Abstract
Both epidemiological and experimental studies indicate that ethanol exposure enhances tumor progression. Ethanol exposure promotes cancer cell invasion and is implicated in tumor metastasis. Metastasis consists of multiple processes involving intravasation and extravasation of cancer cells across the blood vessel walls. The integrity of the vascular endothelial barrier that lines the inner surface of blood vessels plays a critical role in cancer cell intravasation/extravasation. We examined the effects of ethanol on the endothelial integrity in vitro. Ethanol at physiologically relevant concentrations did not alter cell viability but disrupted the endothelial monolayer integrity, which was evident by a decrease in the electric resistance and the appearance of intercellular gaps in the endothelial monolayer. The effect of ethanol was reversible once ethanol was removed. The disruption of the endothelial monolayer integrity was associated with an increased invasion of cancer cells through the endothelial monolayer. Ethanol induced the formation of stress fibers; stabilization of actin filaments by jasplakinolide prevented ethanol-induced disruption of endothelial integrity and cancer cell invasion. VE-cadherin is a critical component of the adherens junctions, which regulates vascular endothelial integrity. Ethanol induced the endocytosis of VE-cadherin and the effect was blocked by jasplakinolide. Our results indicate that ethanol may facilitate cancer metastasis by disrupting the vascular endothelial barrier.
Keywords: alcohol, adherens junctions, carcinogenesis, invasion, migration, stress fibers
Excessive ethanol exposure is associated with an increased risk of a number of human cancers, including oral, pharyngeal, esophageal, hepatic, ovarian, colon, rectal, gastric, and breast and has also been suspected in pancreatic and lung cancers (Boffetta and Hashibe, 2006; Go et al., 2005; Poschl and Seitz, 2004; Rohrmann et al., 2006). Ethanol exposure may also promote the progression of cancer. For example, epidemiological studies show that alcohol consumption is associated with advanced and invasive breast tumors in breast cancer patients and enhanced liver metastasis in colorectal carcinoma patients (Maeda et al., 1998; Vaeth and Satariano, 1998; Weiss et al., 1996). The cellular/molecular mechanisms underlying ethanol-induced tumor promotion, however, remain unclear. The majority of this investigation focuses on the direct effect of ethanol on cancer cells and supports that ethanol can alter the behavior of tumor cells. Ethanol promotes migration/invasion and stimulates the proliferation of breast cancer cells (Aye et al., 2004; Dumitrescu and Shields, 2005; Izevbigie et al., 2002; Ke et al., 2006; Luo and Miller, 2000; Ma et al., 2003; Singletary, 1997; Xu et al., 2010a,b; Yirmiya et al., 1992). However, little attention has been paid to the effect of ethanol on other cell types in cancer microenvironments.
Cancer metastasis consists of highly regulated multiprocesses in which cancer cells first detach from the primary tumor, attach to/invade surrounding tissues, intravasate into blood and/or lymphatic systems, extravasate from the vasculature, and subsequently settle and colonize at the target organs. Each step of these multiprocesses is critical in the metastasis procedure. For example, during the extravasation, blood-borne tumor cells travel along the vascular system, adhere to selective vascular endothelium, and then transmigrate through the vascular wall. Cancer cell intravasation/extravasation is a dynamic process that requires active interaction between cancer cells and vascular endothelia. Endothelia lining the inner surface of blood vessels constitute a selective barrier between the bloodstream and underlying tissues. Changes in the integrity of endothelium are involved in many physiological and pathological processes, including leukocyte and cancer cell extravasation. Both in vivo and in vitro experiments indicate that disruption of the endothelial monolayer causes an increase in endothelial permeability, which accelerates cancer metastasis (Chen et al., 2006; Eum et al., 2004; Wang et al., 2005). Vascular endothelial growth factor (VEGF), as an example, disrupts the endothelial barrier and enhances tumor cell extravasation (Weis et al., 2004a).
In the present study, we hypothesized that ethanol disrupted the vascular endothelial barrier and increased permeability of the endothelial monolayer, resulting in an enhanced invasion of cancer cells through the endothelial monolayer. With in vitro models, human umbilical vein endothelial cells (HUVEC) and bovine pulmonary artery endothelial cells (BPAEC), we demonstrated that ethanol caused a reversible disruption of the endothelial barrier and an enhanced invasion of cancer cells through the endothelial monolayer. Ethanol induced a formation of actin stress fibers and a disassociation of VE-cadherin clustering. Ethanol-induced disruption of endothelial barrier integrity and VE-cadherin clustering may be mediated by the reorganization of the actin cytoskeleton.
MATERIALS AND METHODS
Materials.
Human plasma fibronectin was obtained from Chemicon International (Temecula, CA). Gentamicin sulfate was obtained from ICN Biomedicals, Inc. (Aurora, OH). Sulfosuccinimidyl 2-(biotinamido) ethyl-dithioproprionate (sulfo-NHS-SS-biotin) and NeutrAvidin agarose resin were purchased from Pierce (Rockford, IL). Prolong Gold anti-fade reagent, Alexa Fluor 488 phalloidin, and Alexa Fluor–labeled secondary antibodies were obtained from Invitrogen Molecular Probes (Eugene, OR). Gold-coated electrodes were obtained from Applied Biophysics (Troy, NY). MTT assay kit was purchased from Roche Molecular Biochemicals (Indianapolis, IN). Transwells were purchased from Becton Dickinson Labware (Franklin lakes, NJ). Jasplakinolide was purchased from Enzo Life Sciences (Plymouth Meeting, PA). Polyclonal antibody directed against the extracellular domain (CAY-160840) of VE-cadherin and monoclonal antibody (CD144) directed against human VE-cadherin were purchased from Axxora (San Diego, CA) and Beckman Coulter Company (Fullerton, CA), respectively. Anti-p120-catenin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-catenin antibody and Calcein AM fluorescent dye were obtained from BD Bioscience (San Jose, CA). All other chemicals were obtained from Sigma-Aldrich (St Louis, MO).
Cell culture and treatments.
HUVEC were isolated from fresh human placentas and treated with 1 mg/ml of type I collagenase and grown in Clonetics Endothelial Cell Growth Medium-2 (EGM-2; Lonza, Walkersville, MD). HUVECs were used between passages 3 and 10. BPAEC were provided by Dr Fred Minnear (West Virginia University, Morgantown, WV). BPAEC were cultured in MCDB medium containing 10% fetal bovine serum (FBS) and 50 μg/ml of gentamicin sulfate. Endothelial cells were grown to confluent monolayers before the initiation of experiments. A549 lung cancer, HCT116 colon cancer, and MDA-MB231 breast cancer cells were grown in DMEM medium containing 10% FBS, penicillin (100 U/ml), streptomycin (100 U/ml), at 37°C with 5% CO2. Prior to the experiment of transendothelial migration (TEM), cancer cells were switched to EGM-2 medium. For ethanol treatment experiments, a method utilizing sealed containers was used to maintain ethanol concentrations in the culture medium (Xu et al., 2010a). With this method, ethanol concentrations in the culture medium can be accurately maintained. A physiologically relevant concentration of ethanol (200 mg/dl) was used for most experiments (Luo et al., 1999). For the treatment of jasplakinolide, HUVEC monolayers were pretreated with 50nM jasplakinolide for 4 h and then jasplakinolide was removed by two washes using EGM-2 medium.
