Abstract
Human arsenic exposure is associated with increased risk of skin cancer, and arsenite greatly enhances ultraviolet (UV)-induced skin tumors in a mouse model of carcinogenesis. Inhibition of DNA repair is one proposed mechanism for the observed cocarcinogenicity. We have previously demonstrated that low concentrations of arsenite inhibit poly(ADP-ribose) polymerase (PARP)-1, thus interfering with DNA repair process triggered by UV radiation. Because overactivation of PARP-1 often leads to apoptotic cell death, and unrepaired DNA lesions promote genomic instability and carcinogenesis, we hypothesized that inhibition of PARP-1 by arsenic may promote the survival of potentially “initiated carcinogenic cells,” i.e., cells with unrepaired DNA lesions. In the present study, we tested this hypothesis on UV-challenged HaCat cells. Cells were pretreated with 2μM arsenite for 24 h before UV exposure. Outcome parameters included apoptotic death rate, PARP-1 activation, apoptotic molecules, and retention of DNA lesions. UV exposure induced PARP-1 activation and associated poly(ADP-ribose) production, apoptosis-inducing factor release, cytochrome C release, and caspases activation, which led to apoptotic death in HaCat cells. Pretreatment with 2μM arsenite significantly inhibited UV-induced cell death as well as the associated molecular events. Notably, knockdown of PARP-1 with small interfering RNA completely abolished the antagonism of arsenite. Furthermore, arsenite pretreatment led to long-term retention of UV-induced cyclobutane pyrimidine dimers. Together, these results suggest that low concentration of arsenite reduces UV-induced apoptosis via inhibiting PARP-1, thus promoting the survival of cells with unrepaired DNA lesions, which may be an important mechanism underlying arsenic cocarcinogenic action.
Keywords: arsenic, PARP-1, PARG, DNA damage repair, carcinogenesis
Arsenic is a naturally occurring toxic metalloid found in water, soil, and air. Epidemiological studies have revealed a strong association between arsenic exposure and several human cancers including skin, lung, urinary bladder, kidney, and liver (Kitchin and Conolly, 2010). Multiple mechanisms by which relatively high concentrations of arsenic induce carcinogenesis have been proposed, including induction of DNA damage by reactive oxygen species, endocrine disruption, activation of cell signaling pathways leading to alterations in cell cycle kinetics and proliferative response and epigenetic alterations (Durham and Snow, 2006; International Agency for Research on Cancer, 2004; Kitchin and Conolly, 2010; Kligerman and Tennant, 2007; Shi et al., 2004; Witkiewicz-Kucharczyk and Bal, 2006). In contrast to high concentration of arsenite, relatively low concentration of arsenic is considered a cocarcinogen to potentiate the genotoxicity of other mutagen-carcinogens, including ultraviolet (UV) radiation (Ding et al., 2008, 2009; Maier et al., 2002; Rossman et al., 2004). In mouse models of skin cancer, low concentration of arsenite greatly enhances the incidence and progression of skin malignancies induced by UV radiation (Burns et al., 2004; Rossman et al., 2001, 2004), and increased lung cancer incidence has been reported in areas with low to moderate concentrations of arsenic (< 100 μg/l) (Heck et al., 2009). However, mechanisms underlying arsenic cocarcinogenic action are not entirely understood.
Arsenic inhibition of DNA repair is one proposed mechanism of carcinogenicity (Durham and Snow, 2006; Kligerman and Tennant, 2007; Witkiewicz-Kucharczyk and Bal, 2006). In the process of DNA repair, poly(ADP-ribose) polymerase (PARP)-1 functions as a ‘‘nick sensor’’ and directly participates in the repair of strand breaks and oxidative DNA damage. Recent studies by our lab and others have demonstrated that arsenic can readily bind to the DNA-binding zinc finger motifs of PARP-1 to effectively inhibit PARP-1 activation at low concentrations ranging from 0.01 to 2μM, thereby exacerbating DNA damage induced by UV radiation (Ding et al., 2008, 2009; Hartwig et al., 2003; Qin et al., 2008b; Zhou et al., 2011). Similar inhibitory effects of low concentration of arsenic on PARP-1 have also been reported by other groups (Hartwig et al., 2003; Kitchin and Conolly, 2010; Witkiewicz-Kucharczyk and Bal, 2006). Besides acting as a DNA-repairing enzyme, PARP-1 is also an important modulator of cell death in response to cell injury. Activated PARP-1 transfers ADP-ribosyl moieties from nicotinamide adenine dinucleotide (NAD+) to itself and other proteins, and its excessive activation depletes cellular reserves of NAD+ and its precursor adenosine triphosphate (ATP), leading to cell death via energy failure (Hassa, 2009; Rouleau et al., 2010). The dual role of PARP-1 may result in a procarcinogenesis scenario when the skin is concurrently exposed to the two very common environmental toxins, i.e., UV radiation and arsenite. On one hand, UV radiation results in significant DNA damage and subsequent excessive PARP-1 activation, thus leading to cell death. However, the presence of low concentrations of arsenite could potentially act as an antagonist on UV-induced cell death through inhibiting the overactivation of PARP-1. On the other hand, the inhibition of PARP-1 by arsenite would inevitably result in the retention of unrepaired DNA damage in the injured cells. As a consequence, unrepaired DNA lesions may promote genome instability and mutagenesis, leading to carcinogenesis (Kryston et al., 2011). In this context, promoting the survival of cells with unrepaired DNA lesions by arsenic may account for an important mechanism underlying its cocarcinogenic action.
In this study, we tested this important possibility using the immortalized human keratinocyte cell line HaCat cells. Our results demonstrated that exposure to low concentrations of arsenite inhibited UV-induced apoptosis, leading to the prolonged survival (2 weeks) of cells harboring cyclobutane pyrimidine dimers (CPDs) DNA damage. Furthermore, our results showed that arsenite-induced prosurvival effect was attributed to its inhibition on PARP-1, leading to subsequent inhibition of the activation of caspase cascades and poly(ADP-ribose) (PAR) polymer-apoptosis-inducing factor (AIF) pathways. These results may provide a novel mechanism underlying the cocarcinogenic action of arsenic.
MATERIALS AND METHODS
Cell culture and treatments.
