Abstract
OBJECTIVE
Brain damage is universal in the rare survivor of unwitnessed cardiac arrest. Non-pulsatile-controlled cerebral reperfusion offsets this damage, but may simultaneously cause brain oedema when delivered at the required the high mean perfusion pressure. This study analyses pulsatile perfusion first in control pigs and then using controlled reperfusion after prolonged normothermic brain ischaemia (simulating unwitnessed arrest) to determine if it might provide a better method of delivery for brain reperfusion.
METHODS
Initial baseline studies during isolated brain perfusion in 12 pigs (six non-pulsatile and six pulsatile) examined high (750 cc/min) then low (450 cc/min) fixed flow before and after transient (30 s) ischaemia, while measuring brain vascular resistance and oxygen metabolism. Twelve subsequent pigs underwent 30 min of normothermic global brain ischaemia followed by either uncontrolled reperfusion with regular blood (n = 6) or pulsatile-controlled reperfusion (n = 6) before unclamping brain inflow vessels. Functional neurological deficit score (NDS; score: 0, normal; 500, brain death) was evaluated 24 h post-reperfusion.
RESULTS
High baseline flow rates with pulsatile and non-pulsatile perfusion before and after transient ischaemia maintained normal arterial pressures (90–100 mmHg), surface oxygen levels IN Vivo Optical Spectroscopy (INVOS) and oxygen uptake. In contrast, oxygen uptake fell after 30 s ischaemia at 450 cc/min non-pulsatile flow, but improved following pulsatile perfusion, despite its delivery at lower mean cerebral pressure. Uncontrolled (normal blood) reperfusion after 30 min of prolonged ischaemia, caused negligible INVOS O2 uptake (<10–15%), raised conjugated dienes (CD; 1.75 ± 0.15 A233 mn), one early death, multiple seizures, high NDS (243 ± 16) and extensive cerebral infarcts (2,3,5-triphenyl tetrazolium chloride stain) and oedema (84.1 ± 0.6%). Conversely, pulsatile-controlled reperfusion pigs exhibited normal O2 uptake, low CD levels (1.31 ± 0.07 A233 mn; P < 0.01 versus uncontrolled reperfusion), no seizures and a low NDS (32 ± 14; P < 0.001 versus uncontrolled reperfusion); three showed complete recovery (NDS = 0) and all could sit and eat. Post-mortem brain oedema was minimal (81.1 ± 0.5; P < 0.001 versus uncontrolled reperfusion) and no infarctions occurred.
CONCLUSIONS
Pulsatile perfusion lowers cerebral vascular resistance and improves global O2 uptake to potentially offset post-ischaemic oedema following high-pressure reperfusion. The irreversible functional and anatomic damage that followed uncontrolled reperfusion after a 30-min warm global brain ischaemia interval was reversed by pulsatile-controlled reperfusion, as its delivery resulted in consistent near complete neurological recovery and absent brain infarction.
Keywords: Brain ischaemia, Brain death, Reperfusion, Controlled reperfusion, Neurological recovery, New treatment, Sudden death, Pulsatile perfusion
INTRODUCTION
The brain is the most sensitive organ to ischaemia, which helps explain why current clinical mortality is almost 100% if >10 min elapse before treating sudden death [1], and the rare survivor of an unwitnessed arrest always incurs neurological damage, because irreversible brain damage is thought to follow only 4 min of ischaemia. Moreover, despite improvements in cardiac survival by using emergency cardiopulmonary bypass after witnessed or unwitnessed arrest, substantial neurological damage is sustained by most survivors [1, 2], making absent neurological recovery the evident weak link in treating sudden death.
The genesis of this brain damage is secondary to an ischaemic/reperfusion injury, which follows the conventional delivery of normal blood or ‘uncontrolled reperfusion’. Proof of this concept initially stemmed from studies in the heart, lung and lower extremity [1, 3–5], where the avoidance of cellular and functional damage following prolonged ischaemia was achieved by adopting the method of ‘controlled reperfusion’, which adjusts (controls) the conditions and composition of reflow, thereby excluding the conventional uncontrolled reperfusion method. Further proof followed the application of controlled reperfusion after 30 min of global brain ischaemia. Preliminary studies demonstrated the achievement of nearly complete neurological recovery following this prolonged ischaemic insult, provided that the controlled reperfusate was delivered at a high rather than a low reperfusion pressure [2].
The importance of insuring increased pressure rather than only addressing the flow rate became evident at post-mortem autopsy findings, which confirmed the evolution of brain infarction within the deep and watershed regions if the same 750 cc/min (high flow rate) controlled reflow was delivered at low carotid pressure. Subsequent studies demonstrated that these resistance changes leading to a low reperfusion pressure were most likely linked to the continuation of the higher Isoflurane concentrations used to help lower brain vascular resistance during the management of the catecholamine surge following sudden global brain ischaemia [2, 6]. Analysis of brain vascular resistance, oxygen uptake and cortical saturation in non-ischaemic pigs implied that higher isoflurane concentrations can ‘steal’ flow by redistributing adequate fixed brain inflow away from its intended deeper brain distribution, channelling it into the superficial cortex [6].
Functional recovery at the 30-min time-frame previously thought to cause irreversible damage, thereby suggests that absent regional infarction was avoided by homogeneous delivery of the brain reperfusate during the higher pressure-controlled reperfusion delivery. Unfortunately, increased controlled reperfusion pressure creates the potential dilemma of causing brain oedema, because the reperfused brain (constrained by rigid skull surroundings) is vulnerable to endothelial and cellular damage [1, 2, 4, 5, 7]. These observations indicate a balance must be adopted to sustain the required high pressure needed to provide adequate deep brain-controlled reperfusate distribution, while concurrently limiting the unintended consequences of tissue injury from increased pressure.