Transendothelial electrical resistance.
Endothelial barrier function was measured by electrical resistance across HUVEC and BPAEC monolayers using Electrical Cell-Substrate Impedance Sensing (ECIS) as previously described (Xu et al., 2007). Briefly, endothelial cells were seeded onto ECIS cultureware (0.8 cm2/well) precoated with 0.2% gelatin. The electrical resistance measured was that of those cells located on the small gold electrode (5 × 10−4 cm2) in each of the wells. The culture medium served as the electrolyte; the small gold electrode that was covered by confluent endothelial cells, and a larger gold counter-electrode (∼2 cm2) were connected to a phase-sensitive lock-in amplifier. A constant current of 1 μA was supplied by a 1-V, 4000-Hz alternating current through a 1-MΩ resistor. Changes in voltage between the small electrode and the large counter electrode were monitored by the lock-in amplifier, stored, and then calculated as impedance by the computer. Electrical resistance was measured for 30 min prior to any treatment to stabilize results. Data were presented as changes in the resistive portion of electrical impedance and normalized to the initial value at time 0.
MTT assay.
The MTT assay was employed to determine the number of viable cells in culture. Briefly, the cells were plated into 96-well plates and exposed to ethanol for indicated times. After the treatment, 10 μl of MTT reagent was added into each well, and the plates were incubated at 37°C for 4 h. The cultures were solubilized, and spectrophotometric absorbance was measured at 595 nm using a microtiter plate reader (Beckman Coulter).
Immunofluorescence microscopy.
The procedure for immunofluorescence microscopy has been previously described (Xu et al., 2007). Briefly, cells were seeded on coverslips precoated with fibronectin (10 μg/ml). After treatment, cells were fixed with 3.7% paraformaldehyde for 10 min, washed three times in PBS, and permeabilized with 0.5% Triton X-100 for 5 min. Cells were blocked with 5% BSA and incubated with primary antibodies for 1 h. Following incubation with primary antibodies, cells were washed and treated with Alexa Fluor-labeled secondary antibodies and rinsed with PBS. Coverslips were mounted with Prolong Gold anti-fade reagent, and images were captured using a Zeiss LSM 510 confocal microscope or an Olympus 1X81 inverted fluorescent microscope with the same exposure time, detector gain, and amplifier offset.
TEM assay.
The invasion of cancer cells through endothelial monolayers was measured with a TEM assay (Hordijk et al., 1999; Lee et al., 2003). Briefly, HUVECs were seeded on fibronectin-coated upper chambers of transwells, which were separated from the lower chambers by a porous membrane with pore size of 8 μm. The HUVECs were grown in EGM-2 medium until confluent. Cancer cells were prelabeled with calcein AM at 37°C for 30 min and then washed with EGM-2 medium. Calcein-labeled cancer cells (5 × 104) were added to the upper chamber containing the HUVEC monolayer. For ethanol treatment, ethanol was added to both upper and lower chambers and incubated for 18 h at 37°C. Nonmigrating cells on the apical side of the upper chamber were removed by gentle scraping with cotton tips and washed with PBS. Transendothelial-migrated cells on the other side of the upper chambers were measured at 495/517 nm (Abs/Em) by using a bottom-read microtiter plate reader.
Immunoblotting and immunoprecipitation.
The procedure for immunoprecipitation and immunoblotting has been previously described (Ma et al., 2003; Xu et al., 2010a). Briefly, cells were washed twice in ice-cold PBS, lysed in RIPA buffer (15mM NaCl, 50mM Tris, 1% NP-40, 0.5% sodium deoxycholate, 2mM EGTA, 1mM sodium vanadate, 1mM phenylmethanesulfonyl fluoride, 5 μg/ml aprotinin, and 2 μg/ml leupeptin). Samples were separated by centrifugation at 10,000 rpm for 10 min at 4°C. For immunoprecipitation, equal amounts of proteins (about 500–800 μg) were incubated with anti-VE-cadherin antibody overnight at 4°C, followed by treatment with Protein A/G Plus-agarose beads for 2 h at 4°C. Immunoprecipitates were collected by centrifugation at 10,000 × g for 5 min at 4°C. Samples were washed five times with RIPA buffer, one time with cold PBS and boiled in sample buffer (187.5mM Tri-HCl, pH 6.8, 6% SDS, 30% glycerol, 150mM DTT, and 0.03% bromophenol blue). Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes using a mini-Protean electrophoresis system. Protein blots were probed with indicated primary antibodies, followed by the appropriate horseradish peroxidase-conjugated secondary antibodies and developed by enhanced chemiluminescence.
Assessment of VE-cadherin distribution.
VE-cadherin on the cell surface was quantified using a quantitative biotinylation assay (Le et al., 2002). Briefly, a HUVEC monolayer was incubated at 0°C for 1 h with 1 mg/ml sulfosuccinimidyl 2-(biotinamido) ethyl-dithioproprionate (sulfo-NHS-SS-biotin), a biotin-labeled cell impermeable reagent. Cells were washed once with a blocking reagent (50mM NH4Cl in PBS containing 1mM MgCl2 and 0.1mM CaCl2) and then incubated at 0°C for 10 min to quench free sulfo-NHS-SS-biotin, followed by washes with cold PBS. Cells were lysed in RIPA buffer with protease inhibitors. Equal amounts of protein were incubated with NeutrAvidin agarose beads to collect biotin-labeled proteins and then resolved by SDS-PAGE. VE-cadherin was identified using a rabbit polyclonal antibody directed against the extracellular domain of human VE-cadherin, followed by a horseradish peroxidase–conjugated secondary antibody, and developed by enhanced chemiluminescence.
To evaluate intracellular VE-cadherin, trypsinization of VE-cadherin on the cell surface was performed (Gavard and Gutkind, 2006). Briefly, cells were incubated in trypsin-EDTA at 37°C to cleave away the extracellular domain of VE-cadherin. Trypsin was inactivated by trypsin inhibitors. Cells were collected, centrifuged, and pellets were lysed in RIPA buffer for immunoblotting analysis. In trypsin-resistant fraction, internalized VE-cadherin was identified using a polyclonal antibody directed against the extracellular domain of VE-cadherin.
Statistical analysis.
Differences among treatment groups were tested using ANOVA. Differences in which p was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests.
