Abstract
The vascular toxicity of inhaled agents may be caused by soluble factors that are released into the systemic circulation. To confirm this in a straightforward manner, we obtained plasma from healthy human volunteers before and after exposure to diesel exhaust (DE) and nitrogen dioxide (NO2). Plasma samples were obtained from human volunteers exposed to 100 μg/m3 DE or filtered air for 2 h. A second cohort was exposed to 500 ppb NO2 or filtered air in an identical protocol. Primary human coronary artery endothelial cells (hCAECs) were grown to confluence and treated for 24 h with a 10 or 30% (in media) mixture of plasma obtained before, immediately post or 24 h postexposure to pollutant exposures. Messenger RNA (mRNA) was isolated from hCAECs following the incubation and probed for intracellular cell adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) expression. ICAM-1 mRNA expression was increased by plasma obtained at both timepoints following the NO2 exposures. VCAM-1 was significantly elevated in cells treated with plasma obtained 24 h following diesel exposure and at both timepoints following NO2 exposure. Interleukin-8 protein was elevated in the hCAEC supernatant when cells were incubated with plasma from NO2 exposures. These data indicate that proinflammatory circulating factors are elevated acutely following exposure to both DE and a primary component thereof, NO2. These functional translational assays offer novel approaches to assessing the cardiovascular risk associated with air pollution exposure.
Keywords: diesel, nitrogen dioxide, cardiovascular, air pollution, endothelial activation, ICAM-1, VCAM-1, translational, interleukin-8
The well-established relationship between air pollution and cardiovascular mortality lacks mechanistic clarity. In particular, the pathways that link pulmonary exposure with cardiovascular health effects, such as the progression of atherosclerosis, remain controversial. Three principal hypotheses have been developed to explain the commutation of toxicity from pulmonary exposures to the systemic vasculature, and each has some measure of supportive data. First, direct systemic translocation of particulate matter (PM), for instance, has been shown by several studies, and there is evidence that direct exposure to PM negatively impacts both cardiomyocyte and endothelial function (Miller et al., 2009; Simkhovich et al., 2007). Second, neurally mediated responses have been conjectured in studies of concentrated ambient particulates and diesel exhaust (DE) exposure, where a role for transient receptor potential channels have been established in driving cardiac oxidative stress (Ghelfi et al., 2008; Rhoden et al., 2005) and a heightened sensitivity to cardiac arrhythmias (Hazari et al., 2011). A third likely pathway related to circulating factors, emerging from either soluble components of PM or from molecular mediators released from pulmonary responses, has only been demonstrated in a limited manner (Kido et al., 2011). Unfortunately, there are few experimental approaches that are capable of elucidating cellular mechanisms of this latter pathway.
Inflammatory activation of endothelial cells following stress or injury is a hallmark event in the early stages of atherosclerosis (Zhang et al., 2010). This activation, often induced by cytokines via nuclear factor-kappa beta (NF-kB), leads to the presentation of key proteins involved in the recruitment of leukocytes, including adhesion molecules and chemotactic factors (Collins et al., 1995). Such mechanisms play a role both in the early onset of disease as well as in late stage events such as plaque rupture. Among the major molecules involved, vascular cell adhesion molecule-1 (VCAM-1) and intracellular cell adhesion molecule-1 (ICAM-1) are consistently reported as both early responders and essential to binding of circulating leukocytes.
In the present study, we hypothesized that inhalation of air pollutants could induce circulating proinflammatory factors. We developed a straightforward method to treat primary human coronary artery endothelial cells (hCAECs) with plasma from human subjects exposed to a common source pollutant, diesel engine exhaust, and a primary component thereof, nitrogen dioxide (NO2). We then probed for markers of inflammatory response in those cells and released into the supernatant. Significant responses were observed for individuals exposed to either DE or NO2. Sham exposures (filtered air) in the exact same individuals failed to elicit any response at all. This method for investigating the inflammatory potential of plasma holds a great promise for (1) studying both the relative potency of air pollution mixtures, (2) developing a translational platform for risk extrapolation, and (3) identifying soluble components and biological mechanisms that drive the inflammatory responses.
MATERIALS AND METHODS
Exposures.