The human keratinocyte cell line (HaCat) was generously provided by Dr Mitch Denning (Loyola University Medical Center, Maywood, IL). HaCat cells were cultured in Dulbecco’s modified Eagle’s medium F-12 HAM (DMEM/F12), supplemented with 10% fetal bovine serum (FBS), fourfold final concentration of MEM amino acids, 2mM l-glutamine, and antibiotics (penicillin 100 U/ml and streptomycin 100 μg/ml) (Ding et al., 2009). The cells were cultured at 37°C in a 95% air/5% CO2 humidified incubator. Sodium arsenite solution was sterilized by passing through a 0.22-μm syringe filter and diluted with serum-free DMEM/F12 medium. For all experiments involving arsenite incubation, HaCat cells (60–70% confluence) were rinsed with PBS and placed into serum-free DMEM/F12 medium containing 2μM arsenite for 24 h. For UV exposure, the medium was removed and cells were rinsed three times with PBS then covered with a thin layer of PBS (0.5 ml per well of six-well plate) and placed on ice. Cells were exposed to 5 J/cm2 solar-simulated light using an Oriel 1600 W Watt Solar Ultraviolet Simulator (Oriel Corp., Stratford, CT). This solar simulator produces a high-intensity UV beam in both the UVA (320–400 nm) and UVB (280–320 nm) spectrum. Following UV exposure, the PBS was removed and replaced with cell growth medium, and cells were incubated for indicated times prior to further analysis.
Apoptosis assay.
Apoptosis was assayed using the TACS Annexin V kit (Trevigen, Gaithersburg, MD) 24 h after UV exposure following the protocol supplied by the vendor. Briefly, cell pellets were washed with cold (4°C) PBS, then gently resuspended in the Annexin V incubation reagent at a density of 105–106 cells per 100 μl, and incubated in the dark for 15 min at room temperature. After the incubation, 400 μl of binding buffer from the kit was added to each sample, and then apoptosis were analyzed by flow cytometry.
Detection of cellular NAD+.
Cellular NAD+ was detected using an NAD+/nicotinamide adenine dinucleotide (NADH) quantification kit (BioVision, Mountain View, CA) using the procedures provided by the vendor. Briefly, 2 × 105 cells were washed with cold PBS and pelleted by centrifugation at 2000 × g for 5 min. After the addition of 400 μl of NAD+/NADH extraction buffer, cell pellets were subjected to two freeze/thaw cycles (20 min on dry ice, then 10 min at room temperature). The lysed cells were vortexed for 10 s and centrifuged at 14,000 × g for 5 min, and the resultant supernatants (50 μl) were quantified based on an NAD+ standard curve. The concentration of NAD+ was expressed as ng/mg protein.
Poly(ADP-ribose) glycohydrolase activity assay.
Poly(ADP-ribose) glycohydrolase (PARG) activity was measured by Trevigen's HT Colorimetric PARG Assay Kit (Trevigen). The cells were harvested by trypsinization and centrifugation. After washing in cold PBS, the cells were suspended with 5–10 pellet volumes of cold cell extraction buffer. The cell suspension was incubated on ice, with periodic vortexing, for 30 min. Then the suspension was centrifuged at 10,000 × g for 10 min at 4°C, and the cleared cell lysate was collected for assessing PARG activity following manufacturer’s procedure. Briefly, 25 μl of diluted PARP enzyme (0.008 U/μl) was added to each well of histone-coated strip followed by the addition of 25 μl of PARP cocktail. The strip was incubated at room temperature for 60 min. After washing the wells four times with PBS (containing 0.1% Triton X-100), 50 μl of the following agents: The cell lysates or diluted PARG standards, diluted Strep-HRP80, and TACS-Sapphire colorimetric substrate were sequentially added to each well and incubated at room temperature for 60 min, with an exception for the last agent which was incubated for 10 min in the dark. Before the addition of the next agents, wells were washing four times with PBS. The absorbance was measured at 450 nm. The activity of PARG was calculated by the standard curves.
PARP-1 silencing.
Small interfering RNA (siRNA) transfection was performed as we described previously (Ding et al., 2009; Qin et al., 2008b). Briefly, SMARTpool siRNA specific for human PARP-1 (GenBank accession number: NM001618) was obtained from Dharmacon Research, Inc. (Lafayette, CO). SiGLO RISC-free siRNA (Dharmacon) was used as a control siRNA. HaCaT cells were seeded at a density of 2 × 105 cells per well in six-well plates the day before transfection in DMEM/F12 containing 10% FBS without antibiotics. Transfection of siRNAs was conducted using DharmaFECT transfection reagent (Dharmacon). DharmaFECT reagent was diluted 1:50 in serum-free DMEM/F12 and incubated at room temperature for 5 min. In parallel, 2μM siRNA in 1 × siRNA buffer (Dharmacon) was diluted 1:1 in serum-free DMEM/F12. The two mixtures were combined and incubated for 20 min at room temperature prior to adding to cells. The final siRNA concentration was 100nM. After 24 h, the medium was replaced with complete growth medium. After an additional 48 h, cells were treated with arsenite and/or UV exposure, as indicated in the figure legends. Specific silencing was confirmed by Western blot analysis.
Preparation of cell extracts and Western blot analysis.
Nuclear and cytosolic extracts were prepared using the TransFactor Extraction Kit (Clontech, Mountain View, CA), and mitochondria were isolated using the Mitochondria Isolation Kit (Pierce, Rockford, IL) following the protocol provided by the vendor. Protein concentrations were determined by the Coomassie Plus Protein assay (Pierce). Cell lysate (30 μg of protein) was resolved on an 8–10% SDS-polyacrylamide gel and transferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA) and incubated for 1 h in tris-buffered saline with Tween (TBST) (10mM Tris, pH 8.0, 150mM NaCl, and 0.1% Tween 20) containing 5% nonfat milk at room temperature. The membrane was then incubated overnight at 4°C with the following primary antibodies at indicated dilutions. PARP-1 (1:1000), AIF (1:1000), and Cytochrome C (Cyto C) (1:2000) were obtained from Santa Cruz Biotechnology. Caspase-3 (1:1000), caspase-7 (1:1000), and caspase-9 (1:1000) were obtained from Cell Signaling. After washing with TBST, the membrane was then incubated for 1 h with horseradish peroxidase–conjugated secondary antibody (all from Santa Cruz Biotechnology with a dilution of 1:2000), and signal was detected using the SuperSignal West Pico Chemiluminescence kit (Pierce) on a Kodak Image Station 4000MM. To control for sample loading and protein transfer, the membrane was stripped and reprobed to detect β-actin (1:500) for cytosolic extracts, histone deacetylase 1 (HDAC1) (1:500) for nuclear extracts, or cytochrome c oxidase (COX) IV (1:2000) for mitochondrial extracts. All these three antibodies were from Santa Cruz Biotechnology. The intensities were quantified by KODAK Molecular Imaging Software version 4.0.