RESULTS
Ethanol Disrupts Endothelial Integrity
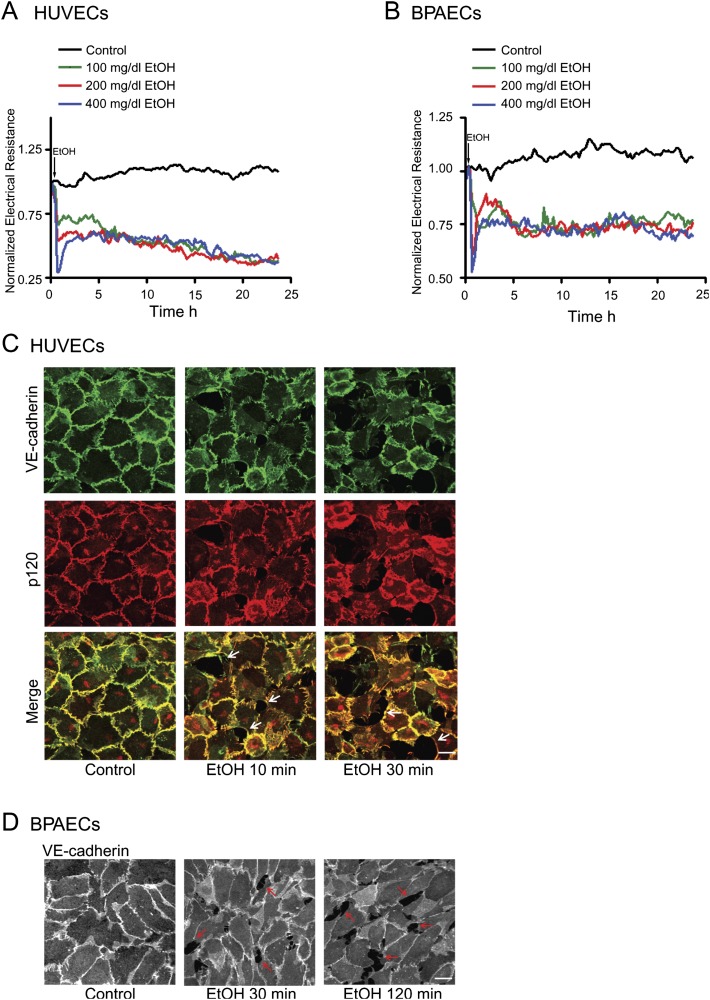
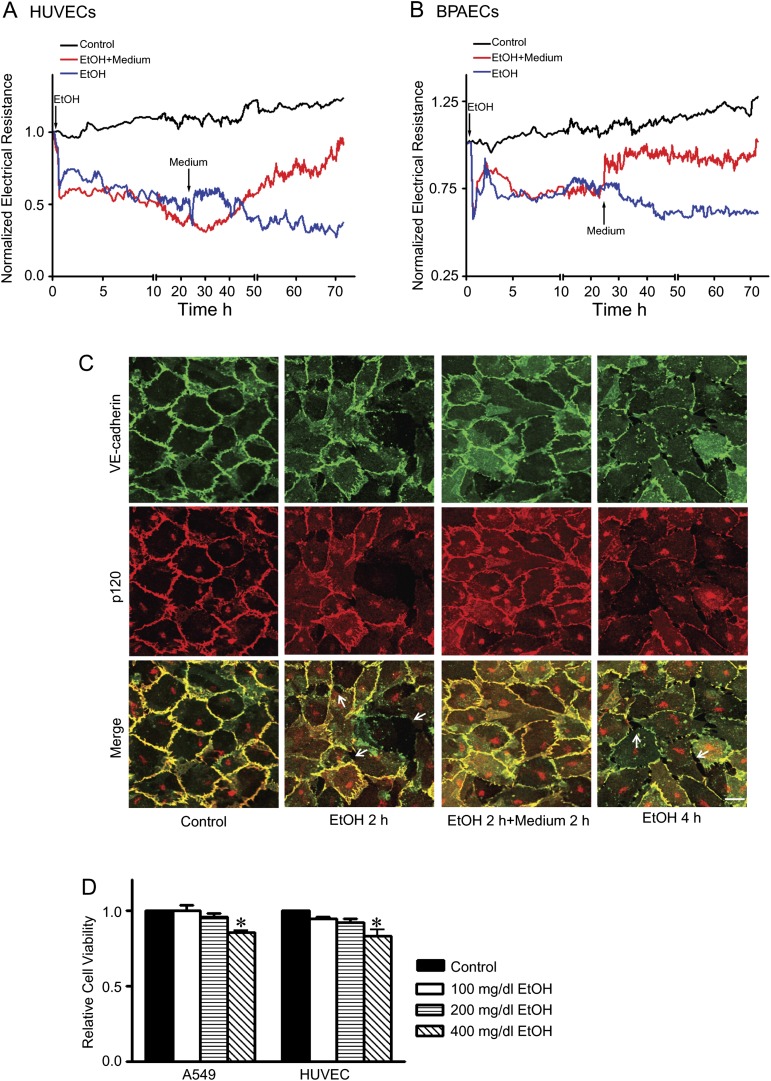
We first tested the effects of ethanol on endothelial integrity in cultured HUVECs and BPAECs. The endothelial cells were grown to confluent monolayers. The integrity of the endothelial monolayers was determined by real-time measurement of transendothelial resistance by an ECIS machine. Ethanol exposure caused a rapid decrease of endothelial electrical resistance in both HUVEC and BPAEC monolayers in a concentration-dependent manner (Figs. 1A and B), indicating a disruption of the endothelial barrier. The alterations in endothelial electrical resistance were confirmed by morphological analysis of immunofluorescence staining of VE-cadherin and p120. VE-cadherin and p120 are components of endothelial adherens junctions and play a critical role in regulating endothelial integrity. Ethanol exposure disrupted cell/cell contacts and generated intercellular gaps in the endothelial monolayer (Figs. 1C and D). Ethanol caused a loss of junctional VE-cadherin and p120 staining, suggesting a disassociation of adherens junctions. The effect of ethanol was evident after 10 min of ethanol exposure and sustained for hours (Figs. 1 and 2A). An increase in cytoplasmic VE-cadherin was observed in HUVECs after 2 h of ethanol exposure (Fig. 2C). To determine whether the effect of ethanol was reversible, we removed the ethanol-containing medium and replaced it with fresh medium. As shown in Figure 2, removal of the ethanol resulted in a recovery of endothelial electrical resistance and the disappearance of intercellular gaps in the endothelial monolayer. After the removal of ethanol for 2 h, VE-cadherin reappeared in cell/cell junctions and cytoplasmic VE-cadherin was reduced (Fig. 2C). The data indicated that ethanol-induced disruption of the endothelial barrier was reversible. MTT assay confirmed that ethanol at the concentrations of 100 or 200 mg/dl did not affect the viability of endothelial cells (Fig. 2D).
FIG. 1.
Effect of ethanol on endothelial integrity. HUVECs and BPAECs were grown to a confluent monolayer and exposed to ethanol (0, 100, 200, or 400 mg/dl). A and B: Electrical resistances on HUVECs or BPAECs were continuously recorded over a 24-h time period. C and D: HUVEC or BPAEC monolayers were exposed to ethanol (0 or 200 mg/dl) for indicated times. The expression of VE-cadherin and p120 was visualized by immunofluorescent staining as described under the Materials and Methods section. Arrows indicate the intercellular gaps. Scale bar = 20 μm. The experiment was replicated three times.
FIG. 2.