All exposures were conducted with approval from the U.S. Environmental Protection Agency Institutional Review Board; subsequent analysis of de-identified plasma was conducted as an exempted protocol with approval from the University of New Mexico Human Research Review Committee. Healthy adults were recruited and exposed as previously reported (Lund et al., 2011); no subjects were on prescription medications during the study. Table 1 highlights key demographic data for the two cohorts used in these studies. Diesel exposures were for 2 h at 100 μg/m3 with intermittent moderate exercise (Lund et al., 2011). DE was generated by a Cummins engine operating at idle conditions using a certified commercial #2 fuel (ChevronPhillips). The principal components of diesel for these exposures were: 106 ± 9 μg PM/m3; 4.7 ppm NO2; 0.8 ppm NO2; 2.8 ppm CO; and 2.4 ppm total hydrocarbons; PM mass mean aerodynamic diameter was 0.10 μm. A more detailed chemical analysis of the diesel exposure atmosphere is provided by Sobus et al. (2008).
TABLE 1.
Subject Demographics
| Diesel-exposed subjects | NO2-exposed subjects | |
| Age | 24.9 ± 4.5 | 25.3 ± 5.5 |
| Gender (M/F) | (3/4) | (5/2) |
| Weight (kg) | 67.2 ± 9.4 | 77.3 ± 13.2 |
| Body mass index (kg/m2) | 23.9 ± 1.9 | 24.9 ± 2.7 |
| Body surface area (m2) | 1.8 ± 0.2 | 1.9 ± 0.2 |
The concentration of NO2 was 500 ppb (slightly less than that in the diesel mixture), also for a duration of 2 h. All subjects underwent both a pollutant (diesel or NO2) and filtered air (control) exposure on separate occasions, permitting pairwise comparisons for all measures; a washout period of at least 4 weeks between exposures was implemented for all subjects. Blood was drawn into EDTA-containing tubes and promptly spun to collect plasma for each subject before exposures and immediately after and 24 h after exposures. Thus, each subject yielded six samples for analysis.
The samples provided for the present study had been banked at −80°C for ∼2 years prior to use. A limited supply of plasma was available from the diesel-exposed cohort, allowing for analysis of a total of seven subjects that had matching diesel and filtered air exposures. Likewise, the NO2 exposure study yielded matched air and NO2-exposed samples from exactly seven individuals.
Cell culture.
hCAECs were obtained from a commercial vendor (Lonza) and maintained according to manufacturer’s recommendations at 37°C in 5% CO2-95% room air with complete microvascular endothelial growth medium-2 (EGM-2 MV) supplemented with 5% fetal bovine serum and antibiotics (gentamycin and amphotericin-B). All experiments were performed between passages 3–6. Upon final plating, approximately 5 × 103 cells were seeded to each well of a 48-well plate (Costar) and grown to confluence, to mimic cell-cell interactions in the vasculature. Assays were batched by individual (i.e., all filtered air and pollutant plasma samples for individuals were run at the same time) to enhance consistency and comparability across studies.
Plasma-conditioned hCAECs.
For the volume-limited diesel exposure samples, plasma was added to the wells at a ratio of 1:9 (10%) with complete EGM-2 MV culture media in duplicate (containing 5% fetal bovine serum). Plasma was added in a similar fashion for NO2 exposure samples at a ratio of 1:9 (10%) or 3:7 (30%) in duplicate. Treated cells were incubated ∼24 h for both studies.
C reactive protein and tumor necrosis factor α–conditioned hCAECs.
Cells were plated and cultured as described above. Confluent cells were treated in duplicate with human recombinant C-reactive protein (CRP) (Sigma) and human recombinant tumor necrosis factor α (TNF-α) (Sigma) for a representative range of concentrations for each compound (1–100 μg/ml and 0.015–1.5 ng/ml, respectively) for 4 or 24 h. To more precisely compare the more modest responses generated by human plasma, we subsequently conducted an additional exposure of hCAECs to CRP at concentrations of 10, 25, 50, 75, and 100 μg/ml for 24 h, which provided a more detailed response range from which to derive a linear model. As TNF-α at 24 h provided some response at all three doses, further interpolations were not conducted for this relationship.
ELISA for supernatant and plasma protein.
Cell supernatants were collected prior to RNA purification and measured using commercially available monocyte chemotactic protein-1 (MCP-1) and interleukin-8 (IL-8) ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Supernatants were diluted as needed using recommended diluent for the assay. The plasma levels of the lectin-like receptor for oxidized low-density lipoprotein (LOX-1) was performed with a commercially available kit (R&D Systems) as previously described (Lund et al., 2011).
RNA purification and quantitative PCR.
