Abstract
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are widespread environmental contaminants found in seafood and dairy products. PCBs and PBDEs are structurally similar chemicals and affect thyroid hormone function and behavior in children and laboratory rodents. Although coexposure frequently exists, the in vivo developmental effects of combined exposure to PCBs and PBDEs on thyroxine (T4) levels are unknown. We examined the effects of PCB and PBDE coexposure from gestational day 6 through postnatal day (p) 21, alone and in combination, on T4 levels in rat offspring. In males, exposure to PCBs and PBDEs at 1.7, 5, 10, 20, 40, and 60 μmol/kg/day induced equivalent and dose-dependent reductions in T4 from p 7 to p 21. Exposure to equimolar mixtures of PCBs and PBDEs at 3.4, 10, 20, 40, and 80 μmol/kg/day additively reduced T4 from p 7 to p 21 in males. In a second series of experiments, we determined sex effects on the mixture exposures and found that coexposure to PCBs and PBDEs had similar additive effects on T4 levels in male and female offspring. This study demonstrates that equimolar exposure to PCBs and PBDEs induces similar reductions in T4 levels and that coexposure to a mixture of PCBs and PBDEs has additive effects on T4 levels. These thyroid hormone effects of coexposure to PCBs and PBDEs are important when considering the cumulative effects of coexposure to multiple environmental thyroid hormone–disrupting agents in risk assessment for developmental disorders.
Keywords: polychlorinated biphenyls, polybrominated diphenyl ethers, thyroxine, developing rats, coexposure, additivity
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are persistent lipophilic environmental contaminants. Coexposure is likely in adults via consumption of food of an animal origin and exposure to household dust (Thomsen et al., 2008). Exposure is also likely in developing offspring because PCBs and PBDEs can cross the blood-placenta barrier and are lipophilic so can be transferred lactationally to enter the fetal and neonatal brain (Bergonzi et al., 2009; Bi et al., 2006; Mazdai et al., 2003). Laboratory and epidemiological data suggest that maternal PCB and PBDE levels are associated with Attention Deficit Hyperactivity Disorder-like behavior and thyroid hormone changes in children and rodents (Holene et al., 1998; Jacobson et al., 1984; Jacobson and Jacobson, 2003; Kodavanti et al., 2010; Stewart et al., 2003, 2005). Reductions in thyroxine (T4), associated with PCBs and PBDEs, are attributed to the fact that both contaminants share a structural homology with T4 and triiodothyronine (T3) (Boas et al., 2006; Brouwer et al., 1998). Essentially, PCBs and PBDEs alter thyroid hormone function because they mimic T4 and T3, encourage T4 excretion by the liver, and alter the efficacy of T4 carrier proteins and the expression of thyroid hormone–regulated proteins. In addition, the hydroxylated metabolites of both PCBs and PBDEs alter thyroid hormone–sensitive pathways and thyroid hormone receptor activity (Boas et al., 2006; Brouwer et al., 1998).
It is well known that PCBs and PBDEs are thyroid hormone–disrupting agents, which reduce T4 levels, but whether coexposure has additive, antagonistic, or synergistic effects is unknown. PCBs and PBDEs individually can influence neuronal activity, white matter development, and motor and cognitive function in laboratory rodents (Costa and Giordano, 2007; Gray et al., 2005; Kodavanti and Derr-Yellin, 2002; Kodavanti et al., 2005, 2010; Miller et al., 2010; Powers et al., 2001; Sagiv et al., 2010; Sharlin et al., 2006). The aforementioned neurological changes may be attributed in part to T4 reductions. Therefore, it is imperative to determine the consequences of coexposure to the contaminants on circulating T4 levels.
Few studies have compared the effects of exposure to equimolar doses of PCBs and PBDEs alone or in combination in vivo. One study found that a single exposure at postnatal day (p) 10 to a mixture of PCBs and PBDEs had agonistic effects on behavior in male rats (Eriksson et al., 2006). Another study by Hallgren and Darnerud (2002) found that a single postnatal exposure on p 7 to a PCB mixture (Aroclor 1254) and the DE-47 PBDE congener had agonistic effects on T4 in female rats. These aforementioned studies are limited in their interpretation because they only used a single dose at a single time point and only one sex. Their results, however, led us to hypothesize that coexposure to a mixture of PCBs and PBDEs could have agonistic effects on T4 levels. Moreover, there is a compelling argument that developmental coexposure to PCBs and PBDEs would have additive effects on T4 levels, because Crofton et al. (2005) found additive effects of short-term coexposure to thyroid-disrupting chemicals on T4 levels in young female Long-Evans (LE) rats. Therefore, we exposed rodents both in utero and during lactation, to equimolar doses of PCBs and PBDEs, alone and in combination and determined the effects of these contaminants on circulating T4 levels. We were interested in T4 in particular because neither PCBs nor PBDEs significantly affect circulating levels of T3 or thyroid-stimulating hormone (Hallgren and Darnerud, 2002; Kodavanti et al., 2010; Sharlin et al., 2006).
MATERIALS AND METHODS
Choice of doses of PCBs and PBDEs.
The PCB mixture we used is also known as the Fox River mix (FRM) and is a formulation of Aroclors 1242, 1248, 1254, and 1260 in a 35:35:15:15 ratio that resembles the PCB congener pattern found in contaminated fish consumed by residents near the Fox River in Wisconsin (Kostyniak et al., 2005). The PBDE mixture we used is also known as DE-71 and consists of a mixture of tri, tetra, penta, and hexabromodiphenyl ethers and was from a sample originally obtained from the Great Lakes Chemical Company (West Lafayette, IN; lot number 755O0K20A). Stock solutions of FRM and DE-71 were prepared in corn oil, using a molecular weight of 296 for PCBs (calculated on the basis of the average weight of each Aroclor that makes up the mixture) and a molecular weight of 564.7 for PBDEs (from the Great Lakes Chemical Company’s Material Safety Data Sheet for DE-71).
