and intergroup differences. Perioperative characteristics of groups were analysed by Student's t-test for continuous data and χ2 or Fisher's exact test for categorical data. P < 0.05 were considered statistically significant.
RESULTS
Baseline haemodynamic and blood gas data were similar in all pigs. No Sham, ischaemic or perfusion pig required a blood transfusion, and the estimated blood loss was <100 ml in all pigs.
Global ischaemia (30 min) studies
All control (Sham) pigs survived and recovered normal neurological score (NDS score 0) at 4 and 24 h, with no evidence of infarction on TTC stain. In ischaemic pigs, post-reperfusion cardiac function was unchanged from baseline (Table 1), but each developed post-reperfusion tachycardia upon emerging from anaesthesia.
Table 1:
Cardiac and pulmonary function
| Heart rate | Arterial systolic pressure | Cardiac output | Arterial blood gases |
|||
|---|---|---|---|---|---|---|
| O2 | CO2 | pH | ||||
| Baseline | 136 ± 17 | 113 ± 8 | 6.0 ± 1.0 | 400 ± 128 | 38 ± 4 | 7.49 ± 0.07 |
| Ischaemia | 181 ± 37 | 132 ± 12 | 5.8 ± 0.6 | 385 ± 58 | 44 ± 4 | 7.40 ± 0.04 |
| Reperfusion | 151 ± 27 | 127 ± 17 | 5.6 ± 0.4 | 402 ± 100 | 37 ± 3 | 7.45 ± 0.07 |
Mean ± SE, no statistical significance (P > 0.05) between baseline, ischaemia and reperfusion. Ischaemia = during 30 min global brain ischaemia, reperfusion = 1 h post-reperfusion.
The sudden interruption of cerebral blood flow caused a catecholamine surge producing severe hypertension (blood pressure systolic rising from ∼120 to ∼300 mmHg) and tachycardia (heart rate >200) within 60–90 s requiring Nitroprusside and esmolol infusions to restore normal haemodynamic stability. These infusions could usually be reduced by 15–20 min, and discontinued by the end of 30 min of ischaemia. With global ischaemia, the INVOS cerebral oximeter system fell to the machine's lowest level of 15%. Pupils dilated and became non-reactive, and carotid artery pressure fell to <12 mmHg, while simultaneous jugular central venous pressure (CVP) was 6–8 mmHg to imply absent collateral blood flow. All parameters (low INVOS, sudden and severe catecholamine surge, dilated pupils and carotid pressure <12 mmHg) had to occur to include an animal in the study.
Six of the eight pigs undergoing 30 min of global brain ischaemia met the complete brain ischaemia criteria. Two other pigs were not included because their catecholamine surge was less severe, INVOS slowly fell to 20–25%, and carotid pressure was higher (15–20 mmHg). ‘On subsequent surgical dissection, these two pigs were found to have a very proximal costo-cervical trunk off the left subclavian artery, with the vascular clamp applied distal to this vessel’.
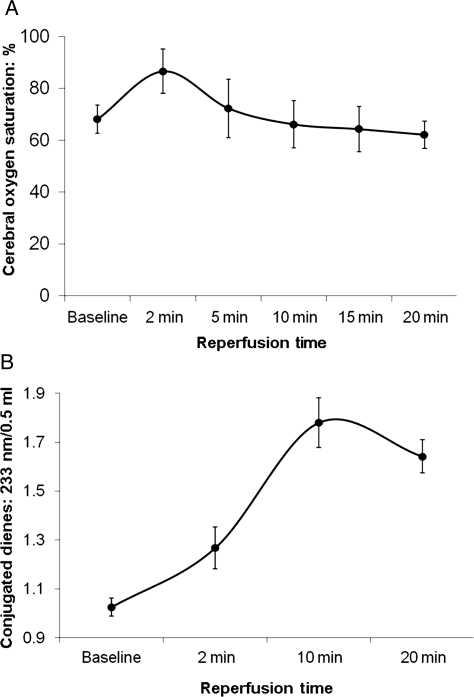
Cerebral reperfusion immediately raised internal jugular vein oxygen saturations at 2, 5, 10, 15 and 20 min (86 ± 8, 85 ± 7, 91 ± 5, 89 ± 7, and 91 ± 5: baseline 65 ± 5%) and brain oximeter readings (INVOS) >85–90% (<10–15% O2 extraction) in all six ischaemic pigs (Fig. 3A). Conjugated Dienes (measuring oxygen radical damage) rose substantially (Fig. 3B) during reperfusion. Post-anaesthesia neurological scores at 24 h in the four surviving pigs was 241 ± 40, indicating severe damage (Sham controls score = 0). Seizures occurred in each ischaemic pig, with 33% (two of six pigs) developing fixed dilated pupils, marked hypertension, bradycardia and absent respiration and succumbing ‘at 13 and 16 hours after reperfusion’.