For the NO2 exposure samples, cell supernatants were collected and hCAECs were washed with PBS, lysed, and immediately cell lysates from duplicate samples were pooled and collected for RNA purification. Total RNA was isolated from pooled samples using RNeasy Mini Prep Kits (Qiagen), and RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems) prior to quantitative real-time PCR (qPCR) assessment of endothelial adhesion markers. Amplification of target message was performed in TaqMan Universal Master Mix following manufacturer’s recommended conditions with TaqMan gene expression assays for ICAM-1 (Hs00164932_m1) and VCAM-1 (Hs01003372_m1) with endogenous TATA box–binding protein (Hs0042762_m1) using Applied Biosystems 7900HT Fast Real-Time PCR System. Relative gene expression was analyzed by the 2−ΔΔCT method (Livak and Schmittgen, 2001) using a relative amount of messenger RNA (mRNA) for each sample normalized to TATA box–binding protein.
Statistics.
All data were tested for normal distributions and with few exceptions fit this assumption. Data for qPCR and plasma/media protein measurements were analyzed by a pairwise two-way ANOVA with Newman-Keuls multiple comparison post hoc testing using GraphPad Prism (v 5.02). Probability values less than 0.05 were considered significant. For the modeling analysis of CRP and TNF-α equivalents, a linear regression (y = mx + b) was determined for the relationship between CRP/TNF-α and ICAM/VCAM expression using GraphPad as well.
RESULTS
Adhesion Molecule Gene Expression Changes
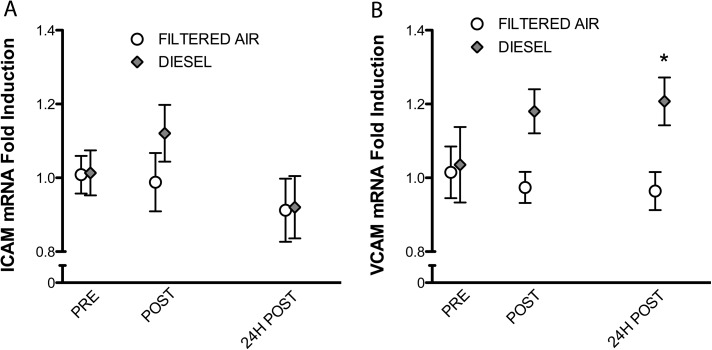
Using qPCR techniques, we probed the relative expression of tandem mRNA markers of endothelial cell inflammatory response, ICAM-1, and VCAM-1, in cells incubated with plasma from before or after inhalation exposure to diesel or NO2 (or their respective controls). Plasma samples obtained following the diesel exposure had no effect on ICAM-1 expression but led to a significant (∼20%) increase in the expression of VCAM-1 as compared with both prediesel exposure samples and pre- and postfiltered air exposure samples (Fig. 1).
FIG. 1.
Impact of diesel emissions inhalation on plasma inflammatory potential. Absolute quantitative PCR for adhesion molecules ICAM-1 and VCAM-1 from endothelial cells incubated for 24 h in media treated with human plasma at 10% per volume. Each value represents the mean ± SE of seven individuals. Significance was determined by a two-way ANOVA with Bonferroni post hoc tests; asterisks (*) indicate difference from control values (p < 0.05).
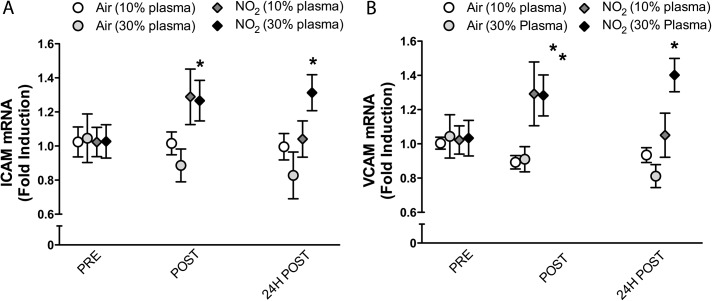
For plasma obtained following NO2 exposure, increased ICAM and VCAM mRNA were evident in endothelial cells incubated with both the immediately postexposure and 24-h postexposure plasma samples (Fig. 2). By treating with either a 1:9 or 3:7 plasma:media ratio, we observed stronger outcomes at the higher concentration, especially for the 24-h timepoint, which suggests that a dose-related phenomenon may be involved. The magnitude of change was slightly greater than that of DE (∼30–40% increase), but the concentration of plasma was essentially threefold greater.