To enable a side-by-side comparison of the effects of PCBs and PBDEs on T4 levels, we exposed dams to equimolar doses of the contaminants, alone and in combination. We chose doses known to induce hypothyroxinemia in developing rodent offspring (Miller et al., 2010). Table 1 describes the doses used in this study, in both mg/kg/day and μmol/kg/day. For experiment 1, which was only conducted in males, there were 48 pregnant dams, with 4 dams per treatment, yielding ∼n = 4 male pups per group for analysis at each time point, i.e., p 7, 14, 21, and 42. In a second experiment, we determined whether sex altered PCB and PBDE effects on T4 and used 30 timed-pregnant dams with 5 per treatment. Because we noted that T4 levels peaked at p 14 in the control offspring, in experiment 2, we included a p 11 time point to determine if there were sex and toxicant interactions at time points when T4 levels are most critical to development.
TABLE 1.
Doses Chosen for Exposures
| Toxicant | Experiment 1: mixtures (mg/kg/day) | Experiment 2: sex (mg/kg/day) |
| PCB | 0.51 mg: 1.7 μmol | |
| 1.5 mg: 5 μmol | ||
| 3 mg: 10 μmol | ||
| 6 mg: 20 μmol | 6 mg: 20 μmol | |
| 12 mg: 40 μmol | 12 mg: 40 μmol | |
| 18 mg: 60 μmol | ||
| PBDE | 0.96 mg: 1.7 μmol | |
| 2.85 mg: 5 μmol | ||
| 5.7 mg: 10 μmol | ||
| 11.4 mg: 20 μmol | 11.4 mg: 20 μmol | |
| 22.8 mg: 40 μmol | 22.8 mg: 40 μmol | |
| 34.2 mg: 60 μmol | ||
| Mixture | 0.51 mg PCBs + 0.96 mg PBDEs: 3.4 μmol | |
| 1.5 mg PCBs + 2.85 mg PBDEs: 10 μmol | ||
| 3 mg PCBs + 5.7 mg PBDEs: 20 μmol | 3 mg PCBs + 5.7 mg PBDEs: 20 μmol | |
| 6 mg PCBs + 11.4 mg PBDEs: 40 μmol | 6 mg PCBs + 11.4 mg PBDEs: 40 μmol |
Note. Equimolar doses of PCBs and PBDEs were chosen for use in this study. The dose of the mixtures enabled comparison with the individual contaminants at the dose contained in the mixture and also at the total dose of the mixture. For experiment 1, there were n = 4 dams per treatment, with ∼n = 4 male pups per treatment group per analysis. For experiment 2, there were n = 5 dams per treatment and ∼n = 5 male and ∼n = 5 female pups per treatment group per analysis.
Animals and exposure paradigm.
Timed-pregnant LE rats were obtained from Harlan. From gestational day 6 until weaning at p 21, timed-pregnant dams were exposed to the contaminants, either alone or in combination, or corn oil, which was the vehicle used to dissolve the contaminants and used to dose the control dams. We administered doses by placing a measured amount of the corn oil contaminant solution onto a cookie, which was fed to each dam on a daily basis. The volume of each dosing mixture in corn oil was adjusted three times per week based on changes in the dam’s weight.
Litters were comprised of 8–14 pups who were culled on p 2 and supplemented within treatments to maintain equal numbers of male and female pups (n = 5 of each) per dam and maintain equal lactational exposure to the contaminants. Control and dosed dams were housed in clear plexiglass cages with stainless steel wire lids in a temperature (21°C–23°C) controlled room and maintained in a sterile pathogen-free environment, on a 12:12-h light:dark cycle (lights on at 7:00 A.M.). Rats were fed ad libitum to PMI (Purina) 5002 test diet ad libitum. Sera for T4 analysis were obtained at the time of sacrifice from each individual pup or dam. Offspring were anesthetized with CO2 and sacrificed between 12 and 2 P.M., in groups on p 7, 11, 14, 21, and 42. All procedures were approved by the Institutional Care and Animal Use Committee at the Wadsworth Center, and all experiments were conducted in a blinded fashion.
Analysis of circulating T4 levels.
For experiment 1, a radioimmunoassay was used to determine total T4 levels in sera and was conducted by Dr Tom Zoeller (University of Massachusetts, Amherst, MA). For the radioimmunoassay, 3–4 pups per group were used from 12 groups at p 7, 14, 21, and 42. Briefly, total T4 levels were measured according to the manufacturer’s instructions using a total T4 RIA kit (ICN Diagnostics, Costa Mesa, CA). The assays were performed at 40% binding with a standard range of 10–200 μg/dl and an intra-assay variation of 3.5%. Samples were measured in duplicate. An ELISA assay was used to quantify total sera T4 levels for experiment 2. To validate this assay, rat and mice sera samples were analyzed independently by Drs Miller and Zoeller using a commercially available T4 ELISA kit (Calbiotech), and the radioimmunoassay kit used in experiment 1 (ICN Diagnostics), respectively. The intra-assay validations gave similar results with < 5% variation.
For the ELISA assay, a standard curve ranged from 0 to 25 μg/dl, T4 starting at 1 μg/dl was used to determine sera T4 levels in 25 μl of sera per rat in triplicate. We diluted standards provided with the kit to allow for a more sensitive range of detection, i.e., to allow for a lower range of detection for the younger offspring. Samples were run according to kit instructions. Briefly, plates were loaded with sera samples, in addition to spiked samples of sera with T4 of a known concentration and the T4 standards. Next, 100 μl of the T4-enzyme conjugate solution was added to all wells, the plate was covered and then incubated for 1 h at room temperature. The ELISA plates were then washed six times and incubated with 100 μl of an horseradish peroxidase-3,3′,5,5′-tetramethylbenzidine reagent for 15 min. After the substrate color was developed, 50 μl of an acidic stopping solution provided with the kit was added to each well, and the mean absorbance of the solution per well was read using a spectrophotometric plate reader at 450 nm within 15 min of stopping the reaction. The concentration of T4 per well was determined by comparing values per sample with the appropriate standard curve. In addition, control samples spiked with T4 were used as positive controls in the assay. For ELISA assays, sera from an n = 5 animals per group were run in triplicate.