Pulsatile reperfusion introduces a possible solution, as this technique could potentially lower mean pressure while concomitantly ensuring an adequate regional brain distribution via its higher ‘peak’ pressure [8–10]. This study initially makes comparisons in non-ischaemic and transiently ischaemic (30 s) pig brains during isolated total brain perfusion to distinguish the effects of pulsatile and non-pulsatile flow at high (750 cc/min) and low (450 cc/min) flow rates in determining vascular resistance and oxygen metabolism. Subsequently, based on our understanding of the (i) importance of higher perfusion pressure [2], (ii) the possible advantage of pulsatile perfusion [8–10], (iii) need for lower isoflurane dose to raise vascular resistance [6] and (iv) required higher reflow rates [7], this study utilizes pulsatile perfusion to determine if we can consistently recover the brain with controlled reperfusion after prolonged (30 min) ischaemia.
As with our other recent studies [2, 7], we used a new model of isolated global brain ischaemia, as it simulates the neurological insult in sudden death, and consistently leads to a severe neurological injury with normal blood reperfusion, but (i) allows us to specifically examine the brain, (ii) avoids the confounding variables of multiple ischaemic organs in sudden death models and (iii) parallels our initial studies of controlled reperfusion in the heart and lung [1, 3–5]. As with prior isolated studies of controlled reperfusion of the heart, lung and lower extremity [1, 3–5], this study will further emphasize the importance of uncovering and then dealing with factors that allow controlled reperfusion to become a unifying concept during the management of the ischaemia/reperfusion injury process.
METHODS
The study protocol was approved by the Institutional Animal Research Committee of the University of California at Los Angeles. All animals received human care in compliance with the 1996 NRC Guide for the Care and Use of Laboratory Animals.
Twenty-four Yorkshire–Duroc pigs (47.5 ± 1.2 kg) were premedicated with 15 mg/kg of ketamine and 0.5 mg/kg of diazepam intramuscularly and received neuromuscular blockade with pancuronium (0.2 mg/kg) during surgical dissection. After endotracheal intubation, anaesthesia was achieved with inhaled isoflurane (1.0–3.0%), and 12–14 bpm were provided by a volume-controlled ventilator (Servo 900C, Siemens Elema, Sweden) at 10–15 ml/kg tidal volume, 4 mmHg positive end-expiratory pressure, with setting adjustment to normalize pH (7.37–7.45) and oxygen (PaO2 = 80–200 mmHg) and carbon dioxide tension (PaCO2 = 35–45 mmHg). Anaesthesia was supplemented with fentanyl (1 μg/kg) every hour as needed.
The surgical preparation was described previously [2, 7]. Briefly, electrocardiography monitored the heart rate and rhythm, and left internal thoracic artery intubation monitored arterial pressure and allowed blood gas samples. Pulmonary artery pressure and cardiac output were determined from a balloon-tipped pulmonary artery catheter (Model 132F5, Baxter Healthcare Corp., Irvine, CA, USA) via the right external jugular vein. Sterile incisions exposed vessels used for cerebral ischaemia (see below). Cefazolin (30 mg/kg, GlaxoSmithKline, Philadelphia, PA, USA) was given perioperatively every 8 h, and saline infused intravenously at 5–10 ml/kg/h. Body temperature was kept at 37°C using a heating pad and lamp as needed. An IN Vivo Optical Spectroscopy (INVOS) cerebral oximeter (INVOS model 4100; Somanetics, Troy, MI, USA) patch was placed on the forehead avoiding the sagittal sinus area, and cerebral oxygen saturation recorded continuously.
Experimental protocol
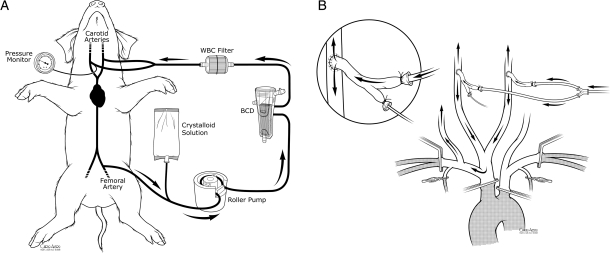
The previously described isolated global brain ischaemia and/or perfusion model was used for all studies [2, 7]. Briefly, carotid artery inflow was occluded by clamping the pig's innominate artery containing left and right carotid and right subclavian artery branches. Vertebral artery inflow was prevented by clamping the left subclavian just distal to the aortic arch, both internal mammary arteries and both subclavian arteries just beyond their mammary arteries. Cerebral perfusion was via both carotid arteries using arterial grafts that were harvested from donor pigs. Inflow carotid pressure and blood sampling was via a catheter placed within a branch of carotid graft. After heparinization (12 000 units), a Quest MPS system (Quest Medical, Allen, TX, USA) drained whole blood from the right femoral artery via a 14-Fr femoral cannula and delivered non-pulsatile flow into both carotid arteries via 10 Fr cannulas (Fig. 1).
Figure 1:
(A) Cerebral perfusion system and (B) method of isolated brain perfusion. The BCD, WBC filter and crystalloid solution bag were used for controlled reperfusion, whereas these three items were removed for the perfusion studies as previously depicted [7] in earlier studies.
Perfusion studies (non-pulsatile versus pulsatile) without prolonged ischaemia
The first ‘step’ was analysis of pulsatile and non-pulsatile perfusion in 12 normal pigs to determine differences in mean and peak pressure and their impact on brain oxygen metabolism.
Non-Pulsatile Perfusion: Six pigs received non-pulsatile flow via the Quest perfusion system.
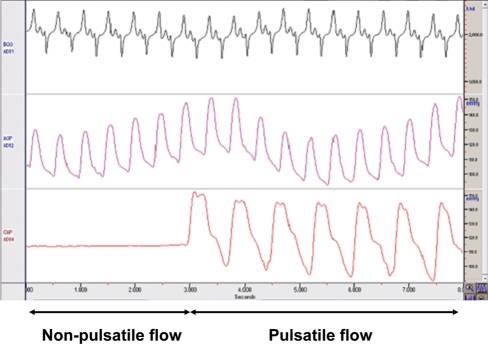
Pulsatile Perfusion: Six pigs received pulsatile flow that was achieved by outfitting the Quest system with a long tube which contained a 36-cc balloon catheter that was connected to an intra-aortic balloon pumping system (IABP; Senko Medical, Tokyo, Japan). Balloon inflow volume and timing were adjusted at a pulse rate of 80 bpm, and this system generated a nearly physiological pulse pressure of 40–60 mmHg (peak to valley) as shown in Fig. 2 that compares pulsatile and non-pulsatile flow.