FIG. 2.
Impact of NO2 inhalation on plasma inflammatory potential. Absolute quantitative PCR for adhesion molecules ICAM-1 and VCAM-1 from endothelial cells incubated for 24 h in media treated with human plasma at 10 or 30% per volume. Each value represents the mean ± SE of seven individuals. Significance was determined by a two-way ANOVA with Bonferroni post hoc tests; asterisks (*) indicate difference from control values (p < 0.05).
Supernatant IL-8 and MCP-1 Changes
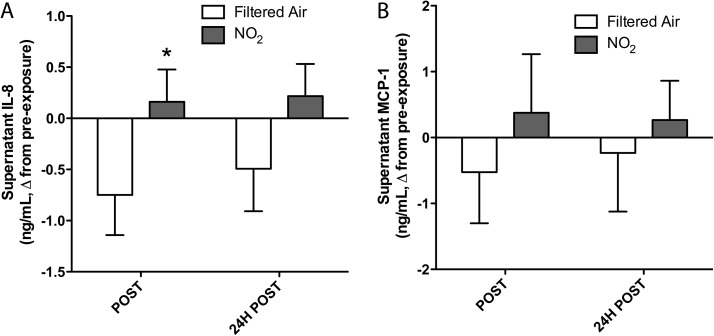
To determine whether proinflammatory stimulus from the plasma after NO2 exposure led to release of chemotactic cytokines, we assessed the levels of IL-8 and MCP-1 in the hCAEC supernatant following the 24-h incubation with plasma samples. IL-8 was increased in a modest, but significant manner (Fig. 3); two-way ANOVA confirmed a potential effect of NO2 exposure (p = 0.035). MCP-1, however, was not significantly altered by the treatment.
FIG. 3.
Difference from controls in supernatant levels of IL-8 (A) and MCP-1 (B) released by endothelial cells following the 24-h incubation with a 30% plasma in media mixture. A two-way ANOVA revealed a significant effect of exposure for IL-8 trends (p = 0.037) but not for MCP-1.
Comparing Responses With Known Inflammatory Mediators
We applied various concentrations of CRP and TNF-α to endothelial cells to derive a relationship between concentrations of these known inflammatory mediators (and risk factors for cardiovascular disease) and the magnitude of increased expression of ICAM-1 and VCAM-1. Concentrations for both mediators were selected based on circulating levels in healthy individuals and patients with clinical cardiovascular disease. Both TNF-α and CRP expectedly increased the mRNA expression of both adhesion molecules (Fig. 4). Interestingly, both mediators induced more potent ICAM responses at the 4-h treatment than the 24 h, which suggests that the responses seen from human plasma samples may have been diminished at 24 h compared with earlier timepoints.
FIG. 4.
Reference risk factors, CRP, and TNF-α, induce ICAM-1 (A–C) and VCAM-1 (D–F) at physiologically relevant concentrations. Treatments of 4 and 24 h were used to assess the temporality of the effects. CRP elevated ICAM and VCAM significantly in the range of 10–100 μg/ml. TNF-α induced significant elevations in ICAM and VCAM at 24 h in the lowest concentration (0.015 ng/ml) and at 4 and 24 h for all higher concentrations. The linear portion of the dose-response range was modeled to compared the relative ICAM-1 (C) and VCAM-1 (F) response of DE or NO2 to that of CRP to derive an estimate of biological equivalents.
As a simple extrapolation, we modeled the relationship between CRP concentration and mRNA response in the linear portion of the 24-h treatment relationship and then derived the relative change in ICAM-1 and VCAM-1 from diesel (12%, n.s., and 21% increase, respectively) and NO2 (31 and 40% increase, respectively) exposures, with the appreciation that these effects were induced with plasma diluted 1:9 (diesel) and 3:7 (NO2) with cell culture media. Based on the linear equations, we estimated that the acute effect of diesel inhalation was proportional to CRP increases of 11–25 μg/ml, whereas the response to NO2 was proportionate to 28–44 μg/ml (Figs. 4C and F). Conducting a similar process for the TNF-α–induced response of ICAM and VCAM, we find that diesel elicited an inflammatory response proportional to 0–16 pg/ml TNF-α, whereas NO2 induced responses comparable to 13–39 pg/ml TNF-α. The 12% response for the diesel effect on ICAM was not significant, but the mean value was used for this exercise. Notably, this level of response is also too low for the linear regression results to predict a positive response.