Computational modeling of additivity using the Bliss model.
To determine whether coexposure to equimolar doses of the contaminants had additive effects, we used the Bliss mathematical modeling approach (Borgert et al., 2005), similar to our previous publication on the effects of PCBs and PBDEs on the dopamine transporter function (Dreiem et al., 2010). Briefly, the Bliss model is useful for determining the predicted additivity of two individual drugs or chemicals and is based on the concept that the fractional response of the system to any one chemical, such as PCBs is FPCB; likewise, the response to PBDEs is FPBDE. Once PCBs are present in the system, such as during coexposure, the fractional response of the system to PBDEs is FPBDEs (1−FPCBs). Thus, the additive response of the system to a mixture containing PCBs and PBDEs is equal to FPCB + FPBDE (1−FPCB). By comparing the predicted additivity of exposure to PCBs and PBDEs, at a particular dose such as 10 μmol/kg/day generated with the Bliss model approach i.e., F10 μmol/kg/day PCB + F10 μmol/kg/day PBDE (1−F10μmol/kg/day PCB), to the actual effect of exposure to the mixture at 20 μmol/kg/day (10 μmol/kg/day PCBs + 10 μmol/kg/day PBDEs), we can determine whether the mixture effects are additive. If the effects are greater than additive (synergistic) then the real values should be statistically greater than the predicted additivity, and if the effects are less than additive, or agonistic, then the real values should be significantly less than the predicted additivity (Borgert et al., 2005).
Statistical analysis of data.
All data were entered into SPSS v 18 for statistical analysis. ANOVA with Dunnett’s test was used to determine statistically significant differences between PCB- and PBDE-dosed and control groups. The Bliss model was used to determine predicted additivity of coexposure to the contaminants at equimolar doses. The Spearman correlation test was used to determine if there was a correlation between the effects of equimolar doses of PCBs and PBDEs on T4 levels in developing male and female offspring. The n reported per analysis is representative of the number of litters. Significance was set at p < 0.05 for all analyses.
RESULTS
Effects of Exposure to Equimolar Doses of PCBs and PBDEs on Circulating T4 Levels
ANOVA revealed that PCB exposure was associated with a reduction in T4 levels compared with the control group at p 7, F = 34.94, p < 0.001, at p 14, F = 44.88, p < 0.001, and at p 21, F = 24.05, p < 0.001. ANOVA revealed that PBDE exposure was also associated with a reduction in T4 levels compared with the control group at p 7 F = 21.43, p < 0.001, at p 14, F = 31.03, p < 0.001, and at p 21, F = 31.54, p < 0.001.
ANOVA with post hoc Dunnett’s correction for multiple group comparisons revealed that exposure to doses of 10, 20, 40, and 60 μmol/kg/day of PCBs reduced T4 levels compared with the control offspring at p 7 (p < 0.05). At p 14, ANOVA with Dunnett’s correction revealed that exposure to 20, 40, and 60 μmol/kg/day doses of PCBs reduced T4 levels compared with controls (p < 0.05) and that 10 μmol/kg/day dose of PCBs produced an almost significant reduction in T4 levels (p = 0.063) compared with the control offspring. At p 21, the 5, 10, 20, 40, and 60 μmol/kg/day doses of PCBs reduced T4 levels compared with the control group (p < 0.05). The maximum reduction in T4 levels associated with PCB exposure was 82% (± 8% SEM) at p 21 after exposure to 60 μmol/kg/day PCBs. With regards to PBDE exposure, ANOVA with Dunnett’s correction revealed that exposure to the 5, 10, 20, 40, and 60 μmol/kg/day doses of PBDEs reduced T4 levels compared with the control offspring at p 7 (p < 0.05). At p 14, the 10, 20, 40, and 60 μmol/kg/day doses of PDBEs also reduced T4 levels compared with control offspring (p < 0.05). At p 21, the 5, 10, 20, 40, and 60 μmol/kg/day doses of PBDEs reduced T4 levels compared with controls (Fig. 1). Similar to PCB exposure, the maximum reduction in T4 levels was seen at p 21, after exposure to 60 μmol/kg/day PBDEs, which produced a ∼75% (± 8% SEM) reduction in T4.
FIG. 1.