Figure 2:
Pulsatile flow—this image shows how pulsatile flow (bottom line – right side) develops a wide pulse pressure, in comparison to mean pressure (bottom line – left side) following delivery by roller pump. Top, ECG; middle, femoral arterial pressure; bottom, carotid arterial pressure.
The following variables were measured:
Cerebral perfusion pressure (CPP): carotid arterial and internal jugular venous pressures were recorded continuously and CPP was defined as the difference between arterial and venous mean pressure at a fixed perfusion flow rate.
Cerebral oxygen saturation: an INVOS cerebral oximeter, using near-infrared light, measured oxygen saturation in the cerebral cortex under the sensor [2, 7, 11].
Cerebral oxygen uptake: Transcranial oxygen uptake was determined by the difference between carotid arterial and jugular venous oxygen content after determining haemoglobin concentration, per cent saturation and dissolved oxygen using the standard formulas described previously [2, 7].
Perfusion protocol
Ischaemia was avoided by simultaneously starting a flow rate of 750 cc/min (either pulsatile or non-pulsatile) with clamping all cerebral inflow, as this perfusion rate maintained the normal mean systemic pressure of 90–100 mmHg. Subsequently, six pigs in each group (pulsatile and non-pulsatile) were evaluated to examine their response to transient 30-s ischaemia at a flow rate of 750 cc/min (high flow), followed by evaluation of a flow rate of 450 cc/min (low flow).
Five minutes after starting perfusion at each flow rate, pump flow was discontinued for 30 s (transient ischaemia) and resumed at that flow rate as CPP, oxygen saturation and transcranial cerebral oxygen uptake were monitored. Measurements of CPP and oxygen saturation were repeated at intervals of 10, 20 and 30 s and 1, 2 and 5 min. Transcranial oxygen uptake, via samples from carotid artery and high jugular vein, was determined at baseline, and after 30 s and 5 min following transient ischaemia.
Verification of complete cerebral isolation at the end of each experiment was achieved by demonstrating a catecholamine surge, decrease in cerebral oxygen saturation (INVOS) to 15% (its minimum expression), carotid artery pressure <12 mmHg and bilateral pupil dilation with occlusion of the inflow vessels and no pump flow. Post-mortem examination confirmed the presence of the internal jugular venous catheter at the jugular bulb.
Prolonged (30-min) ischemia/reperfusion studies
Twelve pigs underwent 30 min of isolated global normothermic brain ischaemia in the manner described previously [2, 7]. Isoflurane was maintained at 1% (or less) in all studies during reperfusion, based on prior findings that higher concentrations caused hypotension by reducing brain vascular resistance [6]. Management of the hypertension and tachycardia that followed the sudden catecholamine surge from interrupting total brain inflow was achieved by delivering titrated infusions of nitroprusside and esmolol as described previously [2, 7].
Uncontrolled reperfusion (normal blood)
Six pigs received normal blood reperfusion by removing all the vascular clamps immediately following the 30 min of global brain ischaemia.
Pulsatile-controlled reperfusion
Six pigs received 20 min of normothermic pulsatile-controlled reperfusion via the carotid artery grafts at a flow rate of 750 cc/min, which was then followed by the removal of vascular clamps to restore normal blood circulation. With the exception of pulsatility, the method of controlled reperfusion in this model has been previously described [2] and is depicted in Fig. 1.
Solution composition
Supplementary data 1 shows the modified controlled brain reperfusion solution and its additives, which simultaneously has similar components to the solution used for controlled reperfusion of the heart, lung and lower extremity [1, 3–5] and was the same solution used in our previous study of controlled brain reperfusion [2]. Systemic ionic calcium levels >0.8 mM/l were maintained by intravenous calcium chloride bolus injections to counteract the fall in calcium caused by the citrate within the modified brain perfusate.
Post-operative care
Thirty minutes following reperfusion, protamine was given, all cannulas were removed and all wounds closed. A small tube was placed in the mediastinum. Extubation was done after each pig regained consciousness and could maintain a normal PaO2 and PaCO2 during spontaneous breathing. The incidence and duration of post-operative seizures was recorded, and IV midazolam (0.1 mg/kg) was delivered and repeated if seizures lasted more than a few minutes. Pigs were monitored for 24 h unless there was premature death. Euthanasia was accomplished using pentobarbital and potassium chloride at the completion of each study.
Haemodynamic data
Haemodynamic measurements were made before induction of ischaemia and after reperfusion by determining cardiac output with triplicate injections of 4°C cold saline (the thermodilution technique) and calculating the cardiac index with standard equations.
Biochemical data
Arterial and internal jugular blood were sampled at baseline and during reperfusion, and to mark oxidant mediated-lipid peroxidation, conjugated diene (CD) levels were determined as described previously [2, 7]. CDs were expressed either as total amount in the venous (internal jugular) sample or as production when flow was known (controlled reperfusion) using the formula: CD production = (venous CD level − Arterial CD level) × perfusion flow
Neurological deficit score
Neurological assessment was performed at 4 and 24 h after reperfusion using a species-specific behaviour scale [2, 7] described previously, and seizure activity was closely evaluated. Neurological deficit score (NDS) evaluates five general neurological examination components with a maximal score of 100 in each category: 0 indicates normal and 500 indicates brain death. NDS was independently assessed by two laboratory team members, and the mean value recorded if different scores were reported.
Tissue oedema and analysis of infarction
After euthanasia, the brain is excised, weighted and placed in the freezer for 10 min. Coronal samples 3–5 mm thick were sliced from the frontal lobe, thalamus and hippocampus, and sagittal samples were sliced from the posterior brain stem and cerebellum. A small sample of tissue from the frontal cortex, hippocampus, cerebellum and brain stem was taken to evaluate tissue oedema (wet to dry weight). These samples were immediately weighted, placed in an oven at 80°C for 24 h and then reweighted: tissue oedema = (wet weight − dry weight/wet weight) × 100. The remaining cut brain samples were placed in 1% TTC (2,3,5-triphenyl tetrazolium chloride) solution at 37°C for 20–30 min to assess for brain infarction.