Reversibility of ethanol’s effect on endothelial cells. (A and B): HUVECs and BPAECs were exposed to ethanol (0 or 200 mg/dl) for 24 h. For some groups, the culture medium was removed at 24 h and replaced with fresh medium containing no ethanol, and cells were grown in this medium for an additional 48 h. Electrical resistances on HUVEC and BPAEC monolayer were continuously recorded. Arrows indicate the time of ethanol exposure and replacement of fresh medium. (C): HUVEC monolayer was exposed to ethanol for 2 h. For some groups, ethanol-containing medium was removed, and cells were washed and replaced with medium containing no ethanol for an additional 2 h. The expression of VE-cadherin and p120 was visualized as described in Figure 1. Arrows indicate the intercellular gaps. Scale bar = 20 μm. (D): A549 and HUVECs were exposed to ethanol (0, 100, 200, or 400 mg/dl) for 24 h, and cell viability was determined with MTT assay as described under the Materials and Methods section. The number of viable cells was presented relative to untreated controls. Each datum point was the mean ± SEM of three independent experiments. * denotes a statistically significant difference from untreated controls (p < 0.05).
Ethanol Increases Cancer Cell Invasion Through the Endothelial Monolayer
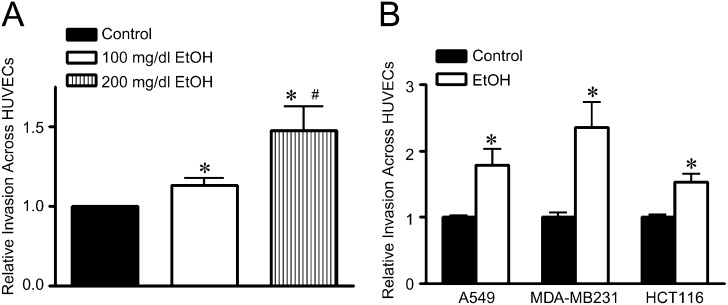
We sought to determine whether ethanol-induced disruption of the endothelial barrier facilitates the invasion of cancer cells across the endothelial monolayer. In this experiment, a confluent monolayer of HUVECs was maintained on fibronectin-coated transwells, and cancer cells were placed on top of the endothelial monolayer in the presence or absence of ethanol. As shown in Figure 3A, ethanol exposure (100–200 mg/dl) caused a concentration-dependent increase in the invasion of human lung adenocarcinoma cells (A549 cells) through the endothelial monolayer. At a higher concentration (400 mg/dl), ethanol still increased cancer cell invasion but to a lesser extent in comparison to the group treated with 200 mg/dl (data not shown). This was likely due to reduced cell viability after exposure to a high concentration of ethanol (Fig. 2C). The increase in cancer cell invasion was also observed in other types of cancer cells, such as breast cancer cells (MDA-MB231) and colon cancer cells (HCT116) (Fig. 3B).
FIG. 3.
Effect of ethanol on cancer cell invasion through the endothelial monolayer. (A): HUVECs were seeded on transwell chambers and grown to a confluent monolayer. Calcein-labeled A549 cells (5 × 104) were placed on top of the HUVEC monolayer and treated with ethanol (0, 100, and 200 mg/dl) for 18 h. After the treatment, the number of A549 cells that invaded and migrated through the endothelial monolayer was measured as described under the Materials and Methods section. (B): The invasion and migration of A549, MDA-MB231, and HCT116 cells through the HUVEC monolayer after ethanol treatment (0 or 200 mg/dl) were determined as described above. * denotes a statistically significant difference from untreated controls. # denotes a significant difference from the group treated with 100 mg/dl ethanol (p < 0.05). These experiments were replicated three times.
Ethanol Induces the Formation of Actin Stress Fibers in Endothelial Cells
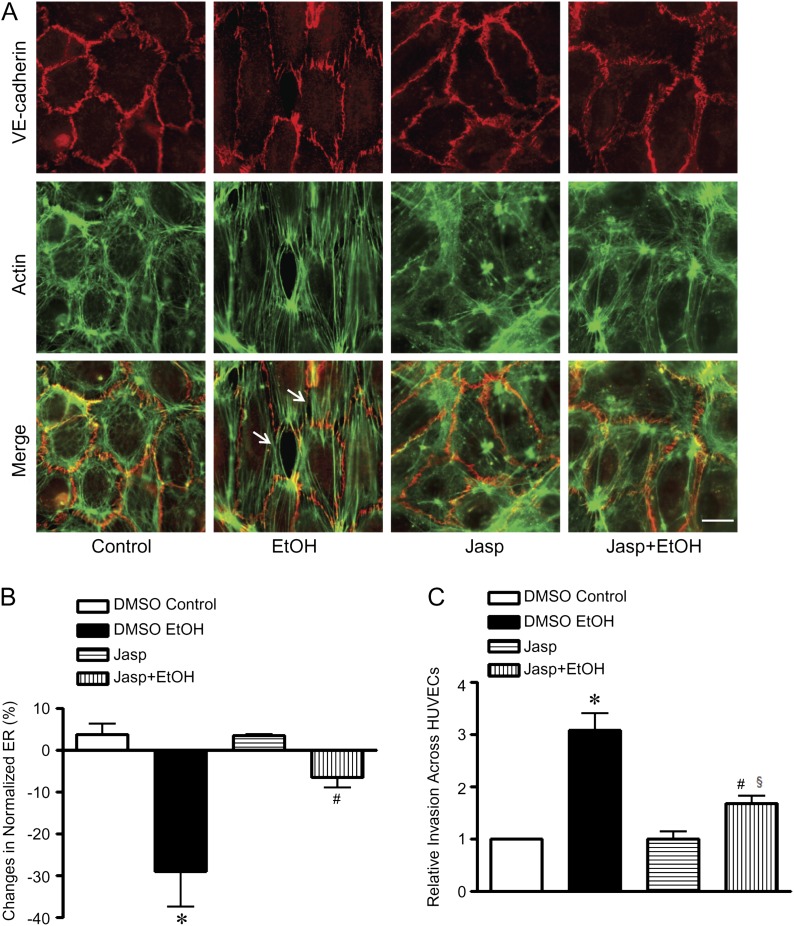
We have demonstrated above that ethanol disrupted the endothelial barrier and facilitated cancer cell invasion. We sought to investigate the underlying mechanism. Dynamic organization of the actin cytoskeleton plays a critical role in endothelial integrity. Both in vivo and in vitro evidence supports that either depolymerization or hyperpolymerization of F-actin increases endothelial permeability (Waschke et al., 2005). In an untreated endothelial monolayer, peripheral actin, also called cortical actin, was the predominant form of the actin cytoskeleton in HUVECs (Fig. 4A). Ethanol exposure rapidly induced the formation of actin stress fibers; the stress fibers were observed after 10 min of ethanol exposure and were accompanied by the appearance of intercellular gaps around the stress fibers. The ethanol-induced stress fiber formation was temporally correlated to the decrease in endothelial electrical resistance, which is shown in Figure 1.
FIG. 4.