Because of the limitations on sample volume, it was not possible to assess CRP levels in the plasma, but plasma levels of TNF-α had been measured as a routine part of these exposure studies. No exposure-related trends were noted, and levels for all subjects across all exposure timepoints averaged 1.33 ± 0.39 pg/ml for the diesel study and 0.75 ± 0.09 pg/ml for the NO2 study. Thus, these low levels would not be likely to account for the effects seen on the hCAECs, in terms of ICAM and VCAM expression.
LOX-1 Levels
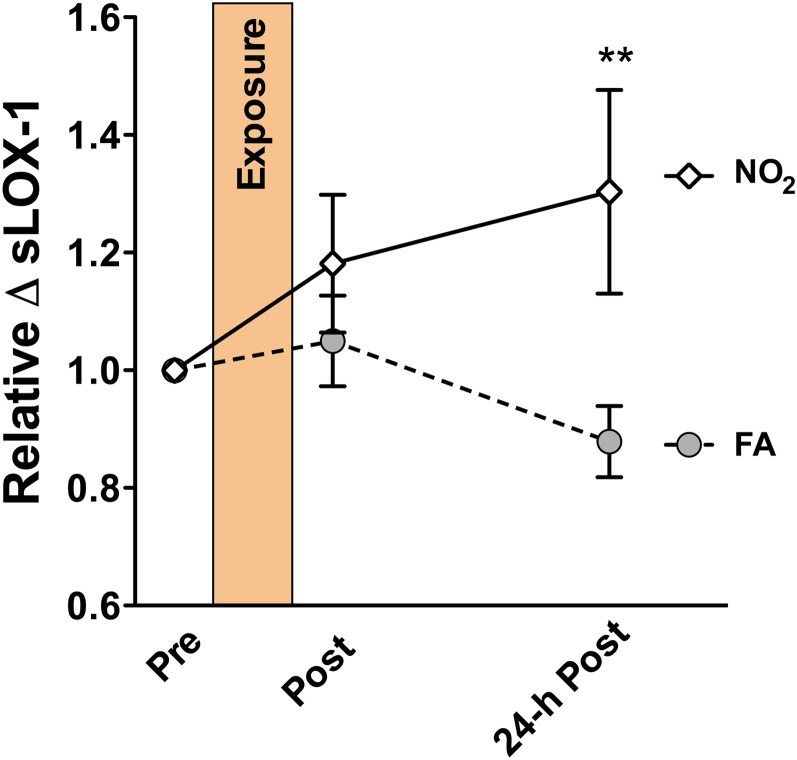
In addition to measuring the plasma-mediated endothelial cell inflammatory markers, we also assessed soluble LOX-1 protein in the plasma, which has been shown to be elevated following DE exposures in humans and following a number of exposures in rodents (Kodavanti et al., 2011; Lund et al., 2011). Consistent with previous studies, we observed a significant interindividual variance in the absolute levels of LOX-1, ranging from roughly 10 pg/ml to well over 2000 pg/ml. However, the influence of NO2 exposure on the relative levels was still evident by considering the relative increase in LOX-1 from preexposure to postexposure. As such, we observed an approximately 30% increase in soluble LOX-1 levels following NO2, paired with a roughly 10% reduction in soluble LOX-1 levels due to filtered air exposure (Fig. 5). This amounts to a significant difference in soluble LOX-1 levels at 24 h following the exposure. These trends, both in terms of the increases related to the inhaled pollutant and decreases related to inhalation of filtered air, are consistent with our previous studies with DE (Lund et al., 2011) and document a complementary in vitro index for cardiovascular risk factors in plasma samples obtained from humans exposed to diesel and NO2.
FIG. 5.
Relative plasma levels of soluble LOX-1 from subjects exposed to air and NO2. Values were normalized to preexposure (baseline) values for each subject. The samples obtained 24-h post-NO2 exposure had significantly (p < 0.01) elevated.
DISCUSSION
Our findings suggest that exposure to diesel or NO2 can cause upregulation of proinflammatory factors in the circulation. As a result, the plasma as a whole has the capacity to activate an early inflammatory response in endothelial cells, typified by increased expression of ICAM and VCAM and release of IL-8, which are consistent with initial events in the development of vascular disease. In parallel, we observed increased levels of LOX-1, which has previously been shown to be elevated following diesel inhalation (Lund et al., 2011). As discussed below, we speculate that the presence of sLOX is representative of an activated pathway, rather than a mediator of toxicity.
