Abstract
OBJECTIVES
Predictors of long-term survival for patients with lung cancer assist in individualizing treatment recommendations. Diffusing capacity (DLCO) is a predictor of complications after resection for lung cancer. We sought to determine whether DLCO is also prognostic for long-term survival after lung resection for cancer.
METHODS
We assessed survival among patients in our prospective database who underwent lung resection for cancer between 1980–2006. Potential prognostic factors for all-cause mortality were evaluated by computing average annual hazard rates, and variables significantly associated with survival were included in multivariable Cox modelling. Multiple imputation was used to address missing values.
RESULTS
Among 854 unique patients, there were 587 deaths. The median follow-up time from surgery was 9.6 years. Predictors of survival included age, stage, performance status, body mass index, history of myocardial infarction, renal function and DLCO. On univariate analysis, the hazard ratio increased incrementally compared with those with a DLCO of ≥80% (70–79%, 1.12; 60–69%, 1.29; <60%, 1.35). On multivariable analysis, DLCO was an independent predictor of long-term survival for all patients (corrected for all other important covariates; HR 1.04 per 10-point decrement; 95% CI 1.00–1.08; P = 0.05). Its prognostic ability for long-term survival was above and beyond its influence on operative mortality.
CONCLUSIONS
DLCO is an independent and clinically important determinant of long-term survival after major lung resection for cancer, a finding that is not generally known. Knowledge of this may help improve selection of patients for lung resection and may help tailor the extent of resection, when possible, in order to appropriately balance operative risk with long-term outcomes.
Keywords: Lung resection, Survival, Diffusing capacity, Lung cancer
INTRODUCTION
Long-term survival in patients with non-small-cell lung cancer (NSCLC) is determined primarily by cancer stage. Additional clinical factors that are well accepted to affect survival include histological subtype, patient age and type of therapy. It is not as well known that physiological parameters such as lung function are determinants of long-term survival in patients with NSCLC. In particular, several reports have identified spirometry, specifically forced expiratory volume in the first second (FEV1), as an independent determinant of overall survival [1–3]. This should not be surprising, as spirometry was initially reported as being related to survival as early as 1846 by Hutchinson, and has been suggested as being useful in developing actuarial tables by the insurance industry for decades [4].
Diffusing capacity of the lung for carbon monoxide (DLCO) has been associated with increased risks of acute morbidity and mortality after major lung resection [5,6]. A prior publication [7] from our group found no conclusive correlation between DLCO and long-term survival. However, a recent report by Liptay et al. [8] identified DLCO as an independent determinant of long-term survival after resection of NSCLC, a finding that should influence algorithms for patient selection for surgical therapy. We evaluated our updated institutional database to determine whether their finding could be replicated.
PATIENTS AND METHODS
A retrospective analysis was performed on the data from consecutive patients undergoing major lung resection on the Thoracic Surgery Service at The University of Chicago from 1980 through 2006. Preoperative, intraoperative and postoperative data were retrospectively collected prior to 1990 and were prospectively collected as part of an institutional review board-approved database from 1990 onwards. This study was approved by the institutional review board and specific patient consent was waived. Operations consisted of lobectomy, bilobectomy, pneumonectomy; combined operations including chest wall resection, bronchoplasty and arterioplasty were included. Patient selection criteria did not include DLCO prior to the late 1980s. Thereafter the selection criteria were relatively unchanged over time, and were based in part on age, performance status, cardiovascular status, spirometry and diffusing capacity. Recently, formal cardiopulmonary exercise testing with measurement of peak oxygen consumption has been used in patients considered to be borderline candidates for resection. The primary decision regarding operability throughout has been based on the surgeons' clinical judgement. For patients who underwent more than one major lung resection during the period of study, only the first operation was used for analysis of outcomes. Patients were managed according to clinical protocols in place at the time of the operations, including the more frequent use of induction therapy (usually two cycles of platinum-based chemotherapy and ≥60 Gy radiation therapy) in more recent years. Operative mortality was defined as mortality during hospitalization for lung resection or within 30 days of resection. Information on the use of postoperative adjuvant therapy was not available. Staging was performed according to the sixth edition of the manual of the American Joint Committee on Cancer (AJCC) [9]. Follow-up was updated in July 2011 via clinical records and the Social Security Death Index.