Effect of jasplakinolide on ethanol-induced disruption of endothelial integrity and cancer cell invasion. (A): HUVECs were pretreated with dimethyl sulfoxide (DMSO) (vehicle control) or jasplakinolide (Jasp, 50nM in DMSO) for 4 h and exposed to ethanol (0 and 200 mg/dl) for 10 min. F-actin was stained with phalloidin labeled with Alexa Fluor 488. The expression of VE-cadherin was detected by immunofluorescent staining as described under the Materials and Methods section. Images were captured using an Olympus inverted fluorescent microscope. Arrows indicate the intercellular gaps. Scale bar = 20 μm. (B): HUVECs were pretreated with DMSO (vehicle control) or Jasp (50nM in DMSO) for 4 h and exposed to ethanol (0 and 200 mg/dl); electrical resistances were recorded and expressed relative to time 0. The maximal decrease was recorded at approximately 10 min. (C): HUVEC monolayer in transwell chambers was pretreated with DMSO or Jasp (50nM in DMSO) for 4 h followed by several washes to remove Jasp from the medium. Calcein-labeled MDA-MB231 cells were placed on top of HUVEC monolayer and treated with ethanol (0 or 200 mg/dl) for 18 h. After the treatment, the number of MDA-MB231 cells that invaded and migrated through the endothelial monolayer was measured as described under the Materials and Methods section. Each datum point was the mean ± SEM of three independent experiments. * denotes a statistically significant difference from controls. # denotes a significant difference from ethanol-treated groups. § denotes a significant difference from jasplakinolide-treated groups (p < 0.05).
To determine whether ethanol-induced stress fiber formation was responsible for the alteration of endothelial integrity, we used jasplakinolide to stabilize actin filaments. Jasplakinolide at low concentrations (100nM or lower) can stabilize the actin cytoskeleton and prevent cytochalasin D–increased endothelial permeability (Waschke et al., 2005). Jasplakinolide (50nM) slightly increased the peripheral actin network but had little effect on endothelial morphology (Fig. 4A). Jasplakinolide blocked ethanol-induced formation of actin stress fibers and intercellular gaps (Fig. 4A). Consistent with the morphological results, jasplakinolide significantly attenuated an ethanol-induced decrease in endothelial electrical resistance (Fig. 4B).
We next sought to determine whether stabilization of the actin cytoskeleton was sufficient to block ethanol-promoted invasion of cancer cells through the endothelial monolayer. As shown in Figure 4C, pretreatment with jasplakinolide partially but significantly inhibited ethanol-stimulated invasion of cancer cells. Together, these data indicated that ethanol-induced actin stress fiber formation may be responsible for the disruption of the endothelial barrier, which facilitates cancer cell invasion.
Ethanol Disrupts Junctional VE-Cadherin and Induces Endocytosis of VE-Cadherin
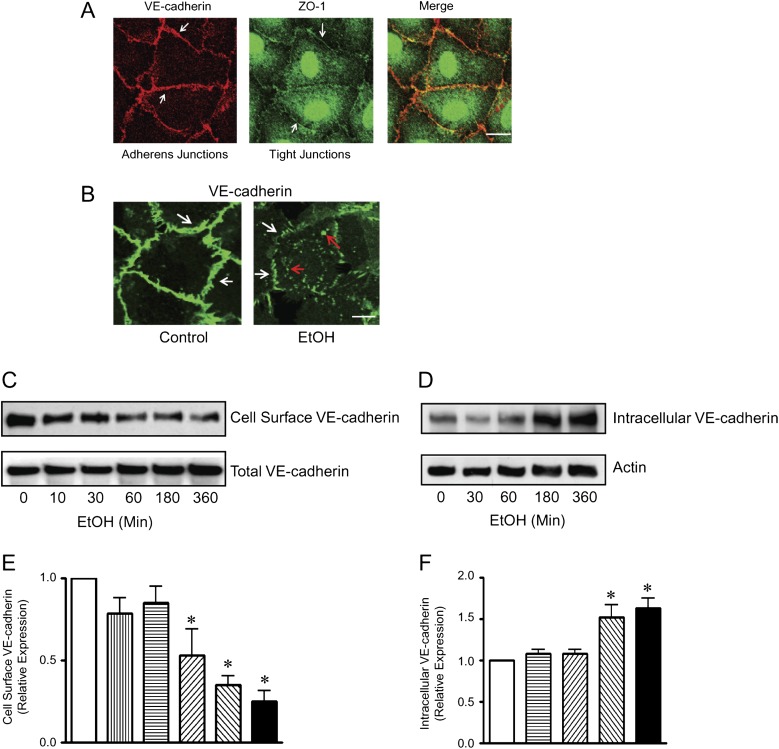
Intercellular junctions, such as adherens junctions and tight junctions, play an important role in intercellular communication and endothelial integrity (Vestweber, 2000). Tight junctions are predominantly expressed in endothelial cells of the blood-brain barrier and of large arteries, whereas adherens junctions are more ubiquitously expressed. We examined the expression of adherens junctions and tight junctions in HUVECs. Consistent with previous findings (Beese et al., 2010), the expression of tight junctions in HUVEC cells was weak and adherens junctions were the predominant form of junction complexes (Fig. 5A). Ethanol exposure induced a rapid loss of VE-cadherin in the intercellular junctions (Figs. 1C and D). Following prolonged exposure to ethanol (2 h or more), the decrease in junctional VE-cadherin staining was accompanied by an accumulation of cytoplasmic VE-cadherin (Fig. 5B), suggesting the endocytosis of VE-cadherin. To further investigate the effect of ethanol on the endocytosis of VE-cadherin, we biotinylated cell surface VE-cadherin and examined the alterations of cell surface and internalized VE-cadherin. As shown in Figures 5C to F, ethanol induced a gradual loss of cell surface VE-cadherin but increased trypsin-resistant intracellular VE-cadherin.
FIG. 5.
Effect of ethanol on VE-cadherin trafficking. (A): Adherens junctions and tight junctions in HUVECs were visualized by VE-cadherin and ZO-1 immunofluorescent staining, respectively, as described under the Materials and Methods section. Scale bar = 10 μm. (B): HUVEC monolayer was exposed to ethanol (0 or 200 mg/dl) for 4 h. VE-cadherin was visualized by immunofluorescence microscopy. Arrows indicate either the junctional or cytoplasmic VE-cadherin. Scale bar = 5 μm. (C): HUVEC monolayer was exposed to ethanol (0 or 200 mg/dl) for indicated times and incubated with a cell impermeable marker sulfo-NHS-SS-biotin at 0°C for 1 h. Cell lysates were collected, and biotin-labeled protein was precipitated as described under the Materials and Methods section. The expression of cell surface VE-cadherin was identified using a polyclonal antibody directed against the extracellular domain of human VE-cadherin. The expression of total VE-cadherin was also examined. (D): HUVEC monolayer was exposed to ethanol (0 or 200 mg/dl) for indicated times and treated with trypsin-EGTA as described under the Materials and Methods section. The cell lysates were collected, and the internalized VE-cadherin (trypsin-resistant) was identified using a polyclonal antibody as described above. The expression of actin was used as a loading control. (E and F): The relative expression of cell surface VE-cadherin (E) and intracellular VE-cadherin (F) was determined by densitometry and normalized to the total VE-cadherin (E) or actin (F), respectively. * denotes a statistically significant difference from untreated controls (p < 0.05). These experiments were replicated three times.