Statistical analysis
Data were expressed as the mean ± SEM. Two-way analysis of variance with the Fisher's least significant difference procedure for post hoc repeated measurements was used to analyse intra- and intergroup differences. Perioperative characteristics of groups were analysed by Student's t-test for continuous data and the χ2 or the Fisher's exact test for categorical data. P–values of <0.05 were considered statistically significant.
RESULTS
Perfusion studies
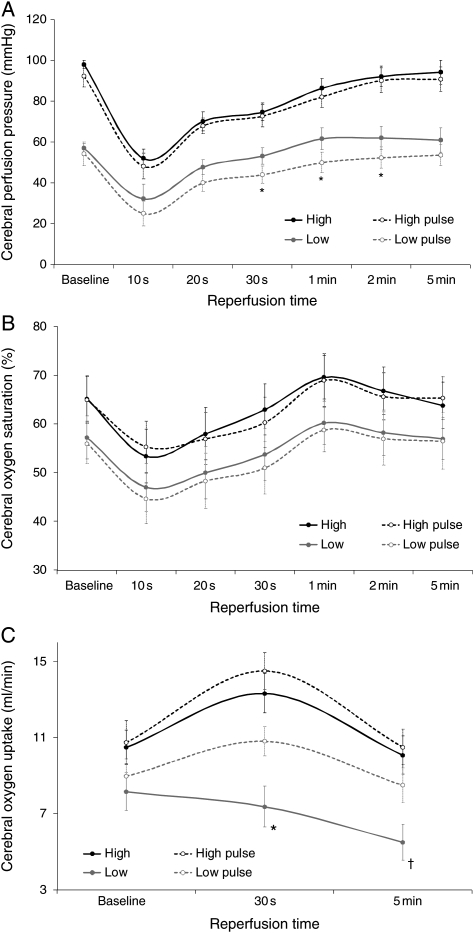
Pulsatile and non-pulsatile perfusion at a fixed flow rate of 750 cc/min resulted in normal mean systemic arterial pressures (90–100 mmHg) and INVOS oximeter (55–70%) levels. Transient ischaemia during pulsatile and non-pulsatile perfusion at these higher flow rates (750 cc/min) did not produce significant changes in mean carotid pressure, INVOS brain saturation or transcranial oxygen uptake (Fig. 3).
Figure 3:
Serial assessments of CPP (A), oxygen saturation (INVOS) (B) and oxygen uptake (C) under non-pulsatile and pulsatile flow at flows of 750 or 450 cc/min. High and low, flow rates; pulse, pulsatile flow. *P < 0.05 low versus low pulse and †P < 0.05 low pulse versus baseline value.
Pulsatile and non-pulsatile perfusion slightly reduced baseline carotid pressure and surface oxygen saturations (INVOS) at the low flow rate of 450 cc/min. These variables also fell following transient global ischaemia (30 s), but both methods resulted in their recovery to baseline by 5 min after ischaemia (Fig. 3). However, pulsatile and non-pulsatile reperfusion after transient ischaemia differed at this low flow rate because the reduced baseline global cerebral O2 consumption was further decreased following reperfusion with non-pulsatile flow, whereas O2 uptake improved with pulsatile flow mimicking the observations at the higher flow rates. Moreover, this decrease in oxygen consumption with low flow (450 cc/min) non-pulsatile perfusion occurred despite a higher perfusion pressure (Fig. 3C).
Completeness of ischaemia was substantiated in each pig at the completion of the perfusion studies validating the isolated brain model. Occlusion of brain inflow resulted in the same catecholamine surge, bilateral pupil dilation and reduction in INVOS and carotid pressure that categorized total brain ischaemia in the prolonged ischaemic studies.
Prolonged ischemia/reperfusion studies
Interrupting cerebral blood flow caused a catecholamine surge in all pigs, producing hypertension and tachycardia within 60–90 s, which required nitroprusside and esmolol to maintain normal haemodynamic stability. These drugs were usually needed for only 15–20 min and could normally be discontinued prior to the end of 30 min ischaemia. With clamping, the INVOS cerebral oximeter abruptly fell to its lowest level of 15%, pupils became dilated and non-reactive and carotid artery pressure fell to <12 mmHg while simultaneous jugular central venous pressure (CVP) was 6–8 mmHg implying the absence of flow. Baseline haemodynamics (blood pressure and cardiac index) and pulmonary function (arterial blood gases and pulmonary pressures) were similar in all pigs, there were no signs of cardiac or pulmonary comprise following cerebral ischaemia or reperfusion and none required a blood transfusion.
Uncontrolled reperfusion
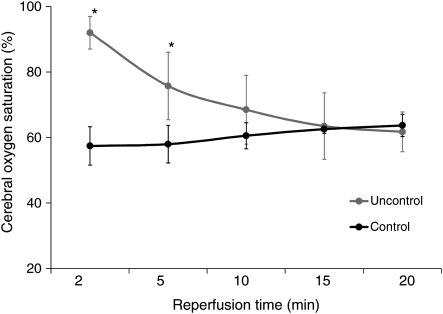
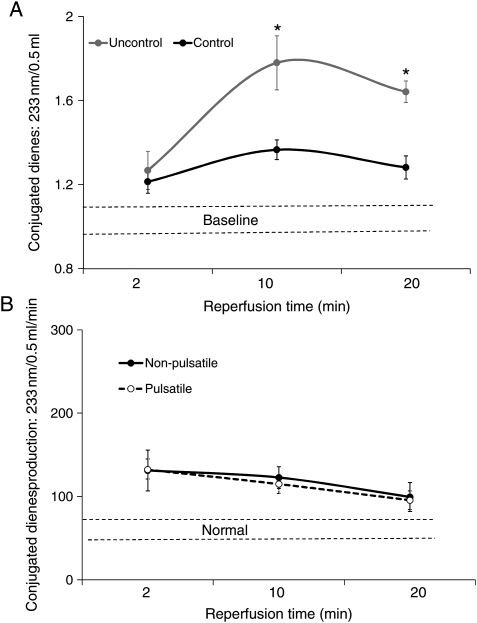
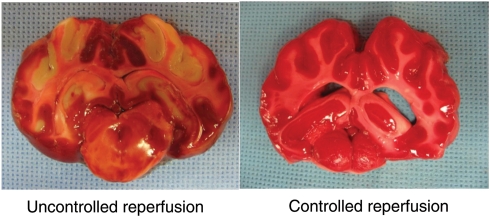
Cerebral reperfusion immediately raised internal jugular vein and brain O2 saturations (INVOS) >85–90% (<10–15% O2 extraction) in all six pigs (Fig. 4). CDs (oxygen radical damage) rose substantially (Fig. 5A) with reperfusion. Post-reperfusion seizures occurred in every pig, and one of the six pigs developed bradycardia, hypertension, fixed dilated pupils, absent respiration and died prior to 24 h. Post-anaesthesia neurological scores at 24 h in the surviving pigs were 243 ± 16, indicating severe damage (Table 1). Post-mortem macroscopic swelling was accompanied by oedema (Table 1) in cortex, cerebellum and hippocampus in all surviving animals. TTC stain (Fig. 6) consistently demonstrated extensive cerebral infarction.