Measurement of CPDs by ELISA.
After UV exposure, cells were cultured in complete growth medium for 14 days, then cellular DNA was isolated using the QIAamp Blood Kit (QIAGEN Inc., Valencia, CA). The concentrations of DNA were measured by a Beckman DU 800 spectrophotometer (Beckman Instruments, Fullerton, CA). ELISA was used to measure CPDs as we described previously (Ding et al., 2008). Briefly, Falcon polyvinylchloride flat-bottom 96-well assay plates (Becton Dickinson Labware, Franklin Lakes, NJ) precoated with 0.003% protamine sulfate (Sigma, St Louis, MO) were incubated with 15 ng purified genomic DNA in PBS at 37°C for 20 h. TDM-2 (Medical & Biological Laboratories Co., Woburn, MA) was used as the antibody for CPDs detection. Subsequently, after incubation with biotinylated F(ab′)2 goat anti-mouse IgG fragments and streptavidin-peroxidase (Zymed, San Francisco, CA), the optical density from o-phenylenediamine at 492 nm was measured using a Spectra Max 340 (Molecular Devices, Sunnyvale, CA).
Data analysis.
Data are presented as means ± SD. Statistical analysis was performed using ANOVA. A value of p < 0.05 was considered statistically significant.
RESULTS
Low Concentration of Arsenite Attenuates UV-Induced Apoptosis
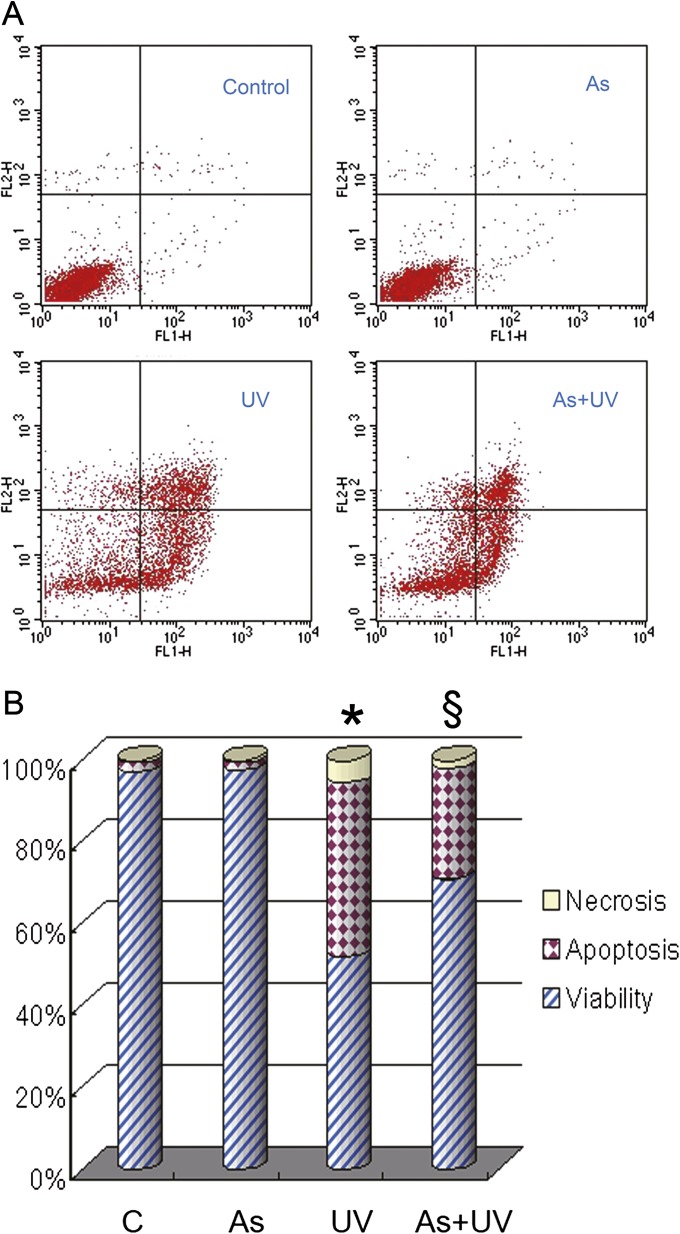
Our recent data have shown that low concentrations of arsenite effectively inhibit PARP-1-mediated DNA repair in UV-challenged HaCat cells (Ding et al., 2008, 2009; Qin et al., 2008b). Besides serving as a protective enzyme to detect and repair DNA damage, PARP-1 also contributes to cell death through overconsumption of NAD+ (depleting ATP pools) when it is excessively activated in response to a variety of cellular stresses such as UV radiation (Molho-Pessach and Lotem, 2007). In this context, we speculated that the presence of low concentration of arsenite might make HaCat cells more resistant to UV-induced death. To test this possibility, we pretreated HaCat cells with 2μM arsenite for 24 h prior to UV exposure. Cell survival and death were analyzed by flow cytometry at 12 h after UV exposure (Figs. 1A and B). As expected, control (no exposure to UV or arsenite) or arsenite alone-treated cells displayed minimal apoptotic or necrotic changes. UV exposure induced a robust apoptotic changes in HaCat cells, as reflected by a 42% apoptotic rate. Meanwhile, UV exposure also induced necrotic changes in cells but in a much smaller portion (6%) compared with apoptotic changes. Notably, pretreatment with arsenite resulted in a significant reductions in both apoptotic and necrotic cell death, with rates of 26 and 2%, respectively (Fig. 1). These results demonstrate that low concentration of arsenite indeed inhibits UV-induced apoptotic cell death.
FIG. 1.
Effect of arsenite on UV-induced apoptosis. HaCat cells were incubated with 2μM arsenite for 24 h before UV exposure. Twelve hours after UV radiation, cell apoptosis was measured by flow cytometry using the TACS Annexin V kit (A). Percentages of cell apoptosis, necrosis, and viability were calculated (B). *p < 0.05 versus control and §p < 0.05 versus UV.