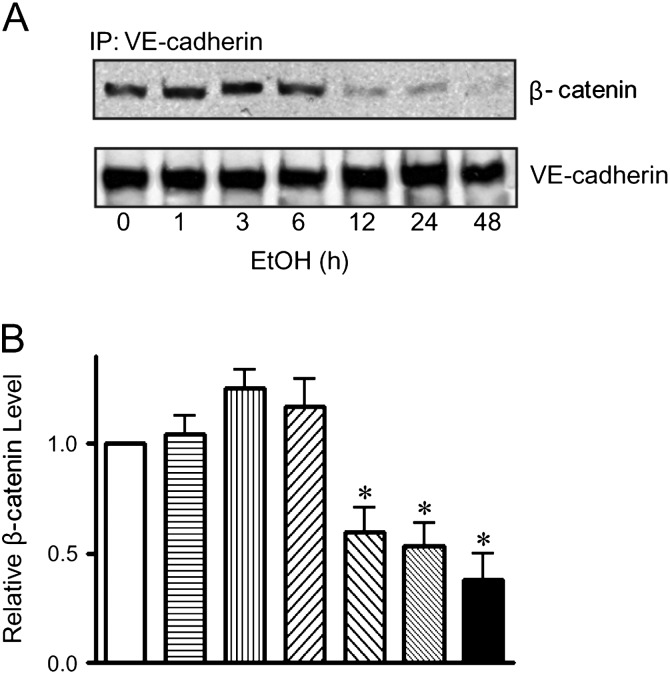
VE-cadherin is linked to the actin cytoskeleton through β- and α-catenin. The stabilization of cadherin/catenin complex also maintains endothelial integrity and inhibits tumor cell extravasation (Weis et al., 2004a). We demonstrated that prolonged ethanol exposure (12 h) disrupted the VE-cadherin/β-catenin association (Figs. 6A and B). The unstable VE-cadherin/β-catenin complex may further increase endothelial permeability and subsequent cancer cell intravasation/extravasation.
FIG. 6.
Effect of ethanol on the association between VE-cadherin and β-catenin. (A): HUVEC monolayer was exposed to ethanol (0 or 200 mg/dl) for indicated times. Cell lysates were collected and immunoprecipitated (IP) with an anti-VE-cadherin antibody, then immunoblotted (IB) with an anti-β-catenin antibody. (B): The association between VE-cadherin and β-catenin was quantified by densitometry. * denotes a statistically significant difference from untreated controls (p < 0.05). The experiment was replicated three times.
Jasplakinolide Antagonizes Ethanol-Induced Loss of Junctional VE-Cadherin
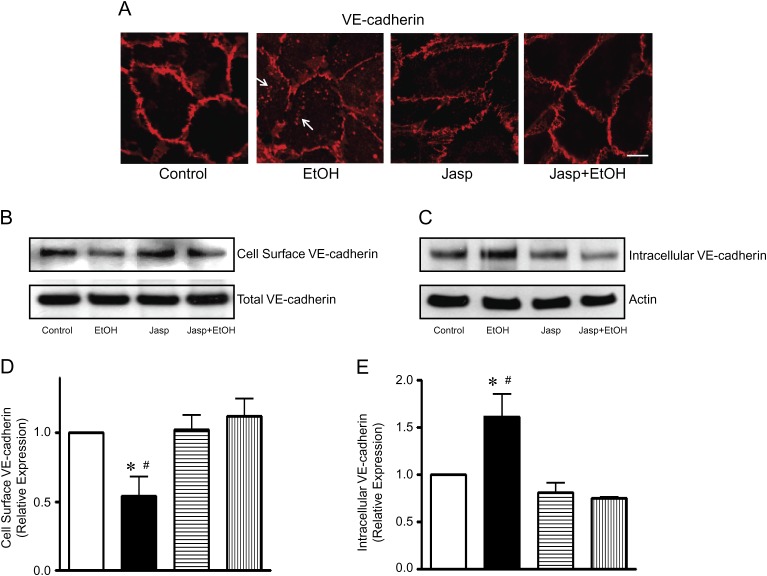
The dynamics of actin cytoskeleton is important for the distribution and function of junctional VE-cadherin (Waschke et al., 2005). We sought to determine whether ethanol-induced rearrangement of actin cytoskeleton mediated the dissociation of junctional VE-cadherin. In this experiment, HUVECs were pretreated with jasplakinolide for 4 h to stabilize actin and then exposed to ethanol. As shown in Figure 7A, ethanol decreased junctional VE-cadherin and increased cytoplasmic VE-cadherin. More importantly, jasplakinolide blocked the translocation of VE-cadherin and maintained cell surface VE-cadherin. This finding was confirmed by the experiment of VE-cadherin biotinylation. Jasplakinolide antagonized ethanol-induced loss of cell surface VE-cadherin and attenuated the increase of trypsin-resistant intracellular VE-cadherin (Figs. 7B to E).
FIG. 7.
Effect of jasplakinolide on ethanol-induced endocytosis of VE-cadherin. (A) HUVEC monolayer was pretreated with jasplakinolide (Jasp, 50nM) for 4 h and then exposed to ethanol (0 or 200 mg/dl) for 4 h. VE-cadherin was visualized by immunofluorescence microscopy. Arrows indicate the cytoplasmic VE-cadherin. Scale bar = 10 μm. (B and C): HUVEC monolayer was pretreated with Jasp (50nM) for 4 h and then exposed to ethanol (0 or 200 mg/dl) for 6 h. The expression of cell surface VE-cadherin (B) and intracellular VE-cadherin (C) was determined as described in Figure. 5. (D and E): The relative expression of cell surface VE-cadherin (D) and intracellular VE-cadherin (E) was determined by densitometry and normalized to the total VE-cadherin (D) or actin (E), respectively. * denotes a statistically significant difference from untreated controls. # denotes a significant difference from jasplakinolide-treated groups (p < 0.05). These experiments were replicated three times.
DISCUSSION
Excessive ethanol exposure has been implicated in tumorigenesis and cancer progression/metastasis. However, the underlying mechanisms remain unclear. Previous studies have focused on cancer cells and demonstrate that ethanol may enhance the aggressiveness of tumor cells. The effect of ethanol on endothelial cells and the implication of ethanol/endothelia interaction receive little attention. In this study, we demonstrate that ethanol disrupts the endothelial barrier and facilitates cancer cell invasion across the barrier. We further show that alterations in the actin cytoskeleton and adherens junctions play a critical role in ethanol-impaired endothelial integrity. This is the first report showing that ethanol promotes cancer cell invasion by disrupting the endothelial barrier. The results imply that ethanol may increase intravasation/extravasation and promote tumor metastasis.
Ethanol-Induced Disruption of Endothelial Integrity
Disruption of the endothelial barrier has been reported to directly facilitate cancer cell intravasation/extravasation (Chen et al., 2006; Eum et al., 2004; Wang et al., 2005), whereas stabilization of the endothelial barrier can suppress intravasation/extravasation (Weis et al., 2004a). Ethanol at physiologically relevant concentrations (100–200 mg/dl) disrupts the endothelial barrier, which is evident by a rapid decrease in electrical resistance of the endothelial monolayer, indicating an increased permeability (Fig. 1). This is confirmed morphologically by the appearance of intercellular gaps in the endothelial monolayer following ethanol exposure (Fig. 1). The disruption of endothelial integrity occurs within 10 min and remains for at least 24 h in the presence of ethanol. Ethanol (100–200 mg/dl) has little effect on cell viability, and the disruption of endothelial integrity is reversible once ethanol is removed (Fig. 2). More importantly, the disruption of endothelial integrity facilitates cancer cell invasion (Fig. 3). The enhanced cancer cell invasion does not appear to be cell type-specific; the effect is observed in lung, breast, and colon cancer cells. We have previously demonstrated that ethanol increases the invasive potential of breast cancer cells (Ma et al., 2003; Xu et al., 2010a). We cannot exclude the possibility that increased invasion across the endothelial monolayer results from enhanced aggressiveness of cancer cells. Our further study using jasplakinolide shows that maintaining endothelial integrity is sufficient to inhibit cancer cell invasion, indicating it is the increased permeability that facilitates cancer cell invasion. The study using jasplakinolide will be discussed in a greater detail later.