Figure 4:
Brain oxygen saturation measured by INVOS during reperfusion. *P < 0.05 versus controlled reperfusion.
Figure 5:
(A) IJV conjugate diene release during reperfusion with controlled and uncontrolled reperfusion. (B) CDs production during controlled reperfusion (known flow) in pigs receiving pulsatile and high-pressure non-pulsatile perfusion reported previously [2]. These values are similar to the high pressure non-pulsatile-controlled reperfusion. *P < 0.001 versus controlled reperfusion.
Table 1:
NDS and brain oedema (percent water) post-reperfusion
| Reperfusion | NDS |
Tissue oedema (%) |
|||
|---|---|---|---|---|---|
| 4 h | 24 h | Cortex | Hippocampus | Cerebellum | |
| Lab Controls | 0 | 0 | 81.5 ± 0.2 | 77.3 ± 0.6 | 81.1 ± 0.3 |
| Uncontrolled | 253 ± 18* | 243 ± 16* | 84.1 ± 0.6* | 81.6 ± 0.7* | 84.8 ± 0.5* |
| Controlled | 70 ± 9 | 32 ± 14 | 81.1 ± 0.5 | 77.8 ± 0.5 | 82.1 ± 0.6 |
*P < 0.001 versus lab controls and high-pressure-controlled reperfusion; low and high refer to flow rates. NDS: neurological deficit score.
Figure 6:
TTC stain showing areas of infarction. On the left, a typical example of the TTC stain in a pig undergoing 30 min of ischaemia followed by uncontrolled reperfusion. Note the marked oedema collapsing the lateral ventricles and infarctions in the basal ganglia and watershed areas of the cortex. In contrast, on the right, a typical section from a pig receiving controlled reperfusion. Note the lack of oedema with preserved ventricles and no evidence of brain infarction.
Controlled reperfusion
Each pig demonstrated normal brain oxygen (INVOS) saturations (55–70%) and similar infusion pressures during reperfusion, and all survived the 24-h observation period. Controlled reperfusion reduced post-reperfusion CDs (Fig. 7A), returning them to normal by the end of reperfusion (Fig. 7B). No seizures developed, and the 24-h neuroscore was 32 ± 14 (Table 1). Three pigs recovered completely (NDS = 0, Supplementary Video 1) and six of the six pigs could sit, eat and drink at 24 h. Post-mortem examination showed minimal brain oedema (Table 1), and no infarction was evident following TTC staining (Fig. 6). These results were almost identical to the pigs in our previous study that received high-pressure non-pulsatile-controlled reperfusion [2], with no statistical difference in global O2 consumption, brain O2 saturations, CDs or tissue oedema (Figs 5 and 7).
Figure 7:
(A and B) Mean CPP and oxygen consumption during controlled pulsatile reperfusion compared with controlled non-pulsatile high-pressure (flow) reperfusion reported previously [2]. These values are similar with both methods.
DISCUSSION
This study demonstrates that the delivery of pulsatile flow with controlled reperfusion at the 750-ml/min flow rate previously shown to provide adequate brain perfusion [7] resulted in ‘consistent’ near complete neurological recovery after 30 min of warm global brain, in contrast to the pressure-related brain salvage that follows non-pulsatile-controlled reperfusion [2]. Simultaneously, pulsatile perfusion avoided the oxidant damage which followed uncontrolled reperfusion with blood, while markedly restricting post-ischaemic oedema and preventing brain infarction. The genesis of this study related to prior observations demonstrating that non-pulsatile-controlled reperfusion resulted in similar functional and anatomic brain salvage if its mean perfusion pressure was >50 mmHg, whereas maldistribution of flow followed lower pressure reperfusion, leading to brain infarction within watershed and deeper brain regions [2]. This raised concern about the development of brain oedema at these higher pressures and thus led to the postulation that pulsatile flow could reduce mean pressure while simultaneously delivering a higher peak pressure.
Pulsatile versus non-pulsatile flow
The first step in this investigation involved improved understanding of comparisons between non-pulsatile and pulsatile flow without prolonged brain ischaemia, because lower non-pulsatile flow rates of 450 ml/min were previously shown to impair reflow oxygen consumption following transient ischaemia of only 30 s [7]. The hallmarks of adequate perfusion during reflow are pressure-dependent, since only 30 s of ischaemia causes maximal brain vasodilatation [12]. Moreover, the conditions of reperfusion must evolve from organ-specific requirements, as prior reports demonstrate that controlled lung reperfusion at a lower pressure of 20–30 mmHg was superior to the higher pressures of ∼50 mmHg required to avoid cardiac reperfusion injury [1, 3–5].