Data were examined for characteristics associated with overall (i.e. all-cause) mortality. Potentially important patient and disease characteristics were identified from prior analyses of this cohort [10,11]. Additional factors previously established as related to survival after lung surgery were also considered. For factors measured on a continuous scale, discrete categories were defined based on clinical risk considerations or previously defined groupings. As a descriptive empirical summary of the relationship of these factors to death, average annual hazard rates (number of deaths divided by person years at risk) were computed [12]. In particular, DLCO was grouped into 10-point increments of clinical interest, forming categories of <60%, 60–69%, 70–79% and ≥80%. The Cox proportional hazards model was used to examine associations with hazard of death for each factor individually [13]. Model diagnostics were used to identify the appropriate functional form (e.g. linear, quadratic, discrete categories, etc.) for covariates and the proportionality assumption [12]. Survival curves by categories of individual covariates were produced using the Kaplan–Meier estimator [14].
In multivariable Cox modelling, all covariates significantly associated with survival in the univariate analyses were included, and hazard ratios with 95% confidence intervals are reported from these models. DLCO was considered a linear continuous variable as well as categorized as previously defined. Because there was missing information among cases for most covariates, the method of van Buuren et al. [15,16] was used to perform multiple imputation. Briefly, in the multiple imputation approach, a probabilistic rule is used to impute possible values for individual missing covariate values. The rule is based on regression models for each covariate, with the other covariates serving as predictors. This process is repeated several times, creating replicates of the original data set with different imputed plausible values for the missing data. Each data set is then analysed in a standard fashion, followed by application of methodology to combine the parameter estimates from each separate analysis and compute appropriate standard errors. Because of the possible influence of stage and induction therapy on DLCO, we examined how the influence of DLCO might be variable by stage in two ways: first, we explored whether there is statistical evidence of a differential DLCO effect per stage (i.e. interaction effect) and also examined the effect of DLCO separately within stage groups. Second, we used stage as a stratification factor in the model, rather than simply adjusting for it, as this permits a different ‘baseline’ risk of death per stage and then estimates the incremental risk of DLCO. We performed bootstrap samples on each imputed data set in order to calculate a bootstrap estimate of the standard error of the DLCO hazard ratio. We then combined the DLCO hazard ratios and their associated bootstrap estimated standard errors across five imputed data sets, using the standard multiple imputation combining rules. Finally, to graphically summarize the effect on survival of specific covariates while accounting for other prognostic covariates, a method based on the Cox model and akin to direct adjustment was used [17,18]. The survival curves produced by this method represent the survival histories by groups defined by a given covariate when all other prognostic covariates are equally distributed between the groups.
RESULTS
Patient demographics and clinical characteristics are listed in Table 1. Eight patients had more than one major lung resection and only the first such resection was used for data analysis. The population is representative of a typical group of patients undergoing surgical therapy for lung cancer. During the period of study, there were significant increases in hypertension rate, mean performance status, obesity incidence and use of induction therapy over time [10,11]. At the same time, immediate preoperative tobacco use and surgery for advanced stages of disease decreased. Surgical details and outcomes are listed in Table 2. The historical number of pneumonectomies is striking, but this rate decreased from 31% in the first decade to 9.6% in the last decade (P < 0.001). The incidence and distribution of other variables are similar to those of other large case series, including the incidence of operative mortality, which decreased from 8.4% in the first decade to 3.7% in the most recent decade (P = 0.054). The median follow-up time from surgery was 115 months. Among the 854 unique patients with follow-up time information, there were 587 deaths. The median survival time was 51.9 months.