Arsenite Inhibits the Mitochondria-Dependent Apoptotic Pathway
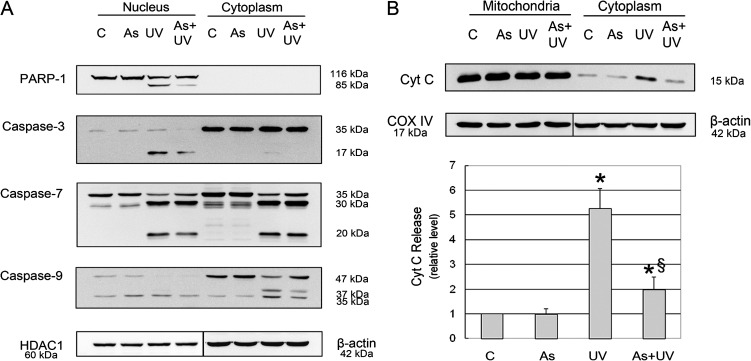
Although excessive PARP-1 activation is generally considered to induce necrotic cell death, our results showed that apoptosis was the major type of cell death in UV-treated cells. Recent reports suggest that PARP-1 can be quickly inactivated by activated caspases, particularly caspase-3 and -7 (Wang et al., 2009), which cleave PARP-1 into 86 and 24 kDa fragments, thus converting otherwise necrotic cell death into caspase-mediated apoptosis (Chaitanya et al., 2010; Hassa, 2009). To demonstrate whether this was the case in UV-induced apoptosis (Fig. 1), we assessed PARP-1 cleavage, caspases activation, and Cyto C release. Because PARP-1 is a nuclear protein and caspases are activated in the cytoplasm, we analyzed their changes in both nuclear and cytoplasmic fractions. As shown in Figure 2, PARP-1 cleavage was observed in the nuclear fraction of UV-treated cells. Concurrently with this change was the activation of mitochondria-dependent caspase activation pathway (Scorrano, 2009), reflected by mitochondrial release of Cyto C, caspase-9, -3, and -7 activation after UV radiation (Fig. 2). Importantly, arsenite pretreatment showed significant antagonism on these molecular changes. These results indicate that arsenite may protect HaCat cells against UV-induced apoptosis through inhibiting the mitochondria-dependent caspase pathway.
FIG. 2.
Effect of arsenite on UV-induced cleavages of PARP-1, caspase-3, -7, and -9 and release of Cyto C. HaCat cells were incubated with 2μM arsenite for 24 h before UV exposure. Twelve hours after UV radiation, nuclear and cytoplasmic cell extracts were prepared. Protein levels of cleaved caspase-3, -7, and -9 were determined by Western blot (A). Six hours after UV radiation, mitochondrial and cytoplasmic extracts were prepared. The release of Cyto C from mitochondria to the cytoplasm was detected by Western blot (B). The band intensities were quantified by KODAK Molecular Imaging Software version 4.0. Data represent the mean ± SD of three independent experiments. *p < 0.05 versus control and §p < 0.05 versus UV.
Arsenite Attenuates UV-Induced AIF Release by Inhibiting PARP-1 Activity
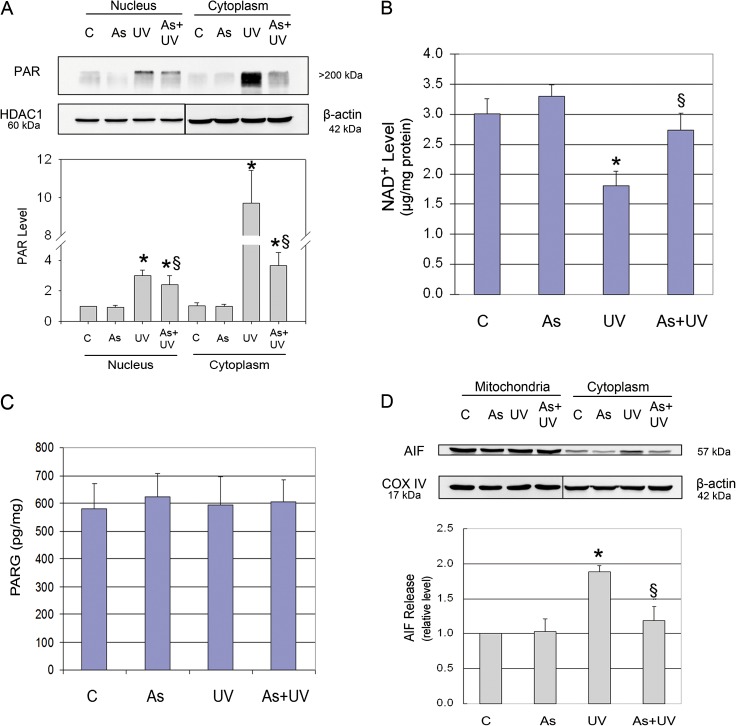
Besides the caspase cascade, AIF is another important downstream effector in PARP-1-mediated cell death (Cregan et al., 2004), and its release is stimulated by PAR, the product of PARP-1 activation (Andrabi et al., 2006; Wang et al., 2009). Therefore, we assessed the impact of UV radiation and arsenite on PAR and AIF. Western blot analysis revealed that control HaCat cells exhibited low basal level of PAR polymer production in both cytosolic and nuclear fractions, which was significantly increased by UV radiation, with approximately 3-fold and 10-fold increases in the cytosolic and nuclear fractions, respectively (Fig. 3A). Because activated PARP-1 consumes NAD+ to produce PAR, we measured intracellular NAD+ levels and found that UV radiation significantly decreased in NAD+ content in HaCat cells (Fig. 3B). PAR polymer can be degraded by the constitutively active PARG, and the level of PAR polymer is dictated by both PARP-1 and PARG activities (Gagne et al., 2006). To rule out the possibility that the observed PAR polymer changes induced by UV or arsenite were due to their respective influence on PARG, we measured the enzymatic activity of PARG in HaCat cells after identical treatments as above. As shown in Figure 3C, UV radiation did not induce significant changes in PARG activity, indicating that PARG did not likely account for the observed PAR polymer changes. To determine whether UV-induced PAR formation led to AIF release from the mitochondria, we detected its level in the mitochondria and cytosol with Western blot analysis. Indeed, we found that AIF was significantly increased in the cytosol of UV-treated cells (Fig. 3D).
FIG. 3.
Effect of arsenite on UV-induced PAR production. HaCat cells were incubated with 2μM arsenite for 24 h before UV exposure. Six hours after UV radiation, the PAR levels in nuclear and cytoplasmic cell extracts and the release of AIF from mitochondria to the cytoplasm were measured by Western blot. The band intensities were quantified by KODAK Molecular Imaging Software version 4.0 (A and D). The NAD+ level was measured using an NAD+/NADH quantification kit (B). PARG activity was measured by Trevigen's HT Colorimetric PARG Assay Kit (C). Data represent the mean ± SD of three independent experiments. *p < 0.05 versus control and §p < 0.05 versus UV.