Figure 3:
(A) INVOS (cerebral oximeter) surface oxygen saturation levels and (B) conjugated diene levels at baseline and in the six ischaemic pigs during the first 20 min of reperfusion. Note: the increased release of conjugated dienes (3B) during reperfusion indicates substantial oxygen free radical formation.
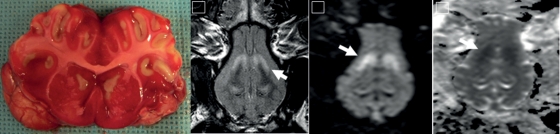
Post-mortem macroscopic swelling was observed and accompanied by oedema in the cortex, cerebellum and hippocampus (Table 2). TTC staining demonstrated cerebral infarction in the basal ganglia and distal cortical regions in all ischaemic pigs (Fig. 4).
Table 2:
Neuro-deficit score and brain oedema (water percentage) post-reperfusion
| Neuro-deficit score |
Tissue oedema |
||||
|---|---|---|---|---|---|
| 4 h | 24 h | Cortex (%) | Hippocampus (%) | Cerebellum (%) | |
| Sham | 0 | 0 | 81.5 ± 0.2 | 77.3 ± 0.6 | 81.1 ± 0.3 |
| Ischaemic | 243 ± 45 | 241 ± 40 | 84.3 ± 0.6 | 81.8 ± 0.9 | 84.6 ± 0.5 |
P < 0.001 ischaemic versus Sham for all values, ischaemic = pigs undergoing 30 min of global brain ischaemia.
Figure 4:
On the left, a typical example of the TTC stain in a pig undergoing 30 min of ischaemia followed by uncontrolled (normal blood) reperfusion. Note the marked oedema collapsing the lateral ventricles and median fissure, as well as infarctions in the basal ganglia and throughout the cortex. On the right, MRI following sudden death produced by 25 min of ventricular fibrillation: (i) T2 sequence, (ii) DWI sequence, (iii) ADC sequence demonstrating basal infarction (arrows) similar to what is seen in the TTC stain.
Flow and perfusion studies
The same model was used for these baseline studies, and we documented its capacity to induce complete global ischaemia at the end of each study using the aforementioned criteria.
Flow studies
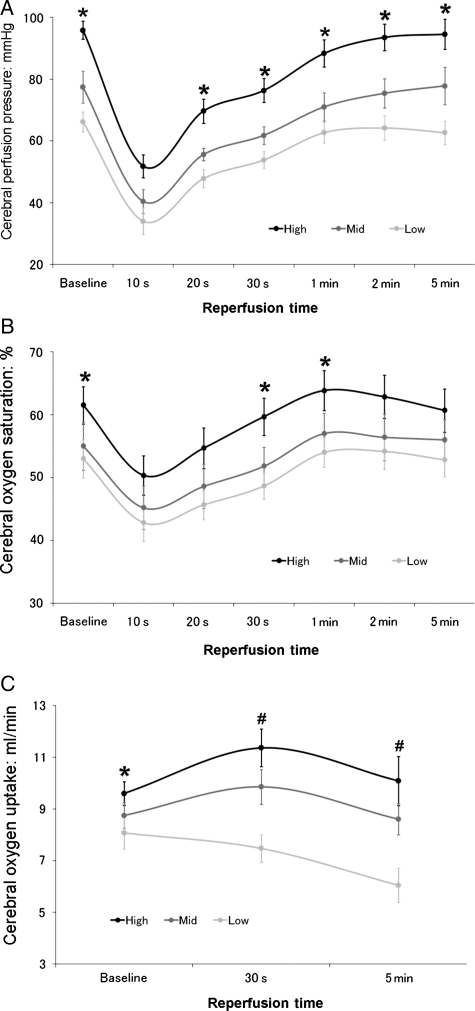
A flow rate of 750 cc/min always reproduced the normal mean systemic arterial pressure of 90–100 mmHg; INVOS levels and global O2 consumption were unchanged, suggesting this high flow insured adequate brain perfusion. Cerebral perfusion pressure and INVOS fell after transient (30 s) ischaemia at flows of 750, 550 and 450 ml/min, but returned to respective baseline control levels after 5 min of reperfusion (Fig. 5). In the low flow group (450 cc/min), baseline pressure and cerebral oxygen saturations (INVOS) were slightly reduced. Global O2 consumption returned to pre transient ischaemic levels in the 750 and 550 cc/min studies. Conversely, the reduced global O2 consumption value at low flow (450 cc/min) became further decreased following 5 min of reperfusion (Fig. 5).