Table 1:
Preoperative characteristics of study population
| Category | Evaluable patients | Mean (SD) or percent affected |
|---|---|---|
| Male gender | 854 | 55% |
| Age at operation (years) | 853 | 63.0 (10.2) |
| Serum creatinine (mg/dl) | 629 | 1.1 (1.0) |
| Serum haemoglobin (g/dl) | 661 | 13.1 (1.6) |
| Serum albumin (g/dl) | 582 | 4.0 (0.4) |
| Hypertension | 848 | 40.6% |
| Body mass index <18.5 | 810 | 4.9% |
| Prior myocardial infarction | 837 | 10.9% |
| Diabetes mellitus | 846 | 14.3% |
| Any tobacco use | 849 | 91.8% |
| Performance status 0–1 | 834 | 83.7% |
| FEV1% | 807 | 81.8 (21.3) |
| DLCO% | 748 | 83.9 (22.2) |
| Pretreatment clinical stage | 853 | |
| I | 53.9% | |
| II | 19.6% | |
| III | 25.0% | |
| IV | 1.5% | |
| Induction chemotherapy | 776 | 8.5% |
| Induction radiation therapy | 783 | 10.6% |
FEV1: forced expiratory capacity in the first second; DLCO: diffusing capacity of the lung for carbon monoxide.
Table 2:
Perioperative characteristics of the study population
| Category | Evaluable patients | Percent affected |
|---|---|---|
| Operation | 854 | |
| Lobectomy | 74.2% | |
| Bilobectomy | 8.0% | |
| Pneumonectomy or completion pneumonectomy | 17.8% | |
| Surgical outcomes | 854 | |
| Mortality | 6.1% | |
| Pulmonary morbidity | 23.3% | |
| Cardiovascular morbidity | 15.8% | |
| Other morbidity | 17.7% | |
| Decade of operation | 854 | |
| 1980–1989 | 30.8% | |
| 1990–1999 | 31.7% | |
| 2000–2006 | 37.5% | |
| Final pathological stage | 853 | |
| 0, I | 58.6% | |
| II | 19.2% | |
| III | 20.9% | |
| IV | 1.3% |
Patient and disease characteristics were evaluated in relation to all-cause mortality risk. Greater age at surgery and more advanced disease stage were associated with increasing mortality risk. Those with more favourable performance status scores, females, persons of normal weight and non-/former smokers tended to have lower mortality risk. Patients with unfavourable creatinine and albumin levels and those with history of myocardial infarction had significantly greater mortality risk. With respect to spirometric function, patients with FEV1 <80% of predicted had a greater mortality risk (70–79%, 1.30; 60–69%, 1.59; <60%, 1.44). Gas exchange capacity, as determined by DLCO, also was related to all-causes mortality, with hazard of death increasing incrementally compared with those with a DLCO of ≥80% (70–79%, 1.12; 60–69%, 1.29; <60%, 1.35).
Considering factors related to mortality jointly, DLCO remained associated with mortality, with a 4% increase in risk of death per 10-point decrement in DLCO (Table 3). The effect of DLCO was independent of the decade of surgery. The interaction test of DLCO and stage did not formally indicate a differential effect of DLCO according to stage; however, separate estimates within stage groups suggested that the effect is small in early-stage patients and larger in late-stage patients, leading to the effect that we saw overall: stage I/II: 1.8% risk increase per 10-point decrement; stage III/IV: 7.4% risk increase per 10-point decrement. Owing to the smaller number of patients and failure events within subsets, statistical significance is reduced in both groups. A more efficient option that provides for different risks of death by stage but does implicitly assume a similar effect per stage is the stratified model. This model indicates a similar but slightly smaller DLCO effect than the model simply adjusting for stage: 3.7% risk increase per 10-point decrement.