Next, we investigated the effect of arsenite on these changes. We found that pretreatment with 2μM arsenite significantly attenuated PAR polymer production in both subcellular fractions in UV-treated cells (Fig. 3A). Along with its inhibition on UV-induced PAR formation, arsenite pretreatment also blocked AIF release in UV-treated cells (Fig. 3D). These results indicate that, besides its inhibition on caspase cascade (Fig. 2), arsenite treatment also inhibits the activation of PAR-AIF signal pathway in UV-treated cells.
PARP-1 Is the Target Protein for Arsenite Attenuation of UV-Induced Apoptosis
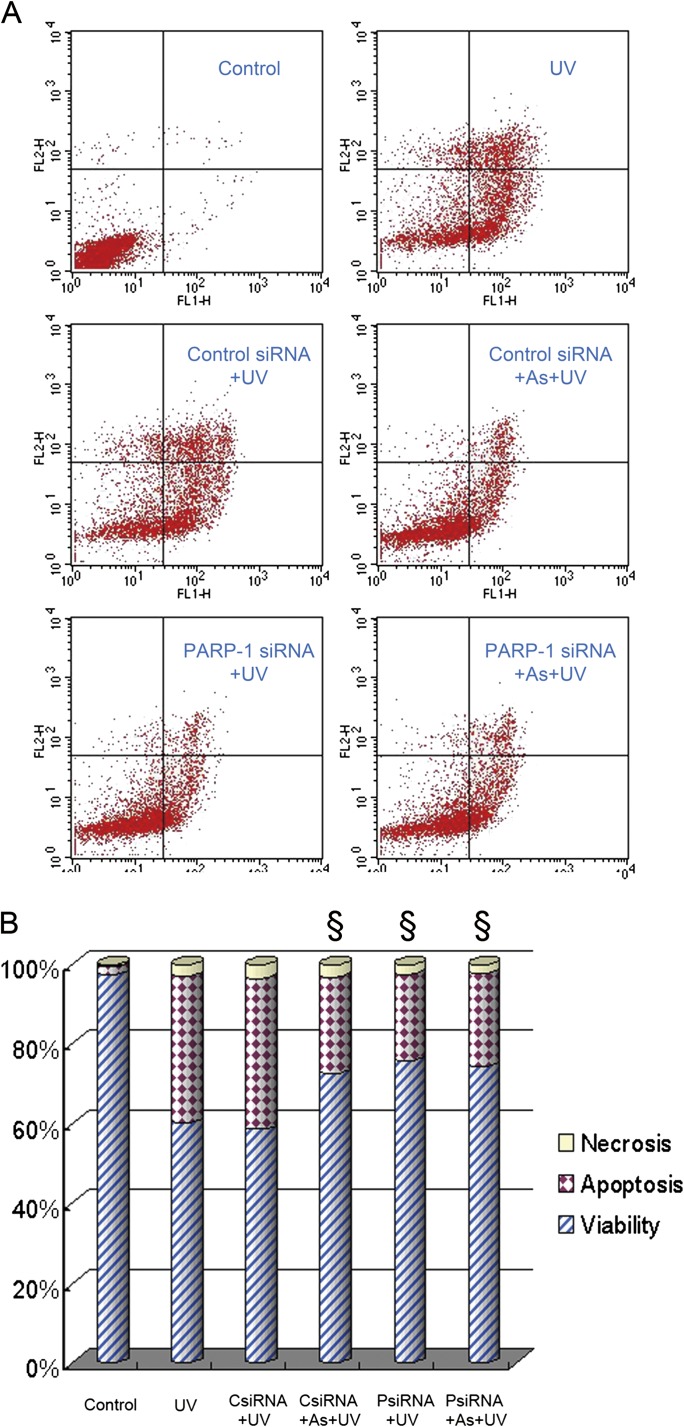
To further establish a causal link between PARP-1 activation and UV-induced apoptosis and to determine whether arsenite protected HaCat cells against apoptotic death through inhibiting PARP-1, we applied siRNA approach to knockdown PARP-1 expression. Consistent with our previous study (Qin et al., 2008a), PARP-1 siRNA effectively (∼90%) knocked down PARP-1 protein expression at 48 h after transfection (Data not shown). As expected, control siRNA had no effect on UV-induced apoptotic death in HaCat cells (Fig. 4). Most importantly, knockdown of PARP-1 with siRNA significantly reduced UV-induced apoptosis, and this protection was comparable to that of arsenite. In addition, when PARP-1 was knockdown by siRNA, pretreatment with 2μM arsenite could not further decrease UV-induced apoptosis. These results indicate that PARP-1 activation contributes to UV-induced apoptosis in HaCat cells, and arsenite indeed acts on PARP-1 to exert its antiapoptotic action.
FIG. 4.
Effect of PARP-1 knockdown on UV-induced apoptosis. HaCat cells were transfected with control or PARP-1 siRNA. Seventy-two hours after transfection, cells were incubated with 2μM arsenite for 24 h before UV exposure. Twelve hours after UV radiation, cell apoptosis was measured by flow cytometry using the TACS Annexin V kit (A). Percentages of cell apoptosis, necrosis, and viability were calculated (B). §p < 0.05 versus UV.