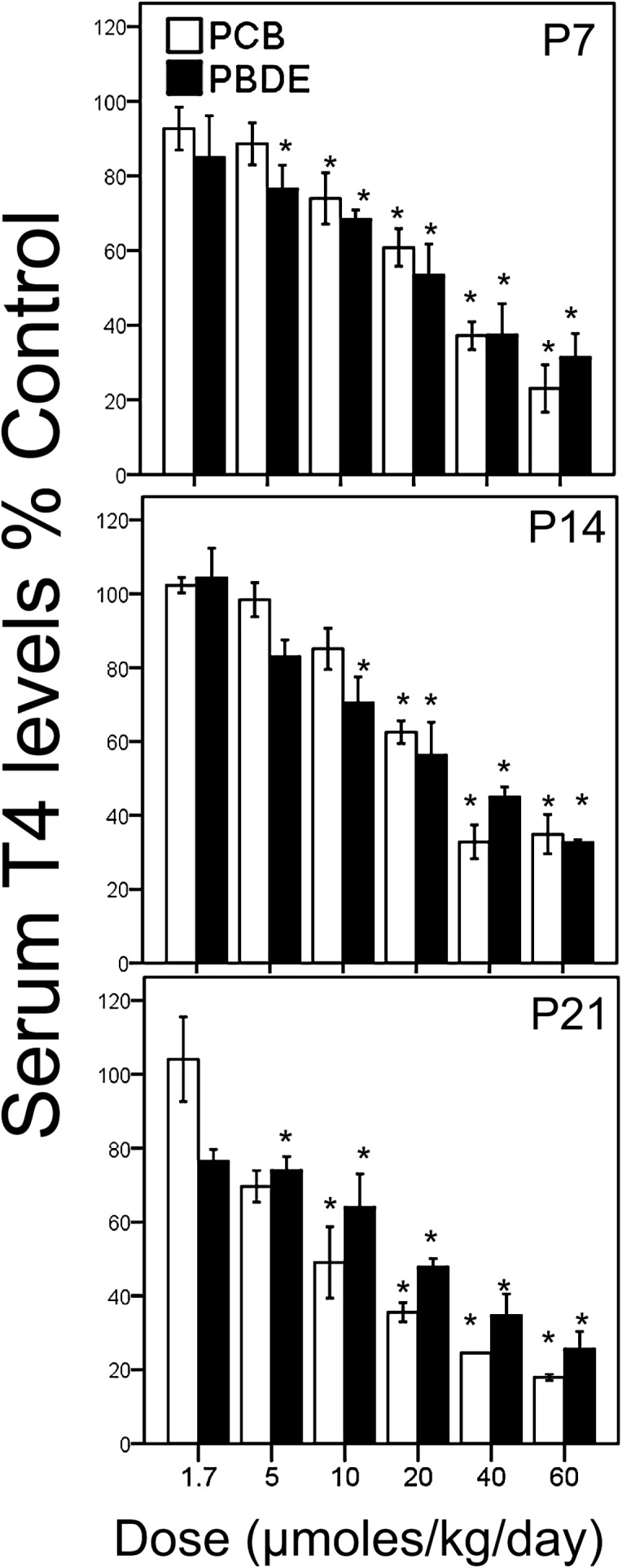
The bar graphs show the serum levels of T4 as a percent of the control levels in male rodent offspring, which were exposed to equimolar doses of PCBs and PBDEs and sacrificed at postnatal days 7, 14, and 21. *p < 0.05 comparing a specific dose of PCBs or PBDEs with the control value at that age. There were no significant differences in T4 levels between the PCB- and PBDE-exposed rodents at the same age or dose. Error bars = ± SEM.
To determine whether the contaminants had similar or different effects on circulating T4 levels, we compared the percent reduction in T4 levels induced by exposure to PCBs or PBDEs at the same dose, using a statistical approach advocated by Nieuwenhuis et al. (2011). ANOVA revealed there were no significant differences in the effects of equimolar doses of PCBs and PBDEs on reducing T4 levels at any dose at p 7, 14, or 21. Furthermore, T4 levels returned to values similar to the controls by p 42 in both PCB- and PBDE-exposed offspring (data not shown).
Effects of Exposure to an Equimolar Mixture of PCBs and PBDEs on Circulating T4 Levels
ANOVA revealed that exposure to a mixture containing equimolar doses of PCBs and PBDEs reduced T4 levels in male offspring compared with control offspring, at p 7 F = 53.77, p < 0.01, at p 14 F = 65.43, p < 0.01, and at p 21 F = 68.08, p < 0.01. ANOVA with Dunnett’s post hoc correction for multiple group comparisons revealed that the 10, 20, 40, 80, and 120 μmol/kg/day doses of the mixture reduced T4 compared with controls at p 7 (p < 0.05). At p 14, ANOVA with Dunnett’s post hoc correction revealed the 10, 20, 40, 80, and 120 μmol/kg/day doses of the mixture also reduced T4 levels compared with controls (p < 0.05). At p 21, ANOVA with Dunnett’s post hoc correction revealed the 3.4, 10, 20, 40, 80, and 120 μmol/kg/day doses of the mixture also reduced T4 levels compared with the controls (p < 0.05).
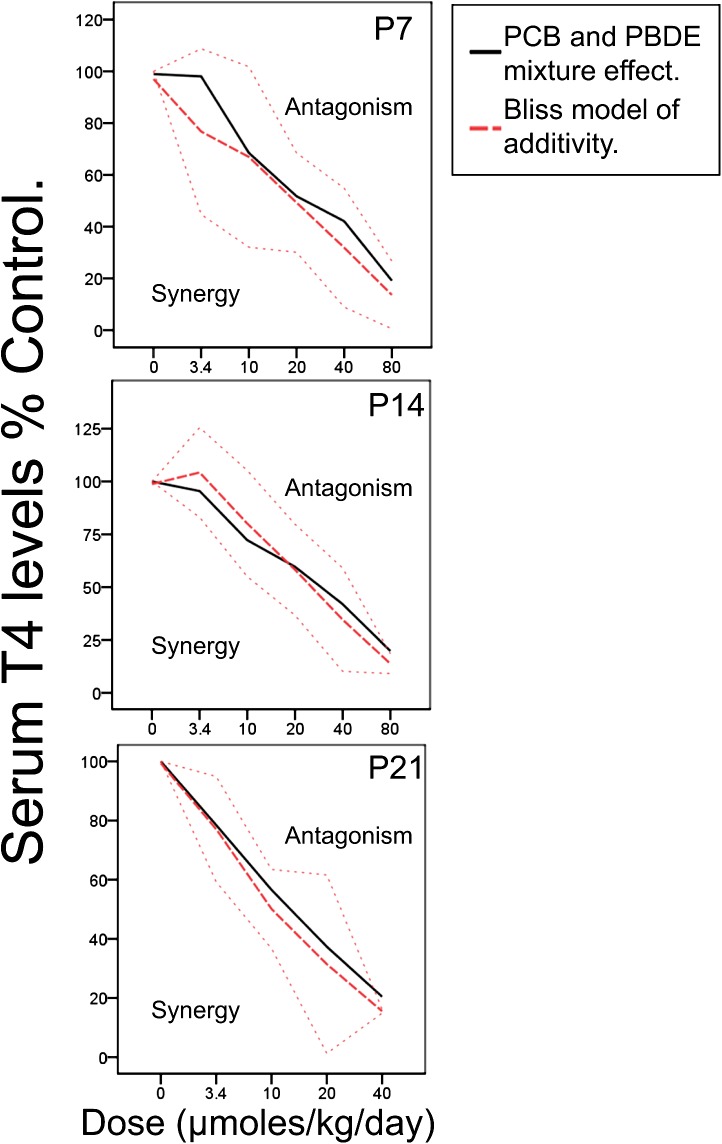
We compared the percent reductions in T4 associated with exposure to the mixture to those predicted using the in silico Bliss model of additivity to determine whether the mixture effects were agonistic, additive, or synergistic and found near identical reductions (Fig. 2). There were no statistical differences between the effects of the mixture and the predicted additive effects on T4 levels using the Bliss model approach. This means that the mixture effects are additive and neither synergistic nor agonistic.