Table 3:
Multivariable analysis of variables associated with hazard of death during long-term follow-up
| All patients |
Excluding operative deaths |
|||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age at operation (per 10 year increase) | 1.33 | 1.22–1.46 | 1.34 | 1.22–1.47 |
| DLCO% (per 10-point decrement) | 1.04 | 1.00–1.08 | 1.03 | 0.99–1.08 |
| FEV1% (per 10-point decrement) | 1.05 | 1.00–1.09 | 1.05 | 1.00–1.09 |
| Performance status 0/1 vs. 2 to 4 | 0.69 | 0.56–0.85 | 0.72 | 0.57–0.90 |
| Stage II vs. I | 1.95 | 1.57–2.42 | 1.94 | 1.54–2.43 |
| Stage III/IV vs. I | 2.78 | 2.28–3.38 | 2.94 | 2.40–3.61 |
| Creatinine (per unit increase) | 1.19 | 1.10–1.29 | 1.19 | 1.09–1.30 |
CI: confidence interval; FEV1: forced expiratory capacity in the first second; DLCO: diffusing capacity of the lung for carbon monoxide.
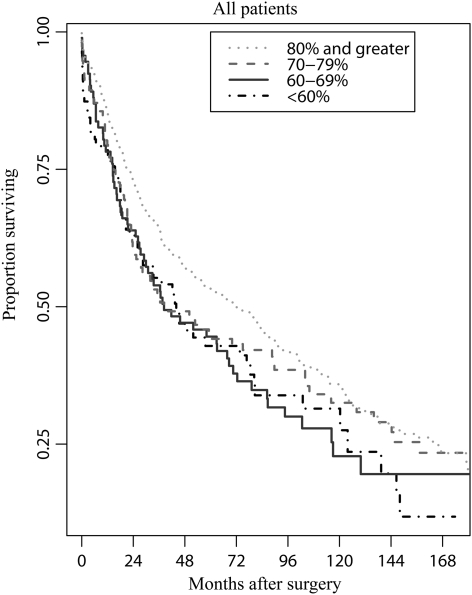
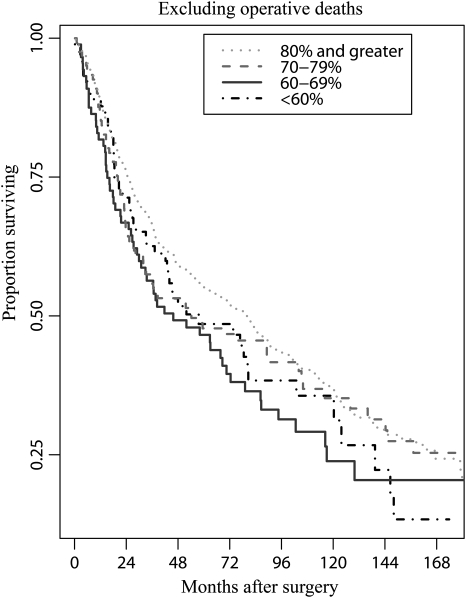
Excluding patients experiencing operative mortality, which is known to be associated with impaired DLCO, somewhat blunted but did not eliminate the association between all-cause mortality and DLCO (Table 3). The results of 1000 bootstrap samples were similar to our primary analysis (95% confidence interval 1.00 to 1.08; P = 0.051). The results suggest that our findings are not sensitive to large-sample approximations. We further examined DLCO to identify discrete risk groups, and in the adjusted model there was a suggestion of distinctly lower mortality risk for those with DLCO of ≥80%. Patients with DLCO < 80% of predicted had an ∼25% greater mortality risk (HR = 1.22, P = 0.03). This risk was similar to that associated with an FEV1 <80% (HR = 1.23, P = 0.02). The relative risks of all-cause mortality related to preoperative DLCO for all patients and for patients who survived the surgical hospitalization are depicted in Figs 1 and 2.
Figure 1:
Survival according to preoperative DCLO for all patients. Unadjusted Kaplan–Meier plots are shown; plots adjusted for other covariates are similar.
Figure 2:
Survival according to preoperative DCLO for patients not experiencing operative mortality. Unadjusted Kaplan–Meier plots are shown; plots adjusted for other covariates are similar.
DISCUSSION
Prediction of outcomes is valuable in making treatment recommendations and discussing those recommendations with patients. Most often, such discussions for surgical patients focus on immediate postoperative outcomes. Historically, there has been some disconnection between factors considered important by surgeons and those considered by patients in determining how acceptable surgical risk is [19]. There is increasing awareness among surgeons that issues such as quality of life and long-term survival are of primary interest to patients and their families. Efforts to incorporate these values into discussions about options for acute intervention are ongoing through the use of tools such as patient decision aids.