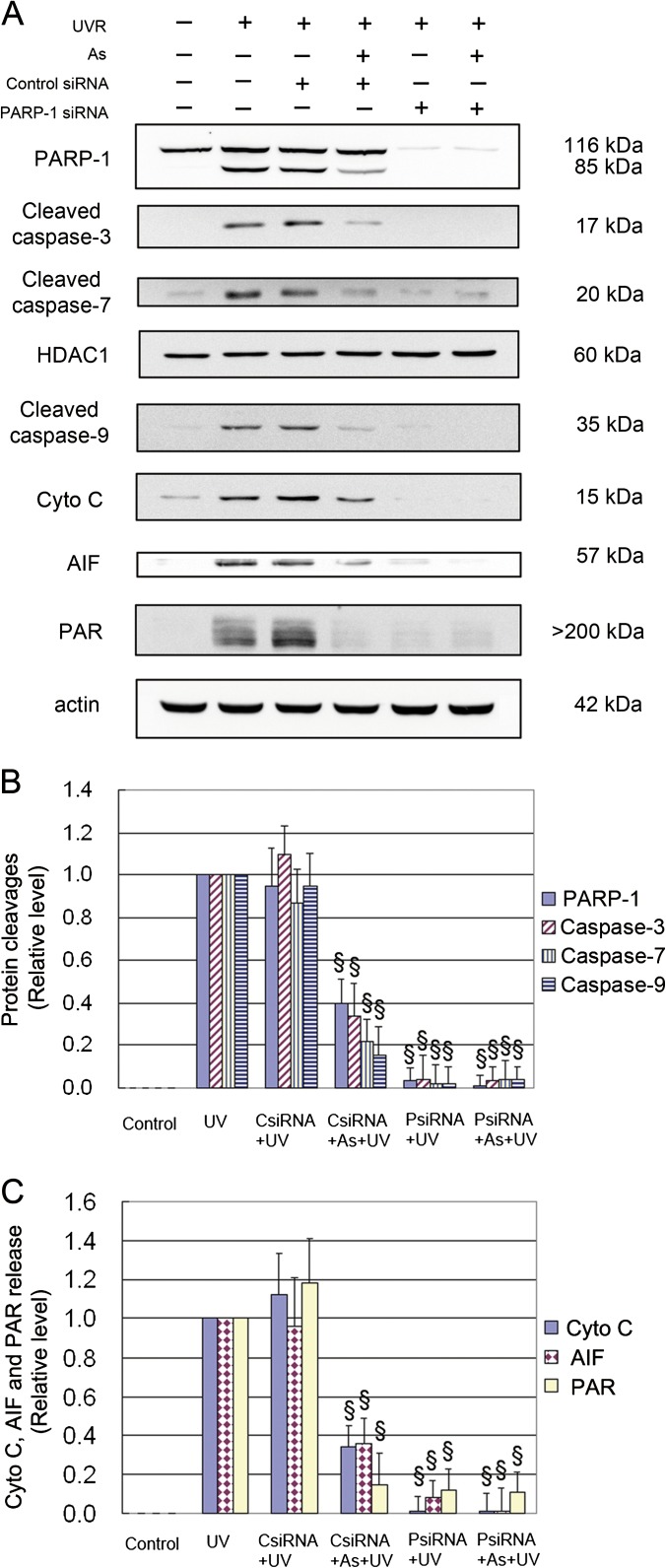
To further determine whether the inhibition of UV-induced activation of caspase and PAR-AIF pathways by arsenite was indeed secondary to its inhibition on PARP-1, we measured the changes of these molecules after knockdown of PARP-1. As shown in Figure 5, knockdown of PARP-1 with siRNA resulted in substantial reductions in UV-induced cleavage of caspase-3, -7, and -9, the release of Cyto C, PAR production, and AIF release, and arsenite pretreatment did not cause any further significant changes on these proteins, indicating that PARP-1 is the predominant target protein.
FIG. 5.
Effect of PARP-1 knockdown on UV-induced PARP-1, caspase cleavages, releases of Cyto C, and AIF and PAR formation. HaCat cells were transfected with control or PARP-1 siRNA. Seventy-two hours after transfection, cells were incubated with 2μM arsenite for 24 h before UV exposure. Cells were collected 6 h after UV radiation for assessment of PAR production and Cyto C and AIF release in cytosolic fraction. Cells were collected 12 h after UV radiation for detection of PARP-1 and caspase cleavages in nuclear extract by Western blot (A). The band intensities were quantified by KODAK Molecular Imaging Software version 4.0 (B and C). Data represent the mean ± SD of three independent experiments. §p < 0.05 versus UV.
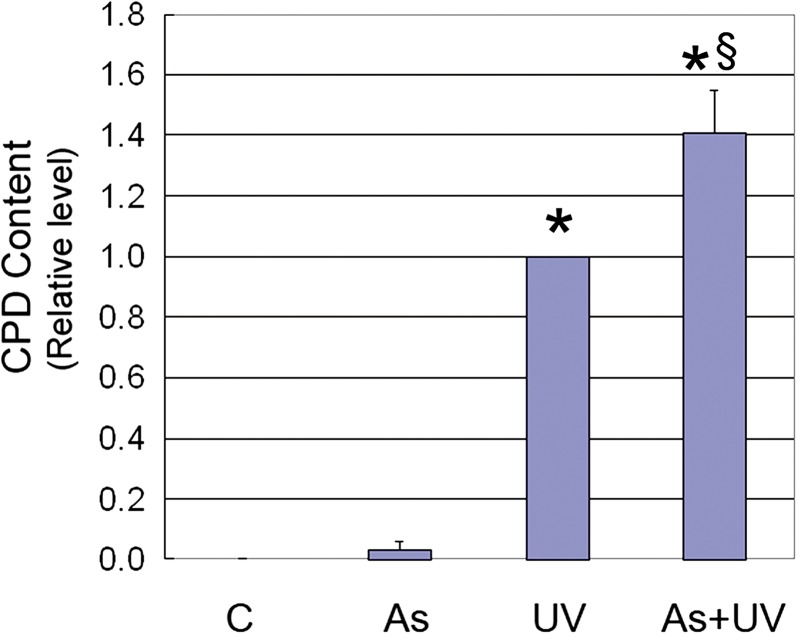
Arsenite Increases Persistent UV-Induced DNA Damages in Survived HaCat Cells
Our data thus far demonstrated that 2μM arsenite could effectively attenuate UV-induced apoptosis through inhibiting PARP-1. However, because PARP-1 also performs a critical role in detecting and repairing DNA damage, the cells spared from UV-induced death by arsenite treatment would likely bear unrepaired DNA lesions induced by UV radiation. To test this possibility, we chose to measure CPDs, a major UV-induced mutagenic lesion, in the survived HaCat cells at 14 days post-UV exposure. As shown in Figure 6, no or little CPDs were observable in control or arsenite-exposed cells, whereas UV exposure caused a dramatic increase in CPDs. Most significantly, cells that had been pretreated with arsenite before UV exposure were found to have 40% greater CPDs than UV alone, and these cells represented the population of cells that had survived the UV exposure but retained the DNA damage. The persistence of CPDs at 14 days post-UV exposure suggests that inhibition of PARP-1 by arsenite, and the resulting resistance to apoptosis, may turn a cell death event into the survival of cells with mutagenic DNA lesions.
FIG. 6.
Effect of arsenite on persistent UV-induced CPDs. Cells were incubated with 2μM arsenite for 24 h before UV exposure. After UV radiation, cells were cultured for 14 days before detection of CPDs by ELISA. Data represent the mean ± SD of three independent experiments. *p < 0.05 versus control and §p < 0.05 versus UV.