FIG. 2.
The line graphs show T4 levels as a percent of control values in offspring exposed to a mixture containing equimolar doses of PCBs and PBDEs, to yield a total dose of 3.4, 10, 20, 40, or 80 μmol/kg/day at p 7, 14, and 21. The predicted additive effect of the mixture using the Bliss model approach is shown in the red dashed line (color available online). The actual effect of the mixture is shown in the black line. Above and below the red dotted lines are ± 2 SDs from the predicted additive effect. Above the red dotted line is where we would expect to find agonistic interactions between the two contaminants and below the red dotted line is where we would expect to find synergistic interactions between the two contaminants (Borgert et al., 2005). There is no difference between the actual effect of exposure to the mixture and the predicted additive effect generated with the Bliss model.
Effects of Coexposure to PCBs and PBDEs on Circulating T4 Levels With Respect to Sex
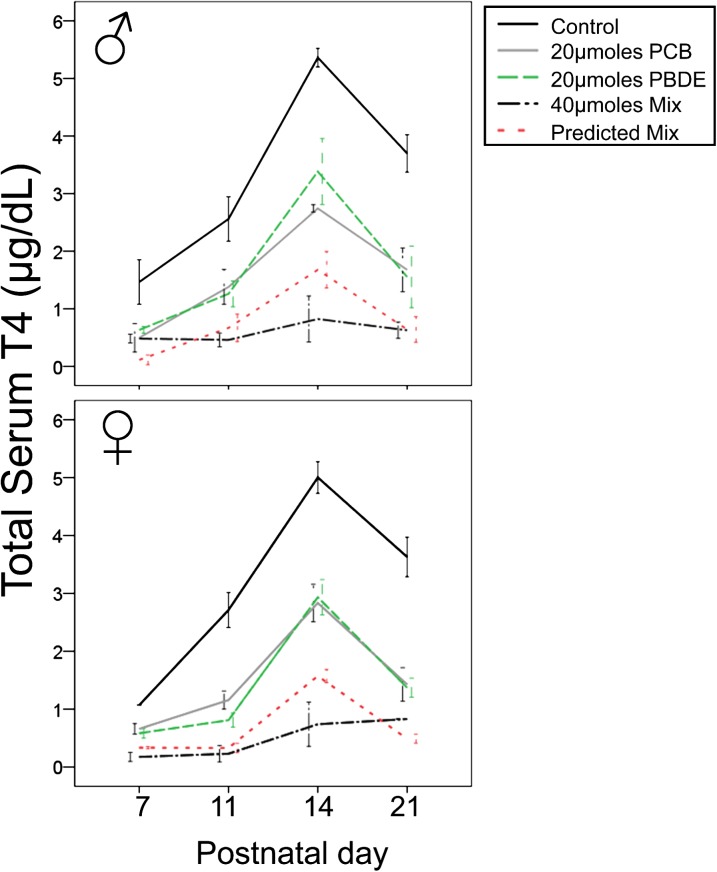
In experiment 2, we determined whether the sex of offspring altered the additive effects of coexposure to a mixture containing equimolar doses of PCBs and PBDEs, using a more refined dosing range (inclusive of p 11). Using the Spearman correlation test, we determined a significant correlation between the effects of 20 μmol/kg/day PCBs and 20 μmol/kg/day PBDEs on T4 levels (p < 0.05) in both male and female offspring across the duration of the experiment, which indicates that PCBs and PBDEs have similar effects on T4 levels in both male and female offspring (Fig. 3). The 40 μmol/kg/day mixture (20 μmol/kg/day PCBs and 20 μmol/kg/day PBDEs) reduced T4 levels significantly more than the individual contaminants at 20 μmol/kg/day (p < 0.05). Moreover, in both male and female offspring, ANOVA revealed no difference between the Bliss model’s predicted additive effects of coexposure to the mixture of 40 μmol/kg/day PCBs and PBDEs and the actual effect of exposure to the mixture.
FIG. 3.
The line graphs show the T4 levels in control-, PCB-, PBDE-, and mixture-exposed male and female offspring from p 7 through p 21. The predicted additivity for coexposure to a mixture containing 20μM/kg/day PCBs and 20μM/kg/day PBDEs is shown by the red dotted line. Error bars = ± SEM.
Body Weight Changes With Respect to PCB and PBDE Exposure
There was a main effect of exposure to PCBs and PBDEs alone and in combination on body weight at ∼p 7, 11, 14, and 21 (p < 0.05). Body weights for male and female offspring from experiment 2 were plotted as a bar graph in Supplementary figure 1. ANOVA with Dunnett’s correction revealed a significant difference between the 20 μmol/kg/day PCB group and controls but not between the 20 μmol/kg/day PBDE groups on body weight.
DISCUSSION
In this study, we firstly present evidence that developmental exposure to equimolar doses of PCBs or PBDEs at doses of 1.7, 5, 10, 20, 40, and 80 μmol/kg/day have similar effects on circulating T4 levels. Secondly, we demonstrate that exposure to a mixture containing equimolar doses of PCBs and PBDEs, 3.4, 10, 20, and 40 μmol/kg/day, has additive effects on circulating T4 levels at p 7, 14, and 21. Moreover, the effects of coexposure to a mixture of the contaminants were similar to the predicted additivity of the contaminants generated with the Bliss model.