Actin Stress Fibers and Endothelial Integrity
Ethanol may disrupt endothelial integrity by numerous mechanisms and the change in the dynamics of the actin cytoskeleton is a potential one. We demonstrate that ethanol exposure decreases cortical actin and induces stress fiber formation (Fig. 4). Many vasoactive mediators induce actin fiber reorganization, resulting in changes in endothelial barrier function. For example, thrombin and histamine induce actin stress fiber formation and increase vascular permeability (Wojciak-Stothard et al., 2001). Sphingosine 1-phosphate (S1P) enhances cortical actin formation and decreases endothelial permeability (Xu et al., 2007). VEGF causes a breakage in the endothelial barrier as well as the stress fiber formation, which accelerates tumor cell extravasation (Weis et al., 2004a). In contrast, VEGF receptor inhibitor stabilizes the endothelial barrier and blocks VEGF-enhanced tumor cell extravasation (Lee et al., 2003; Weis et al., 2004a).
To establish the role of actin cytoskeleton organization in ethanol-mediated disruption of endothelial integrity, we use jasplakinolide to stabilize the actin cytoskeleton in HUVECs. At 100nM or lower concentrations, jasplakinolide can stabilize actin cytoskeleton without affecting resting endothelial permeability and monolayer integrity (Waschke et al., 2005). It has been shown that jasplakinolide at 100nM blocks cytochalasin D–increased endothelial permeability (Waschke et al., 2005). However, a high concentration of jasplakinolide (10μM) increases the content of actin filaments and disrupts the integrity of the endothelial monolayer. We show that jasplakinolide at 50nM inhibits ethanol-induced formation of stress fibers in endothelial cells and significantly attenuates ethanol-increased endothelial permeability (Fig. 4). This is confirmed morphologically by the disappearance of intercellular gaps in the endothelial monolayer following jasplakinolide treatment. This evidence supports that ethanol-induced formation of stress fibers is responsible for increased permeability.
The mechanisms underlying ethanol-induced rearrangement of actin filaments remain unclear. Small GTPases, Rho, Rac, and Cdc42, are essential for actin cytoskeleton organization. Rac and Cdc42 are mainly involved in lamellipodia and filopodia formation, respectively; while Rho promotes the formation of actin stress fibers, inducing actomyosin contraction and focal adhesion formation (Hall, 1998). It is reported that ethanol promotes RhoA-mediated ROCK-1 activation in astrocytes (Minambres et al., 2006). It remains to be investigated whether RhoA is involved in ethanol-induced rearrangement of actin filaments in endothelial cells.
Adherens Junctions and Endothelial Integrity
HUVECs express predominantly adherens junctions. Adherens junctions play a critical role in regulating endothelial barrier integrity (Vestweber, 2000). A recent study shows the assembly of adherens junctions is required to maintain tight junction formation in vitro (Taddei et al., 2008). Vascular endothelial cadherin (VE-cadherin), a major component of endothelial adherens junctions, plays many functional roles in regulating endothelial barrier integrity. VE-cadherin constantly traffics between the cytoplasm membrane and cytosolic compartment via endocytotic and recycling pathways (Bryant and Stow, 2004; Vincent et al., 2004). Trafficking of VE-cadherin has been suggested to be involved in the regulation of endothelial barrier functions and angiogenesis (Alexander et al., 1998; Dejana et al., 2008; Harris and Nelson, 2010). Disruption of Ca2+-dependent hemophilic binding of VE-cadherin on cell-cell contacts induces endocytosis of VE-cadherin and results in increased vascular permeability (Corada et al., 1999; Gavard and Gutkind, 2006). Similarly, VEGF increases endothelial permeability by promoting VE-cadherin endocytosis (Gavard and Gutkind, 2006).
We demonstrate that ethanol decreases cell surface VE-cadherin, which is accompanied by an increase in intracellular VE-cadherin. Because ethanol does not affect total VE-cadherin, we conclude that ethanol causes VE-cadherin endocytosis. We note that the decrease of cell surface VE-cadherin occurs earlier (10 min, Figs. 1, 2, and 5) than the appearance of VE-cadherin in the cytoplasm (2 h, Fig. 5). The reason for this lag is unclear; it may be due to insufficient sensitivity of the assay measuring intracellular VE-cadherin. Local disassociation of VE-cadherin clustering is required for leukocyte transmigration through adherens junctions (Allport et al., 1997). Junctional VE-cadherin disappears during cancer cell extravasation but is reexpressed after transmigration is complete (Lewalle et al., 1997; Sandig et al., 1997). Taken together, ethanol-induced VE-cadherin endocytosis may impair endothelial integrity and facilitate cancer metastasis.
Actin Stress Fibers and Adherens Junctions
Reorganization of the actin cytoskeleton can modulate VE-cadherin localization and function (Hordijk et al., 1999; Lee et al., 2003; Waschke et al., 2005). A recent study indicates that actin stress fibers in adjacent endothelial cells can interact through adherens junctions (Millan et al., 2010). Depolymerization of F-actin by cytochalasin D caused a significant reduction of VE-cadherin–mediated adhesion (Waschke et al., 2005). We demonstrate here that stabilization of actin filaments by jasplakinolide inhibits ethanol-induced VE-cadherin endocytosis (Fig. 7), suggesting that ethanol-induced redistribution of VE-cadherin is initiated by its effect on the actin cytoskeleton.
Junctional VE-cadherin is associated with β-catenin, which is in turn linked to the actin cytoskeleton through α-catenin. Stabilization of the VE-cadherin/β-catenin complex maintains the vascular integrity (Ben-Ze'ev et al., 2000; Weis et al., 2004b). Contrarily, disturbance of the cadherin/catenin complex results in the disruption of the endothelial barrier and facilitates tumor cell extravasation (Weis et al., 2004a). We demonstrate that prolonged ethanol exposure (12 h or longer) disrupts VE-cadherin/β-catenin association; the alteration may impose additional damage to the endothelial barrier.
In summary, ethanol may impair the endothelial barrier by inducing the rearrangement of the actin cytoskeleton and disrupting adherens junctions, which results in increased permeability of endothelia. The alteration in endothelia may facilitate cancer cell intravasation/extravasation during metastasis.
FUNDING
National Institutes of Health (AA017226).
Acknowledgments
We thank Dr Fred Minnear at West Virginia University for assisting in the analysis of electric resistance of endothelial monolayer.