Figure 5:
Serial assessment of cerebral perfusion pressure (A), oxygen saturation (B) and oxygen uptake (C) at flows of 750, 550 or 450 cc/min. *P < 0.05 high versus low flow, #P < 0.05 high and mid versus low flow.
Perfusion distribution studies
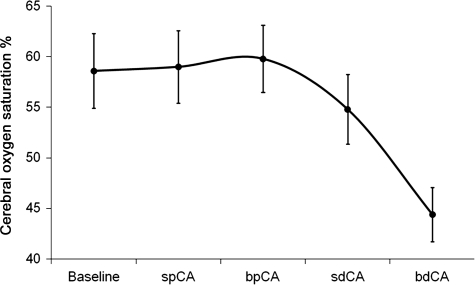
During the fixed flow state of 750 cc/min clamping either the right (or left) proximal carotid artery before the graft anastomosis changed carotid (perfusion) blood pressure by only 7 mmHg from 93 ± 2 to 86 ± 2 mmHg. Clamping both proximal carotid arteries raised perfusion pressure by 14 ± 3 mmHg; INVOS cerebral oximeter readings did not change in either instance (Fig. 6). In contrast, occluding either the distal right or left carotid artery beyond the anastomosis raised perfusion pressure by 27 ± 3 mmHg and slightly lowered INVOS oxygen saturations (Fig. 6). Clamping both distal carotid arteries raised pressure so dramatically that flow reduction from 750 to 232 ± 27 cc/min was required to maintain perfusion pressure at 90–100 mmHg, and oximeter readings (INVOS) fell markedly (Fig. 6) during the 15–30 s of this reduced flow. Clamping the right vertebral artery alone (only done in these acute studies to help determine flow distribution) resulted in flow changes statistically similar to occluding both distal carotid arteries. The vertebral artery was never occluded in subsequent survival studies [2, 12] because of potential problems outlined in the discussion.
Figure 6:
Cerebral oximeter (oxygen saturation) readings (INVOS) with clamping of different cerebral vessels during isolate brain perfusion. CA: carotid artery; sp: single proximal; bp: bilateral proximal; sd: single distal; bd: bilateral distal; proximal and distal (carotid artery) refers to the location of the occluding vascular clamp on the native carotid artery relative to perfusion via the anastomosed vessel.
DISCUSSION
This new minimally invasive model avoids systemic cardiac or pulmonary damage, and causes no neurological changes in Sham pigs. Conversely, its creation of global cerebral ischaemia consistently induces severe anatomic and functional evidence of brain damage following 30 min occlusion of all brain inflow vessels. Markers of cerebral damage include lack of oxygen uptake during reperfusion implying mitochondrial damage [2, 4, 17], pronounced oxygen free radical production (increased conjugated dienes), multiple brain infarcts (TTC staining), marked global oedema, multiple seizures and neurological scores demonstrating the same severe dysfunction as previously observed in sudden death models following 15-25 min of ventricular fibrillation [1, 5]. Indeed, the cerebral injury was so severe, that two of six pigs (33%) died from presumed brain herniation prior to the 24 h NDS measurement. The model's importance compared with sudden death models includes avoidance of the complicating variables of inflammation with CPB, and washout of substances from remote ischaemic organs (heart, lung) that could adversely influence neurological results [1, 2, 5–7].
Determination of global brain ischaemia
The employed monitors of severe global ischaemia perfectly matched the prior manifestations seen in sudden death after CA [1, 5], and assured ischaemia despite not directly measuring cerebral blood flow with microspheres. These manifestations included the abrupt fall of the INVOS cerebral oximeter to its lowest level (15%) [1], lowering of carotid pressure <12 mmHg associated with simultaneous CVP of 6–8 mmHg to imply little if any flow, bilateral pupil dilatation and severe hypertension following a marked catecholamine surge that immediately required nitroprusside and esmolol to restore normal haemodynamics. The need for these drugs was transient, and they could generally be weaned after ∼15–20 min. Conversely, a partially ischaemic brain should continue to stimulate adrenaline, necessitating continued use of these agents.