PBDEs have a greater molecular weight than PCBs. So, it is reasonable to assume that on a per milligram basis, PCBs would be more effective in reducing circulating T4 levels than PBDEs. For example, on a per milligram basis, PCBs affect protein kinase C signaling in cerebellar granular cells more potently than PBDEs (Kodavanti et al., 2005). However, on a per molar basis, PCBs and PBDEs have similar effects on arachidonic acid release in cerebellar granular cells (Kodavanti and Derr-Yellin, 2002). We deemed it appropriate to compare the efficacy of the contaminants on a per molar basis in order to determine which contaminant may be more effective and whether in combination, they induce agonistic, additive, or synergistic effects. In essence, we found a direct correlation between the reductions in T4 associated with PCB exposure and the reductions associated with PBDE exposure. Secondly, we found that the mixture of the contaminants reduced T4 levels to a greater extent than the individual contaminants did, at the dose contained in the mixture; e.g., 40 μmol/kg/day of the mixture was more potent than the individual contaminants at 20 μmol/kg/day. More importantly, at multiple doses and multiple ages, we found that the reductions in T4 levels induced by exposure to a mixture of the contaminants were nearly identical to the predicted additivity of the contaminants, which confirms the mixture had additive effects on T4 levels.
In general, the T4 reductions induced by exposure to PCB, as well as PBDE exposure, were nonlinear; e.g., exposure to the 40 μmol/kg/day of PCBs was not twice as effective as exposure to 20 μmol/kg/day PCBs (Goldey et al., 1995; Kodavanti et al., 2005; Miller et al., 2010; Sharlin et al., 2006). Correspondingly, we noted that a combined dose of 40 μmol/kg/day PCBs and PBDEs was not twice as effective as the individual contaminants at 20 μmol/kg/day. The Bliss modeling approach accounts for nonlinear dose responses (Bliss, 1939). Using this approach, we found that the reductions in T4 levels induced by the mixture were similar to the predicted additivity of the mixture. Previously, using dopaminergic tissues derived from developing rats, we reported that coexposure to equimolar doses of PCBs and PBDEs had additive effects on the inhibition of dopamine reuptake (Dreiem et al., 2010). This study confirms in a different model system that equimolar doses of PCBs and PBDEs have similar effects alone and have additive effects when used in combination. However, it should be noted that by p 90 the circulating T4 levels in both male and female offspring exposed to PBDEs and PCBs alone, and in combination, returned to control levels.
It is worth noting that PCBs and PBDEs induce reductions in circulating T4 levels because they enhance the excretion of T4 by the liver associated with increased activity of the enzyme uridine diphosphate-glucuronosyltransferase (UGT) (Boas et al., 2006; Brouwer et al., 1998; Costa and Giordano, 2007; Crofton et al., 2005). In addition, studies have also noted that PCBs and PBDEs can alter the efficacy of transthyretin, which is a carrier protein for T4 in the blood, in tandem with blunting the sensitivity of the hypothalamic pituitary axis (Boas et al., 2006; Brouwer et al., 1998). It may be worth determining whether the molecular mechanisms associated with T4 alterations after exposure to PCBs and PBDEs, i.e., UGT expression are similar. We used two experimental approaches to determine additivity: the in vitro Loewe mathematical model, which assumes the two chemicals have similar mechanisms and the in silico Bliss model approach, which assumes the two chemicals use independent mechanisms (Bliss, 1939; Borgert et al., 2005). Both model systems produced similar results. It is known that the Bliss and Loewe models of “effect” and “dose” addition, respectively, will produce similar results when the dose-response curves of the two chemicals are parallel and they use similar mechanisms. Therefore, our data showing that PCBs and PBDEs have additive effects in combination, strongly suggests that PCBs and PBDEs induce reductions in circulating T4 via similar mechanisms.
We determined the effects of coexposure to the contaminants with respect to sex, because the previous studies on PCB and PBDE interactions on T4 levels were conducted in either male or female offspring but not both (Hallgren and Darnerud, 2002). We found that exposure to equimolar doses of either PCBs or PBDEs induced similar T4 reductions in male and female offspring. In addition, exposure to a mixture containing equimolar doses of PCBs and PBDEs induced similar additive reductions in T4 levels in both male and female offspring, i.e., there was no sex bias in the effects of the contaminants. Previously, we and other groups have found sex-specific neurological effects of PCBs, independent of sex-specific changes in circulating T4 levels (Miller et al., 2010). Furthermore, exposure to the A1254 PCB mixture can induce sex-specific effects on brain, but not circulating T4 levels, associated with a 44% reduction in T4 in the forebrain in female but not male mice (Morse et al., 1996). Thus, it will be interesting to determine whether the additive effects of coexposure to PCBs and PBDEs on T4 levels yield similar effects on central T4 levels and sex-specific neurological changes associated with thyroid hormone–sensitive pathways (Costa and Giordano, 2007; Gray et al., 2005; Kodavanti and Derr-Yellin, 2002; Kodavanti et al., 2005, 2010; Miller et al., 2010; Powers et al., 2001; Sagiv et al., 2010; Sharlin et al., 2006).