Baseline studies of pulsatile and non-pulsatile flow employed the newly developed in situ brain perfusion model which permitted accurate and prompt detection of brain-specific alterations of oxygen metabolism. These measurements were achieved under circumstances where cardiac haemodynamics were unchanged [2, 7], and other organ ischaemia (sudden death models) were excluded to avoid their potential confounding influence. Documentation of isolated total brain perfusion was confirmed at the completion of each study, since terminal exclusion of arterial inflow produced the same sudden catecholamine surge, bilateral pupil dilation and changes in carotid (<12 mmHg) pressure that characterizes total ischaemia in prior and current studies [2, 7].
Although insight into the preferred pressure that the brain should ‘receive during controlled reperfusion is unknown’, our prior preliminary studies imply that increased pressure is essential to perfuse the deep areas, similar to cardiac reperfusion [2]. Unfortunately, the rigid skull that encases the brain poses a restriction, since the potential for tissue oedema exists because of endothelial damage that accompanies prolonged ischaemia or ischaemia/reperfusion [1–3, 5, 7]. A balance simultaneously exists because ischaemia abolishes cerebral auto-regulation to create a circumstance where reperfusion brain blood flow varies passively with perfusion pressure [8, 10, 13, 14]. Consequently, the higher pressure required to avoid reflow redistribution away from deeper regions becomes counterweighted against the potential to cause tissue oedema and cellular damage if pressure is too high.
Several factors that underlie the pulsatile versus the non-pulsatile delivery method may impact brain blood flow distribution. Pulsatile flow generates a higher haemodynamic energy, maintains better microcirculation and improves cerebral oxygen consumption as well as flow distribution following brief global cerebral ischaemia than non-pulsatile delivery [10, 15, 16]. The capacity of the hydraulic forces of pulsatile flow to better maintain capillary bed patency [10, 16] is related to its ability to have a peak pressure that is higher than the critical closing pressure that retards perfusion. Pulsatile flow also causes less endothelial damage, normalizes nitric oxide release [17] and allows carotid baroreceptors to respond to both static and pulsatile components, whereas non-pulsatile blood flow leads to reflex vasoconstriction [18]. The current investigation evaluates the capacity of pulsatile perfusion to improve deeper tissue brain reperfusion and thus avoid its redistribution into the more superficial cortical regions in a manner that may mirror the recently shown ability of post-ischaemic pulsatile cardiac flow to improve subendocardial perfusion [15, 19].
Changes in carotid pressure at fixed flows were measured to determine alterations in vascular resistance (vasodilation) caused by transient ischaemia of 30 s to parallel studies by Gourley and Heistad [12], who found this brief ischaemic episode caused maximal cerebral reactive hyperemia, coupled by return of oxygen uptake and brain blood flow to normal levels after 5 min of reperfusion. Determination of simultaneous measurements of oxygen saturation by INVOS and total brain oxygen uptake in this study permitted comparisons between pulsatile and non-pulsatile flow on oxygen metabolism, since the evidence of flow redistribution existed when there were differences between them at any fixed flow rate. These changes only occurred during non-pulsatile perfusion at low flow rates of 450 m/min, as the reduced transcranial oxygen consumption failed to recover after ischaemia and became dissociated from surface oxygen saturation (INVOS). These observations indicate maintenance of flow to the brain surface (exhibited by higher INVOS recordings) together with redistribution away from the deeper brain, leading to the reduction in total brain oxygen uptake as shown in Fig. 3. In contrast, pulsatile perfusion at the same lower flow rates raised ‘peak pressure’ while lowering mean pressure and retaining oxygen uptake as venous oxygen extraction increased, thereby implying that the lower vascular resistance maintained proper transcranial flow distribution by insuring better capillary flow despite a lower mean pressure. The subsequent study of 30 min of warm ischaemia was done to determine if brain oedema was also avoided by employing pulsatile perfusion and sets the stage for subsequent studies of controlled reperfusion at lower flow rates than accomplished in these experiments.
Pulsatile flow is distributed to a larger proportion of cortical gray versus white matter than non-pulsatile flow [8]. Moreover, gray matter has higher oxygen demands than white matter, and the disproportionately greater arterio-venous shunting during non-pulsatile flow may further reduce nutrient brain perfusion when comparable cerebral blood flow is delivered with these two methods. Implications for this redistribution during lower flow rates were observed in the current study of transient ischaemia, where oxygen uptake was maintained during pulsatile perfusion and fell during non-pulsatile delivery. The mechanical reason likely relates to the higher peak pressure during pulsatile delivery which maintained greater capillary patency by offsetting critical closure pressure within deeper brain regions. These potential benefits also exist during hypothermia, as reported by Watanabe and Washio [9], who suggested that pulsatile flow during low-flow perfusion would improve cerebral aerobic metabolism, despite flow rates that approached the lower limit of the critical range. Our experiment supports this assumption by also showing a benefit to low-flow pulsatile assistance on oxygen uptake. However, none of these prior studies tested this capacity after prolonged, rather than transient ischaemia.
Precise measurement of total brain oxygen uptake is uncertain, because some of this model's inflow perfused the muscles, bone and soft tissues supplied by the external carotid and other smaller branches [7]. Despite this limitation of not directly measuring only brain inflow, the oxygen uptake calculations most likely primarily reflect changes in brain oxygen metabolism because (i) oxygen uptake by the muscles, bone and soft tissue should be relatively low due and stable to anaesthesia and muscle paralysis, (ii) venous oxygen content samples were taken high in the internal jugular vein at the skull base and (iii) non-pulsatile and pulsatile perfusion were similarly sampled, so any discrepancy would be present in both groups.
Controlled reperfusion
The adverse functional and anatomic brain damage that follows uncontrolled reperfusion after 30 min of warm ischaemia was ‘consistently reversed’ when pulsatile-controlled reperfusion was delivered at a flow rate of 750 ml/min. Such avoidance of neurological changes, absence of oxidant damage, negligible oedema and evasion of any brain infarction differs from findings following non-pulsatile-controlled reperfusion at this same higher flow rate [2], since such similar recovery ‘only followed its delivery at higher mean pressures’. Conversely, lower pressure non-pulsatile-controlled reperfusion resulted in changes similar to uncontrolled reperfusion with severe oxidant damage, multiple seizures, marked brain oedema and cerebral infarctions, despite the maintenance of the high flow rate.