The functional equivalent was the severe neurological dysfunction demonstrated by the NDS, and development of marked global oedema. The anatomic characteristic was evidence of extensive infarction by TTC staining that paralleled magnetic resonance imaging (MRI) findings following sudden death (Fig. 4) [1]. The severity of reperfusion damage resulted in two pigs developing probable herniation prior to 24 h. It is possible that a small amount of collateral cerebral blood flow exists during ischaemia, but it is insignificant, as a substantial brain injury always occurred. We do not believe this should preclude using this model to study brain ischaemia anymore than non-coronary collateral flow, which may supply a small amount of blood to the heart after cross clamping the aorta, invalidates using a model of aortic clamping to study global heart ischaemia [2, 4].
Although the small incisions made the surgical dissection quite difficult, there were no need for transfusions, and it avoids opening the sternum, pleura or pericardium, facilitating post-operative recovery and reducing pain. Using INVOS, carotid pressure and a catecholamine surge to document ischaemia appears valid, as it correctly identified the two pigs that had a proximal costo-cervical artery with persistent vertebral blood flow and incomplete ischaemia. In contrast, when these three modalities predicted global brain ischaemia, there were multiple infarcts, marked cerebral oedema and a high NDS demonstrating significant neurological dysfunction.
Anatomic considerations
Effective use of this model via minimally invasive approaches requires full understanding of the pigs underlying vascular anatomy. The pig's aortic arch has two branches. The first is the innominate or brachiocephalic artery, which bifurcates at the second rib to supply both carotid arteries and the right subclavian vessel, and perfuses the neck, head, shoulders and forelimb (Fig. 1) [18–21]. Occlusion of the innominate within the superior mediastinum interrupts flow to these areas, provided the distal subclavian and internal mammary vessels are also occluded to limit collateral flow (Fig. 1B). The second aortic arch vessel is the left subclavian artery which also originates at the second rib, and its branches supply the head and neck, as well as the thorax and shoulder musculature. Interruption of brain flow requires proximal occlusion to prevent flow through its costo-cervical trunk to the vertebral artery, as well as distally, to limit collateral flow. As with the right side, the large internal mammary artery must also be occluded to prevent retrograde vertebral flow via its external epigastric vessel connections.
Several vessels arise from the subclavian arteries that are important in utilizing this model [18–21]. Starting proximally they include the (i) costo-cervical trunk, (ii) thyrocervical, (iii) internal mammary and (iv) external thoracic arteries. The costo-cervical trunk is the first branch of the subclavian vessel as it arises near the level of the first rib. Its first and most anterior branch is the vertebral, which proceeds cranial to the brain, and its two other branches (deep cervical and dorsal) are small and unimportant. Interrupting vascular flow to the vertebral artery by occluding the subclavian artery proximal and distal to this costo-cervical branch was deemed preferable to directly occluding the verterbral vessels. First, it avoids injury to the phrenic nerve that runs adjacent to the vertebral artery during dissection via this small incision, as such damage would impair post-ischaemic ventilation following extubation. Second, interruption of flow via the costo-cervical vessel will simultaneously interrupt flow via its smaller deep cervical and dorsal branches that may provide collateral flow to the brain. Maintaining an open costo-cervical trunk also provides an advantage that became apparent in the flow studies, as retrograde flow provides right vertebral perfusion via the vascular grafts implanted into the carotid arteries to give posterior brain inflow (Fig. 2B).
Proper costo-cervical trunk identification is important, especially since it may originate more proximally than usual from the left subclavian artery. Placement of the proximal left subclavian occlusion distal to this vessel will permit perfusion via the left vertebral vessel, and this happened in the two excluded pigs that had incomplete cerebral ischaemia, characterized by a reduced catecholamine surge, and higher carotid artery pressure and INVOS readings.