Polybrominated dibenzofurans (PBDFs) and/or polychlorinated dibenzofurans (PCDFs) are established cocontaminants found in some commercial mixtures of PCBs. Both PBDFs and PCDFs have dioxin-like activity and are powerful environmental toxicants, which arguably may contribute to the hypothyroxinemic effects we ascribe to PCBs in our study. Kostyniak et al. (2005) used the aryl hydrocarbon receptor (AhR) reporter assay to determine the dioxin-like activity of the Fox River mixture of PCBs and found that the dioxin toxic equivalent of the FRM was relatively low and barely above the limit of detection (16–35 pg/mg PCBs). Thus, they concluded that the toxicity of the FRM mix was unlikely to be due to dioxin-like contaminants in the mixture. The low concentration of dioxin-like contaminants in the FRM mix unlikely contributes to the hypothyroxinemic responses we describe in this study. It could be argued that PBDFs and PCDFs also contribute to PBDE-induced hypothyroxinemia. However, a study by Peters et al. (2006) found that although PBDEs do bind to the AhR receptor, they do not activate the AhR-AhR nuclear translocator protein-xenobiotic response element complex. Thus, it is very unlikely that activation of AhR-sensitive dioxin–related pathways contributes to hypothyroxinemia in our PBDE dosing study. Moreover, dioxins do not affect the thyroid hormone system as potently as PCBs do. A study by Schantz et al. (1997) found that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and coplanar (dioxin like) PCBs cause only moderate reductions in thyroid hormones. Thus, although dioxin-like contaminants are potent toxicants, it is more likely that the noncoplanar PCBS and PBDEs induce reductions in thyroxine levels, both alone and in combination. Indeed, the hypothyroxinemic effects of PCBs and PBDEs described in our study are most likely independent of activating AhR and thus would be missed by simply using toxic equivalency factors (TEF) to determine toxicity. We suggest that alternative additional means of determining toxicity, via alternative molecular reporter systems, including steroid hormone receptor–binding assays may improve risk assessment measures in the future. A similar argument has been used by Pessah et al. (2006) to propose the dioxin-independent activation of the ryanodine receptor Ca2+ channel complex type 1 as a method to determine toxicity of noncoplanar PCBs to better inform TEFs.
Epidemiological studies suggest that there is a relationship between serum levels of PCB mixtures and individual congeners and alterations in the thyroid hormone system (Koopman-Esseboom et al., 1994; Osius et al., 1999). PCB levels in fatty tissues in the general population are estimated between 15 and 750 ppb (Arrebola et al., 2011; Fernandez et al., 2008; Stellman et al., 1998; Wang et al., 2010). At a 5 μmol/kg/day dose of PCBs, we found significant reductions in circulating T4 levels at p 21. Using data from a previous study on developmental exposure to PCBs, we estimate brain tissue PCB content in rats exposed to 5 μmol/kg/day at ∼500 ppb, which is within the range found in the general population (Miller et al., 2010). PBDE levels in breast tissues of women from California were recently estimated at ∼80 ppb, and studies have found correlations between sera PBDE levels and measures of thyroid hormone dysfunction (Petreas et al., 2011; Zota et al., 2011). Kodavanti et al. (2010) reported that rats exposed ∼50 μmol/kg/day PBDEs, for a similar duration as our study, resulted in 150 ppb of PBDEs in mammary gland tissues. We can estimate that exposure to 5 μmol/kg/day PBDEs in our study would be associated with ∼15 ppb in mammary gland tissues, which is lower than that reported in the women from California (Petreas et al., 2011). We found that exposure to 5 μmol/kg/day doses of PCBs and PBDEs alone and in combination significantly reduced thyroxine levels in rat offspring. Thus, it is reasonable to suggest that our data set implies that coexposures to low levels of PCBs and PBDEs may have similar additive effects on the thyroid hormone system in humans. Moreover, we suggest that it would be useful if epidemiologists use the additive effects of PCBs and PBDEs we report in a rodent model system, as a rationale to use the Bliss model approach in their analysis of the relationships between environmental contaminants and biological end points such as alterations in the thyroid hormone system.
The health consequences of exposures to mixtures of PCBs and PBDEs remain to be fully determined. We have found that similar doses of PCBs alter white matter composition in developing rodents, and other groups have found impaired spatial behavior, changes in hippocampal and cerebellar signaling pathways in similarly treated rodents (Kodavanti et al., 2010; Kodavanti and Derr-Yellin, 2002; Miller et al., 2010; Schantz et al., 1997). It is likely that coexposure to mixtures of contaminants not only has additive effects on circulating thyroid hormone levels, but also has additive effects in impairing the development of neural networks in animal model systems, and by extension may prove to be an additional health hazard in developing infants. More studies to determine the neural consequences of exposures to mixtures of contaminants are needed to help better determine the health impacts of mixtures of contaminants.
PCBs and PBDEs are members of a larger super family of environmental contaminants known as polyhalogenated aromatic hydrocarbons (PHAHs), which also includes furans and dioxins, and are associated with reductions in circulating thyroid hormones. These data illustrate that PCBs and PBDEs have similar effects on circulating thyroxine levels in vivo and that coexposure to a mixture of the contaminants induces additive reductions in circulating T4 levels in developing male and female rat offspring. This study by extension implies that exposure to low levels of PHAHs in combination likely has additive effects on T4 levels, which is important for risk assessment (Crofton et al., 2005).
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported in part by National Institute of Environmental Health Sciences Grant 1R01ES015688-01 to R.F.S.
Supplementary Material
Acknowledgments
We thank Dr Kevin Crofton of the U.S. Environmental Protection Agency for a PBDE sample originally obtained from the Great Lakes Chemical Company (West Lafayette, IN; lot number 755O0K20A). We thank Tom Zoeller (University of Massachusetts, Amherst) for his advice and also assistance validating our T4 ELISA kits with his T4 radioimmunoassay. We also acknowledge assistance from the Biochemistry Core at the Wadsworth Center. The authors declare that there are no conflicts of interest.