References
- Alexander JS, Jackson SA, Chaney E, Kevil CG, Haselton FR. The role of cadherin endocytosis in endothelial barrier regulation: Involvement of protein kinase C and actin-cadherin interactions. Inflammation. 1998;22:419–433. doi: 10.1023/a:1022325017013. [DOI] [PubMed] [Google Scholar]
- Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: A process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J. Exp. Med. 1997;186:517–527. doi: 10.1084/jem.186.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye MM, Ma C, Lin H, Bower KA, Wiggins RC, Luo J. Ethanol-induced in vitro invasion of breast cancer cells: The contribution of MMP-2 by fibroblasts. Int. J. Cancer. 2004;112:738–746. doi: 10.1002/ijc.20497. [DOI] [PubMed] [Google Scholar]
- Beese M, Wyss K, Haubitz M, Kirsch T. Effect of cAMP derivates on assembly and maintenance of tight junctions in human umbilical vein endothelial cells. BMC Cell Biol. 2010;11:68. doi: 10.1186/1471-2121-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A, Shtutman M, Zhurinsky J. The integration of cell adhesion with gene expression: The role of beta-catenin. Exp. Cell Res. 2000;261:75–82. doi: 10.1006/excr.2000.5045. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor vascular permeabilization by vascular-targeting photosensitization: Effects, mechanism, and therapeutic implications. Clin. Cancer Res. 2006;12(3 Pt 1):917–923. doi: 10.1158/1078-0432.CCR-05-1673. [DOI] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008;121(Pt 13):2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35:213–225. doi: 10.1016/j.alcohol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp. Cell Res. 2004;296:231–244. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol. 2005;35:205–211. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Harris ES, Nelson WJ. VE-cadherin: At the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 2010;22:651–658. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J. Cell Sci. 1999;112(Pt 12):1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- Izevbigie EB, Ekunwe SI, Jordan J, Howard CB. Ethanol modulates the growth of human breast cancer cells in vitro. Exp. Biol. Med. (Maywood) 2002;227:260–265. doi: 10.1177/153537020222700406. [DOI] [PubMed] [Google Scholar]
- Ke Z, Lin H, Fan Z, Cai TQ, Kaplan RA, Ma C, Bower KA, Shi X, Luo J. MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int. J. Cancer. 2006;119:8–16. doi: 10.1002/ijc.21769. [DOI] [PubMed] [Google Scholar]
- Le TL, Joseph SR, Yap AS, Stow JL. Protein kinase C regulates endocytosis and recycling of E-cadherin. Am. J. Physiol. Cell Physiol. 2002;283:C489–C499. doi: 10.1152/ajpcell.00566.2001. [DOI] [PubMed] [Google Scholar]
- Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J. Biol. Chem. 2003;278:5277–5284. doi: 10.1074/jbc.M210063200. [DOI] [PubMed] [Google Scholar]
- Lewalle JM, Bajou K, Desreux J, Mareel M, Dejana E, Noel A, Foidart JM. Alteration of interendothelial adherens junctions following tumor cell-endothelial cell interaction in vitro. Exp. Cell Res. 1997;237:347–356. doi: 10.1006/excr.1997.3799. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Ethanol enhances erbB-mediated migration of human breast cancer cells in culture. Breast Cancer Res. Treat. 2000;63:61–69. doi: 10.1023/a:1006436315284. [DOI] [PubMed] [Google Scholar]
- Luo J, West JR, Cook RT, Pantazis NJ. Ethanol induces cell death and cell cycle delay in cultures of pheochromocytoma PC12 cells. Alcohol Clin. Exp. Res. 1999;23:644–656. [PubMed] [Google Scholar]
- Ma C, Lin H, Leonard SS, Shi X, Ye J, Luo J. Overexpression of ErbB2 enhances ethanol-stimulated intracellular signaling and invasion of human mammary epithelial and breast cancer cells in vitro. Oncogene. 2003;22:5281–5290. doi: 10.1038/sj.onc.1206675. [DOI] [PubMed] [Google Scholar]
- Maeda M, Nagawa H, Maeda T, Koike H, Kasai H. Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer. 1998;83:1483–1488. doi: 10.1002/(sici)1097-0142(19981015)83:8<1483::aid-cncr2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, Correas I, Ridley AJ. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minambres R, Guasch RM, Perez-Arago A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J. Cell Sci. 2006;119(Pt 2):271–282. doi: 10.1242/jcs.02723. [DOI] [PubMed] [Google Scholar]
- Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Linseisen J, Boshuizen HC, Whittaker J, Agudo A, Vineis P, Boffetta P, Jensen MK, Olsen A, Overvad K, et al. Ethanol intake and risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Am. J. Epidemiol. 2006;164:1103–1114. doi: 10.1093/aje/kwj326. [DOI] [PubMed] [Google Scholar]
- Sandig M, Voura EB, Kalnins VI, Siu CH. Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil. Cytoskelet. 1997;38:351–364. doi: 10.1002/(SICI)1097-0169(1997)38:4<351::AID-CM5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Singletary K. Ethanol and experimental breast cancer: A review. Alcohol Clin. Exp. Res. 1997;21:334–339. [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Vaeth PA, Satariano WA. Alcohol consumption and breast cancer stage at diagnosis. Alcohol Clin. Exp. Res. 1998;22:928–934. [PubMed] [Google Scholar]
- Vestweber D. Molecular mechanisms that control endothelial cell contacts. J. Pathol. 2000;190:281–291. doi: 10.1002/(SICI)1096-9896(200002)190:3<281::AID-PATH527>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: Adhesion at arm's length. Am. J. Physiol. Cell Physiol. 2004;286:C987–C997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- Wang HS, Hung Y, Su CH, Peng ST, Guo YJ, Lai MC, Liu CY, Hsu JW. CD44 cross-linking induces integrin-mediated adhesion and transendothelial migration in breast cancer cell line by up-regulation of LFA-1 (alpha L beta2) and VLA-4 (alpha4beta1) Exp. Cell Res. 2005;304:116–126. doi: 10.1016/j.yexcr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Waschke J, Curry FE, Adamson RH, Drenckhahn D. Regulation of actin dynamics is critical for endothelial barrier functions. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1296–H1305. doi: 10.1152/ajpheart.00687.2004. [DOI] [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004a;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J. Clin. Invest. 2004b;113:885–894. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss HA, Brinton LA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB, Swanson CA. Epidemiology of in situ and invasive breast cancer in women aged under 45. Br. J. Cancer. 1996;73:1298–1305. doi: 10.1038/bjc.1996.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 2001;114(Pt 7):1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Xu M, Bower KA, Chen G, Shi X, Dong Z, Ke Z, Luo J. Ethanol enhances the interaction of breast cancer cells over-expressing ErbB2 with fibronectin. Alcohol Clin. Exp. Res. 2010a;34:751–760. doi: 10.1111/j.1530-0277.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M, Wang S, Shi X, Ke Z, Luo J. Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol. Cancer. 2010b;9:285. doi: 10.1186/1476-4598-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am. J. Physiol. Cell Physiol. 2007;293:C1309–C1318. doi: 10.1152/ajpcell.00014.2007. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Ben-Eliyahu S, Gale RP, Shavit Y, Liebeskind JC, Taylor AN. Ethanol increases tumor progression in rats: Possible involvement of natural killer cells. Brain Behav. Immun. 1992;6:74–86. doi: 10.1016/0889-1591(92)90061-r. [DOI] [PubMed] [Google Scholar]