A number of factors are known to put lung cancer patients at excess mortality risk long term, including advanced age and cancer stage. Comorbidities that contribute to excess risk include poor performance status and chronic conditions such as coronary artery disease, diabetes and renal insufficiency. Lung spirometric function has long been recognized as a determinant of acute morbidity and mortality after lung resection [20,21], and is recognized as a determinant of all-cause mortality risk in the general population [22].
In contrast, other measures of lung function, including DLCO and assessment of maximum oxygen consumption during exercise (peak VO2), have been used primarily to assess acute postoperative risk after lung resection for cancer [23]. In fact, a prior publication from our group previously failed to demonstrate a relationship between DLCO and long-term survival after major lung resection [7]. Possible reasons for this include the relatively small numbers of patients evaluated in that study, use of dichotomized rather than continuous values for some analyses and lack of multiple imputation techniques for handing missing values. Recently, however, new evidence has come to light that impaired DLCO may contribute to excess mortality risk long-term after lung resection for lung cancer [8]. Because of the potential importance of this finding, we chose to re-evaluate the relationship between DLCO and long-term survival in patients undergoing resection for lung cancer in our patient population. The updated data set used in this study added 10 years of data collection and more than doubled the number of patients in the prior analysis, strengthening the possibility that relevant statistical relationships would be identified.
We identified a number of clinical factors that were associated with an increased risk of long-term mortality after major lung resection in our patient population, including advanced cancer stage, increasing patient age, poor performance status, coronary artery disease, renal insufficiency and poor spirometry. In addition, our analysis confirmed that decreased DLCO is an independent determinant of an increased risk of long-term mortality in patients who have undergone major lung resection for cancer. Our findings were similar to those of Liptay et al. in terms of the magnitude of the effect DLCO had on long-term mortality risk. Our study did not separately analyse cause-specific mortality, and so no comparison between the studies could be made in that regard. The work by Liptay et al. included a limited exploration of the interactions of stage and spirometry with the effects of DLCO. In contrast, our study included a detailed exploration of covariates, including important determinants of survival such as cancer stage, patient age, performance status and so on and conclusively demonstrated that DLCO is an independent determinant of long-term mortality risk in patients after major lung resection for cancer. Liptay et al. identified a preoperative DLCO of 40% as a cutoff value identifying patients at increased mortality risk. In our study, although there was suggestion of a gradient of risk over a range of DLCO values, there appeared to be a clear differential effect of DLCO on mortality risk at a cutoff of 80% predicted. It is not clear how this difference can be reconciled based on available data, but differences in patient characteristics and the basing of their partition on other-cause (i.e. not cancer) deaths only may offer possible explanations.
It is not currently known whether preoperative or postoperative DLCO is more accurate in estimating long-term mortality risk. If the latter is more accurate, it suggests that limited resection rather than standard lobectomy may be considered in selected patients in an effort to achieve the curative effects of surgery without compromising long-term survival for physiological reasons. Such information may help in developing treatment algorithms that include surgical and alternative therapies (radiation therapy, radiofrequency ablation, etc.) for clinical early-stage cancers amenable to different modalities.
There are a number of potential criticisms to the findings in this study. We recognize that acute and long-term outcomes of patients operated over a period lasting almost three decades will vary according to surgical quality and management techniques that improved over time. Our analysis did not demonstrate any effect of the year of surgery on the influence of DLCO on long-term mortality risk. Although the sample size is relatively large, this remains a single-institution experience, with the attendant concerns regarding applicability to other patient populations. In addition, ours is one of few institutions that can provide data regarding DLCO and long-term outcomes after major lung resection over such a long time interval, so validating these results may pose challenges.
In conclusion, we have confirmed that DLCO is an independent prognostic factor for long-term mortality risk in patients who have undergone major lung resection for cancer. This finding should be of use in developing algorithms for determining optimal therapy for patients with early-stage lung cancer, and will be of value to efforts to model outcomes of lung resection. Further work is needed to determine whether standard anatomic resections such as lobectomy, which adversely affect DLCO more than lesser resections, unduly influence long-term mortality risk.