DISCUSSION
Human exposure to high levels of arsenic is associated with increased skin cancer risk (Agency for Toxic Substances and Disease Registry, 2007; Yu et al., 2006a). Generally speaking, rat and mouse models of arsenic carcinogenesis have a lower degree of responsiveness (than the human) and require large exterior doses of arsenic. Arsenic concentrations that do not cause skin tumors in mice enhance UV-induced skin carcinogenesis (Burns et al., 2004; Rossman et al., 2001). These findings, in conjunction with evidence that concentrations of arsenic that do not cause detectable DNA damage significantly amplify the DNA damaging actions of other carcinogens such as UV (Ding et al., 2008, 2009; Maier et al., 2002; Pi et al., 2005; Rossman et al., 2004; Wu et al., 2005), have supported the concept of arsenic as a cocarcinogen.
A growing body of evidence indicates that low concentrations of arsenic inhibit DNA repair processes (Beyersmann and Hartwig, 2008; Durham and Snow, 2006; Hamadeh et al., 2002; Huang et al., 2004; Kligerman and Tennant, 2007; Witkiewicz-Kucharczyk and Bal, 2006), and this inhibitory action on DNA repair has been proposed as one of the mechanisms for arsenic cocarcinogenicity. However, the underlying mechanisms are not well understood. Our earlier work has shown that low concentrations of arsenite effectively inhibit PARP-1-mediated DNA repair in UV-challenged skin cells (Ding et al., 2008, 2009; Qin et al., 2008b). Besides acting as a DNA repair enzyme, PARP-1 is also a key modulator of cell death in response to various stress stimuli (Gagne et al., 2006; Hassa, 2009). In the setting of UV radiation, inhibition of PARP-1 with a low concentration of arsenite may promote cell survival from lethal UV radiation, but the rescued cells very likely contain unrepaired mutagenic DNA lesions, thus enhancing UV-induced carcinogenesis. In the present study, we tested this novel cocarcinogenic hypothesis. Our results demonstrate that pretreatment with arsenite not only renders HaCat cells significantly more resistant to UV-induced apoptotic death but also helps the cells retain greater amount of DNA damage (CPDs) than UV exposure alone. We found that inhibition of PARP-1 by arsenite is the central event mediating these changes. Furthermore, the activation of caspase-independent cascade (PAR-AIF pathway) and mitochondria-dependent caspase cascade (including caspase-9, -3, and -7) was found to mediate UV-induced apoptosis in HaCat cells.
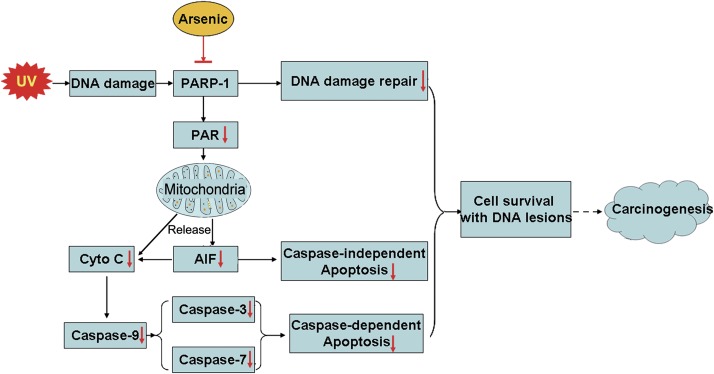
UV radiation causes various DNA damage and subsequent activation of DNA repair processes in skin cells. The balance between DNA damage and its repair dictates the fate of UV-exposed cells (Fig. 7). If all DNA lesions are properly repaired, cells will be fully recovered from UV radiation. On the other end, if DNA damage is too severe to be repaired, the injured cells will be destined to die. Between these two extremes, there is an outcome that injured cells survive from UV radiation, but with unrepaired or not properly repaired DNA lesions. These surviving cells are potentially carcinogenic because unrepaired DNA lesions can act as “substrates” for the production of mutations to promote genome instability and carcinogenesis (Kryston et al., 2011; Lange et al., 2011). In UV-triggered DNA repair, PARP-1 activation is considered the initial step, in which activated PARP-1 senses the damage and initiates the complicated DNA repair process (Woodhouse and Dianov, 2008). In addition, PARP-1 also plays an important role in apoptotic cell death in response to various stress stimuli including UV radiation (Rouleau et al., 2010). Our previous studies have shown that low concentrations of arsenite effectively inhibit PARP-1 through interfering with the zinc figure domains (Ding et al., 2009; Zhou et al., 2011). These findings suggest, as illustrated in Figure 7, that arsenite may promote the survival of UV-exposed cells (by inhibiting PARP-1-mediated apoptosis), but the surviving cells would likely bear greater number of unrepaired DNA lesions (due to inhibition of PARP-1-mediated DNA repair). Our data that PARP-1 knockdown with siRNA significantly reduces apoptotic death in UV-exposed HaCat cells (Fig. 4) confirm the important role of PARP-1 in this process.
FIG. 7.
Scheme depicting the mode of action for the cocarcinogenicity of arsenite: promoting the survival of cells with unrepaired DNA lesion following UV radiation through inhibiting PARP-1.
We further explored how PARP-1 mediated UV-induced apoptosis in HaCat cells. It is well known that activated PARP-1 consumes NAD+ to produce PAR. Several studies have shown that increased PAR levels are associated with increased cell death (Andrabi et al., 2006; Heeres and Hergenrother, 2007; Schreiber et al., 2006; Siegel and McCullough, 2010; Yu et al., 2006b), in which PAR triggers mitochondrial release of AIF and Cyto C to lead to apoptosis in both caspase-dependent and independent manners (Cregan et al., 2004; Yu et al., 2006b). In good agreement with these studies, we found that UV radiation led to increased PAR production and mitochondrial release of AIF and Cyto C in HaCat cells (Figs. 2 and 3). Moreover, we observed that pretreatment of HaCat cells with arsenite significantly inhibited UV-induced apoptosis as well as the activation of the apoptosis-inducing molecules tested above. Notably, when PARP-1 was knocked down with siRNA, arsenite pretreatment could not further decrease the apoptotic response of HaCat cells to UV radiation. These results clearly indicate that arsenite acts on PARP-1 to exert its action for promoting the survival of UV-exposed cells.
In the setting of UV radiation, arsenite promotion of the survival of UV-exposed cells can be problematic because inhibition of PARP-1 by arsenite can lead to the retention of unrepaired DNA lesions in surviving cells. In this study, we tested this possibility by detecting CPDs. CPD is a major cause of mutations identified in ras oncogenes, p53, and PTCH tumor suppressor genes that are implicated in nonmelanoma skin cancers (de Gruijl and Rebel, 2008). Our data demonstrate that arsenite exposure significantly increases the level of persistent UV-induced CPDs (at 14 days) when compared with UV alone. Unrepaired DNA lesions that promote genome instability constitute an important mechanism of carcinogenesis (Kryston et al., 2011; Ziech et al., 2011). In this context, the cells surviving from UV-induced apoptosis due to “protective antagonism” by arsenite could potentially be the carcinogenic cells that would eventually lead to later mutagenesis and tumorigenesis at later stage. This notion is corroborated by the literature reports that UV radiation-induced skin carcinogenesis was greatly increased in mice receiving arsenite in drinking water, with a nearly fivefold increase in tumor number/mouse (Rossman et al., 2001, 2004).