The thyrocervical or inferior cervical trunk normally arises from the dorsal surface at the first rib, opposite the internal mammary, which originates from the ventral inferior surface and is quite large in the pig [18–21]. The thyrocervical origin may be variable, and a more proximal origin may be mistaken for the costo-cervical trunk, which occurred in these studies (two pigs) before more experience was gained. The thyrocervical trunk does not have to be clamped directly as long as the distal subclavian clamp is placed proximal to its origin. However, if the subclavian clamp is placed distal to its origin, then this vessel should be clamped to limit collateral flow from reaching the vertebral artery during ischaemia, and to prevent ‘stealing’ cerebral flow when perfusion is directed into the carotid arteries during flow studies. The final subclavian vessel is the small external thoracic (lateral thoracic) vessel which usually arises distally to the vascular clamp.
Flow studies
We next assessed the relationship between cerebral flow, flow reserve and oxygen metabolism using cerebral reactive hyperaemic responses based on the report of Gourley and Heistad [22]. They found that peak reactive hyperaemia (vasodilatation) in the brain was produced by brief periods of global ischaemia <30 s, and cerebral blood flow and venous oxygen saturation returned to control levels by 5 min. We found something comparable by showing that the cerebral perfusion pressure (which determines vasodilatation in fixed flow studies) and oxygen saturation returned to baseline within 5 min after ischaemia in all groups (Fig. 5). However, in the low flow group, the transcranial O2 uptake was dissociated from surface oxygen saturation (INVOS) and failed to recover, without the expected repayment of oxygen ‘debt’ [1, 17]. This suggests there was not enough flow reserve at lower flows to perfuse all areas of the brain, and that some irreversible damage would occur if the pressure or flow dropped any lower, and maybe even at this flow. This implies that a carotid perfusion pressure of ∼60 mmHg (pressure at flow 450 cc/min) is the threshold pressure for critical closure of some components of the cerebral vasculature under normothermia, and is why we did not test flows below 450 cc/min.
Several baseline values and responses are defined by these studies. First, a flow of 750 ml/min is required to produce a normal mean systemic arterial pressure of 90–100 mmHg. Second, the disparity between INVOS and global O2 uptake between high and low flow groups indicates (i) that higher flows are needed in this model to insure perfusion to all areas of the brain, especially after ischaemia, and (ii) INVOS measurements have limitations as normal levels did not always correlate with global O2 consumption.
There are probably several reasons high flow is needed to produce a normal mean arterial pressure in this model. Although perfusion is via both carotid arteries, this flow perfuses the brain as well as the head and part of the neck, making it impossible to measure the exact brain flow at any given time. To help minimize this limitation, we considered occluding the external carotid arteries, and all branches arising from the common carotid arteries. However, this would require extensive neck dissection, especially to expose the external carotids, as they lay quite deep under the mandible [11, 18–21]. Such a dissection would restrict the capacity to measure post-ischaemic neurological function by potentially lengthening and complicating surgical recovery.
This flow limitation also means we cannot definitively calculate cerebral oxygen consumption. However, we believe our measurement of venous oxygen content reflects primarily brain metabolism as (i) samples were taken high in the internal jugular vein, so this blood is likely only from the brain, and (ii) the oxygen uptake and flow to the muscles, bone and soft tissue should be relatively low, and most important, it should be constant, since the animal is paralyzed and anaesthetized, and the brain is much more sensitive to ischaemia [2, 23]. Therefore, significant changes in oxygen extraction and consumption most likely represent predominate alterations in brain oxygen metabolism. The oxygen data support this contention, as with high flow there was the expected increase in oxygen extraction following transient ischaemia, whereas with lower flow ischaemic areas of the brain were inadequately perfused, resulting in the inability to raise oxygen extraction to repay this energy deficiency [2, 4, 17].
INVOS and brain oxygen uptake
These observations document a lack of correlation of INVOS (cerebral oximeter) and global brain oxygen extraction at lower flows. Although baseline INVOS levels were slightly reduced at 450 cc/min (Fig. 5B), they quickly returned to normal after ischaemia, whereas global oxygen consumption did not show the expected response [2, 17], and actually fell (Fig. 5C) indicating inadequate perfusion and continued brain ischaemia. This discrepancy is most likely due to the way in which INVOS works. The INVOS oximeter uses near infrared spectroscopy to measure oxygen levels immediately beneath the sensors placed on the skull [15, 16]. Unfortunately INVOS is only effective at measuring brain oxygen levels directly under the sensors, usually extending to the first few centimetres of the cerebral cortex, and provides no information concerning oxygen levels in the deep brain [12, 15, 16]. In contrast global oxygen consumption measures all areas.