References
- Arrebola JP, Cuellar M, Claure E, Quevedo M, Antelo SR, Mutch E, Ramirez E, Fernandez MF, Olea N, Mercado LA. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and adipose tissue from Bolivia. Environ. Res. 2011;112:40–47. doi: 10.1016/j.envres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Bergonzi R, Specchia C, Dinolfo M, Tomasi C, De PG, Frusca T, Apostoli P. Distribution of persistent organochlorine pollutants in maternal and foetal tissues: Data from an Italian polluted urban area. Chemosphere. 2009;76:747–754. doi: 10.1016/j.chemosphere.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D, Yu L, Fu J. Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ. Pollut. 2006;144:1024–1030. doi: 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Bliss CI. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939;26:585–615. [Google Scholar]
- Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eur. J. Endocrinol. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- Borgert CJ, Borgert SA, Findley KC. Synergism, antagonism, or additivity of dietary supplements: Application of theory to case studies. Thromb. Res. 2005;117:123–132. doi: 10.1016/j.thromres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Gerlienke SA, Murk J, Klasson-Wehler E, Bergman A, Visser TJ. Interactions of persistent environmental organohalogens with the thyroid hormone system: Mechanisms and possible consequences for animal and human health. Toxicol. Ind. Health. 1998;14:59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Craft ES, Hedge JM, Gennings C, Simmons JE, Carchman RA, Carter WH, Jr, DeVito MJ. Thyroid-hormone-disrupting chemicals: Evidence for dose-dependent additivity or synergism. Environ. Health Perspect. 2005;113:1549–1554. doi: 10.1289/ehp.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Okoniewski RJ, Brosch KO, Miller VM, Seegal RF. Polychlorinated biphenyls and polybrominated diphenyl ethers alter striatal dopamine neurochemistry in synaptosomes from developing rats in an additive manner. Toxicol. Sci. 2010;118:150–159. doi: 10.1093/toxsci/kfq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Fischer C, Fredriksson A. Polybrominated diphenyl ethers, a group of brominated flame retardants, can interact with polychlorinated biphenyls in enhancing developmental neurobehavioral defects. Toxicol. Sci. 2006;94:302–309. doi: 10.1093/toxsci/kfl109. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Kiviranta H, Molina-Molina JM, Laine O, Lopez-Espinosa MJ, Vartiainen T, Olea N. Polychlorinated biphenyls (PCBs) and hydroxy-PCBs in adipose tissue of women in Southeast Spain. Chemosphere. 2008;71:1196–1205. doi: 10.1016/j.chemosphere.2007.09.064. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Gray KA, Klebanoff MA, Brock JW, Zhou H, Darden R, Needham L, Longnecker MP. In utero exposure to background levels of polychlorinated biphenyls and cognitive functioning among school-age children. Am. J. Epidemiol. 2005;162:17–26. doi: 10.1093/aje/kwi158. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav. Brain Res. 1998;94:213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am. J. Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, Rayner JL, Kannan K, Birnbaum LS. Developmental exposure to a commercial PBDE mixture, DE-71: Neurobehavioral, hormonal, and reproductive effects. Toxicol. Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Derr-Yellin EC. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H] arachidonic acid release in rat cerebellar granule neurons. Neurotoxicology. 2002;68:451–457. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol. Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, Morse DC, Weisglas-Kuperus N, Lutkeschipholt IJ, van der Paauw CG, Tuinstra LG, Brouwer A, Sauer PJ. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr. Res. 1994;36:468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJ, Seegal RF, Pessah IN, et al. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Kahnke T, Neu N, Sanchez-Morrissey SR, Brosch K, Kelsey K, Seegal RF. Developmental PCB exposure induces hypothyroxinemia and sex-specific effects on cerebellum glial protein levels in rats. Int. J. Dev. Neurosci. 2010;28:553–560. doi: 10.1016/j.ijdevneu.2010.07.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicol. Appl. Pharmacol. 1996;136:269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: A problem of significance. Nat. Neurosci. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Osius N, Karmaus W, Kruse H, Witten J. Exposure to polychlorinated biphenyls and levels of thyroid hormones in children. Environ. Health Perspect. 1999;107:843–849. doi: 10.1289/ehp.99107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem. Res. Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Peters AK, Nijmeijer S, Gradin K, Backlund M, Bergman A, Poellinger L, Denison MS, Van den Berg M. Interactions of polybrominated diphenyl ethers with the aryl hydrocarbon receptor pathway. Toxicol. Sci. 2006;92:133–142. doi: 10.1093/toxsci/kfj186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreas M, Nelson D, Brown FR, Goldberg D, Hurley S, Reynolds P. High concentrations of polybrominated diphenylethers (PBDEs) in breast adipose tissue of California women. Environ. Int. 2011;37:190–197. doi: 10.1016/j.envint.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers B, Poon E, Sable H, Schantz S. Developmental exposure to PCBs, MeHg, or both: Long-term effects on auditory function. Environ. Health Perspect. 2001;117:1101–1107. doi: 10.1289/ehp.0800428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Seo B, Moshtaghian J, Amin S. Developmental exposure to polychlorinated biphenyls or dioxin: Do changes in thyroid function mediate effects on spatial learning? Amer. Zool. 1997;37:399–408. [Google Scholar]
- Sharlin DS, Bansal R, Zoeller RT. Polychlorinated biphenyls exert selective effects on cellular composition of white matter in a manner inconsistent with thyroid hormone insufficiency. Endocrinology. 2006;147:846–858. doi: 10.1210/en.2005-0778. [DOI] [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, White A, Kemeny M, Busch E, Nafziger AN. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiol. Biomark. Prev. 1998;7:489–496. [PubMed] [Google Scholar]
- Stewart P, Fitzgerald S, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, Hauser P. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ. Health Perspect. 2003;111:1670–1677. doi: 10.1289/ehp.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol. Teratol. 2005;27:771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Knutsen HK, Liane VH, Froshaug M, Kvalem HE, Haugen M, Meltzer HM, Alexander J, Becher G. Consumption of fish from a contaminated lake strongly affects the concentrations of polybrominated diphenyl ethers and hexabromocyclododecane in serum. Mol. Nutr. Food Res. 2008;52:228–237. doi: 10.1002/mnfr.200700123. [DOI] [PubMed] [Google Scholar]
- Wang N, Kong D, Cai D, Shi L, Cao Y, Pang G, Yu R. Levels of polychlorinated biphenyls in human adipose tissue samples from southeast China. Environ. Sci. Technol. 2010;44:4334–4340. doi: 10.1021/es9038775. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ. Sci. Technol. 2011;45:7896–7905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.