FUNDING
This work was supported in part by Department of Health and Human Services grants from the U.S. National Institutes of Health (R01 ES012938, R01 ES015826, and R01 ES15826-03S1) and used the Flow Cytometry Shared Resource Center supported by the University of New Mexico Cancer Center (P30 CA118100).
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2007. [PubMed] [Google Scholar]
- Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D, Hartwig A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008;82:493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: A dose-response study. Environ. Health Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR, Rebel H. Early events in UV carcinogenesis–DNA damage, target cells and mutant p53 foci. Photochem. Photobiol. 2008;84:382–387. doi: 10.1111/j.1751-1097.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Ding W, Hudson LG, Sun X, Feng C, Liu KJ. As(III) inhibits ultraviolet radiation-induced cyclobutane pyrimidine dimer repair via generation of nitric oxide in human keratinocytes. Free Radic. Biol. Med. 2008;45:1065–1072. doi: 10.1016/j.freeradbiomed.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J. Biol. Chem. 2009;284:6809–6817. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham TR, Snow ET. Metal ions and carcinogenesis. EXS. 2006;96:97–130. doi: 10.1007/3-7643-7378-4_5. [DOI] [PubMed] [Google Scholar]
- Gagne JP, Hendzel MJ, Droit A, Poirier GG. The expanding role of poly(ADP-ribose) metabolism: Current challenges and new perspectives. Curr. Opin. Cell Biol. 2006;18:145–151. doi: 10.1016/j.ceb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Hamadeh HK, Trouba KJ, Amin RP, Afshari CA, Germolec D. Coordination of altered DNA repair and damage pathways in arsenite-exposed keratinocytes. Toxicol. Sci. 2002;69:306–316. doi: 10.1093/toxsci/69.2.306. [DOI] [PubMed] [Google Scholar]
- Hartwig A, Blessing H, Schwerdtle T, Walter I. Modulation of DNA repair processes by arsenic and selenium compounds. Toxicology. 2003;193:161–169. doi: 10.1016/j.tox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hassa PO. The molecular “Jekyll and Hyde” duality of PARP1 in cell death and cell survival. Front. Biosci. 2009;14:72–111. doi: 10.2741/3232. [DOI] [PubMed] [Google Scholar]
- Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, Duell EJ. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ. Health Perspect. 2009;117:1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeres JT, Hergenrother PJ. Poly(ADP-ribose) makes a date with death. Curr. Opin. Chem. Biol. 2007;11:644–653. doi: 10.1016/j.cbpa.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Huang C, Ke Q, Costa M, Shi X. Molecular mechanisms of arsenic carcinogenesis. Mol. Cell. Biochem. 2004;255:57–66. doi: 10.1023/b:mcbi.0000007261.04684.78. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Arsenic in drinking-water. IARC Monogr. Eval. Carcinog. Risks Hum. 2004;84:39–267. [Google Scholar]
- Kitchin KT, Conolly R. Arsenic-induced carcinogenesis–oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem. Res. Toxicol. 2010;23:327–335. doi: 10.1021/tx900343d. [DOI] [PubMed] [Google Scholar]
- Kligerman AD, Tennant AH. Insights into the carcinogenic mode of action of arsenic. Toxicol. Appl. Pharmacol. 2007;222:281–288. doi: 10.1016/j.taap.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Lange SS, Takata K, Wood RD. DNA polymerases and cancer. Nat. Rev. Cancer. 2011;11:96–110. doi: 10.1038/nrc2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Schumann BL, Chang X, Talaska G, Puga A. Arsenic co-exposure potentiates benzo[a]pyrene genotoxicity. Mutat. Res. 2002;517:101–111. doi: 10.1016/s1383-5718(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Molho-Pessach V, Lotem M. Ultraviolet radiation and cutaneous carcinogenesis. Curr. Probl. Dermatol. 2007;35:14–27. doi: 10.1159/000106407. [DOI] [PubMed] [Google Scholar]
- Pi J, He Y, Bortner C, Huang J, Liu J, Zhou T, Qu W, North SL, Kasprzak KS, Diwan BA, et al. Low level, long-term inorganic arsenite exposure causes generalized resistance to apoptosis in cultured human keratinocytes: Potential role in skin co-carcinogenesis. Int. J. Cancer. 2005;116:20–26. doi: 10.1002/ijc.20990. [DOI] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. Dual actions involved in arsenite-induced oxidative DNA damage. Chem. Res. Toxicol. 2008a;21:1806–1813. doi: 10.1021/tx8001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Timmins GS, Liu KJ. Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity. Toxicol. Appl. Pharmacol. 2008b;232:41–50. doi: 10.1016/j.taap.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol. Appl. Pharmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: An animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Scorrano L. Opening the doors to cytochrome c: Changes in mitochondrial shape and apoptosis. Int. J. Biochem. Cell Biol. 2009;41:1875–1883. doi: 10.1016/j.biocel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic. Biol. Med. 2004;37:582–593. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Siegel C, McCullough LD. NAD+ depletion or PAR polymer formation: Which plays the role of executioner in ischaemic cell death? Acta Physiol. (Oxf.) 2010;203:225–234. doi: 10.1111/j.1748-1716.2010.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatos. Exp. Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewicz-Kucharczyk A, Bal W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol. Lett. 2006;162:29–42. doi: 10.1016/j.toxlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: An international molecule of mystery. DNA Repair (Amst.) 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Wu F, Burns FJ, Zhang R, Uddin AN, Rossman TG. Arsenite-induced alterations of DNA photodamage repair and apoptosis after solar-simulation UVR in mouse keratinocytes in vitro. Environ. Health Perspect. 2005;113:983–986. doi: 10.1289/ehp.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J. Biomed. Sci. 2006a;13:657–666. doi: 10.1007/s11373-006-9092-8. [DOI] [PubMed] [Google Scholar]
- Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. U.S.A. 2006b;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J. Biol. Chem. 2011;286:22855–22863. doi: 10.1074/jbc.M111.232926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)–induced genetic and epigenetic alterations in human carcinogenesis. Mutat. Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]