This raises questions as to the validity of using INVOS to guide cerebral blood flow during procedures such as open heart or carotid surgery. A flow of 450 cc/min in non-ischaemic pigs resulted in a mean pressure of >60 mmHg, which is often similar to the pressure during CPB, and the INVOS reading were also near normal. In contrast, the global oxygen consumption was reduced, implying insufficient flow to the deep brain. Flow to the brain could easily be worsened by carotid stenosis. Does a CPB pressure of 60 mmHg sometimes fail to adequately perfuse the deep brain, leading to small areas of ischaemia, and could this help explain the high incidence of soft neurological dysfunction following CPB for open heart surgery? [24, 25] Does INVOS provide a false sense of security in monitoring adequacy of brain perfusion? [15, 16].
Perfusion distribution studies
The perfusion studies using vessel occlusion documented adequacy of flow to all areas of the brain. Occluding blood flow to any major vessel caused significant changes, the most significant being occlusion of one or both distal carotids. Occluding one distal carotid artery significantly raised perfusion pressure. However, INVOS changed minimally, implying continued perfusion to that part of the brain by the other carotid. Occluding both distal carotids necessitated lowering the flow (because of high pressure) by approximately two-thirds, confirming that the right vertebral (via its costro-chondral origin) artery receives significant blood flow. To delineate vertebral versus costro-chondral flow, we then clamped the right vertebral artery alone (during carotid perfusion) and normalized pressure. This resulted in flow changes statistically similar to occluding the bilateral distal carotid flow, confirming that most of the costro-chondral flow was indeed vertebral, and that the vertebral receives 25–35% of perfusion. Therefore, vertebral flow to the posterior brain is insured in this model by (i) retrograde carotid flow via the innominate vessel to perfuse the right vertebral artery, which joins the left vertebral artery to form the basilar artery, and (ii) collateral brain flow via the circle of Willis [11, 18–21]. It should also be noted that this vertebral flow was inadequate to perfuse the anterior part of the brain, as INVOS fell substantially after only 30 s, and probably would have fallen further if flow to the carotid arteries had not been restored. Moreover, with the occlusion of both proximal carotids (no vertebral flow) INVOS was unaffected, meaning that either the posterior brain receives sufficient flow from the two carotid arteries, or more likely, that a ‘normal’ INVOS reading does not reflect posterior brain perfusion, and is once again misleading.
In conclusion, this study describes a new model of isolated global brain ischaemia that consistently produces severe global brain ischaemia in a pig, which is similar to the insult that occurs with sudden death, providing us with a reliable model in a large animal for studying controlled reperfusion of the brain in the same fashion used in the heart, lung and lower extremity. It describes the response to variations in cerebral blood flow, establishing baseline values, the normal response to transient brain ischaemia, and documents that all areas of the brain are perfused in this model. Lastly, it demonstrated the discrepancy between INVOS and oxygen consumption in determining adequacy of perfusion, and that higher reperfusion flows are needed after brain ischaemia.
Funding
This work was partially supported by a grant from the National Institutes of Health (R01-HL-71729-04).
Conflict of interest: none declared.
REFERENCES
- 1.Liakopoulos OJ, Allen BS, Buckberg GD, Hristov N, Tan Z, Villablanca JP, et al. Resuscitation after prolonged cardiac arrest: role of cardiopulmonary bypass and systemic hyperkalemia. Ann Thorac Surg. 2010;89:1972–9. doi: 10.1016/j.athoracsur.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 2.Allen BS, Buckberg GD. Studies of isolated global brain ischaemia: I. Overview of irreversible brain injury and evolution of a new concept–redefining the time of brain death. Eur J Cardiothorc Surg. 2012;41:1132–7. doi: 10.1093/ejcts/ezr315. [DOI] [PubMed] [Google Scholar]