Pulsatile-controlled reperfusion at the selected flow rate of 750 ml/min was done because (i) baseline studies demonstrated normal maintenance of oxygen metabolism with pulsatile and non-pulsatile flow at this higher rate, (ii) this flow successfully restored function with high-pressure non-pulsatile-controlled reperfusion [2] and (iii) it allowed testing for worsening of oedema, because an even higher peak pressure existed with pulsatile-controlled reperfusion. Moreover, isoflurane concentrations were kept <1% to avoid the drug-related lowering of vascular resistance [6], while simultaneously offsetting this agents capacity be neuroprotective in the higher doses [6, 20]. Unfortunately, results without anaesthesia cannot be accomplished in any experimental study of isolated brain ischaemia, so that outcomes between controlled and uncontrolled reperfusion can only be compared at similar drug dosages.
The consistent neurological recovery and the absence of brain infarction following pulsatile-controlled reperfusion extends prior observations following high-pressure non-pulsatile-controlled reperfusion [2], thereby documenting that properly controlling the pressure conditions of controlled reflow avoids the irreversible brain damage that 30 min of global brain ischaemia is conventionally thought to produce. This observation establishes a launch position for future studies of longer warm ischaemic intervals and indicates that the maximum time to allow for brain salvage is unknown, but is certainly beyond 30 min of global ischaemia.
The balance between having a sufficient pressure to ensure flow to deeper brain regions must be weighed against the potential to cause oedema that naturally follows ischaemia due to endothelial damage, which can lead to brain herniation if severe [1–5, 7, 13]. Ischaemia abolishes the brains auto-regulation that normally ensures perfusion to all areas over a wide range of pressures. Consequently, post-ischaemic blood flow varies passively with perfusion pressure [6, 8, 13, 14, 16]. This study did not establish the ideal brain reperfusion pressure, but pulsatile flow delivered a higher peak pressure than that achieved previously with non-pulsatile perfusion. Besides pulsatile flow causing less endothelial damage [21], another potential mechanism for the negligible oedema formation despite the higher peak pressure may be the enhanced lymphatic flow that accompanies this natural pulsation sequence, a consequence which might offset brain water gain [22].
It should be noted that the mean pressure with pulsatile flow was statistically the same as the high pressure non-pulsatile group that previously recovered (Fig. 7A), and so it remains unproven if pulsatile flow will allow neurological recovery at lower flows and pressures. Nevertheless the transient ischaemic (perfusion) studies suggest that pulsatile flow provides adequate deep brain perfusion at lower pressures and flows, implying that lower mean pressure will be effective with pulsatile-controlled reperfusion. ‘Steal’ of blood away from deeper areas to the brain surface may also occur when vascular resistance is reduced by high-dose (3%) isoflurane [6], and neurological recovery with pulsatile perfusion was identical to that observed with non-pulsatile reperfusion when vascular resistance was not reduced presumably by isoflurane [2]. Therefore, the superiority of pulsatile perfusion in controlled reperfusion of the brain must still be established by comparing each modality (pulsatile and non-pulsatile) at high and low flow rates (pressures) using similar conditions of <1% isoflurane.
Pulsatile perfusion may allow the intentional use of lower reperfusion flows and mean pressures than non-pulsatile perfusion, thereby reducing the risk of post-ischaemic reperfusion cellular damage. Higher mean pressure raises sheer stress and also releases cytokines and activates vascular endothelial cells [1, 23], which leads to white cell attachment with release of proteases and oxygen-free radicals resulting in further cellular damage [1, 23]. Indeed, studies using endothelial cell culture have shown that high hydrostatic pressure alone activates cytokines causing cellular injury independent of sheer stress [21]. High reperfusion pressure also increases release of vasoactive substances such as endothelin which causes vasoconstriction and hypoperfusion [21, 23, 24]. Nevertheless, until lower flow pulsatile perfusion is tested, its potential advantage remains speculative.
Controlled reperfusion integrates many factors, and its success in each organ involves controlling multiple elements of both the conditions of reperfusion, as well as the composition of the reperfusate [1, 3–5]. In contrast, failure always follows efforts directed toward a single reperfusion component, whether it be in the heart, lung [13–5] or even the brain [25]. This study demonstrates the importance of understanding how to optimize the conditions of reperfusion, since the same reperfusate composition was employed in all our recent studies, but consistent recovery was only observed when the reflow conditions were optimized by employing high flow and pressure [2]. Each organ has specific reperfusion needs, so that brain variables of pressure, duration, temperature, metabolism etc. must also become subsequently clarified, since they may be vastly different from those in the heart and lung [1, 3–5]. Future earmarking of these end points will further define the optimal method of controlled brain reperfusion.
In conclusion, evolution of delivering pulsatile-controlled reperfusion started with baseline studies that showed that pulsatile perfusion lowers cerebral vascular resistance and improves total brain O2 uptake after temporary ischaemia at low-pressure (flow) states, before applying this method after prolonged ischaemia. The consistent avoidance of neurological damage after 30 min of warm brain ischaemia with controlled reperfusion redefines the time of brain death beyond the interval previously thought to produce irreversible injury and lays the groundwork to investigate other aspects of controlled brain reperfusion at longer durations. Moreover, these observations may have broader impact, since other forms of brain ischaemia, such as stroke, probably share the same common injury and will also be helped by controlled reperfusion. Improving the outcome of brain ischaemia could have enormous consequences, since over 450 000 sudden deaths and 731 000 strokes occur annually in the United States, and neurological damage is the No. 1 cause of adult disability in 4 million US stroke survivors [1], reflecting the severe limitations of current conventional treatments.
SUPPLEMENTARY MATERIAL
Supplementary material (Video 1 and Supplement 1) is available at EJCTS online.
Funding
This work was supported by a grant from the National Institutes of Health (R01-HL-71729-04).
Conflict of interest: none declared.