The value of the present ex vivo/in vitro methodology is in its translational nature. It is not feasible to directly obtain endothelial samples from human subjects, outside of invasive biopsies that are typically done only in the presence of a significant clinical indication. Using a homogeneous lineage of hCAECs provided a tightly controlled model that enhanced the study design. The plasma obtained from humans is unavoidably quite variable; yet we were surprised to see highly conserved expression of adhesion molecules in the endothelial cells incubated with the preexposure plasma samples. We derived several details for the assay design from a recent study that found postprandial conditions to be stressful to endothelial cells (Spallarossa et al., 2008); therefore, we suspect that the strict control of the exposure protocol was essential to our being able to ascertain the ICAM-1 and VCAM-1 increases. The format of the assay allowed us to compare both the time frame of adhesion molecule mRNA induction as well as consider a dose response to the plasma; both factors affected the expression. Lastly, the present methodology ensures that the exposure route and concentration of pollutant are biologically relevant, as opposed to incubating endothelial cells directly with particles or gases that are incapable of achieving the systemic concentrations typically required to elicit a biological response.
Expression of major adhesion molecules is central to both early vascular disease initiation as well as progression of advanced plaques (Zhang et al., 2010). Air pollution has been shown to induce rolling and adhering of immune cells in otherwise healthy animals (Nurkiewicz et al., 2006), and numerous studies have documented enhanced vascular lesion inflammation following PM (Sun et al., 2005; Suwa et al., 2002) and diesel inhalation (Bai et al., 2011; Campen et al., 2010). Particle-induced IL-8 release has also been documented when human umbilical vein endothelial cells were exposed to conditioned media from DE particle–exposed macrophages (Shaw et al., 2011). Weldy et al. (2011) recently observed that the supernatant from diesel PM–exposed macrophages could elicit an upregulation of MCP-1 and inducible nitric oxide synthase mRNA in endothelial cells. Collectively, the works by Shaw et al. (2011) and Weldy et al. (2011) highlight the important interactions between secondary reactive intermediates and endothelial cells that likely drive inflammatory responses. Future efforts to discriminate the cells and components in the lung that have the most vital contribution to circulating proinflammatory molecules will naturally be highly valuable.
The present findings are consistent with the idea that the vascular endothelium must be activated to induce the recruitment of leukocytes to the vascular lesion. Moreover, these results imply that a circulating factor must be mediating, at least in part, the endothelial response to air pollutants. For one, neural mechanisms are not involved in the cell culture model used, and secondly, the pulmonary exposure to NO2 eliminates the possibility that bloodborne particulate matter (or chemical components thereof) played a role. In a mouse study, Araujo et al., 2008 clearly demonstrated that high density lipoprotein from particulate matter–exposed mice could be modified in a way that reduced its antiinflammatory properties, but few other studies have addressed humoral factors as mediators of endothelial dysfunction. The present findings, however, are not to the exclusion on neurally mediated mechanisms, which have been reported in a number of compelling studies (Hazari et al., 2011; Rhoden et al., 2005).
The temporal relationship between LOX-1 and endothelial activation suggests that soluble LOX-1 is unlikely to be the driver of the endothelial response, but rather an important cog in the pathway. Other reports have noted a few potential intermediates such as IL-6 after PM instillation (Kido et al., 2011) and IL-1b and IL-6 after exposure to forest fire smoke (Van Eeden et al., 2001), both of which have a high likelihood of driving vascular responses. Had we observed substantial elevations in LOX-1 immediately postexposure, it might be suggestive of this protein as a mediator. However, it seems more likely that LOX-1 is activated by another mediator and released as a soluble form. Previous mechanistic work in mice suggested that LOX-1 plays a key role in mediating the extrapulmonary effects of inhaled diesel and gasoline exhausts, but it was not clear where the LOX-1 was activated or by what ligand (Lund et al., 2011). Given ample amounts of biological sample, it would be somewhat straightforward to mechanistically test the role of the endothelial LOX-1 or other receptors implicated in the vascular response (e.g., TLR4; Kampfrath et al., 2011).