- 3.Boyle EM, Jr, Pohlman TH, Cornejo CJ, Verrier ED. Endothelial cell injury in cardiovascular surgery: ischemia-reperfusion. Ann Thorac Surg. 1996;62:1868–75. doi: 10.1016/s0003-4975(96)00950-2. [DOI] [PubMed] [Google Scholar]
- 4.Allen BS. Pediatric myocardial protection: a cardioplegic strategy is the ‘solution’. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:141–54. doi: 10.1053/j.pcsu.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Trummer G, Foerster K, Buckberg GD, Benk C, Heilmann C, Mader I. Successful resuscitation after prolonged periods of cardiac arrest: a new field in cardiac surgery. J Thorac Cardiovasc Surg. 2010;139:1325–32. doi: 10.1016/j.jtcvs.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 6.Oltean M, Mera S, Olofsson R, Zhu C, Blomgren K, Hallberg E. Transplantation of preconditioned intestinal grafts is associated with lower inflammatory activation and remote organ injury in rats. Transplant Proc. 2006;38:1775–8. doi: 10.1016/j.transproceed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des. 2006;12:2867–73. doi: 10.2174/138161206777947597. [DOI] [PubMed] [Google Scholar]
- 8.Allen BS, Veluz JS, Buckberg GD, Aeberhard E, Ignarro LJ. Deep hypothermic circulatory arrest and global reperfusion injury: avoidance by making a pump prime reperfusate—a new concept. J Thorac Cardiovasc Surg. 2003;125:625–32. doi: 10.1067/mtc.2003.96. [DOI] [PubMed] [Google Scholar]
- 9.Allen BS, Castella M, Buckberg GD, Tan Z. Conditioned blood reperfusion markedly enhances neurologic recovery after prolonged cerebral ischemia. J Thorac Cardiovasc Surg. 2003;126:1851–8. doi: 10.1016/s0022-5223(03)01295-9. [DOI] [PubMed] [Google Scholar]
- 10.Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–51. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 12.Allen BS, Ko Y, Buckberg GD, Sakhai S, Tan Z. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia – the importance of perfusion pressure. Eur J Cardiothorac Surg. 2012;41:1147–54. doi: 10.1093/ejcts/ezr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halldorsson AO, Kronon MT, Allen BS, Rahman S, Wang T. Lowering reperfusion pressure reduces the injury after pulmonary ischemia. Ann Thorac Surg. 2000;69:198–203. doi: 10.1016/s0003-4975(99)01104-2. [DOI] [PubMed] [Google Scholar]
- 14.Kronon M, Bolling KS, Allen BS, Halldorsson AO, Wang T, Rahman S. The importance of cardioplegic infusion pressure in neonatal myocardial protection. Ann Thorac Surg. 1998;66:1358–64. doi: 10.1016/s0003-4975(98)00725-5. [DOI] [PubMed] [Google Scholar]
- 15.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 16.Tobias JD. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235–43. doi: 10.1586/17434440.3.2.235. [DOI] [PubMed] [Google Scholar]
- 17.Allen BS, Okamoto F, Buckberg GD, Leaf J, Bugyi H. Effects of ‘duration’ of reperfusate administration versus reperfusate ‘dose’ on regional functional, biochemical, and histochemical recovery. J Thorac Cardiovasc Surg. 1986;92:594–604. [PubMed] [Google Scholar]
- 18.Sack WO. Essentials of pig anatomy. In: Sack WO, editor. Pig Anatomy and Atlas. Ithaca, NY: Veterinary Textbooks; 1982. [Google Scholar]
- 19.Gilber SG. Pictorial Anatomy of the Fetal Pig. 2nd edn. Seattle: University of Washington Press; 1963. [Google Scholar]
- 20.Wingerd BD. Pig Anatomy and Dissection Guide. 1st edn. St. Paul, MN: Bluedoor Publishing, LLC; 2006. [Google Scholar]
- 21.Miller JS. Fetal Pig Dissection Guide. 3rd edn. Goshen, IN: Goshen College, Department of Biology; 1997. [Google Scholar]
- 22.Gourley JK, Heistad DD. Characteristics of reactive hyperemia in the cerebral circulation. Am J Physiol. 1984;246:H52–8. doi: 10.1152/ajpheart.1984.246.1.H52. [DOI] [PubMed] [Google Scholar]
- 23.Nordstrom CH, Siesjo BK. Effects of phenobarbital in cerebral ischemia. Part I: cerebral energy metabolism during pronounced incomplete ischemia. Stroke. 1978;9:327–35. doi: 10.1161/01.str.9.4.327. [DOI] [PubMed] [Google Scholar]
- 24.Carrascal Y, Guerrero AL. Neurological damage related to cardiac surgery: pathophysiology, diagnostic tools and prevention strategies. Using actual knowledge for planning the future. Neurologist. 2010;16:152–64. doi: 10.1097/NRL.0b013e3181bd602b. [DOI] [PubMed] [Google Scholar]
- 25.Funder KS, Steinmetz J, Rasmussen LS. Cognitive dysfunction after cardiovascular surgery. Minerva Anestesiol. 2009;75:329–32. [PubMed] [Google Scholar]