FUNDING
Support for this research was partially provided by Public Health Service grant NCI P30-CA-14599 from the National Cancer Institute, US Department of Health and Human Services.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr G. Friedel (Gerlingen, Germany): Surprisingly, diffusing capacity has been measured routinely in Chicago since 1980, when no one in Europe wasted any thoughts on diffusing capacity. With your excellent data, you could easily compare your group with a group of patients without malignant disease and show whether a decrease in diffusing capacity also increases the mortality in those patients. The other question is, do you perform the functional staging, as required now? This means that a DLCO below 60% predicted requires a lung function stress test.
I have a short comment. You pose the question whether standard anatomic resections like lobectomy influence quality of life unduly. In malignant disease, long-term survival is the main and first goal of every treatment. The median long-term survival of lung cancer is bad enough and we should have specific reasons to deviate from the proved way. Nevertheless, the work of Dr Ferguson is important and future-oriented because cardiorespiratory function tests will play a major role in the definition and identification of risk groups in the future.
Dr Ferguson: The relationship of diffusing capacity to long-term survival, in a manner similar to that of spirometry, has been demonstrated in patients with COPD, but I don't know that it has been investigated adequately in a normal population. So I think that question deserves further investigation. We are increasingly frequently getting measurements of peak oxygen consumption during exercise when we find patients with either impaired spirometry or impaired diffusing capacity, and find that this is probably a more reliable predictor of operative risk than either of the other two alone. So that's something that we're doing more often, as recommended by the recent joint consensus from the European Respiratory Group and the ESTS.
Dr D. Wood (Seattle, WA, USA): Mark, we have discussed this many times, so you're probably disappointed to see me up again, but I have actually simple questions.
You recognized or made clear the change in outcomes over the long time window of your study. How do you see that this may influence the outcomes? You have substantially different mortality and morbidity in your most recent decade than your earliest decade. The second question is that we have increasingly, largely due to your studies, added DLCO to FEV1 to evaluate patients, but in your study, you show a similar effect on survival. So is FEV1 in fact a reasonable, cheaper, easier surrogate for the same outcome?
Finally, you talked about looking at this with a larger dataset, and I know that DLCO has been one of the most problematic variables collected in the STS General Thoracic Database. I'm not familiar with how frequently it has been obtained, and I wonder if you know how effective the STS database will be in looking at this area.
Dr Ferguson: We have been very concerned about the time factor, as you alluded to, in analysing these data, and so one of the main reasons I engage statisticians rather than doing this myself is to evaluate the relationship of the year of surgery to the outcomes. One of the things that they spend a lot of time on is determining whether the year or decade of surgery influences these outcomes, and it doesn't come close to having an effect. DLCO and FEV1 are fairly independent of each other; there's no close correlation between the two, interestingly. So if you try to draw a regression line and put up all the points, it's basically a circular cloud and the regression line is almost horizontal. And in the statistical analysis, I presented the multivariable analysis, and these were completely independent predictors of outcome. So I think they have an additive effect as opposed to one being a surrogate for the other.
I think the STS database holds considerable promise for trying to validate these data, although in our analysis of diffusing capacity that we finished a few years ago, the DLCO data were complete in only 57% of the patients. I think that percentage is probably going to be increasing, and I think those numbers will be large enough to allow us to analyse long-term outcomes.