Toxicological research on the cardiovascular impact of NO2 is quite limited, despite a number of epidemiological studies that report associations between NO2 and cardiovascular disease outcomes (Wellenius et al., 2005; Zhang et al., 2011). More specifically, NO2 associates with increased incidence in myocardial infarction in a number of reports using a variety of study designs (Rich et al., 2010; Rosenlund et al., 2006; Zanobetti and Schwartz, 2006). In a recent ecological panel study, Delfino et al. (2008) identified a relationship between NO2 (among other copollutants) and circulating CRP and TNF-α in a cohort of individuals with coronary artery disease. Though not always significant, the increase in TNF-α levels ranged from 0.06 to 0.19 ng/ml per interquartile range of NO2 (roughly 14 ppb), which corresponds with a substantial (twofold to fourfold) increase in adhesion molecule expression in cultured coronary endothelial cells in the present study. Quite frequently, the trends related to NO2 are dismissed as a marker of vehicle emissions exposure. The findings of the present study, however, lend a degree of biological plausibility to the consideration of NO2 as an independent driver of adverse cardiovascular outcomes.
In perhaps the most straightforward study on the vascular effects of NO2, Langrish et al. (2010) found no impact on brachial artery reactivity in humans, effectively dispelling any theory that previously observed vascular effects of DE (Mills et al., 2005; Törnqvist et al., 2007) were related to this principal gaseous component. The absence of vasoactivity effects by NO2 seems to be at odds with the current study, but this is only due to our assumption that the mechanisms leading to upregulation of adhesion molecules, presumably through NF-kB activation (Collins et al., 1995; Roebuck, 1999), are related to endothelial nitric oxide synthase uncoupling or related pathways for endothelial dysfunction (Knuckles et al., 2008). These outcomes may not be linked at all with respect to the mechanisms of inhaled NO2 or it may be that subtle increases in adhesion molecule expression may precede clinically observable decrements in nitric oxide synthase function. Alternatively, the present methodology may allow for tighter control of a number of variables, thus enhancing the sensitivity of the assay compared with vasoactivity measures.
We observed that the endothelial cell adhesion molecule response to diluted plasma was equivalent to the effect of 11–44 μg/ml CRP, depending on the end point and exposure in question. Because it is not clear which cytokine mediators of inflammation may be vital in driving the endothelial activation, we chose to compare the pollutant-induced endothelial responses with responses to inflammatory cytokines that have been well characterized in terms of cardiac risk. Although it would be myopic to draw direct parallels between the impact of DE/NO2 exposures expressed as “CRP equivalents” and the actual cardiovascular risk of clinical CRP values, this exercise provides a valuable comparison with a pathophysiological stimulus that is also a known cardiovascular disease risk factor. Because CRP is a risk factor for chronic disease, the acute responses may not be relevant to such risk assessment. However, exposure to diesel or NO2 is likewise not an acute, isolated event, as we are exposed to air pollutants throughout our life span. Further development of this method may be valuable for determining cardiovascular risk of environmental exposures.
Our findings demonstrate a novel methodology to explore the vascular proinflammatory properties in plasma caused by toxicant exposures in humans. Although the present application was limited to two experimental exposures, DE and NO2, the ex vivo/in vitro approach is clearly applicable to other environmental pollutants and possibly even useful for safety testing of food or pharmaceuticals. The limited quantities of banked samples in the present study prohibited any inquiry into the underlying mechanism or putative ligands that drive the endothelial activation. However, the methods and model presented herein are ideal for both “-omic”-style approaches for the identification of such ligands as well as for mechanistic studies into the receptors and downstream pathways that mitigate endothelial cell responses. Future studies may make use of such methods to better characterize risk and susceptibility factors than predispose various populations to adverse outcomes of environmental hazards.
FUNDING
National Institutes of Health (ES014369) and U.S. Environmental Protection Agency STAR Award (R83399001).