Supplementary Material
References
- 1.Allen BS, Buckberg GD. Studies of isolated global brain ischaemia: I. Overview of irreversible brain injury and evolution of a new concept – redefining the time of brain death. Eur J Cardiothorac Surg. 2012;41:1132–7. doi: 10.1093/ejcts/ezr315. [DOI] [PubMed] [Google Scholar]
- 2.Allen BS, Ko Y, Buckberg GD, Sakhai S, Tan Z. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia – the importance of perfusion pressure. Eur J Cardiothorac Surg. 2012;41:1147–54. doi: 10.1093/ejcts/ezr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen BS. The role of leukodepletion in limiting ischemia/reperfusion damage in the heart, lung and lower extremity. Perfusion. 2002;17(Suppl.):11–22. doi: 10.1191/0267659102pf555oa. [DOI] [PubMed] [Google Scholar]
- 4.Allen BS. Pediatric myocardial protection: a cardioplegic strategy is the ‘solution’. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:141–54. doi: 10.1053/j.pcsu.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Buckberg GD, Allen BS. Myocardial protection management during adult cardiac operations. In: Baue AE, Geha AS, Hammond GL, Laks H, Naunheim KS, editors. Glenn's Thoracic and Cardiovascular Surgery. Stamford, CT: Appleton and Lange; 1995. pp. 1653–87. [Google Scholar]
- 6.Allen BS, Buckberg GD, Ko Y, Tan Z. Studies of isolated global brain ischaemia: impact of isoflurane anesthesia on cerebral blood pressure, flow distribution, and oxygen metabolism during isolated global brain perfusion. Resuscitation. in press [Google Scholar]
- 7.Allen BS, Ko Y, Buckberg GD, Sakhai S, Tan Z. Studies of isolated global brain ischaemia: I. A new large animal model of global brain ischaemia and its baseline perfusion studies. Eur J Cardiothorac Surg. 2012;41:1138–46. doi: 10.1093/ejcts/ezr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstadt MP, Tedder M, Hegde SS, Perez-Tamayo RA, Crain BJ, Khian Ha VL, et al. Pulsatile versus nonpulsatile reperfusion improves cerebral blood flow after cardiac arrest. Ann Thorac Surg. 1993;56:453–61. doi: 10.1016/0003-4975(93)90879-m. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Washio M. Pulsatile low-flow perfusion for enhanced cerebral protection. Ann Thorac Surg. 1993;56:1478–81. doi: 10.1016/0003-4975(93)90734-y. [DOI] [PubMed] [Google Scholar]
- 10.Undar A, Masai T, Beyer EA, Goddard-Finegold J, McGarry MC, Fraser CD., Jr Pediatric physiologic pulsatile pump enhances cerebral and renal blood flow during and after cardiopulmonary bypass. Artif Organs. 2002;26:919–23. doi: 10.1046/j.1525-1594.2002.07127.x. [DOI] [PubMed] [Google Scholar]
- 11.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl. 1):i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 12.Gourley JK, Heistad DD. Characteristics of reactive hyperemia in the cerebral circulation. Am J Physiol. 1984;246:H52–8. doi: 10.1152/ajpheart.1984.246.1.H52. [DOI] [PubMed] [Google Scholar]
- 13.Halstead JC, Meier M, Wurm M, Zhang N, Spielvogel D, Weisz D, et al. Optimizing selective cerebral perfusion: deleterious effects of high perfusion pressures. J Thorac Cardiovasc Surg. 2008;135:784–91. doi: 10.1016/j.jtcvs.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 14.O'Dwyer C, Prough DS, Johnston WE. Determinants of cerebral perfusion during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1996;10:54–64. doi: 10.1016/s1053-0770(96)80179-0. [DOI] [PubMed] [Google Scholar]
- 15.Jung JS, Son HS, Lim CH, Sun K. Pulsatile versus nonpulsatile flow to maintain the equivalent coronary blood flow in the fibrillating heart. ASAIO J. 2007;53:785–90. doi: 10.1097/MAT.0b013e31815b2d00. [DOI] [PubMed] [Google Scholar]
- 16.Ji B, Undar A. An evaluation of the benefits of pulsatile versus nonpulsatile perfusion during cardiopulmonary bypass procedures in pediatric and adult cardiac patients. ASAIO J. 2006;52:357–61. doi: 10.1097/01.mat.0000225266.80021.9b. [DOI] [PubMed] [Google Scholar]
- 17.Waaben J, Wulf HC, Wettermark G, Andersen K, Husum B. ATP-content in muscular interstitial fluid during pulsatile and non-pulsatile cardiopulmonary bypass in pigs. Biomed Biochim Acta. 1985;44:1113–8. [PubMed] [Google Scholar]
- 18.Sanderson JM, Wright G, Sims FW. Brain damage in dogs immediately following pulsatile and non-pulsatile blood flows in extracorporeal circulation. Thorax. 1972;27:275–86. doi: 10.1136/thx.27.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassab GS, Kostelec M, Buckberg GD, Covell J, Sadeghi A, Hoffman JI. Myocardial protection in the failing heart: II. Effect of pulsatile cardioplegic perfusion under simulated left ventricular restoration. J Thorac Cardiovasc Surg. 2006;132:884–90. doi: 10.1016/j.jtcvs.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 20.Eger EII, Eisenkraft JB, Weiskopf RB. 2nd edn. The Pharmacology of Inhaled Anesthetics. San Francisco, M.D.: Edmond I Eger II, 2002. [Google Scholar]
- 21.Lefer AM, Lefer DJ. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- 22.McGeown JG, McHale NG, Thornbury KD. The effect of electrical stimulation of the sympathetic chain on peripheral lymph flow in the anaesthetized sheep. J Physiol. 1987;393:123–33. doi: 10.1113/jphysiol.1987.sp016814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle EM, Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg. 1997;63:277–84. doi: 10.1016/s0003-4975(96)01061-2. [DOI] [PubMed] [Google Scholar]
- 24.Novick RJ, Gehman KE, Ali IS, Lee J. Lung preservation: the importance of endothelial and alveolar type II cell integrity. Ann Thorac Surg. 1996;62:302–14. doi: 10.1016/0003-4975(96)00333-5. [DOI] [PubMed] [Google Scholar]
- 25.Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–71. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.