REFERENCES
- 1.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer. 2002;37:95–101. doi: 10.1016/s0169-5002(02)00014-4. doi:10.1016/S0169-5002(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 2.Ploeg AJ, Kappetein AP, van Tongeren RB, Pahlplatz PV, Kastelein GW, Breslau PJ. Factors associated with perioperative complications and long-term results after pulmonary resection for primary carcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:26–9. doi: 10.1016/s1010-7940(02)00655-3. doi:10.1016/S1010-7940(02)00655-3. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T, Sekine Y, Yamada Y, Suzuki H, Yasufuku K, Yoshida S, et al. Long-term surgical outcome in patients with lung cancer and coexisting severe COPD. Thorac Cardiovasc Surg. 2009;57:339–42. doi: 10.1055/s-0029-1185571. doi:10.1055/s-0029-1185571. [DOI] [PubMed] [Google Scholar]
- 4.Petty TL. John Hutchinson's mysterious machine revisited. Chest. 2002;121:219S–23S. doi: 10.1378/chest.121.5_suppl.219s. doi:10.1378/chest.121.5_suppl.219S. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85:1158–65. doi: 10.1016/j.athoracsur.2007.12.071. doi:10.1016/j.athoracsur.2007.12.071. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson MK, Gaissert HA, Grab JD, Sheng S. Pulmonary complications after lung resection in the absence of chronic obstructive pulmonary disease: the predictive role of diffusing capacity. J Thorac Cardiovasc Surg. 2009;138:1297–302. doi: 10.1016/j.jtcvs.2009.05.045. doi:10.1016/j.jtcvs.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Olak J, Ferguson MK. Diffusing capacity predicts operative mortality but not long-term survival after resection for lung cancer. J Thorac Cardiovasc Surg. 1999;117:581–7. doi: 10.1016/s0022-5223(99)70338-7. doi:10.1016/S0022-5223(99)70338-7. [DOI] [PubMed] [Google Scholar]
- 8.Liptay MJ, Basu S, Hoaglin MC, Freedman N, Faber LP, Warren WH, et al. Diffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancer. J Surg Oncol. 2009;100:703–7. doi: 10.1002/jso.21407. doi:10.1002/jso.21407. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th edn. New York, NY: Springer; 2002. pp. 167–81. [Google Scholar]
- 10.Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classification trees and multiple imputation techniques. Eur J Cardiothorac Surg. 2008;34:1085–9. doi: 10.1016/j.ejcts.2008.07.037. doi:10.1016/j.ejcts.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson MK, Vigneswaran WT. Changes in patient presentation and outcomes for major lung resection over three decades. Eur J Cardiothorac Surg. 2008;33:497–501. doi: 10.1016/j.ejcts.2007.12.023. doi:10.1016/j.ejcts.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Klein JP, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. 2nd edn. New York: Springer; 1997. [Google Scholar]
- 13.Cox DR. Regression models and life tables. J Royal Stat Soc (Series B) 1972;34:187–229. [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. doi:10.2307/2281868. [Google Scholar]
- 15.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–41. [Google Scholar]
- 16.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. doi:10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35:437–43. doi: 10.1016/0021-9681(82)90058-3. doi:10.1016/0021-9681(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 18.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–74. doi: 10.1016/0021-9681(82)90019-4. doi:10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 19.Cykert S, Kissling G, Hansen CJ. Patient preferences regarding possible outcomes of lung resection: what outcomes should preoperative evaluations target? Chest. 2000;117:1551–9. doi: 10.1378/chest.117.6.1551. doi:10.1378/chest.117.6.1551. [DOI] [PubMed] [Google Scholar]
- 20.Gaensler EA. Analysis of the ventilatory defect by timed capacity measurements. Am Rev Tuberc. 1951;64:256–78. doi: 10.1164/art.1951.64.3.256. [DOI] [PubMed] [Google Scholar]
- 21.Gaensler EA, Cugell DW, Lindgren I, Verstraeten JM, Smith SS, Strieder JW. The role of pulmonary insufficiency in mortality and invalidism following surgery for pulmonary tuberculosis. J Thorac Surg. 1955;29:163–87. [PubMed] [Google Scholar]
- 22.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the first National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–93. doi: 10.1136/thorax.58.5.388. doi:10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson MK, Lehman AG, Bolliger CT, Brunelli A. The role of diffusing capacity and exercise tests. Thorac Surg Clin. 2008;18:9–18. doi: 10.1016/j.thorsurg.2007.11.001. doi:10.1016/j.thorsurg.2007.11.001. [DOI] [PubMed] [Google Scholar]