Acknowledgments
The manuscript contents are solely the responsibility of the grantee (M.J.C.) and do not necessarily represent the official views of the U.S. Environmental Protection Agency (USEPA). Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
References
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ. Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N, Kido T, Suzuki H, Yang G, Kavanagh TJ, Kaufman JD, Rosenfeld ME, van Breemen C, Eeden SF. Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis. 2011;216:299–306. doi: 10.1016/j.atherosclerosis.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD, McDonald JD. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(-/-) mice. Toxicol. Appl. Pharmacol. 2010;242:310–317. doi: 10.1016/j.taap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ. Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol. Sci. 2008;102:328–336. doi: 10.1093/toxsci/kfn005. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ. Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T, Tamagawa E, Bai N, Suda K, Yang HH, Li Y, Chiang G, Yatera K, Mukae H, Sin DD, et al. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am. J. Respir. Cell Mol. Biol. 2011;44:197–204. doi: 10.1165/rcmb.2009-0427OC. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol. Appl. Pharmacol. 2008;230:346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Thomas R, Ledbetter AD, Schladweiler MC, Shannahan JH, Wallenborn JG, Lund AK, Campen MJ, Butler EO, Gottipolu RR, et al. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environ. Health Perspect. 2011;119:312–318. doi: 10.1289/ehp.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Lundbäck M, Barath S, Söderberg S, Mills NL, Newby DE, Sandström T, Blomberg A. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal. Toxicol. 2010;22:192–198. doi: 10.3109/08958370903144105. [DOI] [PubMed] [Google Scholar]
- doi: 10.1006/meth.2001.1262. Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC, Campen MJ. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am. J. Respir. Crit. Care Med. 2011;184:82–91. doi: 10.1164/rccm.201012-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadoke PW, et al. Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ. Health Perspect. 2009;117:611–616. doi: 10.1289/ehp.0800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ. Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, González-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim. Biophys. Acta. 2005;1725:305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Zhang J, Kamat L, Wilson AC, Kostis JB. Triggering of transmural infarctions, but not nontransmural infarctions, by ambient fine particles. Environ. Health Perspect. 2010;118:1229–1234. doi: 10.1289/ehp.0901624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: Differential activation and binding of the transcription factors AP-1 and NF-kappaB. Int. J. Mol. Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- Rosenlund M, Berglind N, Pershagen G, Hallqvist J, Jonson T, Bellander T. Long-term exposure to urban air pollution and myocardial infarction. Epidemiology. 2006;17:383–390. doi: 10.1097/01.ede.0000219722.25569.0f. [DOI] [PubMed] [Google Scholar]
- Shaw CA, Robertson S, Miller MR, Duffin R, Tabor CM, Donaldson K, Newby DE, Hadoke PW. Diesel exhaust particulate–exposed macrophages cause marked endothelial cell activation. Am. J. Respir. Cell Mol. Biol. 2011;44:840–851. doi: 10.1165/rcmb.2010-0011OC. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Marjoram P, Kleinman MT, Kloner RA. Direct and acute cardiotoxicity of ultrafine particles in young adult and old rat hearts. Basic Res. Cardiol. 2007;102:467–475. doi: 10.1007/s00395-007-0681-0. [DOI] [PubMed] [Google Scholar]
- Sobus JR, Pleil JD, Madden MC, Funk WE, Hubbard HF, Rappaport SM. Identification of surrogate measures of diesel exhaust exposure in a controlled chamber study. Environ. Sci. Technol. 2008;42:8822–8888. doi: 10.1021/es800813v. [DOI] [PubMed] [Google Scholar]
- Spallarossa P, Garibaldi S, Barisione C, Ghigliotti G, Altieri P, Tracchi I, Fabbi P, Barsotti A, Brunelli C. Postprandial serum induces apoptosis in endothelial cells: Role of polymorphonuclear-derived myeloperoxidase and metalloproteinase-9 activity. Atherosclerosis. 2008;198:458–467. doi: 10.1016/j.atherosclerosis.2007.11.030. [DOI] [PubMed] [Google Scholar]
- doi: 10.1001/jama.294.23.3003. Sun, Q., Wang, A., Jin, X., Natanzon, A., Duquaine, D., Brook, R. D., Aguinaldo, J. G., Fayad, Z. A., Fuster, V., Lippmann, M., Chen, L. C., et al. (2005). Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA294, 3003–3010. [DOI] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J. Am. Coll. Cardiol. 2002;39:935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- Törnqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Söderberg S, Newby DE, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10) Am. J. Respir. Crit. Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Weldy CS, Wilkerson HW, Larson TV, Stewart JA, Kavanagh TJ. Diesel particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genes. Toxicol. In Vitro. 2011;25:2064–2073. doi: 10.1016/j.tiv.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am. J. Epidemiol. 2005;161:1030–1036. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J. Epidemiol. Community Health. 2006;60:890–895. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Defelice AF, Hanig JP, Colatsky T. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol. Pathol. 2010;38:856–871. doi: 10.1177/0192623310378866. [DOI] [PubMed] [Google Scholar]
- Zhang P, Dong G, Sun B, Zhang L, Chen X, Ma N, Yu F, Guo H, Huang H, Lee YL, et al. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One. 2011;6:e20827. doi: 10.1371/journal.pone.0020827. [DOI] [PMC free article] [PubMed] [Google Scholar]